Abstract
Introduction
The prevalence of tobacco use in the population with schizophrenia is enormously high. Moreover, nicotine dependence is found to be associated with symptom severity and poor outcome in patients with schizophrenia. The neurobiological mechanisms that explain schizophrenia-nicotine dependence comorbidity are not known. This study systematically reviews the evidence highlighting the contribution of nicotinic acetylcholine receptors (nAChRs) to nicotine abuse in schizophrenia.
Methods
Electronic data bases (Medline, Google Scholar, and Web of Science) were searched using the selected key words that match the aims set forth for this review. A total of 275 articles were used for the qualitative synthesis of this review.
Results
Substantial evidence from preclinical and clinical studies indicated that dysregulation of α7 and β2-subunit containing nAChRs account for the cognitive and affective symptoms of schizophrenia and nicotine use may represent a strategy to remediate these symptoms. Additionally, recent meta-analyses proposed that early tobacco use may itself increase the risk of developing schizophrenia. Genetic studies demonstrating that nAChR dysfunction that may act as a shared vulnerability factor for comorbid tobacco dependence and schizophrenia were found to support this view. The development of nAChR modulators was considered an effective therapeutic strategy to ameliorate psychiatric symptoms and to promote smoking cessation in schizophrenia patients.
Conclusions
The relationship between schizophrenia and smoking is complex. While the debate for the self-medication versus addiction vulnerability hypothesis continues, it is widely accepted that a dysfunction in the central nAChRs represent a common substrate for various symptoms of schizophrenia and comorbid nicotine dependence.
Keywords: schizophrenia, smoking, comorbidity, nAChRs, cognition, reward
1. Introduction
1.1. Association between cigarette smoking and schizophrenia
Schizophrenia is a chronic and debilitating mental illness that affects 1% of the world population (Saha et al., 2005). People diagnosed with schizophrenia display a combination of positive (hallucinations, delusions and thought impairments), negative (diminished emotional expression, loss of motivation and psychosocial impairments) and cognitive (disorganized thought, attention and working memory deficits, and executive dysfunction) symptoms (Wong and Van Tol, 2003). The psychotic symptoms have an episodic pattern and can be controlled by antipsychotic drugs, but the negative and cognitive symptoms tend to show limited improvement and tend to persist despite treatment with these drugs (Bowie and Harvey, 2006; Hill et al., 2010; Keefe and Fenton, 2007; Tandon and Jibson, 2002). Although extensive evidence indicates alterations in several neurotransmitters systems including dopamine (DA), glutamate, and GABA (Howes and Kapur, 2009; Lewis et al., 1999; Moghaddam and Krystal, 2012), the etiology and pathophysiology of schizophrenia still remains unclear.
Patients with schizophrenia generally have a higher incidence of substance use disorders as compared to the normal individuals (Volkow, 2009). The most frequently abused substances in subjects suffering from schizophrenia include tobacco, cannabis, alcohol and cocaine (Green, 2005; Thoma and Daum, 2013; Winklbaur et al., 2006). Interestingly, nicotine -which is the main psychoactive ingredient of tobacco, is the most prevalent drug abused by patients with schizophrenia. The incidence of tobacco smoking in individuals with schizophrenia is estimated to be 80–90% versus 20–30% in the general population (de Leon and Diaz, 2005; George and Krystal, 2000). Moreover, smokers with schizophrenia consume more cigarettes and extract higher nicotine from their cigarettes as compared to normal smokers (Olincy et al., 1997; Strand and Nyback, 2005). Additionally, patients with schizophrenia experience greater reinforcing effects of smoking and more severe withdrawal symptoms during abstinence than smokers without schizophrenia (Diaz et al., 2006; Spring et al., 2003; Weinberger et al., 2007). Thus, subjects with schizophrenia are more vulnerable to develop nicotine dependence and smoking cessation in these individuals remains very challenging. Nicotine dependence is also found to be associated with symptom severity and poor outcome in patients with schizophrenia (Krishnadas et al., 2012). Furthermore, heavy smoking in these individuals makes them highly susceptible to develop various disorders such as cardiovascular diseases, lung cancer, and pulmonary conditions, resulting in premature mortality (Hennekens, 2007; Kelley et al., 2011; Shanmugam et al., 2007; Wehring et al., 2012). Therefore, a fundamental understanding of the neurobiological mechanisms that contribute to comorbid nicotine use in schizophrenia is essential to develop therapeutic strategies to improve smoking cessation, treatment outcome and life span of patients suffering from schizophrenia. Although comorbid nicotine use is also known to exist with other psychiatric conditions such as anxiety-related disorders, depression, bipolar disorders and post-traumatic stress disorders (Kutlu et al., 2015; Lasser et al., 2000), the discussion for this review is restricted to schizophrenia only.
The causal mechanisms explaining the widespread smoking behavior among subjects with schizophrenia have remained complex. However, there have been two overarching theories proposed to explain widespread smoking behavior among subjects with schizophrenia. The first one is the self-medication hypothesis that posits that excessive nicotine use represents a form of self-medication strategy to alleviate the negative and cognitive symptoms of the disorder (Adler et al., 1998; Kumari and Postma, 2005; Leonard et al., 2007; Sacco et al., 2004). The remediation of schizophrenia symptoms by nicotine is proposed to involve modulation of neurotransmitter signaling that is implicated in schizophrenia pathology (Kumari and Postma, 2005). Higher comorbidity of smoking is also suggested to represent a strategy to combat the aversive effects of antipsychotic medication and this perspective complements the self-medication hypothesis (Barr et al., 2008; Kumari and Postma, 2005). However, there is evidence of nicotine consumption in first-episode psychotic subjects that are not exposed to any antipsychotic medication (McEvoy et al., 1999), which indicates that this view may not completely explain higher smoking rates in patients with schizophrenia. The second one is the shared vulnerability hypothesis that proposes that shared genetic/environmental factors and neurological deficits inherent to the pathophysiology of schizophrenia make subjects with schizophrenia more vulnerable to tobacco use (Chambers, 2009; Chambers et al., 2001). Based on this model, the shared genetic and neurobiological factors produce nicotine-sensitive cognitive deficits, sensitized reinforcing effects of nicotine, heightened craving during abstinence and increased impulsivity in the schizophrenia population increasing their susceptibility to tobacco smoking at the initiation, maintenance and relapse stages of addiction. Unlike the self-medication hypothesis, this model does not assume any benefits underlying the comorbidity and points only towards neurobiological mechanisms or outcomes that are detrimental. While the available evidence from human and animal studies presents merits and limitations of both models, neither one of those has been confirmed to solely explain the strong association between smoking and schizophrenia. Regardless of how comorbidity develops, a fundamental understanding of the neurobiological mechanisms that contribute to comorbid nicotine use in schizophrenia is essential to develop therapeutic strategies to improve smoking cessation, treatment outcome and life span of patients suffering from schizophrenia.
1.2. Objectives
In this review, we focus on neuronal nicotinic acetylcholine receptors (nAChRs) as a common modulator for functional alterations in schizophrenia and nicotine dependence and provide a framework for how these receptors account for the comorbidity between the two conditions. Additionally, emerging views that early tobacco use may itself increase the risk of developing schizophrenia are discussed in the light of nAChR dysregulation that may act as a shared vulnerability factor for comorbid tobacco dependence in schizophrenia. A particular emphasis is placed on therapeutic strategies that rectify nAChR dysfunction to ameliorate psychiatric symptoms and promote smoking cessation in schizophrenia patients in this review.
2. Method
2.1. Process
Two independent reviewers (V.P. and M.G.K.) searched Medline, Google Scholar and web of science from their inception until January 2016 according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) criteria (Liberati et al., 2009). Electronic data bases were searched using the following key words in various permutations: smoking, schizophrenia, nicotine, nAChRs, cognition, negative symptoms, positive symptoms, genetics, addiction, neurobiology, antipsychotics, nAChR ligands, addiction, dependence and relapse. The search criteria was specified in advance without publication date, publication status and language restriction.
2.2. Eligibility criteria
The search for this qualitative review included full text research articles, review articles, and clinical trials/case reports that matched the aims set forth for this review. Exclusion criteria included dissertations, book reviews and journal articles where the full text was not available. Titles and abstracts of articles relevant to the topic were screened and all duplicate records were eliminated. No date restrictions were applied. A total of 2359 records were identified during the search process and following exclusions based on the above criterion, the abstract of 488 articles was scrutinized. The full text of articles available through either institutional access or open/free electronic access was retrieved and evaluated once the abstracts matched the inclusion. The total number of articles selected for the qualitative synthesis of this review was 275.
3. Results
3.1. neuronal nAChRs
nAChRs are a family of ligand-gated ionotropic receptors that mediate fast synaptic transmission throughout the central nervous system by altering cation channel (Na2+/Ca2+/K+) fluxes. These receptors belong to one of the two classes of cholinergic receptors activated by the endogenous neurotransmitter acetylcholine (ACh). The other type of cholinergic receptors stimulated by ACh is muscarinic receptors, which are G protein-coupled receptors. Nicotine exerts its behavioral and neurochemical effects by binding specifically to neuronal nAChRs and therefore, the discussion here will focus only on these cholinergic receptors. Neuronal nAChRs are pentameric structures that are formed from a combination of five membrane-spanning units consisting of nine isoforms of α subunits (α2-α10) and three isoforms of β subunits (β2-β4), and arranged either as a heteromeric or homomeric assemblies (Dani and Bertrand, 2007; Gotti et al., 2009). Both pre- and post-synaptic localization of these receptors have been reported (Lambe et al., 2003; Levy and Aoki, 2002; Lin et al., 2010; Mansvelder et al., 2002; Nashmi and Lester, 2006). Moreover, presynaptic nAChRs exist as both auto- and heteroreceptors and are known to modulate the release of multiple neurotransmitters including the monoamines, ACh, glutamate and GABA either directly or by interacting with other co-existing receptors at the terminals (Marchi and Grilli, 2010; Sher et al., 2004; Wonnacott, 1997). Within the mammalian brains, α7 and α4β2 nAChRs are the most predominant and widely distributed receptors among the nAChR family. α4β2 nAChRs possess high affinity for nicotine and have been shown to slowly desensitize and upregulate following continued nicotine exposure (Buisson and Bertrand, 2001; Fenster et al., 1999; Govind et al., 2009; Marks et al., 1983; Schwartz and Kellar, 1983; Yates et al., 1995). On the other hand, α7 nAChRs are characterized by fast activation/desensitization and enhanced Ca2+ permeability but possess low affinity for nicotine (Castro and Albuquerque, 1995; Papke and Porter Papke, 2002; Seguela et al., 1993; Sharma and Vijayaraghavan, 2008). By virtue of higher Ca2+ permeability, α7 nAChRs are also known to facilitate downstream signaling pathways such as ERK1/2 and CREB (Bitner et al., 2007; Bitner et al., 2010). Though α4β2 nAChRs have also been shown to gate Ca2+ (Karadsheh et al., 2004; Tapia et al., 2007).
Animal studies utilizing transgenic mice have implicated α4β2 and α7 nAChRs receptors in various behavioral processes. β2 nAChR null mutant mice did not self-administer nicotine (Picciotto et al., 1998); however, re-expression of this receptor subunit in the ventral tegmental area (VTA) restored the nicotine self-administration response (Pons et al., 2008). The rewarding effects of nicotine tested using the conditioned place preference paradigm were also not observed in mice lacking the β2 subunits of nAChRs (Walters et al., 2006). Because β2 subunit-containing nAChRs mediate the reinforcing and rewarding effects of nicotine, α4β2 nAChRs are suggested to contribute to nicotine addiction-like behaviors (Dani and De Biasi, 2001; Changeux 2010). This nAChR subtype has also been associated with the regulation of mood and anxietylike behavior. For example, targeted deletion of α4 subunits of the nAChRs specifically from dopaminergic neurons decreased sensitivity to the anxiolytic effects of nicotine (McGranahan et al., 2011). On the other hand, knock in mice that harbored a leucine to serine mutation in the α4 nAChR gene displayed higher anxiety (Labraca et al., 2001). Reducing nAChR signaling through α4β2 nAChRs has been proposed to be a strategy to combat mood and anxiety disorders (Picciotto et al., 2015; Shytle et al., 2002). Besides exerting effects on reward and affective processes, nAChRs have been involved in regulating a number of cognitive processes. Mice with the genetic deletion of α7 or β2 nAChR subunits displayed deficits in attention, spatial discrimination, working memory and executive functions (Fernandes et al., 2006; Granon et al., 2003; Guillem et al., 2011; Levin et al., 2009; Young et al., 2007; 2011). Nicotine-induced enhancement of sensorimotor gating was not observed in α7 nAChR mutant mice (Azzopardi et al., 2013). Chronic nicotine produced impairments in reversal learning and these deficits were not observed in β2 nAChR heterozygous mice (Cole et al., 2015). Moreover, β2 nAChR knockout mice lacked nicotine withdrawal-related deficits in contextual fear conditioning (Portugal et al., 2008). Collectively, these findings indicated that endogenous ACh signaling by both α7 and β2 nAChR receptors is important to maintain cognitive performance under baseline conditions. Moreover, these nAChRs also mediate the effects of nicotine on cognitive functions.
3.2. nAChR dysregulation in schizophrenia
Postmortem evaluations of the brains of patients with schizophrenia show reduced expression of the low-affinity α7 nAChRs in multiple brain regions including the hippocampus, reticular nucleus of the thalamus, and cortex (Court et al., 1999; Freedman et al., 1995; Guan et al., 1999; Kunii et al., 2015; Leonard et al., 2007; Olincy and Stevens, 2007). However, the mRNA and protein levels of α7 nAChR were found to be similar between smokers with schizophrenia and healthy smokers indicating an aberrant assembly or trafficking of these receptors in schizophrenia that may be corrected by smoking (Mexal et al., 2010). On the other hand, autoradiographic studies indicated an absence of the upregulation of the high-affinity α4β2 nAChRs in the cortex, striatum and hippocampus of smokers with schizophrenia, which is typically seen in normal smokers (Breese et al., 2000; Durany et al., 2000). Recent human neuroimaging studies employing positron emission tomography (PET) technique also show reduced β2*-nAChR availability in smokers with schizophrenia as compared to smokers without schizophrenia (Brašić et al., 2012) but higher upregulation in comparison to non-smoker schizophrenia patients (Esterlis et al., 2014). These findings suggested that either desensitization or turnover of α4β2 nAChRs is abnormal in subjects with schizophrenia. Accumulating evidence from genetic studies points towards an association between schizophrenia and polymorphism in the α7 nAChR subunit (CHRNA7) gene (Freedman et al., 2001; Leonard et al., 2000; Leonard et al., 1998a). Moreover, a recent study by Kunii et al. (2015) reported overexpression of the chimeric gene α7 nAChRs (CHRFAM7A) that is considered to be a dominant negative regulator of α7 nAChR function in the dorsolateral prefrontal cortex (PFC) of patients with schizophrenia. A genetic interaction between the α4 and β2 subunits and schizophrenia has also been reported suggesting a possible link between schizophrenia and α4β2 nAChRs (De Luca et al., 2006; Voineskos et al., 2007). Together these studies indicate that both the high- and low-affinity nAChRs are dysregulated in schizophrenia and higher nicotine use may represent an attempt to restore nAChR function in schizophrenia. However, it must be noted that, chronic administration of antipsychotic drugs in rats affected nAChR expression in different brain regions (Terry et al., 2003; 2005). Therefore, it is possible that some of the effects on nAChR densities in smokers with schizophrenia may occur as consequence of interactions between nicotine and antipsychotic drugs. Table 1 provides a list of studies evaluating nAChR density in smoker and non-smoker schizophrenia patients.
Table 1.
