Abstract
Background and Objective
The Rapid Test Study was a real-time comparison of point-of-care (POC) HIV tests to determine their abilities to detect early HIV infection.
Study Design
Men and transgender persons reporting sex with men in the prior year were recruited at the Public Health – Seattle & King County STD Clinic, Gay City Health Project, and University of Washington Primary Infection Clinic. Study tests included the OraQuick ADVANCE Rapid HIV-1/2 Antibody Test performed on oral fluids and tests performed on fingerstick whole blood specimens including OraQuick, Uni-Gold Recombigen HIV Test, Determine HIV-1/2 Ag/Ab Combo, and INSTI HIV-1 Rapid Antibody Test. Specimens from subjects with negative results were sent for EIA and nucleic acid amplification testing. McNemar's exact tests compared the numbers of HIV-infected subjects detected.
Results
Between February 2010 and August 2014, there were 3438 study visits. Twenty-four subjects had discordant POC results with at least one reactive and one non-reactive test, including one subject with a reactive Determine p24 antigen. OraQuick performed on oral fluids identified fewer persons compared to all fingerstick tests. OraQuick performed on fingerstick whole blood detected fewer persons compared to the Determine Combo antibody component (p=.008) and Combo overall (p=.004), and there was a trend when compared to INSTI (p=.06). The Determine Combo specificity was 98.99%.
Conclusions
As reported by others, Determine Combo underperforms compared to laboratory-based testing, but it did detect one acute infection. If these results are validated, the specificity of Determine Combo may limit its usefulness in populations with lower HIV incidence.
Keywords: HIV testing, rapid HIV test, oral fluids
Background
Early diagnosis of human immunodeficiency virus type 1 (HIV) infection is critical. Over the last two decades, there has been increasing appreciation specifically for the need to routinize use of HIV tests able to detect HIV infection during the antibody-negative “window period” [1]. This emphasis is because of the contribution of acute HIV infection (AHI) to onward transmission [2, 3] and because of the challenges in identifying symptomatic AHI [4, 5]. Beginning in 2001, early adopters among public health departments created pooled HIV nucleic acid amplification testing (NAAT) programs which increased HIV case-finding by approximately 5–10% [6–9]}.
In 2010, the United States Food and Drug Administration (FDA) approved the first laboratory-based 4th generation antigen-antibody chemiluminescent microparticle immunoassay (CMIA) for the detection of HIV, including AHI. Two additional 4th generation antigen-antibody assays received FDA approval in 2011 and 2015. These combination assays can detect both anti-HIV-1/2 antibodies and HIV p24 antigen, which is present in blood plasma within a week after HIV RNA can first be detected [1, 10]. Data suggest that 80%–94% of cases of AHI identified by pooled NAAT programs would be detected by laboratory-based 4th generation testing at a fraction of the cost and time [11–13]. In August 2013, the FDA approved the first 4th generation point-of-care (POC) HIV test, the Alere Determine HIV-1/2 Ag/Ab Combo (Determine Combo). Data from plasma seroconversion panels suggested that the Determine Combo would detect the majority of AHI cases [14–16]; however studies using fingerstick whole blood specimens in real-time have identified few antigen-positive cases [17–19].
Objectives
In 2010, we began a prospective, cross-sectional study to compare the ability of different POC and laboratory-based HIV tests, all performed on fresh specimens from the same individuals, to detect acute and early infection in real-time [20, 21]. This report describes the final findings from the project.
Study Design
Population
Men and transgender persons reporting sex with men in the prior year were recruited when seeking HIV testing at the Public Health – Seattle & King County (PHSKC) Sexually Transmitted Disease (STD) Clinic or Gay City Health Project or when referred to the University of Washington Primary Infection Clinic (PIC). At the STD Clinic, a full-time research staff member tested men and transgender persons seeking HIV testing only and those referred by clinicians. At Gay City, all counselors participated, and the study was offered primarily to men considered to be at higher risk for HIV acquisition; this included men with symptoms of acute infection, who reported sex with an HIV-infected partner, or who had a condom break or had no recollection of events during or after a sexual exposure. Subjects at the STD Clinic and Gay City could participate quarterly. Study enrollment was offered to persons referred to the PIC [22] for suspected or confirmed diagnosis of AHI in order to enrich the analysis with persons with early infection. Subjects at the PIC could participate repeatedly until all POC tests were reactive. Subjects with false-positive test results were excluded from subsequent study participation.
The University of Washington Institutional Review Board approved this study, and all participants gave verbal consent. Participants received $20 in compensation for the single visit.
