Abstract
BACKGROUND
New approaches to ablation of atrial fibrillation (AF) include focal impulse and rotor modulation (FIRM). Studies of this technology with short-term follow-up have shown favorable outcomes.
OBJECTIVE
The purpose of this study was to characterize the long-term results of FIRM ablation in a cohort of patients treated at 2 academic medical centers.
METHODS
All FIRM-guided ablation procedures (n = 43) at UCLA Medical Center and Virginia Commonwealth University Medical Center performed between January 2012 and October 2013 were included for analysis. During AF, FIRM software constructed phase maps from unipolar atrial electrograms to identify putative AF sources. These sites were targeted for ablation, along with pulmonary vein isolation in 77% of patients.
RESULTS
AF was paroxysmal in 56%, and 67% had prior AF ablation. All patients had rotors identified (mean 2.6 ± 1.2 per patient, 77% in LA). Prespecified acute procedural end-point was achieved in 47% of patients (n = 20): AF termination in 4, organization in 7, >10% slowing of AF cycle length in 9. Acute complications occurred in 4 patients (9.3%). At 18 ± 7 months of follow-up, 37% were free from documented recurrent AF after a 3-month blanking period; 21% were free from documented atrial tachyarrhythmias and off antiarrhythmic drugs. Multivariate analysis did not reveal any significant predictors of AF recurrence, including pattern of AF, acute procedural success, or prior failed ablation.
CONCLUSION
Long-term clinical results after FIRM ablation in this cohort of patients showed poor efficacy, different from previously published studies. Randomized studies are needed to evaluate the efficacy and clinical utility of this ablation approach for treating AF.
Keywords: Arrhythmia, Atrial fibrillation, Clinical electrophysiology, Catheter ablation
Introduction
Atrial fibrillation (AF) is a common arrhythmia resulting in significant symptoms in many patients and is associated with increased risk of stroke and mortality. For relief of AF symptoms, catheter ablation has been shown to be more effective than antiarrhythmic drug therapy.1 The cornerstone of AF ablation is electrical isolation of the pulmonary veins (PVs) from the left atrium (LA) at the ostial or antral level, sometimes with the addition of lesions targeting arrhythmogenic atrial substrate or triggers outside the PVs. Currently, the optimal lesion set for catheter ablation of AF is unknown. Long-term clinical outcomes, especially for persistent AF, have been disappointing, considering the expense and risk of LA ablation procedures.2,3 Recent studies have raised doubts about the benefits of empiric atrial substrate ablation beyond pulmonary vein isolation (PVI), even for treatment of persistent AF in which such lesion sets were previously thought to be necessary.4
To improve these results, one approach is identification and targeting of patient-specific localized sources of AF. Various methods of mapping during AF have been developed, targeting regions of maximum dominant frequency,5 high Shannon entropy,6 and focal or rotational sources from body surface mapping.7 One such method is focal impulse and rotor modulation (FIRM), a proprietary computational spatiotemporal phase mapping algorithm that identifies potential rotors. This approach is now commercially available as an AF mapping product (Rhythm View, Topera Medical, Abbott, Abbott Park, IL). In a previous publication, we reported quantitative analysis of FIRM-identified rotor sites and acute results of catheter ablation at those sites.8 Although previously published clinical outcomes of FIRM ablation have been favorable, independent results with long-term follow-up are lacking.9,10 In this study, we sought to describe long-term clinical outcomes of FIRM ablation at 2 academic medical centers.
Methods
Patient population
The institutional review boards at the University of California, Los Angeles, and Virginia Commonwealth University Medical Center approved retrospective review of these data. Consecutive patients undergoing FIRM-guided ablation for paroxysmal or persistent AF at these institutions between January 2012 and October 2013 were included in the study. Patients included underwent FIRM-guided ablation alone (FIRM-only) or FIRM-guided ablation followed by PVI (FIRM+PVI). Previous publications included data from 1 of these patients,11 11 of these patients,10 and 23 of these patients,8 although none reported long-term outcomes as in this study.