Postmortem studies evaluating nAChR densities in smoker and non-smoker schizophrenia patients.
| nAChRs | Imaging Technique | Comparison | Brain Regions | Effects | Study |
|---|---|---|---|---|---|
| α7 nAChRs |
α-bungarotoxin (αBGT) autoradiography |
SZ vs. normal |
hippocampus, thalamus and cortex |
reduced α7 nAChRs density |
Court et al. 1999; Freedman et al., 1995 Guan et al. 1999 |
| Fluorescent in- situ hybridization |
SZ vs. normal |
dorsolateral PFC |
overexpression of the chimeric gene for α7 nAChRs (CHRFAM7A) |
Kunii et al, 2015 | |
| α4β2nAChRs | [3H]-epibatidine binding; [3H]cytisine binding |
SZ smokers vs. normal smokers |
cortex, striatum and hippocampus |
reduced α4β2 nAChRs density |
Breese et al., 2000; Durany et al., 2000 |
| PET (2-[18F]FA) | SZ smokers vs. normal smokers |
thalamus | reduced β2- nAChR availability |
Brašić et al. 2012 | |
| PET ([123I]5-IA) | SZ smokers vs. SZ non- smokers |
frontal cortex, parietal cortex, and striatum |
higher β2-nAChR upregulation |
Esterlis et al., 2014 | |
| SZ smokers vs. normal smokers during early abstinence |
frontal cortex, parietal cortex, and thalamus |
lower β2-nAChR availability |
D’Souza et al., 2012 |
Abbreviations: nAChR: nicotinic acetylcholine receptor; PFC, prefrontal cortex; SZ, schizophrenia.
3.3. Nicotinic modulation of cognitive and affective functions in schizophrenia
3.3.1. nAChRs and sensory processing deficits in schizophrenia
Neurocognitive dysfunction is a critical and enduring aspect of schizophrenia that may involve deficits in both bottom-up and top-down sensory processes (Javitt, 2009). Sensory gating is a fundamental brain mechanism to filter out irrelevant sensory information from the environment and this process occurs during the preattentive phase of information processing (Swerdlow et al., 1999). Gating functions are commonly assessed using the suppression of auditory P50 response or prepulse inhibition of the startle response. The P50 auditory-evoked potential is a positive waveform recorded in the EEG approximately 50 ms after an auditory click stimulus. The presentation of the second (test) stimulus, normally activate inhibitory brain mechanisms to minimize repetitive nonessential noise. This gating process results in diminished amplitude of the P50 component of the evoked response to the second stimulus relative to the first. Prepulse inhibition can be assessed by measuring a reduction in the eye blink response to a strong startling (acoustic) stimulus preceded shortly by a stimulus of subthreshold intensity. Like P50 wave suppression, prepulse inhibition of startle response is a measure of the capacity to suppress processing of or responding to a noise. Patients with schizophrenia display less ability to gate or suppress the response to the second stimulus in both measures (Adler et al., 1985; Braff et al., 2001a; Braff et al., 2001b; Braff et al., 1999; Brockhaus-Dumke et al., 2008; Chen et al., 2011; Devrim-Üçok et al., 2008; Freedman et al., 2000). This loss of gating function or pre-attentive buffering capacity is suggested to contribute to sensory overload, cognitive fragmentation and thought disorder in schizophrenia (Croft et al., 2001; Venables, 1960). Eye movements represent a way by which an individual exercises active selection over complex visual environment and eye-tracking abnormalities in schizophrenia may represent deficits in visuomotor integration (O’Driscoll and Callahan, 2008) that may influence bottom-up and top-down processing in vision perception (Gilbert and Sigman, 2007; Levy et al., 2010). Individuals with schizophrenia display deficits in multiple components of the eye-tracking response including the failure of eye velocity to match target velocity, increased frequency of saccadic eye movements and reduced acceleration of pursuit initiation (Avila et al., 2006; Levy et al., 2000; Levy et al., 2010; Ross et al., 1998; Sweeney et al., 1999). It has been suggested that eye-tracking deficits in schizophrenia may reflect a loss of cortical inhibitory mechanisms (Avila et al., 2003).
Deficits in the early stage sensory information processing have been linked to the CHRNA7 polymorphism (Freedman, 2014; Leonard et al., 1998b). Smokers with schizophrenia exhibited marked improvement in P50 sensory gating immediately after smoking a cigarette while this effect was not observed in normal smokers (Adler et al., 1998). Prepulse inhibition of the acoustic startle response improved in patients with schizophrenia when they smoked 10-min prior to testing or under smoking satiation conditions as compared to non-smoking patients (Kumari et al., 2001; Woznica et al., 2009). Likewise, eye-tracking abnormalities measured using smooth-pursuit and anti-saccade response were improved with cigarette smoking (Olincy et al., 2003; Olincy et al., 1998), nicotine nasal spray (Avila et al., 2003; Sherr et al., 2002), and nicotine patch (Depatie et al., 2002) in patients with schizophrenia. Furthermore, the administration of partial α7 nAChR agonists such as DMXB-A and tropisetron increased the inhibition of P50 auditory gating in schizophrenia (Koike et al., 2005; Olincy et al., 2006). Collectively, these findings suggest that higher nicotine use in patients with schizophrenia may represent an attempt to normalize sensory processing deficits by keeping the low-affinity nAChR activated. This view is also supported by animal studies. For example, administration of α7 nAChR antagonist α-bungarotoxin produced a profound reduction in auditory sensory gating similar to that observed in schizophrenia (Luntz-Leybman et al., 1992). DBA/2J mice that carry a polymorphism in the noncoding region of the CHRNA7 gene and express reduced α7 nAChRs in the hippocampus exhibited deficits in hippocampal (P20/N40; a rodent analog of human P50) sensory gating (Stevens et al., 1998) and prepulse inhibition of startle (Connolly et al., 2003). Moreover, intragastric administration of DMXB-A, a specific agonist for the α7 nAChRs, normalized sensory processing deficits in mice (Simosky et al., 2001). α7 nAChRs are abundant on GABAergic interneurons in the cortex (Krenz et al., 2001) and hippocampus (Heckers and Konradi, 2002), and these interneurons are disrupted in schizophrenia (Gonzalez-Burgos and Lewis, 2008; Lewis et al., 2005; Nakazawa et al., 2012). As GABA-mediated presynaptic regulation of glutamate release is critical for the gating of sensory response (Dutar and Nicoll, 1988), nicotine-mediated improvement in early information processing deficits in schizophrenia may be due to restoration of GABA transmission in these brain regions (Wing et al., 2012). Although deficits in prepulse inhibition were not observed in α7 nAChR knockout mice, these mice did show impairments in attention (Young et al., 2007). It has been suggested that neurobiological mechanisms underlying prepulse inhibition and P50 gating measures differ between schizophrenia patients and healthy control subjects (Oranje et al., 2006). Additionally, the neurochemical modulation of auditory gating measures of N40 suppression and prepulse inhibition diverge in rats (Swerdlow et al., 2006). Therefore, prepulse inhibition in rodent models may not serve as a sole indicator of sensory processing deficits in schizophrenia and α7 nAChR knockout mice must be evaluated in the context of other behaviors relevant to schizophrenia (Powell et al., 2009).
3.3.2. nAChRs and higher cognitive functions
Deficits in sensory gating are suggested to contribute to disrupted higher-order cognitive processes including attention, working memory and cognitive control (Lijffijt et al., 2009; Smucny et al., 2013; Yadon et al., 2009), and these functions were improved with smoking in patients with schizophrenia (Lohr and Flynn, 1992; Myers et al., 2004; Sacco et al., 2005). Moreover, withdrawal from smoking worsened performance in a visuospatial working memory task in smokers with schizophrenia (George et al., 2002). α7 nAChR knockout mice were impaired on measures of sustained attention as assessed using the 5-choice serial reaction time task (Young et al., 2007). Moreover, these mutants exhibited performance deficits under baseline conditions in the odor span task, delayed non-match to sample task, win-shift task and attention set-shifting task (Fernandes et al., 2006; Levin et al., 2009; Young et al., 2007; 2011) indicating that perturbations in α7 nAChR signaling influence a wide range of executive processes. Administration of α7 nAChR full/partial agonists or positive allosteric modulators (PAMs) exerted beneficial effects on attention, working and recognition memory, and cognitive flexibility in normal rodents and aged non-human primates (Boess et al., 2007; Bitner et al., 2007; Callahan et al., 2013; Levin et al., 1999; Nikiforuk et al., 2016; Rezvani et al., 2009; Tietje et al., 2008). On the other hand, systemic or intracranial infusions of α7 nAChR antagonist produced impairments in attention and working memory in normal rats (Chan et al., 2007; Hahn et al., 2011; Levin et al., 2002). α7 nAChR modulators have also been shown to improve attention and cognitive flexibility in animal models used to study cognitive symptoms of schizophrenia such as the developmental models (neonatal rat ventral hippocampus lesion, th(tk-)/th(tk-) mice), models of NMDA glutamate receptor hypofunction (MK801, phencyclidine, ketamine, kynurenic acid) and inbred mouse model (DBA/2J) with poor sensory gating (Alexander et al., 2012; Barak et al., 2009; Brooks et al., 2012; Hauser et al., 2009; Jones et al., 2014; McLean et al., 2012). Higher rate of smoking with higher nicotine extraction in schizophrenia patients has been suggested to activate the low-affinity α7 nAChRs (Young and Geyer, 2013). Additionally, evidence from clinical trials of drugs acting at the α7 nAChRs indicated improvement in attentional capacities in patients suffering from schizophrenia (Lieberman et al., 2013; Olincy et al., 2006). Together, these findings indicate that nicotine’s procognitive effects in smokers with schizophrenia are partly mediated by α7 nAChRs.
Chronic administration of α4β2 nAChR agonists also exerted beneficial effects on attention in both healthy adults and adults suffering from ADHD (Apostol et al., 2012; Wilens and Decker, 2007). Although direct clinical evidence of the involvement of α4β2 nAChRs in the cognitive symptoms of schizophrenia is lacking, smoking-induced activation of these high-affinity nAChRs in subjects with schizophrenia is also hypothesized to exert procognitive effects (D’Souza and Markou, 2012). In this context, animal studies utilizing mice with the genetic deletion of β2 nAChR subunits reported deficits in attentional processing and conflict resolution (Granon et al., 2003; Guillem et al., 2011). Moreover, pharmacological stimulation of α4β2 nAChRs by specific ligands facilitated baseline attentional performance and performance under conditions of higher attentional load (Howe et al., 2010; McGaughy et al., 1999; Mohler et al., 2010) while antagonism of these receptors produced deficits in attention and working memory (Chan et al., 2007; Hahn et al., 2011; Levin et al., 2002). Table 2 provides a summary of animal and human studies on the effects of genetic and pharmacological manipulations of α7 and α4β2 nAChRs on cognitive processes as it relates to schizophrenia.
Table 2.
Animals studies evaluating the contributions of α7 and α4β2 nAChRs in the cognitive processes affected in schizophrenia.
| nAChRs | Manipulation | Species | Model | Effects | Study | |
|---|---|---|---|---|---|---|
|
Genetic studies |
α7 nAChRs |
α7 subunit deletion |
mice | normal animals | impaired attention, short-term memory, working memory, decision making, cognitive flexibility |
Fernandes et al., 2006; Levin et al., 2009; Young et al., 2007; 2011 |
|
α4β2 nAChRs |
β2 subunit deletion |
mice | normal animals | deficits in attentional processing and conflict resolution |
Granon et al., 2003; Guillem et al., 2011 |
|
| Antagonists |
α7 nAChRs |
α-bungarotoxin, methyllycaconitine |
rats | normal animals | reduced auditory sensory gating and impaired working memory |
Luntz-Leybman et al., 1992; Chan et al., 2007; Hahn et al., 2011; Levin et al., 2002 |
|
α4β2 nAChRs |
dihydro-β- erythroidine |
rats | normal animals | impaired working memory |
Chan et al., 2007; Levin et al., 2002 |
|
|
Full agonists |
α7 nAChRs |
A-582941, TC- 5619, PNU- 282987 |
mice, rats | SZ model (transgenic th(tk−)/th(tk−) mice/ DBA/2 mice/ MK801) |
reversed sensory gating and attentional set-shifting deficit in SZ model |
Bitner et al., 2007; Hauser et al., 2009; Jones et al., 2014 |
| ABBF, A-582941, AR-R17779 |
mice, rats, non- human primates |
normal animals |
enhanced sensory gating, working memory, and recognition memory in normal animals |
Bitner et al., 2007; Boess et al., 2007; Levin et al., 1999; Tietje et al., 2008 |
||
|
α4β2 nAChRs |
S38232, ABT- 418, ABT-594 |
rats | normal animals | enhanced attention |
Howe et al., 2010; McGaughy et al., 1999; Mohler et al., 2010 |
|
|
Positive allosteric modulators (PAMs) |
α7 nAChRs |
PNU-120596, CCMI, galantamine |
rats, non- human primates |
SZ model in rats (KYN/ PCP/ ketamine), aged rats and rhesus monkeys |
normalized attentional set-shifting and recognition memory in SZ models, improved spatial and working memory in aged animals |
Alexander et al., 2012; Callahan et al., 2013; McLean et al., 2012; Nikiforuk et al., 2016 |
|
Partial agonists |
α7 nAChRs |
R3487/MEM3454 | rats | normal animals | enhanced attention | Rezvani et al., 2009 |
| DMXB-A (GTS21), SSR180711 |
mice, rats | SZ model (NVH/ MK- 801/ DBA/2 mice) |
normalized attentional set- shifting, latent inhibition, and sensory processing |
Barak et al., 2009; Brooks et al., 2012; Jones et al., 2014; Simosky et al., 2001 |
Abbreviations: KYN, kynurenic acid; NVH, neonatal ventral hippocampus; PCP, phencyclidine; SZ, schizophrenia; th(tk−)/th(tk−), dominant negative mutant of the fibroblast growth factor receptor-1 in tyrosine hydroxylase (TH)-expressing neurons.