HIV Testing
Study testing included one POC test performed on oral fluids (OraQuick ADVANCE Rapid HIV-1/2 Antibody Test, OraSure Technologies) and three POC tests each performed on separate fingerstick whole blood specimens: OraQuick, Determine Combo (Alere Inc.), and either the Uni-Gold Recombigen HIV Test (Uni-Gold, Trinity Biotech) or INSTI HIV-1 Rapid Antibody Test (INSTI, bioLytical) (Figure 1). The switch from Uni-Gold to INSTI occurred in spring 2013 when INSTI became the standard-of-care POC test used at the two clinical sites. Determine Combo was not FDA-approved at the start of the study; the manufacturer provided devices for investigational use beginning ten months after the start of enrollment with occasional interruptions in supply. During the course of this study the manufacturer of Determine Combo changed their production procedures for tests distributed in the United States.
Figure 1. Study-specific and standard HIV testing, by study site.
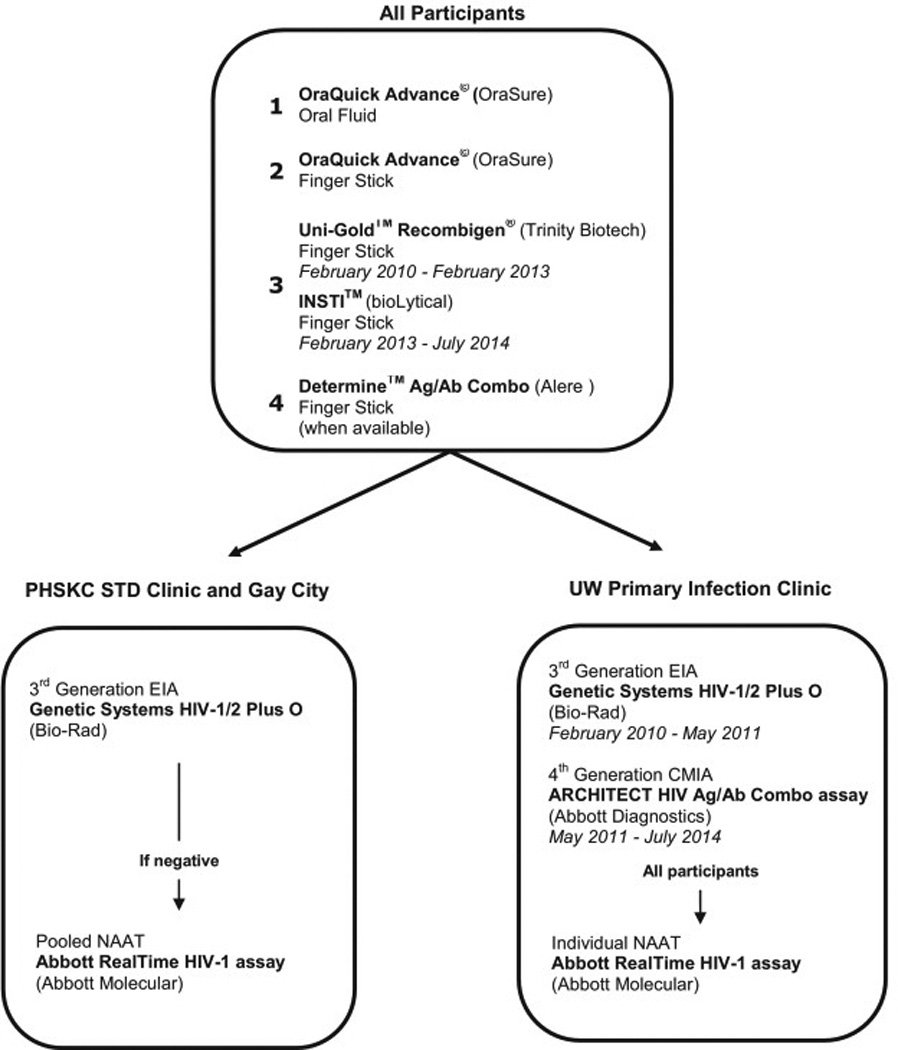
Figure 1 shows the HIV tests that occurred at each of the three participating study sites and the changes that occurred over the duration of the study. EIA: enzyme immunoassay, CMIA: chemiluminescent microparticle immunoassay, NAAT: nucleic acid amplification testing
At the STD Clinic and Gay City, participants with concordant negative POC results had serum specimens sent to the PHSKC laboratory for a 3rd generation enzyme immunoassay (EIA) and pooled NAAT as previously described [5]. Specimens from PIC participants were tested using the 3rd generation GS HIV-1/HIV-2 Plus O antibody EIA (Bio-Rad) until May 2011 and the 4th generation ARCHITECT HIV Ag/Ab Combo assay (ARCHITECT, Abbott Diagnostics Division) thereafter. HIV RNA testing was performed for all PIC participants on individual plasma specimens using the Abbott RealTime HIV-1 RNA assay (Abbott Molecular Inc), regardless of EIA or ARCHITECT result.