Electrophysiologic study and catheter ablation
Antiarrhythmic medications were discontinued in paroxysmal AF patients for at least 5 half-lives (60 days for amiodarone) before the ablation procedure but were left to operator discretion in persistent AF patients. All patients took oral anticoagulants for at least 30 days before ablation. Warfarin was uninterrupted warfarin, and dabigatran, rivaroxaban, or apixaban was held for 24 hours preoperatively. Transesophageal echocardiography was performed in all cases to rule out left atrial (LA) thrombus immediately before the procedure. Catheters were advanced from the femoral and jugular veins to the His-bundle position, coronary sinus, and via double transseptal access to the LA. The 64-pole basket catheter (Constellation, Boston Scientific, Natick, MA) was advanced through a long sheath (8.5Fr SL0 or SL1, Daig Medical, Minnetonka, MN) to map activation during AF in both atria, with optimal electrode contact and maximal coverage of the entire chamber, using an electrode from a quadripolar catheter situated in the superior vena cava as a unipolar reference.
Intracardiac echocardiography was used to guide transseptal puncture and catheter position in the LA. Electroanatomic mapping was performed using the EnSite Velocity system (NavX, St. Jude Medical, Minneapolis, MN). Heparin was infused before deployment of the basket catheter to achieve activated clotting time >300 seconds. Unipolar electrograms were recorded for analysis as described in previous publications.9
If patients were in sinus rhythm at the beginning of the procedure, atrial burst pacing was performed, beginning at cycle length of 500 ms down to 180 ms until AF was induced. If AF was not sustained for at least 5 minutes, isoproterenol was infused and pacing was repeated as necessary to induce sustained AF.
FIRM mapping was performed as described elsewhere, and the phase maps obtained with this system can be viewed in prior publications.11–13 Representatives from Topera Medical were present for all cases and helped determine the presence and location of rotor sites. FIRM sources in both the atria were targeted using an irrigated radiofrequency (RF) ablation catheter (ThermoCool, Biosense-Webster, Diamond Bar, CA) at 25 to 35 W, maximum temperature 42°C. Tissue adjacent to the identified rotor region was targeted for ablation, using fluoroscopy and electroanatomic mapping. Energy was applied until loss of local electrogram and drop in impedance at the site (20–40 seconds per lesion). All electrically active tissue within a 1- to 2-cm radius of the rotor core was ablated until AF terminated, organized to another tachycardia, or the entire rotor region had been ablated (5–10 minutes of RF). Whenever AF organized into atrial flutter or tachycardia, this was treated with conventional mapping and ablation. If AF terminated to sinus rhythm either spontaneously or during ablation, it was reinduced with pacing and another FIRM map was created. If AF persisted, each additional rotor site was similarly ablated until no additional rotor sites could be detected. Cardioversion was performed for patients remaining in AF after ablation of all FIRM-identified rotor sites. After FIRM ablation was completed, most patients underwent PVI with the aid of a circular mapping catheter (Optima, St. Jude Medical, St. Paul, MN; or Lasso, Biosense-Webster) to verify loss of LA–PV conduction. No patients underwent additional substrate ablation, such as roof or mitral isthmus lines, and non-PV sources were not routinely targeted, given the length of the procedure. Application of RF energy directly on the esophagus was avoided, and ablation was stopped for any esophageal temperature rise >1°C.
Acute procedural end-points
For patients initially in AF, the primary procedural end-point was termination, organization, or slowing of AF (>10% increase in cycle length) during the procedure. For patients initially in sinus rhythm, noninducibility after ablation was also required because these patients frequently showed spontaneous termination even before ablation. Procedural safety end-point included adverse events within 30 days of the procedure.