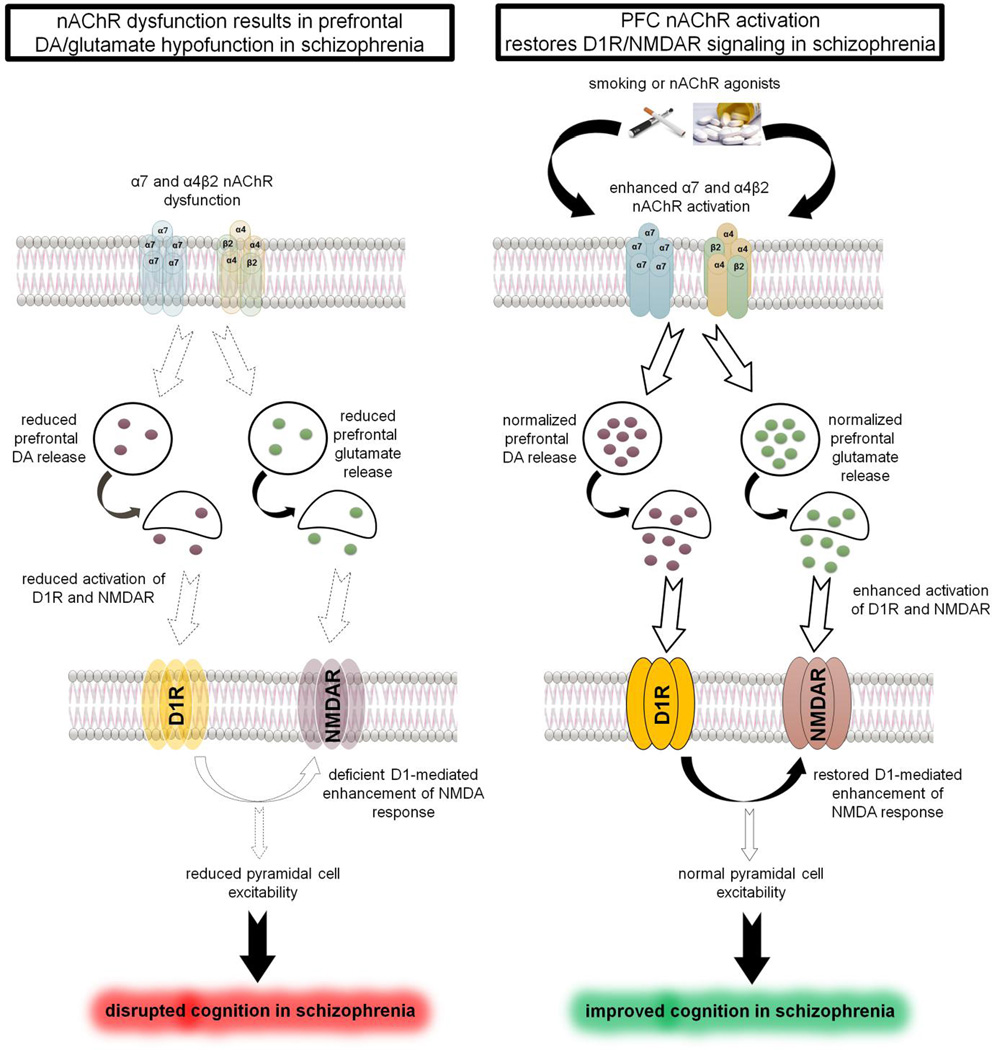
DA deficiency and disrupted D1 receptors signaling in the PFC is linked to the attention, working memory and executive control deficits in schizophrenia (Goldman-Rakic et al., 2004; Brisch et al., 2014). Both α7 and β2 subunit-containing nAChRs are known to exist on dopaminergic terminals in the PFC and modulate DA release (Cao et al., 2005; Livingstone et al., 2010; Livingstone et al., 2009). Additionally, nAChRs also regulate prefrontal glutamate release primarily from the thalamocortical terminals, which is critical for attentional processing (Konradsson-Geuken et al., 2009; Lambe et al., 2003; Parikh et al., 2010; Parikh et al., 2008; Sarter and Polone, 2011). Furthermore, aberrations in NMDA glutamate receptor-mediated signaling have been proposed to be one of the underlying mechanisms contributing to the cognitive symptoms of schizophrenia (Moghaddam and Javitt, 2012). D1 receptor activation also facilitates NMDA responsiveness via intracellular Ca2+ and protein kinase A, and synergistic interactions between these two receptors are critical for normal cortical pyramidal cell excitability and PFC function (Sarantis et al., 2009; Tseng and O’Donnell, 2004). As noted above, intracortical infusions of both α4β2 and α7 nAChR antagonists impaired working memory in rats (Chan et al., 2007). Therefore, stimulation of prefrontal nAChRs either by nicotine or specific α4β2/α7 nAChR agonists may normalize DA-glutamate release and restore D1/NMDA receptor signaling and synergism to ameliorate some of the cognitive deficits in patients with schizophrenia (see Figure 1).
Figure 1.
Cartoon depicting the role of prefrontal nAChRs in cognitive deficits of schizophrenia. Left Panel: nAChR dysfunction in schizophrenia produces deficient DA and glutamate release in the PFC resulting in diminished D1 and NMDA receptor signaling. Reduced synergy between D1 and NMDA receptor disrupts PFC excitability and cognitive functions. Right Panel: Smoking (nicotine) or activation α4β2 and/or α7 nAChRs increases DA/glutamate release in the PFC and restore synergistic interactions between the DA and glutamate signaling to normalize PFC function and improve cognition in schizophrenia.
3.3.3. nAChRs and reward function in schizophrenia
Negative symptoms of schizophrenia such as lack of motivation to pursue goals, anhedonia and reduced emotional reactivity to affective stimuli are ascribed to deficits in reward processing (Gold et al., 2008; 2012). Although direct evidence for the role of brain reward systems in comorbidity of nicotine dependence and schizophrenia is lacking, human studies have suggested that smokers with schizophrenia find smoking more reinforcing as compared to normal smokers (Ahnallen et al., 2012; Spring et al., 2003). Moreover, smokers with schizophrenia reported more severe craving during abstinence and smoking lapse latency in these individuals correlated with baseline depression and nicotine withdrawal symptom severity (Tidey et al., 2014). These findings indicate that negative affect in schizophrenia patients disproportionately increase the reward value of nicotine making them less likely to quit smoking.
The high-affinity α4β2 nAChRs are known to modulate reward-motivated behaviors and play a critical role in nicotine addiction (Changeux, 2010). Moreover, chronic nicotine-induced neuroadaptations underlying dependence involves desensitization and long-lasting upregulation of α4β2 nAChRs, which may contribute to heightened nicotine reward sensitivity and increase relapse vulnerability (Cosgrove et al., 2009; Hilario et al., 2012; Kenny and Markou, 2006; Picciotto et al., 2008). Vulnerability to nicotine dependence is related to high nicotine sensitivity (Pomerleau et al., 1993). Genetic studies have identified several chromosomal regions and specific polymorphisms that may account for individual differences in nicotine sensitivity (Li et al., 2008; Portugal and Gould, 2008). One genetic variant that has been implicated in altering nicotine sensitivity in mice is the naturally occurring T529A polymorphism in CHRNA4, the gene that encodes the nAChR α4 subunit. This polymorphism involves a substitution of threonine to alanine at position 529 of the α4 subunit that results in enhanced α4β2 nAChR function (Butt et al., 2003; Dobelis et al., 2002; Kim et al., 2003). Wilking and colleagues (Wilking et al., 2010) found that CHRNA4 A529 knock-in mice exhibited greater sensitivity to the hypothermic effects of nicotine. Moreover, these animals did not develop conditioned place preference to nicotine, further illustrating that the A529 polymorphism affected the sensitivity to the rewarding properties of nicotine by altering the α4β2 nAChR function. Although this missense polymorphism has not been reported in humans, an association study of the CHRNA4 gene polymorphism and heavy smoking among schizophrenia patients showed a significant association between the CHRNA4 rs3746372 allele 1 and the number of cigarettes smoked daily (Voineskos et al., 2007). Thus, it appears that α4β2 nAChR function appears to be a critical regulator of nicotine reward sensitivity.
Recent human PET/SPECT imaging studies that employed a β2 nAChR agonist radiotracer [123I]5-IA-85380 showed not only lower α4β2 nAChR availability in smokers with schizophrenia as compared to normal smokers but also a negative correlation between the receptor availability and negative symptoms (D’Souza et al., 2012; Esterlis et al., 2014). Smoking cigarettes high in nicotine content reduced negative symptoms of schizophrenia more than denicotinized cigarettes (Smith et al., 2002). However, short-term smoking abstinence did not worsen the negative symptoms of schizophrenia (D’Souza et al., 2012). It is therefore possible that although nicotine intake may restore affective function in schizophrenia, the negative symptoms are not the primary force driving the urge to smoke in patients with schizophrenia.
Extensive evidence suggests that the reinforcing effects of nicotine primarily involve the activation of mesolimbic DA neurons in the ventral tegmental area (VTA) and consequent release of DA in striatal synapses via the α4β2 nAChRs (Dani and De Biasi, 2001; Laviolette and Van Der Kooy, 2004; Leslie et al., 2013). Therefore, a dysregulation of α4β2 nAChR function in schizophrenia and higher smoking rates in individuals suffering from this disorder may be related to reduced nicotine sensitivity and represent an approach to restore DA reward function by activating these nAChRs. The low-affinity α7 nAChRs are known to be localized on the glutamatergic afferents in the VTA and regulate DA release in the nucleus accumbens, and the desensitization of these receptors is suggested to motivate nicotine self-administration (Brunzell and McIntosh, 2012). The α7 nAChR mutant mice exhibited impaired performance on the appetitive learning task (Keller et al., 2005); however, the motivation to obtain natural reward was not affected in these mice (Young et al., 2011). Moreover, the administration of α7 agonist or genetic deletion of α7 nAChR subunit did not affect nicotine reward and reinforcement (Grottick et al., 2000; Pons et al., 2008). Based on these studies, it appears that perhaps α7 nAChRs are not directly involved in regulating reward function and may not contribute to altered reward sensitivity in schizophrenia patients.
3.4. Early nicotine use and risk of schizophrenia: nAChR dysfunction as a shared vulnerability factor
A recent meta-analysis of prospective case-control studies on tobacco users indicated that nicotine use by itself may be associated with increased risk for psychosis and early age of onset of psychosis as compared to non-smokers (Gurillo et al., 2015). The study found that the overall prevalence of smoking in subjects with first episode of psychosis was three times higher (odds ratio 3.22) as compared to non-smokers. Moreover, this analysis reported that daily smokers developed psychiatric illness a year earlier and the risk of developing new psychotic disorders in smokers was higher (overall risk ratio 2.18) as compared to non-smokers. Another recent study that used Cox proportional hazard regression model to investigate the association between smoking and time to schizophrenia diagnosis in Swedish population found that the risk for schizophrenia was substantially higher in heavy smokers as compared to the light smokers (Kendler et al., 2015). Although these data do not establish causal links between smoking and schizophrenia, genetic studies have implicated multiple nAChR genes for a possible association between the two conditions. For example, a genetic variation in CHRNA7 has been associated with increased vulnerability for nicotine dependence phenotype in those individuals that are diagnosed with schizophrenia (De Luca et al., 2004; Saccone et al., 2010). Another genome wide-association study of schizophrenia identified a genetic loci on chromosome 15 that contain a cluster of α3α5β4 nAChR subunit-containing genes CHRNA5/A3/B4 (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014); this cluster has also been associated with nicotine dependence and early age at onset of smoking (Berrettini et al., 2008; Gage and Munafò, 2015; Hartz et al., 2012; Saccone et al., 2007; Saccone et al., 2008). Moreover, a study conducted in transgenic mice overexpressing the CHRNA5/A3/B4 genomic cluster found increased acquisition of nicotine self-administration (Gallego et al., 2012). Therefore, it is plausible that a genetic defect in nAChRs makes individuals more susceptible to heavy smoking at an early age, which could increase the risk of developing schizophrenia, though this needs further examination. It is noteworthy that transgenic mice overexpressing the human CHRNA5/A3/B4 genomic cluster exhibited impairments in novelty recognition memory and chronic nicotine reversed these deficits (Molas et al., 2014). Likewise, the beneficial effects of nicotine on visual attention were not observed in α5 nAChR knockout mice (Bailey et al., 2010). Whether heavy nicotine use in adolescents represent a strategy to reverse cognitive deficits that might have emerged due to disrupted CHRNA5/A3/B4 function remains to be investigated.
If smoking predicts the subsequent risk for schizophrenia, the important question that needs an answer would be what causes adolescent cigarette smoking. Impulsivity is a multifaceted construct that includes lack of inhibitory control, lack of forethought, and inability to delay gratification (see Evenden, 1999; Whiteside and Lynam, 2001 for reviews). An animal study utilizing rats trained to self-administer nicotine and perform a delayed reward task found that impulsive choice predicted a diminished ability to inhibit nicotine seeking during abstinence (Diergaarde et al., 2008). The same study also reported that impulsive action assessed using the 5-choice serial reaction time task was associated with higher rates of nicotine self-administration. Another study that compared the effects of acute nicotine in rats performing the go/no-go and delayed reinforcement tasks found that nicotine produced both behavioral disinhibition and heightened sensitivity to delayed gratification (Kolokotroni et al., 2014). Likewise, numerous human studies indicated that different components of impulsivity may contribute to increased smoking initiation and inability to quit smoking. For instance, smokers discounted the value of delayed money or the uncertainty associated with obtaining money more than the non-smokers (Audrain-McGovern et al., 2009; Bickel et al., 1999; Mitchell, 1999; Reynolds et al., 2004). In the go/no-go task, the behavioral accuracy and the amplitudes of no-go event related potentials (N2 and P300) were lower in adolescent and young adult smokers indicative of impulsive action and deficits in response inhibition in these individuals (Luijten et al., 2011; Yin et al., 2015). Assessment of impulse control function using the oculomotor task also showed higher antisaccade errors in smokers (Spinella, 2002; Powell et al., 2010). Likewise, measures of trait impulsivity evaluated through the self-reported personality test, the Barret Impulsivenes Scale, found a strong association between the total impulsivity score and measures of nicotine dependence (Chase and Hogarth, 2011; Flory and Manuck, 2009). As smokers with schizophrenia were found to be more impulsive than non-smokers with schizophrenia (Wing et al., 2011), it is conceivable that trait impulsivity in adolescents may determine smoking initiation while disinhibition impulsiveness lead to smoking maintenance that eventually increases susceptiblity to develop schizophrenia-like pathology.
Stimulation of α4β2 nAChRs has been linked to nicotine-induced deficits in premature responding, a measure of impulsive choice, in animal studies using either a 5-choice or a 3-choice serial reaction time task (Blondel et al., 2000; Grottick and Higgins, 2000; Tsutsui-Kimura et al., 2010). Additionally, intracerebroventricular infusion of α4β2 nAChR antagonist suppressed impulsive behavior in normal rats that have not been given nicotine further supporting the hypothesis that activation of α4β2 nAChRs may provoke impulsivity in normal individuals that do not suffer from any psychiatric condition (Tsutsui-Kimura et al., 2010). Nicotine-induced impulsivity is hypothesized to involve DA release in the corticolimbic circuits triggered by the activation of α4β2 nAChRs (Ohmura et al., 2012). As impulsivity traits are evident during adolescence (Romer, 2010), and reduced striatal DA D2/D3 receptor function has been linked to trait impulsivity (Dalley et al., 2007), it is possible that dysregulated mesolimbic DA signaling may contribute to increased smoking initiation and restore dopaminergic signaling via α4β2 nAChRs. It must be noted that α7 nAChRs are also known to regulate behavioral inhibition (Keller et al., 2005). Likewise, a polymorphism in CHRNA5/A3/B4 gene cluster is also linked to impulsivity (Stephens et al., 2012). Thus, an early defect in the function of these receptors may also contribute to smoking initiation. However, nicotine use by itself may aggravate impulsivity and reward sensitivity presumably by upregulation of α4β2 nAChRs that leads to smoking maintenance. Moreover, tolerance to the behavioral effects of nicotine as a consequence of nAChR desensitization and inactivation eventually leads to smoking maintenance in schizophrenia-susceptible adolescents. Chronic nicotine use in these individuals may produce maladaptive changes in brain circuits and neurotransmitter dysregulation making them more sensitive to withdrawal-related worsening of the cognitive and affective symptoms. Thus a vicious cycle between smoking, impulsivity and withdrawal-related cognitive deficits is maintained that might put an individual at risk of developing schizophrenia (see proposed model in Figure 2). This view is consistent with a recent study conducted in rats that reported that baseline levels of impulsive choice behavior is an important determinant of the effects of chronic nicotine-induced aggravation of impulsivity and compulsive nicotine use (Kayir et al., 2014).
Figure 2.
A hypothetical model illustrating the central role of nAChRs and trait impulsivity in increasing the risk of schizophrenia in adolescent nicotine users. Susceptibility mechanisms such as a defective nAChR system (e.g. CHRNA7, CHRNA5/A3/B4 polymorphism) or D2/D3 receptor hypofunction may make adolescents more impulsive and engage in smoking initiation. Nicotine use by itself may aggravate impulsivity by upregulating the high-affinity α4β2 nAChRs leading to smoking maintenance. Sustained nicotine use initiates a vicious cycle in schizophrenia-susceptible adolescents that involves tolerance to the behavioral effects of nicotine, maladaptive circuit and neurochemical changes, enhanced sensitivity to withdrawal-related worsening of the cognitive and affective symptoms and higher relapse rates. Thus, nicotine use maintained in adolescent smokers may eventually put them at higher risk of developing schizophrenia.