Specimens tested in the PHSKC laboratory were stored at −70°C for purposes of quality control and future retesting as indicated. When specimens were available, aliquots from frozen serum specimens from persons with acute and early HIV infection were thawed and rescreened with Determine Combo according to manufacturer’s instructions. These results are presented separately from the main analysis.
Data Analysis
Chart reviews were conducted for all participants with discordant test results in order to confirm an HIV infection or false positive test result. Participants with a reactive EIA and positive Western blot or detectable HIV RNA were considered to have confirmed HIV infection. McNemar's exact tests were used to compare the numbers of HIV-positive persons detected by the different tests. Estimates of sensitivity and specificity were generated for STD Clinic participants only, as these participants were more likely to be representative of HIV-negative men and transgender persons seeking HIV testing and because the electronic database allowed for more certainty regarding the identification of persons with any reactive test result. All analyses were performed using Stata13 software (StataCorp LP, College Station, TX).
Results
Between February 22, 2010 and August 1, 2014, there were 3438 study visits; 3407 visits were by men, 24 visits were by transgender women, and seven visits were by transgender men. Minority representation paralleled the race/ethnicity of clients at these sites [23].
One hundred and forty participants tested HIV-positive through the study (Table 1). One hundred HIV-infected participants had concordant reactive POC test results. Six participants had non-reactive results on all POC tests but a reactive EIA (Table 2b). Eleven participants were acutely infected (EIA-negative/NAAT-positive); one of these participants had a reactive p24 antigen result on Determine Combo (Tables 2a and 2b). Twenty-three other HIV-infected participants had discordant results with at least one reactive and one non-reactive POC antibody test result (Table 2a).
Table 1.
Distribution of point-of-care and laboratory HIV test results among study participants, Seattle, 2010–2014
| STD Clinic n=2189 |
Gay City n = 1215 |
PIC n=341 |
Total n=34382 |
|
|---|---|---|---|---|
| HIV-negative | 21213 | 1176 | 1 | 3298 |
| Total HIV Positive | 68 (3.2%) | 39 (3.2%) | 33 | 140 |
| Concordant Reactive POC Tests | 51 (75.0%) | 31 (79.5%) | 18 | 100 |
| Discordant POC Antibody Tests | 7 (10.3%) | 3 (7.6%) | 13 | 23 |
| All POC Tests Negative/EIA-Positive | 2 (2.9%) | 4 (17.9%) | 0 | 64 |
| Acute (EIA-Negative / NAAT-Positive) | 85 (11.9%) | 1 (2.6%) | 26 | 11 |
PIC: University of Washington Primary Infection Clinic; POC: point-of-care; EIA: enzyme immunoassay; NAAT: nucleic acid amplification test
Participants at the UW PIC were referred because of suspicion or recent diagnosis of acute HIV infection.
Number of study visits. Subjects at the PHSKC STD Clinic and Gay City could participate quarterly.
Includes one participant with reactive EIA, indeterminate Western blot, and negative NAAT
Includes five participants screened by Determine Combo
Includes one participant with positive p24 antigen of four participants screened by Determine Combo
These participants had a negative Determine Combo, reactive ARCHITECT, negative Multispot HIV-1/HIV-2 Rapid Test and Western blot, and HIV RNA levels of 33,000 and 72,000 copies/mL.
Table 2.