Long-term follow-up
Patients were seen in the outpatient clinic at 1, 3, and 6 months, and then every 6 months postprocedure. ECG was performed at each visit and for any symptoms suggesting arrhythmia. Although not mandated by protocol, 7-day ambulatory rhythm monitoring at 3, 6, and 12 months was encouraged to detect asymptomatic recurrence. Antiarrhythmic drugs were discontinued at 3 months postprocedure for asymptomatic patients. Oral anticoagulation was discontinued after 3 months for those with CHA2DS2Vasc score of zero but was continued for the other patients. AF recurrence was defined as any office ECG showing AF or any episode of AF lasting >30 seconds detected on ambulatory rhythm monitoring after the 90-day blanking period. Atrial tachyarrhythmia was defined as >30 seconds of atrial flutter or atrial tachycardia at least 170 bpm.
Statistical analysis
Frequencies and proportions were used to describe categorical variables, whereas means and standard deviations were used to describe continuous variables. Associations between categorical variables were assessed using χ2 tests of independence and Fisher exact tests. Independent-samples t tests were used to test for differences among continuous measures between patients grouped by long-term clinical success (successful vs unsuccessful). Kaplan–Meier estimates of the time to freedom from recurrent AF were graphed. Effects of type of AF, prior ablation, and FIRM vs FIRM+PVI on time to freedom from AF were estimated using Cox proportional hazards regression models. Two-tailed P < .05 was considered significant. STATA statistical analysis software was used for all analyses. Power analysis was not performed because this descriptive study included all available FIRM ablation cases at the 2 participating medical centers.
Results
Patient characteristics
During the study period, 43 FIRM-guided ablation procedures for AF were performed and were included in the analysis (Table 1). Most patients were male (74%), had paroxysmal AF (56%), and had previously failed AF ablation (67%). Mean duration of AF before the index procedure was 7.3 ± 5.4 years, and CHA2DS2VaSC score was 1.7 ± 1.1. Normal LA size was seen in 28%, mild or moderate LA enlargement in 47%, and severe LA enlargement in 26%. At the time of ablation, 31 patients (72%) were on antiarrhythmic drugs, which were held before the procedure according to protocol.
Table 1.
Baseline characteristics
| All patients (n = 43) | Clinical success (n = 16) | AF recurrence (n = 27) | P value | |
|---|---|---|---|---|
| Age | 61 ± 11 | 62 ± 11 | 61 ± 12 | .79 |
| Male gender (%) | 32 (74) | 13 (81) | 19 (70) | .43 |
| Paroxysmal pattern of AF (%) | 24 (56) | 7 (44) | 17 (63) | .22 |
| AF duration (years from diagnosis) | 7.3 ± 5.4 | 7.9 ± 5.6 | 6.9 ± 5.3 | .56 |
| Prior AF ablation (%) | 29 (67) | 11 (69) | 18 (67) | .89 |
| No. previous AF ablations | 1.2 ± 1.0 | 1.3 ± 1.0 | 1.1 ± 1.1 | .55 |
| No. failed antiarrhythmic drugs | 1.6 ± 0.8 | 1.7 ± 0.9 | 1.6 ± 0.7 | .69 |
| CHA2DS2-Vasc score | 1.7 ± 1.1 | 1.5 ± 1.1 | 1.9 ± 1.2 | .28 |
| Left ventricular ejection fraction (%) | 59 ± 6.4 | 59 ± 5.0 | 59 ± 7.1 | 1 |
Values are given as no. (%) or mean ± SD.
AF = atrial fibrillation.