It is intriguing that patients with first episode psychosis have smoked for an average of 5.3 years prior to the onset of psychosis (Myles et al., 2012). If nAChR dysregulation and impulsivity leading to higher smoking in adolescents is causal for schizophrenia, it remains to be answered why the onset of psychosis in smokers is delayed for many years. One possibility is that prolonged exposure to high nicotine concentrations might produce maladaptive changes in dopaminergic signaling by altering the nAChR function in impulsive individuals. This view is supported by the evidence that nicotine increases striatal DA D2 receptor sensitivity (Novak and Seeman, 2010), which has been proposed to be an underlying pathogenic mechanism for psychosis (Howes and Kapur, 2009; Seeman and Seeman, 2014). How prolonged nicotine use produces a transition from the striatal hypodopaminergic function that might occur due to disrupted nAChR signaling to a hyperdopaminergic D2 state observed in psychosis remains to be investigated. To summarize, whether smoking has causal role in schizophrenia and what mechanisms might underlie the causal association remains an interesting and a very important question. Although the presented evidence to establish that early cigarette smoking and schizophrenia might have causal relationships is limited, it is becoming apparent that self-medication may not entirely explain schizophrenia-nicotine dependence comorbidity and shared vulnerability factors need to be considered to study these relationships. Future longitudinal and prospective studies with large sample sizes are required to test the hypothesis that high nicotine use during adolescence produces psychosis.
3.5. Targeting nAChRs to attenuate symptoms of schizophrenia and schizophrenia- smoking comorbidity
Evidence from animal and human studies have indicated that α7 nAChRs may serve as a therapeutic targets for improving cognition, memory, and sensory gating deficits in schizophrenia (Bitner et al., 2007; Freedman et al., 2008; Hajos and Rogers, 2010; Marquis et al., 2011; Rezvani et al., 2009; Young and Geyer, 2013). Numerous α7 nAChR agonists have shown to exert procognitive effects in preclinical models and some of them are currently in clinical development for cognitive symptoms of schizophrenia. Phase II clinical trials involving the treatment of schizophrenia patients with a partial α7 nAChR agonist GTS-21 (DMXB-A) showed improvements in P50 sensory gating function, attention, working memory and negative symptoms in schizophrenia patients (Freedman et al., 2008; Olincy and Freedman, 2012; Olincy et al., 2006). An early proof of concept phase II clinical trial conducted in patients with schizophrenia with EVP-6124 (encenicline), a new selective α7 nAChR partial agonist, reported positive effects on mismatch negativity, P300 potentials, and on various measures of cognition such as visual attention, non-verbal working memory and decision-making (Preskorn et al., 2014). Another partial α7 nAChR agonist tropisetron improved auditory sensory gating and exerted attention-enhancing effects in patients with schizophrenia (Shiina et al., 2010; Zhang et al., 2012). Moreover, the α7 nAChR full agonist TC-5619 was also found to exert beneficial effects on working memory performance in smokers with schizophrenia (Lieberman et al., 2013). However, a recent phase 2 clinical trial conducted in patients with schizophrenia comprised of both tobacco and non-tobacco users showed that TC-5619 did not improve scores on the scale for the assessment of Subject Global Impression-Cognition and the negative symptoms (Walling et al., 2015). It is noteworthy that α7 nAChR partial agonists such as encenicline, RG3487 and tropisetron, have also been reported to improve negative symptoms of schizophrenia (Keefe et al., 2015; Noroozian et al., 2013; Olincy and Freedman, 2012; Umbricht et al., 2014). Although the underlying neurobiological mechanism for the efficacy of these ligands for affective symptoms remain poorly understood, these ligands also possess serotonin 5HT3 receptor antagonistic activity and it is possible that these beneficial effects are linked to the normalization of 5HT3 signaling in schizophrenia. However, the presence of α7 nAChRs in brain regions known to be involved in regulating emotion, motivation and goal-directed behavior could also plausibly relate the affective symptoms of schizophrenia to α7 nAChR dysfunction. Future research is warranted to establish a link between α7 nAChRs and the negative symptoms of schizophrenia and whether targeting these receptors would effectively treat these symptoms.
An alternative therapeutic approach to control cognitive symptoms of schizophrenia is to develop PAMs of α7 nAChRs. PAMs bind to an allosteric site distinct from the binding site of the endogenous ligand and do not directly activate the receptor but potentiate the effects of the endogenous ligand. In this case, a key advantage of this approach would be that α7 nAChR modulation will occur only in the presence of the endogenous ligand ACh; therefore, the temporal and spatial integrity of neurotransmission is preserved (Bertrand et al., 2008). Moreover, α7 PAMs enhance the activity elicited by agonists (ACh or nicotine) either by increasing the gating process (type 1 PAMs) or by reducing the receptor desensitization (type II PAMs) (Gill-Thind et al., 2015). Thus, the use α7 PAMs may not only amplify the behavioral/cognitive effects produced by α7 nAChR activation but also prevent the tolerance to these effects that may occur due to the desensitization of these receptors (Uteshev, 2014). In this regard, galantamine, a cholinesterase inhibitor that is also a α7 nAChR-PAM has shown neurocognitive improvement in subjects with schizophrenia (Buchanan et al., 2008; Schubert et al., 2006). A number of type I and type II PAMs have shown beneficial effects on sensory gating, working memory and executive functions in animal models (reviewed in (Beinat et al., 2015; Terry Jr et al., 2015). However, a recent phase I clinical trial with JNJ-39393406, an α7 nAChR PAM, did not reveal any beneficial effects on P50 sensory gating and other electrophysiological markers of early information processing in regularly smoking patients with schizophrenia (Winterer et al., 2013). Future clinical studies with PAMs will determine whether these modulators offer any potential advantages over the orthosteric α7 nAChR ligands.
As discussed above, there is a plethora of evidence from preclinical studies indicating the efficacy of α4β2 nAChR agonists in exerting procognitive effects. Clinical studies conducted with α4β2 nAChR-selective agonists such as AZD3480, ABT-418 and ABT-089 showed improvements in attentional performance in adults with ADHD (Potter et al., 2014; Wilens et al., 1999; Wilens et al., 2006). Varenicline, which is an α4β2 nAChR partial agonist and a full agonist for α7 nAChRs (Mihalak et al., 2006), has shown significant improvements in cognitive performance of smokers with schizophrenia on the Wisconsin Card Sorting Task and the Digitial Symbol Substitution Test (Shim et al., 2012) as well as visuospatial working memory performance measured by the Spatial Delayed Response Task (Wing et al., 2013). Moreover, this drug was effective for smoking cessation in smokers with schizophrenia (Pachas et al., 2012; Williams et al., 2012). Varenicline is proposed to control affective symptoms of schizophrenia and reduce craving for smoking by stimulating DA release via α4β2 nAChRs (Coe et al., 2005). Additionally, its procognitive effects may be related to its ability to stimulate α7 nAChRs (Mackowick et al., 2014). A recent study found that maintenance therapy with varenicline prolonged tobacco abstinence rates in patients with schizophrenia (Evins et al., 2014). Moreover, schizophrenia patients with lower baseline symptoms of affective flattening were more likely to achieve smoking abstinence and demonstrated larger increases in reward sensitivity during varenicline treatment (Dutra et al., 2012). Table 3 depicts the results of clinical trials of nAChR agonists and PAMs in patients with schizophrenia.
Table 3.
Clinical studies assessing the effects of nAChR ligands on cognitive and negative symptoms, and tobacco cessation in schizophrenia patients.
| Ligand | Specificity | Stage | Effects | Study |
|---|---|---|---|---|
|
GTS-21 (DMXB-A) |
α7 nAChR partial- agonist |
Phase II clinical trial |
improved P50 sensory gating function, attention, working memory and negative symptoms |
Freedman et al., 2008; Olincy et al., 2006; Olincy and Freedman, 2012 |
|
EVP-6124 (encenicline) |
α7 nAChR partial- agonist |
Phase II clinical trial |
exerted beneficial effects on mismatch negativity, P300 potentials, visual attention, non- verbal working memory decision- making and negative symptoms |
Keefe et al., 2015; Preskorn et al., 2014; |
| Tropisetron | α7 nAChR partial- agonist |
Phase II clinical trial |
improved auditory sensory gating, attention and negative symptoms |
Noroozian et al., 2013; Shiina et al., 2010; Zhang et al., 2012 |
| RG3487 | α7 nAChR partial- agonist |
Phase II clinical trial |
improved negative symptom assessment scores |
Umbricht et al., 2014 |
| TC-5619 |
α7 nAChR full agonist |
Exploratory Trial |
enhanced working memory performance |
Lieberman et al., 2013 |
| Phase II clinical trial |
no effects on negative symptoms or Subject Global Impression- Cognition |
Walling et al., 2015 | ||
| Galantamine |
α7 nAChR PAM |
Phase III clinical trial |
improved attention and memory |
Buchanan et al., 2008; Schubert et al., 2006 |
|
JNJ- 39393406 |
α7 nAChR PAM |
Phase I clinical trial |
no beneficial effects on P50 sensory gating and other electrophysiological markers of early information processing |
Winterer et al., 2013 |
| varenicline | α4β2 nAChR partial- agonist |
Phase III clinical trial |
improved cognitive flexibility, visuoperceptual processing, and visuospatial working memory performance |
Shim et al., 2012; Wing et al., 2013 |
| Phase III clinical trial |
prolonged tobacco abstinence rates, increased in reward sensitivity, reduced negative symptoms |
Dutra et al., 2012; Evins et al., 2014; Pachas et al., 2012; Williams et al., 2012 |
Abbreviations: PAM, positive allosteric modulator.
Based on the existing evidence, it appears that both α7 and α4β2 nAChR ligands may hold promise as potential therapeutic targets in alleviating the negative and cognitive symptoms of schizophrenia. Additionally, targeting α4β2 nAChRs may also be beneficial in reducing smoking relapse in schizophrenia patients. At this point it is not known whether partial nAChR agonists would be better in clinical efficacy than full agonists as the procognitive efficacy of full α7 nAChR agonist (TC-5619) has yielded mixed results in schizophrenia patients. Likewise, it remains unclear whether mixed α4β2/α7 agonists would be superior to agonists targeting specific nAChR subtype. Rapid nAChRs desensitization is another issue with orthosteric agonists that may limit some of the clinical effects of these drugs and current evidence is limited demonstrating that PAMs would be more effective than the former. Ongoing investigation with newer nAChR ligands and results from pending clinical trials will possibly address these issues.
4. Conclusions
The research on the relationship between schizophrenia and smoking is complex and has remained inconclusive. While the debate for the self-medication versus addiction vulnerability hypothesis continues, it is widely accepted that dysfunction in central nAChRs represent a common substrate for various symptoms of schizophrenia and comorbid nicotine dependence. A growing number of studies have found evidence that there may be an interaction between the genetic and environmental factors that underlie deficient nAChR signaling (Wing et al., 2012). Nicotine is known to exert both positive and negative effects on patients with schizophrenia. Moreover, the highly addictive properties and the development of tolerance following prolonged treatment limit its use as a drug candidate. There is now convincing evidence that the development of nAChR subtype-specific agonists represents a viable therapeutic option to treat schizophrenia symptoms and minimize nicotine use in subjects with schizophrenia. Another goal of utilizing this strategy would be to improve smoking cessation to circumvent smoking-related health hazards in smokers with schizophrenia. The negative and cognitive symptoms precede the onset of psychosis (Addington et al., 2003; Simon et al., 2007; Yung and McGorry, 1996) and there is a temporal link between the initiation of smoking and the prodromal phase of schizophrenia (Riala et al., 2005). Moreover, the current antipsychotic drugs are not very successful in alleviating these symptoms. Therefore, specific nAChR ligands appear to be reasonable candidates for minimizing the use of nicotine during the earlier course of the schizophrenia pathology and eventually improving the treatment outcome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington J, van Mastrigt S, Addington D. Patterns of premorbid functioning in first-episode psychosis: initial presentation. Schizophrenia Research. 2003;62(1):23–30. doi: 10.1016/s0920-9964(02)00408-5. [DOI] [PubMed] [Google Scholar]
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, Flach K, Nagamoto H, Bickford P, Leonard S, Freedman R. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bulletin. 1998;24(2):189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Adler LE, Waldo MC, Freedman R. Neurophysiologic studies of sensory gating in schizophrenia: comparison of auditory and visual responses. Biological Psychiatry. 1985;20(12):1284–1296. doi: 10.1016/0006-3223(85)90113-1. [DOI] [PubMed] [Google Scholar]
- Ahnallen CG, Liverant GI, Gregor KL, Kamholz BW, Levitt JJ, Gulliver SB, Pizzagalli DA, Koneru VK, Kaplan GB. The relationship between reward-based learning and nicotine dependence in smokers with schizophrenia. Psychiatry Research. 2012;196(1):9–14. doi: 10.1016/j.psychres.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander KS, Wu H-Q, Schwarcz R, Bruno JP. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology. 2012;220(3):627–637. doi: 10.1007/s00213-011-2539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol G, Abi-Saab W, Kratochvil CJ, Adler LA, Robieson WZ, Gault LM, Pritchett YL, Feifel D, Collins MA, Saltarelli MD. Efficacy and safety of the novel α4β2 neuronal nicotinic receptor partial agonist ABT-089 in adults with attention-deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled crossover study. Psychopharmacology. 2012;219(3):715–725. doi: 10.1007/s00213-011-2393-2. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug and Alcohol Dependence. 2009;103(3):99–106. doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila MT, Hong LE, Moates A, Turano KA, Thaker GK. Role of anticipation in schizophrenia-related pursuit initiation deficits. Journal of Neurophysiology. 2006;95(2):593–601. doi: 10.1152/jn.00369.2005. [DOI] [PubMed] [Google Scholar]
- Avila MT, Sherr JD, Hong E, Myers CS, Thaker GK. Effects of nicotine on leading saccades during smooth pursuit eye movements in smokers and nonsmokers with schizophrenia. Neuropsychopharmacology. 2003;28(12):2184–2191. doi: 10.1038/sj.npp.1300265. [DOI] [PubMed] [Google Scholar]
- Azzopardi E, Typlt M, Jenkins B, Schmid S. Sensorimotor gating and spatial learning in α7-nicotinic receptor knockout mice. Genes, Brain and Behavior. 2013;12(4):414–423. doi: 10.1111/gbb.12038. [DOI] [PubMed] [Google Scholar]
- Bailey CD, De Biasi M, Fletcher PJ, Lambe EK. The nicotinic acetylcholine receptor α5 subunit plays a key role in attention circuitry and accuracy. The Journal of Neuroscience. 2010;30(27):9241–9252. doi: 10.1523/JNEUROSCI.2258-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S, Arad M, De Levie A, Black MD, Griebel G, Weiner I. Pro-cognitive and antipsychotic efficacy of the α7 nicotinic partial agonist SSR180711 in pharmacological and neurodevelopmental latent inhibition models of schizophrenia. Neuropsychopharmacology. 2009;34(7):1753–1763. doi: 10.1038/npp.2008.232. [DOI] [PubMed] [Google Scholar]
- Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, Deckersbach T, Kelly JF, Freudenreich O, Goff DC. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology. 2008;33(3):480–490. doi: 10.1038/sj.npp.1301423. [DOI] [PubMed] [Google Scholar]
- Beinat C, Banister SD, Herrera M, Law V, Kassiou M. The Therapeutic Potential of α7 Nicotinic Acetylcholine Receptor (α7 nAChR) Agonists for the Treatment of the Cognitive Deficits Associated with Schizophrenia. CNS Drugs. 2015;29(7):529–542. doi: 10.1007/s40263-015-0260-0. [DOI] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V. α-5/α-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Molecular Psychiatry. 2008;13(4):368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand C, Bonafos B, Tremblay M, Ferry A, Chatonnet A. Effect of fluoxetine on neuromuscular function in acetylcholinesterase (AChE) knockout mice. Chemico-Biological Interactions. 2008;175(1–3):113–114. doi: 10.1016/j.cbi.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146(4):447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bitner RS, Bunnelle WH, Anderson DJ, Briggs CA, Buccafusco J, Curzon P, Decker MW, Frost JM, Gronlien JH, Gubbins E, Li J, Malysz J, Markosyan S, Marsh K, Meyer MD, Nikkel AL, Radek RJ, Robb HM, Timmermann D, Sullivan JP, Gopalakrishnan M. Broad-spectrum efficacy across cognitive domains by alpha7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways. The Journal of Neuroscience. 2007;27(39):10578–10587. doi: 10.1523/JNEUROSCI.2444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitner RS, Bunnelle WH, Decker MW, Drescher KU, Kohlhaas KL, Markosyan S, Marsh KC, Nikkel AL, Browman K, Radek R. In vivo pharmacological characterization of a novel selective α7 neuronal nicotinic acetylcholine receptor agonist ABT-107: preclinical considerations in Alzheimer’s disease. Journal of Pharmacology and Experimental Therapeutics. 2010;334(3):875–886. doi: 10.1124/jpet.110.167213. [DOI] [PubMed] [Google Scholar]
- Blondel A, Sanger DJ, Moser PC. Characterisation of the effects of nicotine in the five-choice serial reaction time task in rats: antagonist studies. Psychopharmacology. 2000;149(3):293–305. doi: 10.1007/s002130000378. [DOI] [PubMed] [Google Scholar]
- Boess FG, De Vry J, Erb C, Flessner T, Hendrix M, Luithle J, Methfessel C, Riedl B, Schnizler K, van der Staay FJ, van Kampen M, Wiese WB, Koenig G. The novel alpha7 nicotinic acetylcholine receptor agonist N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-7-[2-(methoxy)phenyl]-1-benzofuran-2-carboxamide improves working and recognition memory in rodents. Journal of Pharmacology and Experimental Therapeutics. 2007;321:716–725. doi: 10.1124/jpet.106.118976. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Harvey PD. Schizophrenia from a neuropsychiatric perspective. Mount Sinai Journal of Medicine. 2006;73(7):993–998. [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Light GA, Sprock J, Perry W, Cadenhead KS, Swerdlow NR. Impact of prepulse characteristics on the detection of sensorimotor gating deficits in schizophrenia. Schizophrenia Research. 2001a;49(1–2):171–178. doi: 10.1016/s0920-9964(00)00139-0. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001b;156(2–3):234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff DL, Swerdlow NR, Geyer MA. Symptom correlates of prepulse inhibition deficits in male schizophrenic patients. The American Journal of Psychiatry. 1999;156(4):596–602. doi: 10.1176/ajp.156.4.596. [DOI] [PubMed] [Google Scholar]
- Brašić JR, Cascella N, Kumar A, Zhou Y, Hilton J, Raymont V, Crabb A, Guevara MR, Horti AG, Wong DF. Positron emission tomography experience with 2-[18F] fluoro-3-(2 (s)-azetidinylmethoxy) pyridine (2-[18F] fa) in the living human brain of smokers with paranoid schizophrenia. Synapse. 2012;66(4):352–368. doi: 10.1002/syn.21520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, Marks MJ, Collins AC, Leonard S. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23(4):351–364. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Brisch R, Saniotis A, Wolf R, Bielau H, Bernstein HG, Steiner J, Bogerts B, Braun K, Jankowski Z, Kumaratilake J, Henneberg M, Gos T. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Frontiers in Psychiatry. 2014;5:47. doi: 10.3389/fpsyt.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Mueller R, Faigle U, Klosterkoetter J. Sensory gating revisited: relation between brain oscillations and auditory evoked potentials in schizophrenia. Schizophrenia Research. 2008;99(1):238–249. doi: 10.1016/j.schres.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Brooks JM, Pershing ML, Thomsen MS, Mikkelsen JD, Sarter M, Bruno JP. Transient inactivation of the neonatal ventral hippocampus impairs attentional set-shifting behavior: reversal with an α7 nicotinic agonist. Neuropsychopharmacology. 2012;37(11):2476–2486. doi: 10.1038/npp.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, McIntosh JM. Alpha7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia. Neuropsychopharmacology. 2012;37(5):1134–1143. doi: 10.1038/npp.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Conley RR, Dickinson D, Ball MP, Feldman S, Gold JM, McMahon RP. Galantamine for the treatment of cognitive impairments in people with schizophrenia. The American Journal of Psychiatry. 2008;165(1):82–89. doi: 10.1176/appi.ajp.2007.07050724. [DOI] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human α4β2 nicotinic acetylcholine receptor function. The Journal of Neuroscience. 2001;21(6):1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt CM, Hutton SR, Stitzel JA, Balogh SA, Owens JC, Collins AC. A polymorphism in the α4 nicotinic receptor gene (Chrna4) modulates enhancement of nicotinic receptor function by ethanol. Alcoholism: Clinical and Experimental Research. 2003;27(5):733–742. doi: 10.1097/01.ALC.0000067973.41153.BC. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Hutchings EJ, Kille NJ, Chapman JM, Terry AV. Positive allosteric modulator of alpha 7 nicotinic-acetylcholine receptors, PNU-120596 augments the effects of donepezil on learning and memory in aged rodents and non-human primates. Neuropharmacology. 2013;67:201–212. doi: 10.1016/j.neuropharm.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y-J, Surowy CS, Puttfarcken PS. Different nicotinic acetylcholine receptor subtypes mediating striatal and prefrontal cortical [3 H] dopamine release. Neuropharmacology. 2005;48(1):72–79. doi: 10.1016/j.neuropharm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Castro NG, Albuquerque EX. alpha-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophysical Journal. 1995;68(2):516. doi: 10.1016/S0006-3495(95)80213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA. A Nicotine Challenge to the Self-Medication Hypothesis in a Neurodevelopmental Animal Model of Schizophrenia. Journal of Dual Diagnosis. 2009;5(2):139–148. doi: 10.1080/15504260902869808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biological Psychiatry. 2001;50(2):71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WK, Wong PT-H, Sheu F-S. Frontal cortical α7 and α4β2 nicotinic acetylcholine receptors in working and reference memory. Neuropharmacology. 2007;52(8):1641–1649. doi: 10.1016/j.neuropharm.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Changeux J-P. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nature Reviews Neuroscience. 2010;11(6):389–401. doi: 10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- Chase HW, Hogarth L. Impulsivity and symptoms of nicotine dependence in a young adult population. Nicotine & Tobacco Research. 2011;13(12):1321–1325. doi: 10.1093/ntr/ntr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X-s, Li C-b, Smith RC, Xiao Z-p, Wang J-j. Differential sensory gating functions between smokers and non-smokers among drug-naive first episode schizophrenic patients. Psychiatry Research. 2011;188(3):327–333. doi: 10.1016/j.psychres.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. Journal of Medicinal Chemistry. 2005;48(10):3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Cole RD, Poole RL, Guzman DM, Gould TJ, Parikh V. Contributions of β2 subunit-containing nAChRs to chronic nicotine-induced alterations in cognitive flexibility in mice. Psychopharmacology. 2015;232(7):1207–1217. doi: 10.1007/s00213-014-3754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly PM, Maxwell CR, Kanes SJ, Abel T, Liang Y, Tokarczyk J, Bilker WB, Turetsky BI, Gur RE, Siegel SJ. Inhibition of auditory evoked potentials and prepulse inhibition of startle in DBA/2J and DBA/2Hsd inbred mouse substrains. Brain Research. 2003;992(1):85–95. doi: 10.1016/j.brainres.2003.08.035. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Batis J, Bois F, Maciejewski PK, Esterlis I, Kloczynski T, Stiklus S, Krishnan-Sarin S, O’Malley S, Perry E. β2-nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Archives Of General Psychiatry. 2009;66(6):666–676. doi: 10.1001/archgenpsychiatry.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court J, Spurden D, Lloyd S, McKeith I, Ballard C, Cairns N, Kerwin R, Perry R, Perry E. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: alpha-bungarotoxin and nicotine binding in the thalamus. Journal of Neurochemistry. 1999;73(4):1590–1597. doi: 10.1046/j.1471-4159.1999.0731590.x. [DOI] [PubMed] [Google Scholar]
- Croft RJ, Lee A, Bertolot J, Gruzelier JH. Associations of P50 suppression and desensitization with perceptual and cognitive features of “unreality” in schizotypy. Biological Psychiatry. 2001;50(6):441–446. doi: 10.1016/s0006-3223(01)01082-4. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Esterlis I, Carbuto M, Krasenics M, Seibyl J, Bois F, Pittman B, Ranganathan M, Cosgrove K, Staley J. Lower ss2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia. The American Journal of Psychiatry. 2012;169(3):326–334. doi: 10.1176/appi.ajp.2011.11020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2012;62(3):1564–1573. doi: 10.1016/j.neuropharm.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315(5816):1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annual Review of Pharmacology and Toxicology. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacology Biochemistry and Behavior. 2001;70(4):439–446. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophrenia Research. 2005;76(2–3):135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- De Luca V, Wong AH, Muller DJ, Wong GW, Tyndale RF, Kennedy JL. Evidence of association between smoking and alpha7 nicotinic receptor subunit gene in schizophrenia patients. Neuropsychopharmacology. 2004;29(8):1522–1526. doi: 10.1038/sj.npp.1300466. [DOI] [PubMed] [Google Scholar]
- De Luca V, Zai G, Tharmalingam S, de Bartolomeis A, Wong G, Kennedy JL. Association study between the novel functional polymorphism of the serotonin transporter gene and suicidal behaviour in schizophrenia. European Neuropsychopharmacology. 2006;16(4):268–271. doi: 10.1016/j.euroneuro.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Depatie L, O’Driscoll GA, Holahan AL, Atkinson V, Thavundayil JX, Kin NN, Lal S. Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology. 2002;27(6):1056–1070. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- Devrim-Üçok M, Keskin-Ergen HY, Üçok A. P50 gating at acute and post-acute phases of first-episode schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32(8):1952–1956. doi: 10.1016/j.pnpbp.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Diaz F, Rendon D, Velásquez D, Susce M, de Leon J. Datapoints: Smoking and smoking cessation among persons with severe mental illnesses. Psychiatric Services (Washington, DC) 2006;57(4):462. doi: 10.1176/ps.2006.57.4.462. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeming in rats. Biological Psychiatry. 2008;63(3):301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Dobelis P, Marks MJ, Whiteaker P, Balogh SA, Collins AC, Stitzel JA. A polymorphism in the mouse neuronal α4 nicotinic receptor subunit results in an alteration in receptor function. Molecular Pharmacology. 2002;62(2):334–342. doi: 10.1124/mol.62.2.334. [DOI] [PubMed] [Google Scholar]
- Durany N, Zochling R, Boissl KW, Paulus W, Ransmayr G, Tatschner T, Danielczyk W, Jellinger K, Deckert J, Riederer P. Human post-mortem striatal alpha4beta2 nicotinic acetylcholine receptor density in schizophrenia and Parkinson’s syndrome. Neuroscience Letters. 2000;287(2):109–112. doi: 10.1016/s0304-3940(00)01144-7. [DOI] [PubMed] [Google Scholar]
- Dutar P, Nicoll R. A physiological role for GABAB receptors in the central nervous system. Nature. 1988;332:156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Dutra SJ, Stoeckel LE, Carlini SV, Pizzagalli DA, Evins AE. Varenicline as a smoking cessation aid in schizophrenia: effects on smoking behavior and reward sensitivity. Psychopharmacology. 2012;219(1):25–34. doi: 10.1007/s00213-011-2373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterlis I, Ranganathan M, Bois F, Pittman B, Picciotto MR, Shearer L, Anticevic A, Carlson J, Niciu MJ, Cosgrove KP. In Vivo Evidence for β 2 Nicotinic Acetylcholine Receptor Subunit Upregulation in Smokers as Compared With Nonsmokers With Schizophrenia. Biological Psychiatry. 2014;76(6):495–502. doi: 10.1016/j.biopsych.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden J. Impulsivity: a discussion of clinical and experimental findings. Journal of Psychopharmacology. 1999;13(2):180–192. doi: 10.1177/026988119901300211. [DOI] [PubMed] [Google Scholar]
- Evins AE, Cather C, Pratt SA, Pachas GN, Hoeppner SS, Goff DC, Achtyes ED, Ayer D, Schoenfeld DA. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. The Journal of the American Medical Association. 2014;311(2):145–154. doi: 10.1001/jama.2013.285113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RA. Upregulation of surface α4β2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. The Journal of Neuroscience. 1999;19(12):4804–4814. doi: 10.1523/JNEUROSCI.19-12-04804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, Hoyle E, Dempster E, Schalkwyk LC, Collier DA. Performance deficit of alpha7 nicotinic receptor knockout mice in a delayed matching-to-place task suggests a mild impairment of working/episodic-like memory. Genes Brain and Behavior. 2006;5:433–440. doi: 10.1111/j.1601-183X.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- Flory JD, Manuck SB. Impulsiveness and cigarette smoking. Psychosomatic Medicine. 2009;71(4):431. doi: 10.1097/PSY.0b013e3181988c2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R. alpha7-nicotinic acetylcholine receptor agonists for cognitive enhancement in schizophrenia. Annual Review of Medicine. 2014;65:245–261. doi: 10.1146/annurev-med-092112-142937. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adams C, Adler LE, Bickford PC, Gault J, Harris JG, Nagamoto HT, Olincy A, Ross RG, Stevens KE. Inhibitory neurophysiological deficit as a phenotype for genetic investigation of schizophrenia. American Journal of Medical Genetics. 2000;97(1):58–64. doi: 10.1002/(sici)1096-8628(200021)97:1<58::aid-ajmg8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biological Psychiatry. 1995;38(1):22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Freedman R, Leonard S, Gault JM, Hopkins J, Cloninger CR, Kaufmann CA, Tsuang MT, Farone SV, Malaspina D, Svrakic DM, Sanders A, Gejman P. Linkage disequilibrium for schizophrenia at the chromosome 15q13-14 locus of the alpha7-nicotinic acetylcholine receptor subunit gene (CHRNA7) American Journal of Medical Genetics. 2001;105(1):20–22. [PubMed] [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, Allensworth D, Guzman-Bonilla A, Clement B, Ball MP, Kutnick J, Pender V, Martin LF, Stevens KE, Wagner BD, Zerbe GO, Soti F, Kem WR. Initial phase 2 trial of a nicotinic agonist in schizophrenia. The American Journal of Psychiatry. 2008;165(8):1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage SH, Munafò MR. Smoking as a causal risk factor for schizophrenia. The Lancet Psychiatry. 2015;2(9):778–779. doi: 10.1016/S2215-0366(15)00333-8. [DOI] [PubMed] [Google Scholar]