| a: Results of 24 HIV-infected participants with discordant point-of-care HIV test results | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Last neg HIV test |
OraQuick OF |
OraQuick FS |
Uni-Gold | INSTI | Determine | 3rd or 4th gen EIA1 |
Western blot band pattern | HIV RNA (copies/mL) |
|||
| Ag | Ab | ||||||||||
| 1 | 2 mo | — | — | — | ND | + | — | 3rd | — | Negative | 5.8 million |
| 2 | 4 yr | + | + | — | ND | ND | 3rd | + | 24, 31, 40, 55, 120 | 141,000 | |
| 3 | 2 yr | — | + | + | ND | ND | 3rd | + | 24, 31, 40, 55, 160 | 128,000 | |
| 4 | 2 yr | — | + | + | ND | ND | 3rd | + | 18, 24, 31, 40, 51, 55, 120, 160 | 25,000 | |
| 5 | NA | — | — | + | ND | ND | 3rd | + | 24, 51, 55, 160 | 12.8 million | |
| 6 | NA | — | — | + | ND | — | + | 3rd | + | 24, 40, 55, 160 | 21,000 |
| 7 | 1 yr | — | + | — | ND | — | + | 4th | Ab+ | 24, 51, 55 | 719,000 |
| 8 | 1 yr | — | + | + | ND | — | + | 4th | Ab+ | 24, 31, 55, 160 | 436,000 |
| 9 | 6 mo | — | + | + | ND | — | + | 4th | Ab+ | 24, 55, 160 | 33,000 |
| 10 | 2 mo | — | + | + | ND | — | + | 4th | Ab+ | 24, 55, 160 | 9000 |
| 11 | 3 mo | — | + | + | ND | — | + | 4th | Ab+ | 18, 24, 55, 160 | 32,000 |
| 12 | 2 mo | — | + | + | ND | — | + | 4th | Ab+ | 24, 160 | 94,000 |
| 13 | 2 mo | — | — | + | ND | — | + | 3rd | + | 18, 24, 31, 41, 51, 55, 65, 120, 160 | ND |
| 14 | 2 yr | — | — | + | ND | — | + | 3rd | + | 18, 24, 31, 40, 51, 55, 65, 120, 160 | ND |
| 15 | 4 mo | — | — | ND | + | — | + | 3rd | + | 24, 55, 160 | ND |
| 16 | 3 mo | — | — | ND | + | — | + | 3rd | + | 24, 51, 55, 160 | 347,000 |
| 17 | 7 mo | — | — | ND | + | — | + | 3rd | + | 18, 24, 65, 160 | 110,000 |
| 18 | 5 mo | — | — | ND | + | — | + | 3rd | + | 24, 51, 55, 160 | 62,000 |
| 19 | 4 mo | — | + | ND | + | — | + | 3rd | + | 18, 24, 31, 41, 51, 55, 65, 120, 160 | 7000 |
| 20 | 8 mo | — | + | ND | + | — | + | 4th | Ab+ | 24, 51, 55, 65, 120, 160 | 70,000 |
| 21 | NA | — | + | ND | + | — | + | 4th | Ab+ | 24, 55, 160 | 7000 |
| 22 | 2 mo | — | + | ND | + | — | + | 4th | Ab+ | Negative | 323,000 |
| 23 | 2 mo | — | + | ND | + | — | + | 4th | Ab+ | 160 | 316,000 |
| 24 | NA | — | — | ND | + | — | + | 4th | Ag+ | 24 | 4.4 million |
| b: Results of 16 HIV-infected participants with antibody-negative/NAAT-Positive or POC-negative/EIA-positive HIV test results1 | |||||||||||
| Last neg HIV test |
OraQuick OF |
OraQuick FS |
Uni-Gold | INSTI | Determine | 3rd or 4th gen EIA2 |
Western blot band pattern | HIV RNA (copies/mL) |
|||
| Ag | Ab | ||||||||||
| 1 | 7 mo | — | — | — | ND | — | — | 3rd | + | Negative | 9.0 million |
| 2 | 8 mo | — | — | — | ND | — | — | 3rd | + | Negative | 587,000 |
| 3 | 5 mo | — | — | — | ND | — | — | 3rd | + | 18, 41, 51 | ND |
| 4 | 8 mo | — | — | NA | NA | — | — | 3rd | + | Negative | 5.8 million |
| 5 | NA | — | — | — | ND | ND | 3rd | + | NA | NA | |
| 6 | 2 mo | — | — | ND | — | — | — | 3rd | + | Negative | 8.5 million |
| 7 | 6 mo | — | — | — | ND | ND | 3rd | — | ND | 5.4 million | |
| 8 | 1 yr | — | — | — | ND | ND | 3rd | — | ND | >10 million | |
| 9 | 3 mo | — | — | — | ND | ND | 3rd | — | ND | 59,000 | |
| 10 | 1.5 mo | — | — | — | ND | ND | 3rd | — | ND | >10 million | |
| 11 | 2 yr | — | — | — | ND | ND | 3rd | — | ND | 306,000 | |
| 12 | 2 mo | — | — | — | ND | — | — | 3rd | — | ND | 25,000 |
| 13 | 3 mo | — | — | ND | — | — | — | 3rd | — | ND | 8,000 |
| 14 | 4 mo | — | — | ND | — | — | — | 3rd | — | ND | 1.1 million |
| 15 | NA | — | — | — | ND | — | — | 4th | Ag+ | negative | 33,000 |
| 16 | NA | — | — | ND | — | — | — | 4th | Ag+ | negative | 72,000 |
OF: oral fluids; FS: fingerstick; Ag: antigen; Ab: antibody; EIA: enzyme immunoassay; ND: not done; NA: not available
Results from participants #1–12 were previously reported [Stekler, J Clin Virol, 2013]
The ARCHITECT assay was considered to be reactive for antibody if the Multispot HIV-1/HIV-2 Rapid Test, performed for confirmatory testing, was reactive. It was considered to be reactive for p24 antigen if the Multispot was non-reactive.