Acute procedural results
At the beginning of the procedure, 51% of patients (n = 22) were in normal sinus rhythm and required rapid pacing for AF induction; 10 of these patients (45%) also required isoproterenol. Rotors or focal sources were seen in all patients, with a mean of 2.0 ± 1.0 in the LA (range 0–5) and 0.6 ± 0.8 in the right atrium (range 0–2). Patients with persistent AF had significantly more right atrial rotors than did paroxysmal AF patients (1.0 vs 0.3, P <.01), whereas the number of LA rotors was similar (2.1 vs 1.8, P = .78). PVI was not performed in 10 of 43 of patients, either because PVs were already completely isolated from prior ablation (n = 9) or the patient refused PVI (n = 1). At least 1 PV reconnection was identified in the majority of patients who had undergone prior PVI (20/29 [69%]), and these veins were reisolated. Average procedure length was 314 ± 82 minutes, with mean 55 ± 24 minutes of fluoroscopy time, and 39 ± 18 minutes of RF ablation time (Table 2). Acute procedural success was seen in 20 of 43 patients (47%), with termination of AF in 4 (9%), organization to another tachyarrhythmia in 7 (16%), and AF cycle length prolongation in 9 (21%). Acute success was achieved in 12 of 22 patients with induced AF (55%) and in 8 of 21 patients with spontaneous AF (38%). Among patients who began the procedure in AF, rotor ablation and PVI resulted in AF termination in 2 patients (9%) and organization of AF in 2 patients (9%). Cardioversion was required to restore normal sinus rhythm in 81% of patients in AF at the beginning of the procedure. Major complications were seen in 4 of 43 patients (9.3%), including 2 heart failure exacerbations requiring hospitalization, 1 cardiac tamponade requiring drainage, and 1 fatal atrioesophageal fistula, which was previously reported.8
Table 2.
Acute procedural results
| All patients (n = 43) | Clinical success (n = 16) | Atrial fibrillation recurrence (n = 27) |
P value | |
|---|---|---|---|---|
| No. left atrial rotors targeted | 2.0 ± 1.0 (0–5) | 1.8 ± 0.9 (1–4) | 2.1 ± 1.1 (0–5) | .36 |
| No. right atrial rotors targeted | 0.6 ± 0.8 (0–2) | 0.7 ± 0.7 (0–2) | 0.6 ± 0.8 (0–2) | .68 |
| Procedural time (minutes) | 314 ± 82 (144–489) | 336 ± 70 (227–452) | 301 ± 88 (144–489) | .18 |
| Fluoroscopy time (minutes) | 55 ± 24 (14–126) | 58 ± 27 (27–126) | 53 ± 23 (14–01) | .52 |
| Total radiofrequency ablation time (minutes) | 39 ± 18 (12–72) | 41 ± 16 (18–68) | 37 ± 20 (12–72) | .50 |
| Acute procedural success | 20 (47%) | 10 (63%) | 10 (37%) | .11 |
Values are given as no. (%) or mean ± SD.
Long-term clinical outcomes
At 18 ± 7 months of follow up, recurrent atrial tachyarrhythmia was documented in 30 of 43 patients (70%), consisting of AF in 27 and atrial tachycardia or flutter in 3. Figure 1 shows Kaplan–Meier curves of freedom from AF in paroxysmal and persistent AF patients, which were not significantly different. A total of 9 of 43 patients (21%) were free from recurrent atrial tachyarrhythmias and off antiarrhythmic drugs at last follow-up, 6 of 24 (25%) with paroxysmal AF and 3 of 19 (16%) with persistent AF (Figure 2). Among patients with acute procedural success (n = 20), 30% were free from recurrent atrial tachyarrhythmias off antiarrhythmic drugs at last follow-up. No baseline demographic characteristic, paroxysmal vs persistent pattern of AF, or prior ablation predicted AF recurrence after ablation (Tables 1 and 2), nor did acute procedural success (including AF termination, organization, or slowing) or concomitant PVI.
Figure 1.