- Gallego X, Molas S, Amador-Arjona A, Marks MJ, Robles N, Murtra P, Armengol L, Fernández-Montes RD, Gratacòs M, Pumarola M. Overexpression of the CHRNA5/A3/B4 genomic cluster in mice increases the sensitivity to nicotine and modifies its reinforcing effects. Amino acids. 2012;43(2):897–909. doi: 10.1007/s00726-011-1149-y. [DOI] [PubMed] [Google Scholar]
- George TP, Krystal JH. Comorbidity of psychiatric and substance abuse disorders. Current Opinion in Psychiatry. 2000;13(3):327–331. [Google Scholar]
- George TP, Vessicchio JC, Termine A, Sahady DM, Head CA, Pepper WT, Kosten TR, Wexler BE. Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology. 2002;26(1):75–85. doi: 10.1016/S0893-133X(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M. Brain states: top-down influences in sensory processing. Neuron. 2007;54(5):677–696. doi: 10.1016/j.neuron.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Gill-Thind JK, Dhankher P, D’Oyley JM, Sheppard TD, Millar NS. Structurally similar allosteric modulators of α7 nicotinic acetylcholine receptors exhibit five distinct pharmacological effects. Journal of Biological Chemistry. 2015;290(6):3552–3562. doi: 10.1074/jbc.M114.619221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Barch DM, Carter CS, Dakin S, Luck SJ, MacDonald AW, Ragland JD, Ranganath C, Kovacs I, Silverstein SM. Clinical, functional, and intertask correlations of measures developed by the Cognitive Neuroscience Test Reliability and Clinical Applications for Schizophrenia Consortium. Schizophrenia Bulletin. 2012;38(1):144–152. doi: 10.1093/schbul/sbr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophrenia bulletin. 2008;34(5):835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology. 2004;174(1):3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophrenia Bulletin. 2008;34(5):944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochemical Pharmacology. 2009;78(7):703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Govind AP, Vezina P, Green WN. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochemical Pharmacology. 2009;78(7):756–765. doi: 10.1016/j.bcp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granon S, Faure P, Changeux J-P. Executive and social behaviors under nicotinic receptor regulation. Proceedings of the National Academy of Sciences. 2003;100(16):9596–9601. doi: 10.1073/pnas.1533498100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AI. Schizophrenia and comorbid substance use disorder: effects of antipsychotics. Journal of Clinical Psychiatry. 2005;66(Suppl 6):21–26. [PubMed] [Google Scholar]
- Grottick A, Higgins G. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behavioural Brain Research. 2000;117(1):197–208. doi: 10.1016/s0166-4328(00)00305-3. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, Higgins GA. Evidence that nicotinic α7 receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. Journal of Pharmacology and Experimental Therapeutics. 2000;294(3):1112–1119. [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Blennow K, Nordberg A. Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport. 1999;10(8):1779–1782. doi: 10.1097/00001756-199906030-00028. [DOI] [PubMed] [Google Scholar]
- Guillem K, Bloem B, Poorthuis RB, Loos M, Smit AB, Maskos U, Spijker S, Mansvelder HD. Nicotinic acetylcholine receptor β2 subunits in the medial prefrontal cortex control attention. Science. 2011;333(6044):888–891. doi: 10.1126/science.1207079. [DOI] [PubMed] [Google Scholar]
- Gurillo P, Jauhar S, Murray RM, MacCabe JH. Does tobacco use cause psychosis? Systematic review and meta-analysis. The Lancet Psychiatry. 2015;2(8):718–725. doi: 10.1016/S2215-0366(15)00152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Selective nicotinic receptor antagonists: effects on attention and nicotine-induced attentional enhancement. Psychopharmacology. 2011;217(1):75–82. doi: 10.1007/s00213-011-2258-8. [DOI] [PubMed] [Google Scholar]
- Hajos M, Rogers BN. Targeting alpha7 nicotinic acetylcholine receptors in the treatment of schizophrenia. Current Pharmaceutical Design. 2010;16(5):538–554. doi: 10.2174/138161210790361434. [DOI] [PubMed] [Google Scholar]
- Hartz SM, Short SE, Saccone NL, Culverhouse R, Chen L, Schwantes-An T-H, Coon H, Han Y, Stephens SH, Sun J. Increased genetic vulnerability to smoking at CHRNA5 in early-onset smokers. Archives of General Psychiatry. 2012;69(8):854–860. doi: 10.1001/archgenpsychiatry.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Konradi C. Hippocampal neurons in schizophrenia. Journal of Neural Transmission. 2002;109(5–6):891–905. doi: 10.1007/s007020200073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekens CH. Aspirin in the treatment and prevention of cardiovascular disease: current perspectives and future directions. Current Atherosclerosis Reports. 2007;9(5):409–416. doi: 10.1007/s11883-007-0053-0. [DOI] [PubMed] [Google Scholar]
- Hilario MR, Turner JR, Blendy JA. Reward sensitization: effects of repeated nicotine exposure and withdrawal in mice. Neuropsychopharmacology. 2012;37(12):2661–2670. doi: 10.1038/npp.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Bishop JR, Palumbo D, Sweeney JA. Effect of second-generation antipsychotics on cognition: current issues and future challenges. Expert Review of Neurotherapeutics. 2010;10(1):43–57. doi: 10.1586/ern.09.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser TA, Kucinski A, Jordan KG, Gatto GJ, Wersinger SR, Hesse RA, Stachowiak EK, Stachowiak MK, Papke RL, Lippiello PM, Bencherif M. TC-5619: an alpha7 neuronal nicotinic receptor-selective agonist that demonstrates efficacy in animal models of the positive and negative symptoms and cognitive dysfunction of schizophrenia. Biochemical Pharmacology. 2009;78:803–812. doi: 10.1016/j.bcp.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe WM, Ji J, Parikh V, Williams S, Mocaer E, Trocme-Thibierge C, Sarter M. Enhancement of attentional performance by selective stimulation of alpha4beta2(*) nAChRs: underlying cholinergic mechanisms. Neuropsychopharmacology. 2010;35(6):1391–1401. doi: 10.1038/npp.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophrenia Bulletin. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Sensory processing in schizophrenia: neither simple nor intact. Schizophrenia Bulletin. 2009;35(6):1059–1064. doi: 10.1093/schbul/sbp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KM, Mcdonald IM, Bourin C, Olson RE, Bristow L, Easton A. Effect of alpha 7 nicotinic acetylcholine receptors agonists on attentional set shifting impairments in rats. Psychopharmacology. 2014;231(4):673–683. doi: 10.1007/s00213-013-3275-6. [DOI] [PubMed] [Google Scholar]
- Karadsheh MS, Shah MS, Tang X, Macdonald RL, Stitzel JA. Functional characterization of mouse alpha4beta2 nicotinic acetylcholine receptors stably expressed in HEK293T cells. Journal of Neurochemistry. 2004;91:1138–1150. doi: 10.1111/j.1471-4159.2004.02801.x. [DOI] [PubMed] [Google Scholar]
- Kayir H, Semenova S, Markou A. Baseline impulsive choice predicts the effects of nicotine and nicotine withdrawal on impulsivity in rats. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2014;48:6–13. doi: 10.1016/j.pnpbp.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Fenton WS. How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophrenia Bulletin. 2007;33(4):912–920. doi: 10.1093/schbul/sbm046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Meltzer HA, Dgetluck N, Gawryl M, Koenig G, Moebius HJ, Lombardo I, Hilt DC. Randomized, Double-Blind, Placebo-Controlled Study of Encenicline, an α7 Nicotinic Acetylcholine Receptor Agonist, as a Treatment for Cognitive Impairment in Schizophrenia. Neuropsychopharmacology. 2015;40(13):3053–3060. doi: 10.1038/npp.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley RE, DasMahapatra P, Wang J, Chen W, Srinivasan SR, Fernandez C, Xu J, Martin-Schild S, Berenson GS. Prevalence of atherosclerotic plaque in young and middle-aged asymptomatic individuals: the Bogalusa Heart Study. Southern Medical Journal. 2011;104(12):803–808. doi: 10.1097/SMJ.0b013e318236c35c. [DOI] [PubMed] [Google Scholar]
- Keller JJ, Keller AB, Bowers BJ, Wehner JM. Performance of alpha7 nicotinic receptor null mutants is impaired in appetitive learning measured in a signaled nose poke task. Behavioural Brain Research. 2005;162:43–152. doi: 10.1016/j.bbr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Lönn SL, Sundquist J, Sundquist K. Smoking and schizophrenia in population cohorts of Swedish women and men: a prospective co-relative control study. The American Journal of Psychiatry. 2015;171(11):1092–1100. doi: 10.1176/appi.ajp.2015.15010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31(6):1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Kim H, Flanagin BA, Qin C, Macdonald RL, Stitzel JA. The mouse Chrna4 A529T polymorphism alters the ratio of high to low affinity α4β2 nAChRs. Neuropharmacology. 2003;45(3):345–354. doi: 10.1016/s0028-3908(03)00167-9. [DOI] [PubMed] [Google Scholar]
- Koike K, Hashimoto K, Takai N, Shimizu E, Komatsu N, Watanabe H, Nakazato M, Okamura N, Stevens KE, Freedman R, Iyo M. Tropisetron improves deficits in audiotry P50 suppression in schizophrenia. Schizophrenia Research. 2005;76(1):67–72. doi: 10.1016/j.schres.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Kolokotroni KZ, Rodgers RJ, Harrison AA. Trait differences in response to chronic nicotine and nicotine withdrawal in rats. Psychopharmacology. 2014;231(3):567–580. doi: 10.1007/s00213-013-3270-y. [DOI] [PubMed] [Google Scholar]
- Konradsson-Geuken Å, Gash CR, Alexander K, Pomerleau F, Huettl P, Gerhardt GA, Bruno JP. Second-by-second analysis of alpha 7 nicotine receptor regulation of glutamate release in the prefrontal cortex of awake rats. Synapse. 2009;63(12):1069–1082. doi: 10.1002/syn.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenz I, Kalkan D, Wevers A, de Vos RA, Steur ENJ, Lindstrom J, Pilz K, Nowacki S, Schütz U, Moser N. Parvalbumin-containing interneurons of the human cerebral cortex express nicotinic acetylcholine receptor proteins. Journal of Chemical Neuroanatomy. 2001;21(3):239–246. doi: 10.1016/s0891-0618(01)00112-0. [DOI] [PubMed] [Google Scholar]
- Krishnadas R, Jauhar S, Telfer S, Shivashankar S, McCreadie RG. Nicotine dependence and illness severity in schizophrenia. The British Journal of Psychiatry. 2012;201(4):306–312. doi: 10.1192/bjp.bp.111.107953. [DOI] [PubMed] [Google Scholar]
- Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neuroscience & Biobehavioral Reviews. 2005;29(6):1021–1034. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Sharma T. Influence of cigarette smoking on prepulse inhibition of the acoustic startle response in schizophrenia. Human Psychopharmacology. 2001;16(4):321–326. doi: 10.1002/hup.286. [DOI] [PubMed] [Google Scholar]
- Kunii Y, Zhang W, Xu Q, Hyde TM, McFadden W, Shin JH, Deep-Soboslay A, Ye T, Li C, Kleinman JE. CHRNA7 and CHRFAM7A mRNAs: Co-Localized and Their Expression Levels Altered in the Postmortem Dorsolateral Prefrontal Cortex in Major Psychiatric Disorders. The American Journal of Psychiatry. 2015;171(11):1122–1130. doi: 10.1176/appi.ajp.2015.14080978. [DOI] [PubMed] [Google Scholar]
- Kutlu MG, Parikh V, Gould TJ. Nicotine addiction and psychiatric disorders. International Review of Neurobiology. 2015;124:171–208. doi: 10.1016/bs.irn.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labraca C, Schwarz J, Deshpande P, Schwarz S, Nowak MW, Fonck C, Nashmi R, Kofuji P, Dang H, Shi W, Fidan M, Khakh BS, Chen Z, Bowers BJ, Boulter J, Wehner JM, Lester HA. Point mutation mice with hypersensitive alpha 4 nicotinic receptors show dopaminergic deficits and increased anxiety. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(5):2786–2791. doi: 10.1073/pnas.041582598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28(2):216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. The Journal of the American Medical Association. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Van Der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nature Reviews Neuroscience. 2004;5(1):55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- Leonard S, Breese C, Adams C, Benhammou K, Gault J, Stevens K, Lee M, Adler L, Olincy A, Ross R. Smoking and schizophrenia: abnormal nicotinic receptor expression. European Journal of Pharmacology. 2000;393(1):237–242. doi: 10.1016/s0014-2999(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Leonard S, Gault J, Adams C, Breese CR, Rollins Y, Adler LE, Olincy A, Freedman R. Nicotinic receptors, smoking and schizophrenia. Restorative Neurology and Neuroscience. 1998a;12(2–3):195–201. [PubMed] [Google Scholar]
- Leonard S, Gault J, Moore T, Hopkins J, Robinson M, Olincy A, Adler LE, Cloninger CR, Kaufmann CA, Tsuang MT, Faraone SV, Malaspina D, Svrakic DM, Freedman R. Further investigation of a chromosome 15 locus in schizophrenia: analysis of affected sibpairs from the NIMH Genetics Initiative. American Journal of Medical Genetics. 1998b;81(4):308–312. doi: 10.1002/(sici)1096-8628(19980710)81:4<308::aid-ajmg6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Leonard S, Mexal S, Freedman R. Smoking, genetics and schizophrenia: evidence for self medication. Journal of Dual Diagnosis. 2007;3(3–4):43–59. doi: 10.1300/J374v03n03_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie FM, Mojica CY, Reynaga DD. Nicotinic receptors in addiction pathways. Molecular Pharmacology. 2013;83(4):753–758. doi: 10.1124/mol.112.083659. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bettegowda C, Blosser J, Gordon J. AR-R17779, and alpha7 nicotinic agonist, improves learning and memory in rats. Behavioural Pharmacology. 1999;10:675–680. doi: 10.1097/00008877-199911000-00014. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bradley A, Addy N, Sigurani N. Hippocampal alpha 7 and alpha 4 beta 2 nicotinic receptors and working memory. Neuroscience. 2002;109(4):757–765. doi: 10.1016/s0306-4522(01)00538-3. [DOI] [PubMed] [Google Scholar]
- Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, Strauss M, Avery J, Nicholson J, Rose JE. Nicotinic alpha7- or beta2-containing receptor knockout: effects on radial-arm maze learning and long-term nicotine consumption in mice. Behavioural Brain Research. 2009;196:207–213. doi: 10.1016/j.bbr.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DL, Lajonchere CM, Dorogusker B, Min D, Lee S, Tartaglini A, Lieberman JA, Mendell NR. Quantitative characterization of eye tracking dysfunction in schizophrenia. Schizophrenia Research. 2000;42(3):171–185. doi: 10.1016/s0920-9964(99)00122-x. [DOI] [PubMed] [Google Scholar]
- Levy DL, Sereno AB, Gooding DC, O’Driscoll GA. Eye tracking dysfunction in schizophrenia: characterization and pathophysiology. Current Topics in Behavioral Neuroscience. 2010;4:311–347. doi: 10.1007/7854_2010_60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy RB, Aoki C. α7 nicotinic acetylcholine receptors occur at postsynaptic densities of AMPA receptor-positive and-negative excitatory synapses in rat sensory cortex. The Journal of Neuroscience. 2002;22(12):5001–5015. doi: 10.1523/JNEUROSCI.22-12-05001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature Reviews Neuroscience. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Pierri JN, Volk DW, Melchitzky DS, Woo TU. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biological Psychiatry. 1999;46(5):616–626. doi: 10.1016/s0006-3223(99)00061-x. [DOI] [PubMed] [Google Scholar]
- Li MD, Lou X-Y, Chen G, Ma JZ, Elston RC. Gene-gene interactions among CHRNA4, CHRNB2, BDNF, and NTRK2 in nicotine dependence. Biological Psychiatry. 2008;64(11):951–957. doi: 10.1016/j.biopsych.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux P, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of Internal Medicine. 2009;151(4):W-65–W-94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Dunbar G, Segreti AC, Girgis RR, Seoane F, Beaver JS, Duan N, Hosford DA. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38(6):968–975. doi: 10.1038/npp.2012.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Lane SD, Meier SL, Boutros NN, Burroughs S, Steinberg JL, Moeller FG, Swann AC. P50, N100, and P200 sensory gating: relationships with behavioral inhibition, attention, and working memory. Psychophysiology. 2009;46(5):1059–1068. doi: 10.1111/j.1469-8986.2009.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Vicini S, Hsu F-C, Doshi S, Takano H, Coulter DA, Lynch DR. Axonal α7 nicotinic ACh receptors modulate presynaptic NMDA receptor expression and structural plasticity of glutamatergic presynaptic boutons. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(38):16661–16666. doi: 10.1073/pnas.1007397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone PD, Dickinson JA, Srinivasan J, Kew JN, Wonnacott S. Glutamate- dopamine crosstalk in the rat prefrontal cortex is modulated by alpha7 nicotinic receptors and potentiated by PNU-120596. Journal of Molecular Neuroscience. 2010;40(1–2):172–176. doi: 10.1007/s12031-009-9232-5. [DOI] [PubMed] [Google Scholar]