The participant with acute HIV infection who had a reactive Determine Combo is shown as #1 in Table 2a.
The ARCHITECT assay was considered to be reactive for p24 antigen if the Multispot was non-reactive.
Of the 24 total HIV-positive participants with discordant POC test results (including the individual with a reactive p24 antigen result on Determine Combo), OraQuick performed on oral fluids identified one (4%) and OraQuick performed on fingerstick whole blood identified 14 (58%) of the 24 participants. Uni-Gold detected 14 (82%) of 17 HIV-positive participants, INSTI detected all 10 (100%) HIV-positive participants, and Determine Combo detected all 20 (100%) HIV-positive participants who were screened during the portion of the study period each device was in use. OraQuick performed on oral fluid detected significantly fewer HIV-infected persons compared to OraQuick performed on fingerstick whole blood (p=.0002), Uni-Gold (p=.006), INSTI (p=.002), and Determine Combo (p=.0001). There were significantly fewer HIV-infected persons detected by OraQuick performed on fingerstick whole blood compared to the Determine Combo antibody component (p=.008) and Determine Combo overall (p=.004), and there was a trend when compared to INSTI (p=.06). The difference in the number of persons detected by Determine Combo compared to the 3rd generation EIA was not significant (p=.2).
The sensitivities and specificities of the different screening tests as performed at the STD Clinic are shown in Table 3. Of the fifteen individuals with false-positive Determine test results, eight had false-positive antibody results and seven had false-positive antigen results. The specificities of Determine Combo before and after the change in manufacturing process in 2011 were 99.4% (156 of 157) and 98.9% (1312 of 1326), respectively (p=.6).
Table 3.
Sensitivity and specificity of screening HIV tests compared to a strategy including pooled HIV NAAT, among PHSKC STD Clinic participants only.
| # tests | Sensitivity (95% CI) compared to all cases |
Sensitivity (95% CI) compared to EIA+ cases |
Specificity (95% CI) | |
|---|---|---|---|---|
| OraQuick (oral fluid) | 2180 | 51/68 = 75.0% (63.0–84.7) | 51/60 = 85.0% (73.4–92.9) | 2109/2112 = 99.86% (99.59–99.97) |
| OraQuick (fingerstick) | 2175 | 53/68 = 77.9% (66.2–87.1) | 53/60 = 88.3% (77.4–95.2) | 2107/2107 = 100% (99.82–100) |
| Uni-Gold | 1614 | 45/53 = 84.9% (72.4–93.3) | 45/47 = 95.7% (85.5–99.5) | 1561/1561 = 100% (99.76–100) |
| INSTI | 559 | 11/15 = 73.3% (44.9–92.2) | 11/13 = 84.6% (54.6–98.1) | 543/544 = 99.82% (98.98–100) |
| Determine Combo | 1523 | 34/40 = 84.6% (70.2–94.3) | 33/36* = 91.7% (77.5–98.2) | 1468/1483 = 98.99% (98.34–99.43) |
| GS HIV-1/HIV-2 Plus O antibody (EIA) | 2161 | 58/66 = 87.9% (77.5–94.6) | 2091/2095 = 99.81% (99.51–99.95) |
This numerator and denominator do not include the participant who tested EIA-negative but p24 Ag-positive on Determine Combo.
Note: estimates cannot be directly compared, as not all POC tests were used on all participants.