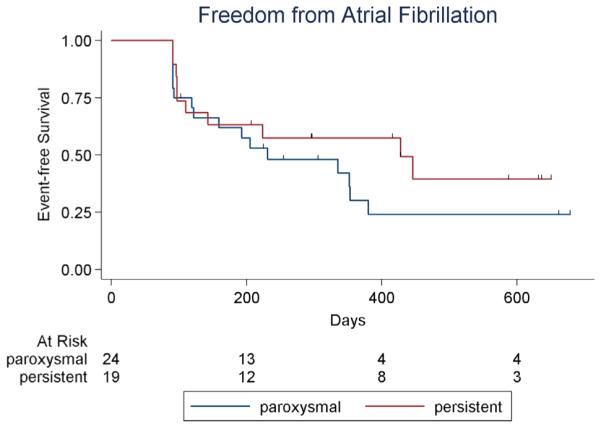
Kaplan–Meier plot of atrial fibrillation-free survival after FIRM ablation. Hatch marks indicate censored patients. Bottom row shows number of patients at risk. Blanking period is 90 days.
Figure 2.
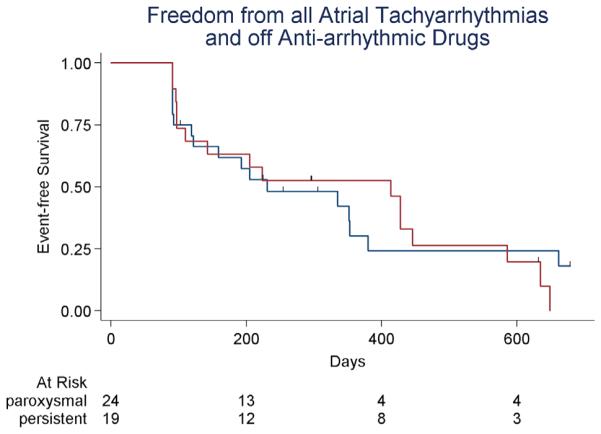
Kaplan–Meier plot of atrial tachyarrhythmia-freesurvival off antiarrhythmic drugs after FIRM ablation. Hatch marks indicate censored patients. Bottom row shows number of patients at risk. Blanking period is 90 days.
Discussion
This is the first independent study of FIRM-guided rotor ablation outcomes with rigorous, long-term clinical follow-up. The major findings in 43 patients at 2 academic centers are as follows. (1) Despite identification of rotors by the FIRM technology in every patient, ablation of these sites resulted in a low rate of AF termination or organization (25%). (2) Among patients with AF as the initial rhythm, termination during ablation was seen in 9%. (3) Single-procedure success rate after mean 18 months of follow-up was 30% free from documented AF recurrence and 21% free from atrial tachyarrhythmias and off antiarrhythmic drugs. A rapid falloff in success was seen after 6 months.
Outcomes with catheter ablation of persistent AF using empiric lesion sets, even when extensive, have been disappointing. Single-procedure success rates of only 20% to 30% have been reported even in experienced, high-volume centers.2,14 In order to move beyond purely anatomic ablation and tailor lesion sets to individual patient substrate, several methods of mapping activation during AF have been proposed, including ablation of complex fractionated atrial electrograms,15 areas of maximum dominant frequency on spectral analysis,16 and sites with high Shannon entropy.6 To date, none of these has been shown to improve outcomes over anatomic approaches in a randomized controlled study. The RADAR-AF randomized trial showed no improvement in outcomes when ablation of high-frequency sites was added to circumferential PVI for persistent AF.5 In the STAR AF 2 randomized trial, substrate ablation in addition to PVI was associated with a trend toward higher recurrence rates than PVI alone.4
Building on demonstrations of stable microreentrant rotational activity in tissue preparations17 and isolated animal hearts,18 Narayan et al proposed that human AF might be sustained by spatially and temporally stable rotors that could be identified with a phase-mapping algorithm and targeted for ablation. In 2012, the CONFIRM study generated considerable excitement in the field because FIRM seemed to offer a method of effectively treating AF with limited, patient-specific ablation.9 Other publications also showed favorable results, although they generally combined rotor ablation with PVI and did not include control groups.10
More recently, several investigators found that rotor ablation failed to achieve acute and long-term outcomes similar to those seen in the CONFIRM study. Tilz et al19 observed AF termination in only 11 of 24 patients (46%), all but 1 of whom had induced rather than spontaneous AF, and 24% AF recurrence during a short follow-up of 6 months post-blanking. Kuklik et al20 found significant technical limitations with the 64-pole basket catheter, which was able to map only 43% of LA surface area. Schade et al21 observed that after pure rotor ablation based on FIRM mapping (no PVI), early recurrences of AF or atrial tachyarrhythmias were seen in 67% of patients. Finally, Gianni et al22 performed FIRM-only ablation in 30 patients with persistent AF and found no AF termination during ablation, as well as 80% recurrence rate within 4 months.