- Livingstone PD, Srinivasan J, Kew JN, Dawson LA, Gotti C, Moretti M, Shoaib M, Wonnacott S. α7 and non-α7 nicotinic acetylcholine receptors modulate dopamine release in vitro and in vivo in the rat prefrontal cortex. European Journal of Neuroscience. 2009;29(3):539–550. doi: 10.1111/j.1460-9568.2009.06613.x. [DOI] [PubMed] [Google Scholar]
- Lohr JB, Flynn K. Smoking and schizophrenia. Schizophrenia Research. 1992;8(2):93–102. doi: 10.1016/0920-9964(92)90024-y. [DOI] [PubMed] [Google Scholar]
- Luijten M, Little M, Franken IHA. Deficits in inhibitory control in smokers during a go/nogo task: An investigation using event-related brain potentials. PLoS One. 2011;6(4):e18898. doi: 10.1371/journal.pone.0018898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luntz-Leybman V, Bickford PC, Freedman R. Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Research. 1992;587(1):130–136. doi: 10.1016/0006-8993(92)91437-j. [DOI] [PubMed] [Google Scholar]
- Mackowick KM, Barr MS, Wing VC, Rabin RA, Ouellet-Plamondon C, George TP. Neurocognitive endophenotypes in schizophrenia: Modulation by nicotinic receptor systems. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2014;52:79–85. doi: 10.1016/j.pnpbp.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33(6):905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Marchi M, Grilli M. Presynaptic nicotinic receptors modulating neurotransmitter release in the central nervous system: functional interactions with other coexisting receptors. Progress in Neurobiology. 2010;92(2):105–111. doi: 10.1016/j.pneurobio.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. Journal of Pharmacology and Experimental Therapeutics. 1983;226:817–825. [PubMed] [Google Scholar]
- Marquis KL, Comery TA, Jow F, Navarra RL, Grauer SM, Pulicicchio C, Kelley C, Brennan JA, Roncarati R, Scali C, Haydar S, Ghiron C, Terstappen GC, Dunlop J. Preclinical assessment of an adjunctive treatment approach for cognitive impairment associated with schizophrenia using the alpha7 nicotinic acetylcholine receptor agonist WYE-103914/SEN34625. Psychopharmacology (Berl) 2011;218(4):635–647. doi: 10.1007/s00213-011-2357-6. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Freudenreich O, Wilson WH. Smoking and therapeutic response to clozapine in patients with schizophrenia. Biological Psychiatry. 1999;46(1):125–129. doi: 10.1016/s0006-3223(98)00377-1. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Decker MW, Sarter M. Enhancement of sustained attention performance by the nicotinic acetylcholine receptor agonist ABT-418 in intact but not basal forebrain-lesioned rats. Psychopharmacology (Berl) 1999;144(2):175–182. doi: 10.1007/s002130050991. [DOI] [PubMed] [Google Scholar]
- McGranahan TM, Patzlaff NE, Grady SR, Heinemann SF, Booker T. α4β2 nicotinic acetylcholine receptors on dopaminergic neurons mediate nicotine reward and anxiety relief. The Journal of Neuroscience. 2011;31(30):10891–10902. doi: 10.1523/JNEUROSCI.0937-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean SL, Idris NF, Grayson B, Gendle DF, Mackie C, Lesage AS, Pemberton DJ, Neill JC. PNU-120596, a positive allosteric modulator of α7 nicotinic acetylcholine receptors, reverses a sub-chronic phencyclidine-induced cognitive deficit in the attentional set-shifting task in female rats. Journal of Psychopharmacology. 2012;26(9):1265–1270. doi: 10.1177/0269881111431747. [DOI] [PubMed] [Google Scholar]
- Mexal S, Berger R, Logel J, Ross RG, Freedman R, Leonard S. Differential regulation of alpha7 nicotinic receptor gene (CHRNA7) expression in schizophrenic smokers. Journal of Molecular Neuroscience. 2010;40(1–2):185–195. doi: 10.1007/s12031-009-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70(3):801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37(1):4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Krystal JH. Capturing the angel in “angel dust”: twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophrenia Bulletin. 2012;38(5):942–949. doi: 10.1093/schbul/sbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler EG, Franklin SR, Rueter LE, Fox GB, Decker MW, Browman KE. ABT-594 improves performance in the 5-choice serial reaction time task under conditions of increased difficulty, sub-chronic dosing, and in poorly-performing subjects. Pharmacology Biochemistry and Behavior. 2010;95(2):146–157. doi: 10.1016/j.pbb.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Molas Casacuberta S, Gener T, Güell J, Martín M, Ballesteros Yáñez I, Sanchez Vives MV, Dierssen M. Hippocampal changes produced by overexpression of the human CHRNA5/A3/B4 gene cluster may underlie cognitive deficits rescued by nicotine in transgenic mice. Acta Neuropathologica Communications. 2014;2(1):147. doi: 10.1186/s40478-014-0147-1. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CS, Robles O, Kakoyannis AN, Sherr JD, Avila MT, Blaxton TA, Thaker GK. Nicotine improves delayed recognition in schizophrenic patients. Psychopharmacology (Berl) 2004;174(3):334–340. doi: 10.1007/s00213-003-1764-8. [DOI] [PubMed] [Google Scholar]
- Myles N, Newall HD, Curtis J, Nielssen O, Shiers D, Large M. Tobacco use before, at, and after first-episode psychosis: a systematic meta-analysis. Journal of Clinical Psychiatry. 2012;73(4):468–475. doi: 10.4088/JCP.11r07222. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62(3):1574–1583. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi R, Lester HA. CNS localization of neuronal nicotinic receptors. Journal of Molecular Neuroscience. 2006;30(1):181–184. doi: 10.1385/JMN:30:1:181. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Kos T, Hołuj M, Potasiewicz A, Popik P. Positive allosteric modulators of alpha 7 nicotinic acetylcholine receptors reverse ketamine-induced schizophrenia-like deficits in rats. Neuropharmacology. 2016;101:389–400. doi: 10.1016/j.neuropharm.2015.07.034. [DOI] [PubMed] [Google Scholar]
- Noroozian M, Ghasemi S, Hosseini SM, Modabbernia A, Khodaie-Ardakani MR, Mirshafiee O, Farokhnia M, Tajdini M, Rezaei F, Salehi B, Ashrafi M, Yekehtaz H, Tabrizi M, Akhondzadeh S. A placebo-controlled study of tropisetron added to risperidone for the treatment of negative symptoms in chronic and stable schizophrenia. Psychopharmacology. 2013;228(4):595–602. doi: 10.1007/s00213-013-3064-2. [DOI] [PubMed] [Google Scholar]
- Novak G, Seeman P. Hyperactive mice show elevated D2High receptors, a model for schizophrenia: Calcium/calmodulin-dependent kinase II alpha knockouts. Synapse. 2010;64(10):794–800. doi: 10.1002/syn.20786. [DOI] [PubMed] [Google Scholar]
- O’Driscoll GA, Callahan BL. Smooth pursuit in schizophrenia: a meta-analytic review of research since 1993. Brain and Cognition. 2008;68(3):359–370. doi: 10.1016/j.bandc.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Ohmura Y, Tsutsui-Kimura I, Yoshioka M. Impulsive behavior and nicotinic acetylcholine receptors. Journal of Pharmacological Sciences. 2012;118(4):413–422. doi: 10.1254/jphs.11r06cr. [DOI] [PubMed] [Google Scholar]
- Olincy A, Freedman R. Handbook of Experimental Pharmacology. 213. 2012. Nicotinic mechanisms in the treatment of psychotic disorders: a focus on the alpha7 nicotinic receptor; pp. 211–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, Ellis J, Zerbe GO, Leonard S, Stevens KE. Proof-of-concept trial of an α7 nicotinic agonist in schizophrenia. Archives of General Psychiatry. 2006;63(6):630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Olincy A, Johnson LL, Ross RG. Differential effects of cigarette smoking on performance of a smooth pursuit and a saccadic eye movement task in schizophrenia. Psychiatry Research. 2003;117(3):223–236. doi: 10.1016/s0165-1781(03)00022-2. [DOI] [PubMed] [Google Scholar]
- Olincy A, Ross RG, Young DA, Roath M, Freedman R. Improvement in smooth pursuit eye movements after cigarette smoking in schizophrenic patients. Neuropsychopharmacology. 1998;18(3):175–185. doi: 10.1016/S0893-133X(97)00095-X. [DOI] [PubMed] [Google Scholar]
- Olincy A, Stevens KE. Treating schizophrenia symptoms with an alpha7 nicotinic agonist, from mice to men. Biochemical Pharmacology. 2007;74(8):1192–1201. doi: 10.1016/j.bcp.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olincy A, Young DA, Freedman R. Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biological Psychiatry. 1997;42(1):1–5. doi: 10.1016/S0006-3223(96)00302-2. [DOI] [PubMed] [Google Scholar]
- Oranje B, Geyer MA, Bocker KB, Leon Kenemans J, Verbaten MN. Prepulse inhibition and p50 suppression: Commonalities and dissociations. Psychiatry Research. 2006;143:147–158. doi: 10.1016/j.psychres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Pachas GN, Cather C, Pratt SA, Hoeppner B, Nino J, Carlini SV, Achtyes ED, Lando H, Mueser KT, Rigotti NA, Goff DC, Evins AE. Varenicline for Smoking Cessation in Schizophrenia: Safety and Effectiveness in a 12-Week, Open-Label Trial. Journal of Dual Diagnosis. 2012;8(2):117–125. doi: 10.1080/15504263.2012.663675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Porter Papke JK. Comparative pharmacology of rat and human α7 nAChR conducted with net charge analysis. British Journal of Pharmacology. 2002;137(1):49–61. doi: 10.1038/sj.bjp.0704833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Ji J, Decker MW, Sarter M. Prefrontal beta2 subunit-containing and alpha7 nicotinic acetylcholine receptors differentially control glutamatergic and cholinergic signaling. The Journal of Neuroscience. 2010;30(9):3518–3530. doi: 10.1523/JNEUROSCI.5712-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Man K, Decker MW, Sarter M. Glutamatergic contributions to nicotinic acetylcholine receptor agonist-evoked cholinergic transients in the prefrontal cortex. The Journal of Neuroscience. 2008;28(14):3769–3780. doi: 10.1523/JNEUROSCI.5251-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Progress in Neurobiology. 2008;84(4):329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Lewis AS, van Schalkwyk GI, Mineur YS. Mood and anxiety regulation by nicotinic acetylcholine receptors: a potential pathway to modulate aggression and related behavioral states. Neuropharmacology. 2015;96(Pt B):235–243. doi: 10.1016/j.neuropharm.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux J-P. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391(6663):173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Collins AC, Shiffman S, Pomerleau CS. Why some people smoke and others do not: new perspectives. Journal of Consulting and Clinical Psychology. 1993;61(5):723. doi: 10.1037//0022-006x.61.5.723. [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh J, Changeux J, Maskos U, Fratta W. Crucial role of α4 and α6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. The Journal of Neuroscience. 2008;28(47):12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Genetic variability in nicotinic acetylcholine receptors and nicotine addiction: converging evidence from human and animal research. Behavioural Brain Research. 2008;193(1):1–16. doi: 10.1016/j.bbr.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter AS, Dunbar G, Mazzulla E, Hosford D, Newhouse PA. AZD3480, a novel nicotinic receptor agonist, for the treatment of attention-deficit/hyperactivity disorder in adults. Biological Psychiatry. 2014;75(3):207–214. doi: 10.1016/j.biopsych.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Powell J, Dawkins L, West R, Powell J, Pickering A. Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors. Psychopharmacology. 2010;212(4):537–549. doi: 10.1007/s00213-010-1975-8. [DOI] [PubMed] [Google Scholar]
- Powell SB, Zhou X, Geyer MA. Prepulse inhibition and genetic mouse models of schizophrenia. Behavioural Brain Research. 2009;204(2):282–294. doi: 10.1016/j.bbr.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn SH, Gawryl M, Dgetluck N, Palfreyman M, Bauer LO, Hilt DC. Normalizing effects of EVP-6124, an alpha-7 nicotinic partial agonist, on event-related potentials and cognition: a proof of concept, randomized trial in patients with schizophrenia. Journal of Psychiatric Practice. 2014;20:12–24. doi: 10.1097/01.pra.0000442935.15833.c5. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behavioural Processes. 2004;65:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Kholdebarin E, Brucato FH, Callahan PM, Lowe DA, Levin ED. Effect of R3487/MEM3454, a novel nicotinic α7 receptor partial agonist and 5-HT3 antagonist on sustained attention in rats. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33(2):269–275. doi: 10.1016/j.pnpbp.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Riala K, Hakko H, Isohanni M, Pouta A, Räsänen P. Is initiation of smoking associated with the prodromal phase of schizophrenia? Journal of Psychiatry and Neuroscience. 2005;30(1):26. [PMC free article] [PubMed] [Google Scholar]
- Romer D. Adolescent risk taking, impulsivity, and brain development: Implications for prevention. Developmental Psychobiology. 2010;52(3):263–276. doi: 10.1002/dev.20442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RG, Harris JG, Olincy A, Radant A, Adler LE, Freedman R. Familial transmission of two independent saccadic abnormalities in schizophrenia. Schizophrenia Research. 1998;30(1):59–70. doi: 10.1016/s0920-9964(97)00133-3. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Bannon KL, George TP. Nicotinic receptor mechanisms and cognition in normal states and neuropsychiatric disorders. Journal of Psychopharmacology. 2004;18(4):457–474. doi: 10.1177/0269881104047273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, Jatlow PI, Wexler BE, George TP. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Archives of General Psychiatry. 2005;62(6):649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Schwantes-An TH, Wang JC, Grucza RA, Breslau N, Hatsukami D, Johnson EO, Rice JP, Goate AM, Bierut LJ. Multiple cholinergic nicotinic receptor genes affect nicotine dependence risk in African and European Americans. Genes Brain and Behavior. 2010;9(7):741–750. doi: 10.1111/j.1601-183X.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, Pergadia ML, Loukola A, Broms U, Montgomery GW, Wang JC, Agrawal A, Dick DM, Heath AC, Todorov AA. Genetic linkage to chromosome 22q12 for a heavy-smoking quantitative trait in two independent samples. The American Journal of Human Genetics. 2007;80(5):856–866. doi: 10.1086/513703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, Saccone NL, Swan GE, Madden PA, Goate AM, Rice JP, Bierut LJ. Systematic biological prioritization after a genome-wide association study: an application to nicotine dependence. Bioinformatics. 2008;24(16):1805–1811. doi: 10.1093/bioinformatics/btn315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Medicine. 2005;2(5):e141. doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantis K, Matsokis N, Angelatou F. Synergistic interactions of dopamine D1 and glutamate NMDA receptors in rat hippocampus and prefrontal cortex: involvement of ERK1/2 signaling. Neuroscience. 2009;163(4):1135–1145. doi: 10.1016/j.neuroscience.2009.07.056. [DOI] [PubMed] [Google Scholar]