The five acutely infected (EIA-negative/NAAT-positive) participants who were screened but not detected by Determine Combo (Table 1) had a median HIV RNA level of 33,000 (range 8000–1,100,000) copies/mL (Five other acutely infected participants were screened during a period when Determine Combo was not available to the study.). The five POC-negative/EIA-positive participants screened but not detected by Determine Combo had a median HIV RNA level of 7.2 million (range 586,000–9,000,000 million) copies/mL. Five specimens were available for additional testing using frozen serum stored in the PHSKC laboratory. Of these, two had previously tested Determine Combo-negative; one that had been reactive by EIA and had HIV RNA of 8.5 million copies/mL retested p24 antigen-positive, and one that had been non-reactive by EIA and had an HIV RNA of 8000 copies/mL retested Determine Combo-negative. Frozen serum specimens from three EIA-negative/NAAT-positive persons who had not initially been screened by Determine Combo were also tested; one tested p24 antigen-positive (HIV RNA 5.3 million copies/mL) and two tested Determine Combo-negative (HIV RNA >10 million and 59,000 copies/mL).
Discussion
These data reinforce and further illuminate our previously published data which showed that POC tests fail to diagnose many HIV-infected persons with early infection and confirm that testing performed on oral fluids is less accurate than testing on fingerstick whole blood [11, 21]. These results are also consistent with data from worldwide studies that found that Determine Combo failed to identify most acute infections among high risk populations and produced more false-positive test results than other POC tests. Despite the disappointing sensitivity for acute HIV infection, Determine Combo was not significantly different from the 3rd generation EIA in detecting infections and as good as (and in one comparison significantly better than) other POC HIV antibody tests performed on fingerstick whole blood.
There are four major considerations for testing programs to decide which HIV tests to deploy. The first consideration is whether there are technical or staffing limitations that prohibit the use of laboratory-based tests, or whether the population targeted for testing has low rates of follow-up. Point-of-care (POC) tests have one advantage over laboratory-based HIV tests in that more persons receive results [24, 25], although this may or may not translate into greater likelihood of linkage to care among persons testing newly HIV-positive [26, 27]. Programs should next consider whether the population being tested has a high HIV incidence and short inter-test intervals, both of which increase the likelihood of testing in the window period and decrease the clinical sensitivity of HIV antibody tests. Recognition of the importance of AHI led to recommendations in the new HIV diagnostic testing algorithm to use 4th generation assays for HIV screening [28]. All other issues being equal, programs should use the most sensitive test possible. In contrast, there might be programs for which the specificity of the HIV test is the most important characteristic because of significant negative consequences of providing false positive test results without results of supplemental testing, e.g. low prevalence labor and delivery settings. Programs should, of course, consider whether the target population has a preference. However, our experience suggests caution in making assumptions, as our work has shown that MSM and transgender persons familiar with HIV testing may prefer oral fluid as a specimen collection method but have more trust in the ability of tests performed on venipuncture specimens to provide accurate results [20]. Finally, one must consider the financial resources and priorities of the program. Clearly there is no HIV testing strategy that is one size fits all.
Our study had several limitations. Not all participants were screened using the identical panel of POC devices because of changes in the standard of care POC test in the clinic and because of supply issues with Determine Combo prior to FDA-approval. Because POC tests were performed concurrently, point estimates for the sensitivity of less-sensitive POC tests may be overestimated by operator interpretation as faint lines were read in the context of strongly reactive tests. As with prior testing projects in Seattle, our results are likely generalizable to other populations with high HIV incidence and frequent testing, factors that produce a high likelihood of testing during early infection. However, our study results may not be generalizable to populations with lower incidence or less frequent testing.
In conclusion, these findings confirm that currently there is no adequate POC substitute for laboratory-based 4th generation testing or HIV NAAT in high-incidence populations and support prior recommendations that programs should not use oral fluid specimens when POC testing is offered to high-risk populations. The future FDA approval of a POC NAAT [29] could be highly desirable for implementation in multiple settings, including HIV testing in populations like ours to detect AHI and reduce ongoing HIV transmission, for PrEP clinics to limit the acquisition of HIV drug resistance when PrEP is initiated during AHI [30, 31], for researchers and vaccine developers interested in studying the earliest host-virus interactions, and possibly even for home self-testing in order to limit the potential harms of “point-of-sex” testing. Until then, the Determine Combo and other 4th generation POC tests in development may be the best POC tests to use in the highest incidence populations, but, if these results are validated in future studies, the lower specificity of Determine Combo may limit its usefulness in populations with lower incidence and prevalence.
Highlights.
Point-of-care HIV tests fail to detect many persons with early HIV infection.
Oral fluid identifies fewer persons with HIV infection compared to fingerstick.
Some differences were seen among the fingerstick point-of-care tests.
Determine Combo detected only one case of acute HIV infection.
Specificity of Combo may be a limitation to use in low prevalence settings.
Acknowledgments
We would like to thank all of the study participants at the three sites and the study counselors at the Gay City Health Project. We thank Dr. Bernie Branson for his review of the manuscript.