Why did ablation of FIRM-identified rotors fail to terminate AF or prevent recurrence in most patients? In our study, both acute and long-term results of rotor ablation were markedly different from those of the CONFIRM study. One possible explanation is that the data used to generate the FIRM maps and identify rotor sites were inadequate because of poor contact by the basket catheter electrodes and clustering of splines, resulting in incomplete sampling of the atrial surface, as we described in a previous publication.8 However, the proprietary phase-mapping algorithm purported to identify rotors in every single patient, even though the signal quality was often too poor or the atrial coverage insufficient to generate a solution. Another possibility is that rotors were not temporally stable or meandered over too large an area to be eliminated by focal ablation of ~2 cm2, in keeping with the findings of multiple investigators using phase mapping and other methods.7,23 Moreover, it remains unclear whether functional rotors can be effectively ablated.24 Finally, it is still a matter of active scientific debate whether stable rotors are responsible for maintenance of human AF, rather than a passive epiphenomenon of disorganized fibrillatory activation.25,26 This is in line with the finding that quantitative analysis of electrograms from patients undergoing FIRM ablation failed to identify characteristics that would be expected if rotors were present.8 Therefore, the area targeted for ablation by the software may not be related to the pathophysiologic mechanisms underlying the arrhythmia.
In our study, most patients had PVI in addition to rotor ablation, yet the recurrence rates were higher than would be expected from PVI alone in a mixed paroxysmal–persistent cohort. This might have resulted from selection bias, as many patients were referred for FIRM ablation because they were deemed poor candidates for conventional ablation, and 67% had previously failed ablation (compared to 42% of FIRM-guided patients in the CONFIRM study.) Moreover, a larger proportion of patients in our study (95%) had previously failed at least 1 antiarrhythmic drug compared to CON (44%), suggesting the possibility of a less favorable atrial substrate. These were the initial cases performed at both centers, and it is possible that better outcomes would be achieved with more experience. However, 11 of the 24 cases at UCLA were performed by an experienced operator and developer of the system. Another potential explanation is that because rotor ablation was performed first and usually lasted several hours, PVI was less thorough at the end of a long procedure. This is supported by the relatively modest amount of RF delivered (39 ± 18 minutes) for combined rotor ablation and PVI. Finally, there is the potential that rotor ablation could be proarrhythmic by creating islands of atrial scar tissue that could facilitate macroreentrant circuits. However, this is less likely considering that most recurrences were AF rather than atrial tachycardia or flutter.
Study limitations
Limitations of this study include the relatively small sample size and lack of a control group. These procedures were performed before the wide availability of contact force sensing catheters, which might have improved the efficacy of rotor ablation. Another limitation is that postprocedure rhythm monitoring was less intense than in many prospective trials; however, arrhythmia recurrence was still documented at a high rate. Finally, because the majority of patients in this study underwent both PVI and FIRM-guided ablation, we could not assess the independent effects of rotor ablation, only the outcomes after a combined ablation strategy.