- Sarter M, Paolone G. Deficits in attentional control: cholinergic mechanisms and circuitry-based treatment approaches. Behavioral Neuroscience. 2011;125(6):825–835. doi: 10.1037/a0026227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science. 1983;220:214–216. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- Schubert MH, Young KA, Hicks PB. Galantamine improves cognition in schizophrenic patients stabilized on risperidone. Biological Psychiatry. 2006;60(6):530–533. doi: 10.1016/j.biopsych.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Seeman MV, Seeman P. Is schizophrenia a dopamine supersensitivity psychotic reaction? Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2014;48:155–160. doi: 10.1016/j.pnpbp.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. The Journal of Neuroscience. 1993;13(2):596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam G, Bhutani S, Khan DA, Brown ES. Psychiatric considerations in pulmonary disease. Psychiatric Clinics of North America. 2007;30(4):761–780. doi: 10.1016/j.psc.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Sharma G, Vijayaraghavan S. Nicotinic receptors containing the α7 subunit: a model for rational drug design. Current Medicinal Chemistry. 2008;15(28):2921. doi: 10.2174/092986708786848703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher E, Chen Y, Sharples T, Broad L, Benedetti G, Zwart R, McPhie G, Pearson K, Baldwinson T, De Filippi G. Physiological roles of neuronal nicotinic receptors subtypes: new insights on the nicotinic modulation of neurotransmitter release, synaptic transmission and plasticity. Current Topics in Medicinal Chemistry. 2004;4(3):283–297. doi: 10.2174/1568026043451393. [DOI] [PubMed] [Google Scholar]
- Sherr JD, Myers C, Avila MT, Elliott A, Blaxton TA, Thaker GK. The effects of nicotine on specific eye tracking measures in schizophrenia. Biological Psychiatry. 2002;52(7):721–728. doi: 10.1016/s0006-3223(02)01342-2. [DOI] [PubMed] [Google Scholar]
- Shiina A, Shirayama Y, Niitsu T, Hashimoto T, Yoshida T, Hasegawa T, Fukami G. A randomised, double-blind, placebo-controlled trial of tropisetron in patients with schizophrenia. Annals of General Psychiatry. 2010;9(1):1–10. doi: 10.1186/1744-859X-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J-C, Jung D-U, Jung S-S, Seo Y-S, Cho D-M, Lee J-H, Lee S-W, Kong B-G, Kang J-W, Oh M-K. Adjunctive varenicline treatment with antipsychotic medications for cognitive impairments in people with schizophrenia: a randomized double-blind placebo-controlled trial. Neuropsychopharmacology. 2012;37(3):660–668. doi: 10.1038/npp.2011.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shytle R, Silver A, Lukas R, Newman M, Sheehan D, Sanberg P. Nicotinic acetylcholine receptors as targets for antidepressants. Molecular Psychiatry. 2002;7(6):525–535. doi: 10.1038/sj.mp.4001035. [DOI] [PubMed] [Google Scholar]
- Simon AE, Cattapan-Ludewig K, Zmilacher S, Arbach D, Gruber K, Dvorsky DN, Roth B, Isler E, Zimmer A, Umbricht D. Cognitive functioning in the schizophrenia prodrome. Schizophrenia Bulletin. 2007;33(3):761–771. doi: 10.1093/schbul/sbm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Kem WR, Freedman R. Intragastric DMXB-A, an alpha7 nicotinic agonist, improves deficient sensory inhibition in DBA/2 mice. Biological Psychiatry. 2001;50(7):493–500. doi: 10.1016/s0006-3223(01)01093-9. [DOI] [PubMed] [Google Scholar]
- Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002;27(3):479–497. doi: 10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- Smucny J, Olincy A, Eichman LC, Lyons E, Tregellas JR. Early sensory processing deficits predict sensitivity to distraction in schizophrenia. Schizophrenia Research. 2013;147(1):196–200. doi: 10.1016/j.schres.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinella M. Correlations between orbitofrontal dysfunction and tobacco smoking. Addiction Biology. 2002;7:381–384. doi: 10.1080/1355621021000005964. [DOI] [PubMed] [Google Scholar]
- Spring B, Pingitore R, McChargue DE. Reward value of cigarette smoking for comparably heavy smoking schizophrenic, depressed, and nonpatient smokers. The American Journal of Psychiatry. 2003;160(2):316–322. doi: 10.1176/appi.ajp.160.2.316. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Kem WR, Mahnir VM, Freedman R. Selective alpha7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology (Berl) 1998;136(4):320–327. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- Stephens SH, Hoft NR, Schlaepfer IR, Young SE, Corley RC, McQueen MB, Hopfer C, Crowley T, Stallings M, Hewitt J, Ehringer MA. Externalizing behaviors are associated with SNPs in the CHRNA5/CHRNA3/CHRNB4 gene cluster. Behavior Genetics. 2012;42:402–414. doi: 10.1007/s10519-011-9514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand JE, Nyback H. Tobacco use in schizophrenia: a study of cotinine concentrations in the saliva of patients and controls. European Psychiatry. 2005;20(1):50–54. doi: 10.1016/j.eurpsy.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Luna B, Haas GL, Keshavan MS, Mann JJ, Thase ME. Pursuit tracking impairments in schizophrenia and mood disorders: step-ramp studies with unmedicated patients. Biological Psychiatry. 1999;46(5):671–680. doi: 10.1016/s0006-3223(99)00132-8. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Cross-species Studies of Sensorimotor Gating of the Startle Reflex. Annals of the New York Academy of Sciences. 1999;877(1):202–216. doi: 10.1111/j.1749-6632.1999.tb09269.x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Shoemaker JM, Light GA, Braff DL, Stevens KE, Sharp R, Breier M, Neary A, Auerbach PP. Convergence and divergence in the neurochemical regulation of prepulse inhibition of startle and n40 suppression in rats. Neuropsychopharmacology. 2006;31:506–515. doi: 10.1038/sj.npp.1300841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon R, Jibson MD. Extrapyramidal side effects of antipsychotic treatment: scope of problem and impact on outcome. Annals of Clinical Psychiatry. 2002;14(2):123–129. doi: 10.1023/a:1016811222688. [DOI] [PubMed] [Google Scholar]
- Tapia L, Kuryatov A, Lindstrom J. Ca2+ permeability of the (alpha4)3(beta2)2 stoichiometry greatly exceeds that of (alpha4)2(beta2)3 human acetylcholine receptors. Molecular Pharmacology. 2007;71:769–776. doi: 10.1124/mol.106.030445. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Callahan PM, Hernandez CM. Nicotinic ligands as multifunctional agents for the treatment of neuropsychiatric disorders. Biochemical Pharmacology. 2015;97(4):388–398. doi: 10.1016/j.bcp.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV, Jr, Gearhart DA, Mahadik SP, Warsi S, Davis LW, Waller JL. Chronic exposure to typical or atypical antipsychotics in rodents: temporal effects on central α7 nicotinic acetylcholine receptors. Neuroscience. 2005;136:519–529. doi: 10.1016/j.neuroscience.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Hill WD, Parikh V, Waller JL, Evans DR, Mahadik SP. Differential effects of haloperidol, risperidone, and clozapine exposure on cholinergic markers and spatial learning performance in rats. Neuropsychopharmacology. 2003;28:300–309. doi: 10.1038/sj.npp.1300039. [DOI] [PubMed] [Google Scholar]
- Thoma P, Daum I. Comorbid substance use disorders in schizophrenia: A selective overview of neurobiological and cognitive underpinnings. Psychiatry and Clinical Neurosciences. 2013;67:367–383. doi: 10.1111/pcn.12072. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Colby SM, Xavier EM. Effects of smoking abstinence on cigarette craving, nicotine withdrawal, and nicotine reinforcement in smokers with and without schizophrenia. Nicotine and Tobacco Research. 2014;16(3):326–334. doi: 10.1093/ntr/ntt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietje KR, Anderson DJ, Bitner RS, Blomme EA, Brackemeyer PJ, Briggs CA, Browman KE, Bury D, Curzon P, Drescher KU, Frost JM, Fryer RM, Fox GB, Gronlien JH, Hakerud M, Gubbins EJ, Halm S, Harris R, Helfrich RJ, Kohlhaas KL, Law D, Malysz J, Marsh KC, Martin RL, Meyer MD, Molesky AL, Nikkel AL, Otte S, Pan L, Puttfarcken PS, Radek RJ, Robb HM, Spies E, Thorin-Hagene K, Waring JF, Ween H, Xu H, Gopalakrishnan M, Bunnelle WH. Preclinical Characterization of A-582941: A Novel α7 Neuronal Nicotinic Receptor Agonist with Broad Spectrum Cognition-Enhancing Properties. CNS Neuroscience and Therapeutics. 2008;14(1):65–82. doi: 10.1111/j.1527-3458.2008.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. The Journal of Neuroscience. 2004;24(22):5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui-Kimura I, Ohmura Y, Izumi T, Yamaguchi T, Yoshida T, Yoshioka M. Nicotine provokes impulsive-like action by stimulating α4β2 nicotinic acetylcholine receptors in the infralimbic, but not in the prelimbic cortex. Psychopharmacology. 2010;209(4):351–359. doi: 10.1007/s00213-010-1804-0. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Keefe RS, Murray S, Lowe DA, Porter R, Garibaldi G, Santarelli L. A randomized, placebo-controlled study investigating the nicotinic α7 agonist, RG3487, for cognitive deficits in schizophrenia. Neuropsychopharmacology. 2014;39(7):1568–1577. doi: 10.1038/npp.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uteshev VV. The therapeutic promise of positive allosteric modulation of nicotinic receptors. European Journal of Pharmacology. 2014;727:181–185. doi: 10.1016/j.ejphar.2014.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables P. The effect of auditory and visual stimulation on the skin potential response of schizophrenics. Brain. 1960;83(1):77–92. doi: 10.1093/brain/83.1.77. [DOI] [PubMed] [Google Scholar]
- Voineskos S, De Luca V, Mensah A, Vincent JB, Potapova N, Kennedy JL. Association of alpha4beta2 nicotinic receptor and heavy smoking in schizophrenia. Journal of Psychiatry and Neuroscience. 2007;32(6):412–416. [PMC free article] [PubMed] [Google Scholar]
- Volkow ND. Substance use disorders in schizophrenia - clinical implications of comorbidity. Schizophrenia Bulletin. 2009;35(3):469–472. doi: 10.1093/schbul/sbp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling D, Marder SR, Kane J, Fleischhacker WW, Keefe RS, Hosford DA, Dvergsten C, Segreti AC, Beaver JS, Toler SM, Jett JE, Dunbar GC. Phase 2 Trial of an Alpha-7 Nicotinic Receptor Agonist (TC-5619) in Negative and Cognitive Symptoms of Schizophrenia. Schizophrenia Bulletin (epub ahead of print) 2015 doi: 10.1093/schbul/sbv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux J-P, Martin B, Damaj MI. The β2 but not α7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology. 2006;184(3–4):339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Wehring HJ, Liu F, McMahon RP, Mackowick KM, Love RC, Dixon L, Kelly DL. Clinical characteristics of heavy and non-heavy smokers with schizophrenia. Schizophrenia Research. 2012;138(2):285–289. doi: 10.1016/j.schres.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Sacco KA, Creeden CL, Vessicchio JC, Jatlow PI, George TP. Effects of acute abstinence, reinstatement, and mecamylamine on biochemical and behavioral measures of cigarette smoking in schizophrenia. Schizophrenia Research. 2007;91(1):217–225. doi: 10.1016/j.schres.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30(4):669–689. [Google Scholar]
- Winklbaur B, Ebner N, Sachs G, Thau K, Fischer G. Substance abuse in patients with schizophrenia. Dialogues in Clinical Neuroscience. 2006;8:37–43. doi: 10.31887/DCNS.2006.8.1/bwinklbaur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Gallinat J, Brinkmeyer J, Musso F, Kornhuber J, Thuerauf N, Rujescu D, Favis R, Sun Y, Franc MA, Ouwerkerk-Mahadevan S, Janssens L, Timmers M, Streffer JR. Allosteric alpha-7 nicotinic receptor modulation and P50 sensory gating in schizophrenia: a proof-of-mechanism study. Neuropharmacology. 2013;64:197–204. doi: 10.1016/j.neuropharm.2012.06.040. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ, Bostic J, Prince J, Monuteaux MC, Soriano J, Fine C, Abrams A, Rater M. A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. The American Journal of Psychiatry. 1999;156(12):1931–1937. doi: 10.1176/ajp.156.12.1931. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Decker MW. Neuronal nicotinic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: focus on cognition. Biochemical Pharmacolology. 2007;74(8):1212–1223. doi: 10.1016/j.bcp.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Verlinden MH, Adler LA, Wozniak PJ, West SA. ABT-089, a neuronal nicotinic receptor partial agonist, for the treatment of attention-deficit/hyperactivity disorder in adults: results of a pilot study. Biological Psychiatry. 2006;59(11):1065–1070. doi: 10.1016/j.biopsych.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Wilking JA, Hesterberg KG, Crouch EL, Homanics GE, Stitzel JA. Chrna4 A529 knockin mice exhibit altered nicotine sensitivity. Pharmacogenetics and Genomics. 2010;20(2):121. doi: 10.1097/FPC.0b013e3283369347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Anthenelli RM, Morris CD, Treadow J, Thompson JR, Yunis C, George TP. A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. Journal of Clinical Psychiatry. 2012;73(5):654–660. doi: 10.4088/JCP.11m07522. [DOI] [PubMed] [Google Scholar]
- Wing VC, Bacher I, Sacco KA, George TP. Neuropsychological performance in patients with schizophrenia and controls as a function of cigarette smoking status. Psychiatry Research. 2011;188(3):320–326. doi: 10.1016/j.psychres.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing VC, Wass CE, Bacher I, Rabin RA, George TP. Varenicline modulates spatial working memory deficits in smokers with schizophrenia. Schizophrenia Research. 2013;149(1):190–191. doi: 10.1016/j.schres.2013.06.032. [DOI] [PubMed] [Google Scholar]
- Wing VC, Wass CE, Soh DW, George TP. A review of neurobiological vulnerability factors and treatment implications for comorbid tobacco dependence in schizophrenia. Annals of the New York Academy of Sciences. 2012;1248:89–106. doi: 10.1111/j.1749-6632.2011.06261.x. [DOI] [PubMed] [Google Scholar]
- Wong AH, Van Tol HH. The dopamine D4 receptors and mechanisms of antipsychotic atypicality. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27(7):1091–1099. doi: 10.1016/j.pnpbp.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends in Neurosciences. 1997;20(2):92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Woznica AA, Sacco KA, George TP. Prepulse inhibition deficits in schizophrenia are modified by smoking status. Schizophrenia Research. 2009;112(1):86–90. doi: 10.1016/j.schres.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Yadon CA, Bugg JM, Kisley MA, Davalos DB. P50 sensory gating is related to performance on selected tasks of cognitive inhibition. Cognitive, Affective and Behavioral Neuroscience. 2009;9(4):448–458. doi: 10.3758/CABN.9.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates SL, Bencheri M, Fluhler EN, Lippiello PM. Up-regulation of nicotinic acetylcholine receptors following chronic exposure of rats to mainstream cigarette smoke or α4β2 receptors to nicotine. Biochemical Pharmacology. 1995;50(12):2001–2008. doi: 10.1016/0006-2952(95)02100-0. [DOI] [PubMed] [Google Scholar]
- Yin J, Yuan K, Feng D, Cheng J, Li Y, Cai C, Bi Y, Sha S, Shen X, Zhang B, Xue T, Qin W, Yu D, Lu X, Tian J. Inhibition control impairments in adolescent smokers: Electrophysiological evidence from a Go/NoGo study. Brain Imaging and Behavior (Epub ahead of print) 2015 doi: 10.1007/s11682-015-9418-0. [DOI] [PubMed] [Google Scholar]
- Young JW, Crawford N, Kelly JS, Kerr LE, Marston HM, Spratt C, Finlayson K, Sharkey J. Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. European Neuropsychopharmacology. 2007;17(2):145–155. doi: 10.1016/j.euroneuro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Evaluating the role of the alpha-7 nicotinic acetylcholine receptor in the pathophysiology and treatment of schizophrenia. Biochemical Pharmacology. 2013;86(8):1122–1132. doi: 10.1016/j.bcp.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Meves JM, Tarantino IS, Caldwell S, Geyer MA. Delayed procedural learning in α7-nicotinic acetylcholine receptor knockout mice. Genes Brain and Behavior. 2011;10(7):720–733. doi: 10.1111/j.1601-183X.2011.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophrenia Bulletin. 1996;22(2):353–370. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Liu L, Liu S, Hong X, Chen DC, Xiu MH, Kosten TR. Short-term tropisetron treatment and cognitive and P50 auditory gating deficits in schizophrenia. The American Journal of Psychiatry. 2012;169(9):974. doi: 10.1176/appi.ajp.2012.11081289. [DOI] [PubMed] [Google Scholar]