This study was supported by NIH R01 MH-83630, U01 AI-38858, UM1 AI-068618, and P30 AI12 27757. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Alere provided Determine™ HIV 1/2 Ag/Ab Combo tests and controls throughout the duration of the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None.
Ethical approval
Ethical approval provided by University of Washington, Human Subjects Division (IRB #36711).
References
- 1.Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 2.Powers KA, Ghani AC, Miller WC, Hoffman IF, Pettifor AE, Kamanga G, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011;378:256–268. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollingsworth TD, Pilcher CD, Hecht FM, Deeks SG, Fraser C. High Transmissibility During Early HIV Infection Among Men Who Have Sex With Men-San Francisco, California. J Infect Dis. 2014 doi: 10.1093/infdis/jiu831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257–264. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 5.Stekler JD, Baldwin HD, Louella MW, Katz DA, Golden MR. ru2hot?: A public health education campaign for men who have sex with men to increase awareness of symptoms of acute HIV infection. Sex Transm Infect. 2013 doi: 10.1136/sextrans-2012-050730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stekler J, Swenson PD, Wood RW, Handsfield HH, Golden MR. Targeted screening for primary HIV infection through pooled HIV-RNA testing in men who have sex with men. AIDS. 2005;19:1323–1325. doi: 10.1097/01.aids.0000180105.73264.81. [DOI] [PubMed] [Google Scholar]
- 7.Pilcher CD, Fiscus SA, Nguyen TQ, Foust E, Wolf L, Williams D, et al. Detection of acute infections during HIV testing in North Carolina. N Engl J Med. 2005;352:1873–1883. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- 8.Klausner JD, Grant RM, Kent CK. Detection of acute HIV infections. N Engl J Med. 2005;353:631–633. doi: 10.1056/NEJM200508113530620. author reply 631–633. [DOI] [PubMed] [Google Scholar]
- 9.Priddy FH, Pilcher CD, Moore RH, Tambe P, Park MN, Fiscus SA, et al. Detection of acute HIV infections in an urban HIV counseling and testing population in the United States. J Acquir Immune Defic Syndr. 2007;44:196–202. doi: 10.1097/01.qai.0000254323.86897.36. [DOI] [PubMed] [Google Scholar]
- 10.Masciotra S, McDougal JS, Feldman J, Sprinkle P, Wesolowski L, Owen SM. Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections. J Clin Virol. 2011;52(Suppl 1):S17–S22. doi: 10.1016/j.jcv.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Stekler JD, Swenson PD, Coombs RW, Dragavon J, Thomas KK, Brennan CA, et al. HIV testing in a high-incidence population: is antibody testing alone good enough? Clin Infect Dis. 2009;49:444–453. doi: 10.1086/600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chavez P, Wesolowski L, Patel P, Delaney K, Owen SM. Evaluation of the performance of the Abbott ARCHITECT HIV Ag/Ab Combo Assay. J Clin Virol. 2011;52(Suppl 1):S51–S55. doi: 10.1016/j.jcv.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Pandori MW, Hackett J, Jr, Louie B, Vallari A, Dowling T, Liska S, et al. Assessment of the ability of a fourth-generation immunoassay for human immunodeficiency virus (HIV) antibody and p24 antigen to detect both acute and recent HIV infections in a high-risk setting. J Clin Microbiol. 2009;47:2639–2642. doi: 10.1128/JCM.00119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel P, Bennett B, Sullivan T, Parker MM, Heffelfinger JD, Sullivan PS, et al. Rapid HIV screening: missed opportunities for HIV diagnosis and prevention. J Clin Virol. 2012;54:42–47. doi: 10.1016/j.jcv.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilcher CD, Louie B, Facente S, Keating S, Hackett J, Jr, Vallari A, et al. Performance of rapid point-of-care and laboratory tests for acute and established HIV infection in San Francisco. PLoS One. 2013;8:e80629. doi: 10.1371/journal.pone.0080629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masciotra S, Luo W, Youngpairoj AS, Kennedy MS, Wells S, Ambrose K, et al. Performance of the Alere Determine HIV-1/2 Ag/Ab Combo Rapid Test with specimens from HIV-1 seroconverters from the US and HIV-2 infected individuals from Ivory Coast. J Clin Virol. 2013;58(Suppl 1):e54–e58. doi: 10.1016/j.jcv.