Future research in this area should be directed toward better understanding the mechanisms underlying AF maintenance and assessing the independent contribution of rotor ablation to clinical outcomes. Uncontrolled retrospective studies, like most of the published literature on rotor ablation, will not provide this evidence, particularly when patients undergo additional ablation lesion sets such as PVI. Randomized trials will be particularly useful, especially those in which 1 of the treatment arms is treated only with rotor ablation. The FIRMAT-PAF trial randomly assigned patients with paroxysmal AF to FIRM-only ablation vs PVI. However, that study was terminated because of lack of enrollment (Clinicaltrials.gov, accessed August 27, 2015). The REAFFIRM trial is currently enrolling patients with persistent AF for randomization to standard PVI vs rotor ablation and PVI. This study will be less compelling because 1 arm will undergo more extensive ablation than the other.
Conclusion
In a cohort of patients at 2 medical centers treated with FIRM-guided rotor ablation and PVI, we observed a low rate of acute AF termination and a high rate of recurrence during long-term follow-up. Prospective, randomized controlled trials with this technology are needed to clarify its role as an ablation strategy.
CLINICAL PERSPECTIVES.
Long-term outcomes after catheter ablation for atrial fibrillation (AF) have been disappointing. There has been increasing interest in new technologies for mapping AF to guide ablation, with promising early results. One such approach is focal impulse and rotor mapping (FIRM), a proprietary phase-mapping algorithm that identifies rotors sustaining AF. Here we report the acute and long-term outcomes in 43 patients with AF (56% paroxysmal, 44% persistent) using FIRM technology to guide ablation. We found a low rate of AF termination (9%) or organization (16%) with rotor ablation, with a 9% procedural complication rate. During mean 18 months of follow-up, recurrent AF was documented in 63% of patients; only 21% were free of recurrent atrial arrhythmias and off antiarrhythmic drugs. This technology and others that identify new targets for catheter ablation of AF should be rigorously examined in randomized controlled trials before widespread incorporation into clinical practice. Until then, anatomic approaches such as antral pulmonary vein isolation, as well as patient-specific strategies such as trigger elimination, should continue to be the primary strategies for AF ablation.
Acknowledgment
We thank Andy Lin, UCLA Institute for Digital Research and Education, for assistance with statistical analysis.
ABBREVIATIONS
- AF
atrial fibrillation
- FIRM
focal impulse and rotor mapping
- LA
left atrium
- PV
pulmonary vein
- PVI
pulmonary vein isolation
- RF
radiofrequency
References
- 1.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–166. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 3.Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–38. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 4.Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 5.Atienza F, Almendral J, Ormaetxe JM, et al. Comparison of radiofrequency catheter ablation of drivers and circumferential pulmonary vein isolation in atrial fibrillation: a noninferiority randomized multicenter RADAR-AF trial. J Am Coll Cardiol. 2014;64:2455–2467. doi: 10.1016/j.jacc.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 6.Ganesan AN, Kuklik P, Lau DH, Brooks AG, Baumert M, Lim WW, Thanigaimani S, Nayyar S, Mahajan R, Kalman JM, Roberts-Thomson KC, Sanders P. Bipolar electrogram Shannon entropy at sites of rotational activation: implications for ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2013;6:48–57. doi: 10.1161/CIRCEP.112.976654. [DOI] [PubMed] [Google Scholar]
- 7.Haissaguerre M, Hocini M, Denis A, et al. Driver domains in persistent atrial fibrillation. Circulation. 2014;130:530–538. doi: 10.1161/CIRCULATIONAHA.113.005421. [DOI] [PubMed] [Google Scholar]