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg NE, Kamanga G, Phiri S, Nsona D, Pettifor A, Rutstein SE, et al. Detection of acute HIV infection: a field evaluation of the determine(R) HIV-1/2 Ag/Ab combo test. J Infect Dis. 2012;205:528–534. doi: 10.1093/infdis/jir789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duong YT, Mavengere Y, Patel H, Moore C, Manjengwa J, Sibandze D, et al. Poor performance of the determine HIV-1/2 Ag/Ab combo fourth-generation rapid test for detection of acute infections in a National Household Survey in Swaziland. J Clin Microbiol. 2014;52:3743–3748. doi: 10.1128/JCM.01989-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conway DP, Holt M, McNulty A, Couldwell DL, Smith DE, Davies SC, et al. Multi-centre evaluation of the Determine HIV Combo assay when used for point of care testing in a high risk clinic-based population. PLoS One. 2014;9:e94062. doi: 10.1371/journal.pone.0094062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Neal JD, Golden MR, Branson BM, Stekler JD. HIV nucleic acid amplification testing versus rapid testing: it is worth the wait. Testing preferences of men who have sex with men. J Acquir Immune Defic Syndr. 2012;60:e117–e120. doi: 10.1097/QAI.0b013e31825aab51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stekler JD, O'Neal JD, Lane A, Swanson F, Maenza J, Stevens CE, et al. Relative accuracy of serum, whole blood, and oral fluid HIV tests among Seattle men who have sex with men. J Clin Virol. 2013;58(Suppl 1):e119–e122. doi: 10.1016/j.jcv.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stekler JD, Wellman R, Holte S, Maenza J, Stevens CE, Corey L, et al. Are there benefits to starting antiretroviral therapy during primary HIV infection? Conclusions from the Seattle Primary Infection Cohort vary by control group. Int J STD AIDS. 2012;23:201–206. doi: 10.1258/ijsa.2011.011178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz DA, Dombrowski JC, Swanson F, Buskin SE, Golden MR, Stekler JD. HIV intertest interval among MSM in King County, Washington. Sex Transm Infect. 2013;89:32–37. doi: 10.1136/sextrans-2011-050470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutchinson AB, Branson BM, Kim A, Farnham PG. A meta-analysis of the effectiveness of alternative HIV counseling and testing methods to increase knowledge of HIV status. AIDS. 2006;20:1597–1604. doi: 10.1097/01.aids.0000238405.93249.16. [DOI] [PubMed] [Google Scholar]
- 25.Spielberg F, Branson BM, Goldbaum GM, Lockhart D, Kurth A, Rossini A, et al. Choosing HIV Counseling and Testing Strategies for Outreach Settings: A Randomized Trial. J Acquir Immune Defic Syndr. 2005;38:348–355. [PubMed] [Google Scholar]
- 26.Keller S, Jones J, Erbelding E. Choice of Rapid HIV testing and entrance into care in Baltimore City sexually transmitted infections clinics. AIDS Patient Care STDS. 2011;25:237–243. doi: 10.1089/apc.2010.0298. [DOI] [PubMed] [Google Scholar]
- 27.Delaney K, Knoble T, Rurangirwa J, Facente S, Janson M, Scheer S, et al. Using a Rapid HIV Testing Algorithm to Improve the Accuracy of HIV Testing, Receipt of Test Results, and Linkage to Care: Results of a Demonstration Project in 2 US Cities. 18th Conference on Retroviruses and Opportunistic Infections; Feb 27–Mar 2, 2011; Boston, MA. [abstract #132LB] [Google Scholar]
- 28.Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm - United States, 2011–2013. MMWR Morb Mortal Wkly Rep. 2013;62:489–494. [PMC free article] [PubMed] [Google Scholar]
- 29.Jani IV, Meggi B, Mabunda N, Vubil A, Sitoe NE, Tobaiwa O, et al. Accurate early infant HIV diagnosis in primary health clinics using a point-of-care nucleic acid test. J Acquir Immune Defic Syndr. 2014;67:e1–e4. doi: 10.1097/QAI.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 30.Liegler T, Abdel-Mohsen M, Bentley LG, Atchison R, Schmidt T, Javier J, et al. HIV-1 drug resistance in the iPrEx preexposure prophylaxis trial. J Infect Dis. 2014;210:1217–1227. doi: 10.1093/infdis/jiu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehman DA, Baeten JM, McCoy CO, Weis JF, Peterson D, Mbara G, et al. Risk of Drug Resistance Among Persons Acquiring HIV Within a Randomized Clinical Trial of Single-or Dual-Agent Preexposure Prophylaxis. J Infect Dis. 2015;211:1211–1218. doi: 10.1093/infdis/jiu677. [DOI] [PMC free article] [PubMed] [Google Scholar]