- 8.Benharash P, Buch E, Frank P, Share M, Tung R, Shivkumar K, Mandapati R. Quantitative analysis of localized sources identified by focal impulse and rotor modulation mapping in atrial fibrillation. Circ Arrhythm Electrophysiol. 2015;8:554–561. doi: 10.1161/CIRCEP.115.002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller JM, Kowal RC, Swarup V, et al. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J Cardiovasc Electrophysiol. 2014;25:921–929. doi: 10.1111/jce.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shivkumar K, Ellenbogen KA, Hummel JD, Miller JM, Steinberg JS. Acute termination of human atrial fibrillation by identification and catheter ablation of localized rotors and sources: first multicenter experience of focal impulse and rotor modulation (FIRM) ablation. J Cardiovasc Electrophysiol. 2012;23:1277–1285. doi: 10.1111/jce.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narayan SM, Krummen DE, Enyeart MW, Rappel WJ. Computational mapping identifies localized mechanisms for ablation of atrial fibrillation. PloS One. 2012;7:e46034. doi: 10.1371/journal.pone.0046034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narayan SM, Krummen DE, Rappel WJ. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:447–454. doi: 10.1111/j.1540-8167.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tilz RR, Rillig A, Thum AM, Arya A, Wohlmuth P, Metzner A, Mathew S, Yoshiga Y, Wissner E, Kuck KH, Ouyang F. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol. 2012;60:1921–1929. doi: 10.1016/j.jacc.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 15.Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 16.Atienza F, Almendral J, Jalife J, Zlochiver S, Ploutz-Snyder R, Torrecilla EG, Arenal A, Kalifa J, Fernandez-Aviles F, Berenfeld O. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm. 2009;6:33–40. doi: 10.1016/j.hrthm.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidenko JM, Kent PF, Chialvo DR, Michaels DC, Jalife J. Sustained vortex-like waves in normal isolated ventricular muscle. Proc Natl Acad Sci U S A. 1990;87:8785–8789. doi: 10.1073/pnas.87.22.8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000;101:194–199. doi: 10.1161/01.cir.101.2.194. [DOI] [PubMed] [Google Scholar]
- 19.Tilz RR, Lin T, Rillig A, Scholz A, Heeger CH, Metzner A, Mathew S, Wissner E, Ouyang F, Kuck KH. Nine month outcomes following focal impulse and rotor modulation for the treatment of atrial fibrillation using the novel 64-pole basket catheter. Europace. 2015;17:iii16. [Google Scholar]
- 20.Kuklik P, Van Hunnik A, Zeemering S, Maesen B, Pison L, Crijns HJ, Willems S, Schotten U. Technical challenges of rotor identification during atrial fibrillation using phase singularity detection. Europace. 2015;17:ii20. [Google Scholar]
- 21.Schade A, Halbfass P, Mueller P, Nentwich K, Roos M, Steinborn F, Szoelloesi GA, Pavlov P, Deneke T. FIRM only ablation in patients with persistent atrial fibrillation: acute and medium term results. Europace. 2015;17:iii68. [Google Scholar]
- 22.Gianni C, Di Biase L, Deneke T, et al. Acute and short-term outcomes in persistent and long-standing persistent patients undergoing rotors only ablation. Heart Rhythm. 2015;12:P001–P058. [Google Scholar]
- 23.Habel N, Znojkiewicz P, Thompson N, et al. The temporal variability of dominant frequency and complex fractionated atrial electrograms constrains the validity of sequential mapping in human atrial fibrillation. Heart Rhythm. 2010;7:586–593. doi: 10.1016/j.hrthm.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Xie F, Qu Z, Garfinkel A. Dynamics of reentry around a circular obstacle in cardiac tissue. Phys Rev. 1998;58:6355–6358. [Google Scholar]
- 25.Narayan SM, Jalife J. CrossTalk proposal: rotors have been demonstrated to drive human atrial fibrillation. J Physiol. 2014;592:3163–3166. doi: 10.1113/jphysiol.2014.271031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allessie M, de Groot N. CrossTalk opposing view: Rotors have not been demonstrated to be the drivers of atrial fibrillation. J Physiol. 2014;592:3167–3170. doi: 10.1113/jphysiol.2014.271809. [DOI] [PMC free article] [PubMed] [Google Scholar]


