Summary
Background
Infant adiposity better predicts childhood obesity/metabolic risk than weight, but technical challenges fuel controversy over the accuracy of adiposity estimates.
Objective
We prospectively measured adiposity(%fat) in term newborns(NB) at 2wk (n=41) and 1yr (n=30).
Methods
% fat was measured by dual X-ray absorptiometry(DXA), PEAPOD, and skinfolds(SF). DXAs were analyzed using Hologic Apex software 3.2(DXAv1) and a new version 5.5.2(DXAv2).
Results
NB %fat by DXAv2 was 55% higher than DXAv1 (14.2%vs9.1%), 45% higher than SF (9.8%), and 36% higher than PEAPOD (10.4%). Among NB, Pearson correlations were 0.73–0.89, but agreement (Intraclass correlations) poor between DXAv2 and DXAv1 (0.527), SF (0.354), and PEAPOD (0.618). At 1yr, %fat by DXAv2 was 51% higher than DXAv1 (33.6%vs22.4%), and twice as high compared to SF (14.6%). Agreement was poor between DXAv2 and DXAv1 (0.204), and SF (0.038). The absolute increase in %fat from 2wk to 1yr was 19.7% (DXAv2), 13.6% (DXAv1) and only 4.8% by SF.
Conclusion
Analysis of the same DXA scans using new software yielded considerably higher adiposity estimates at birth and 1 year compared to the previous version. Using different modalities to assess body composition longitudinally is problematic. Standardization is gravely needed to determine how early life exposures affect childhood obesity/metabolic risk.
Keywords: Infant Body Composition, Dual X-ray Absorptiometry, Air Displacement Plethysmography, Anthropometry, Childhood Obesity Risk, Intra-uterine Environment
Introduction
Adiposity at birth and during the first two years of life is increasingly recognized as an important predictor of childhood obesity and future metabolic syndrome1–3. In the U.S, 27% of children enter kindergarten already obese or overweight4. Increased adiposity at birth is more common in infants born to obese mothers as is their risk of childhood obesity.5,6, 7. It is clear that birth weight (BW) alone corrected for gestational age or ponderal index (mass/height3), although useful for large populations, are inadequate estimates for determining fat mass (FM) relative to fat-free mass (FFM) in infants2, 8, 9. Furthermore, although anthropometric measures of relative fat mass (%fat) using skinfolds (SF) suffer from limited reproducibility and precision in infants and children10, 11, they are the most common estimate utilized in large populations due to their low cost and widespread availability. While adiposity at birth has predictive power for development of future metabolic disease, infants triple their FM during the first year of life, suggesting that developmental changes in adiposity have important implications for the origins of pediatric obesity and metabolic diseases1, 2. In fact, our group recently demonstrated that newborns of obese mothers with gestational diabetes mellitus (GDM) also have increased intrahepatic fat at birth measured by nuclear magnetic resonance (NMR) spectroscopy, potentially setting the stage for childhood non-alcoholic fatty liver disease12.
A reference method for assessing body composition is the 4-compartment model which measures total body mass (scale weight), total body water (TBW, by deuterium dilution), bone mineral content (BMC, by dual x-ray absorptiometry [DXA]), and total body potassium (TBK, by 40K counts). The 4-compartmental method may be the most accurate for measuring body composition during early infancy and childhood because of compositional changes in FFM (e.g., decreasing TBW, increasing bone mineral content) throughout development. Normative data in infants from birth to 2 years (n=76) have been developed using this model13, but the technique is difficult and time consuming for larger clinical trials. Thus, the use of DXA alone has been an attractive alternative for assessing body composition in infants because, in addition to BMC, it measures FM and non-bone FFM. Crucial for its use in longer-term follow-up, DXA demonstrates an excellent safety profile with negligible radiation exposure. However, concerns remain about its accuracy in infancy due to changes during chemical maturation of the body as BMC increases and TBW decreases11, 14, 15, and it requires the infant to lie still. Due its simplicity, there has been increasing and widespread use of air displacement plethysmography (ADP). ADP does not require a still infant, takes <5 minutes to perform, and emits no ionizing radiation. Infant ADP (PEAPOD) measures only body volume and calculates the %fat using assumed densities of FM and FFM. It cannot accommodate infants >10kg (≤8 kg without pediatric tray) and therefore cannot be used much beyond 6 months of age. There also appear to be substantial differences between %fat and FFM when measured by PEAPOD compared to DXA (Lunar v11-30.062) at 6 months15.
Although the technology to scan infants using DXA has advanced over the last 20 years, many of the systems validated against direct carcass analysis that were used to create reference data are no longer in production. Furthermore, there are currently three different DXA manufacturers (Hologic, GE Lunar, and Norland), each with multiple models and a wide variety of software versions, making it nearly impossible to compare an individual’s data to reference data. While differences among scanners are well known, differences within an instrument but using different analysis software versions are less studied. One study in young children and adolescents (>1yr of age and <40kg) found significant differences in FM measured on a Hologic (Discovery model) when analyzed by two different software versions (v12.1 vs. v11.2); infants <1yr of age were not studied14.
In 2014, Hologic released new Infant Whole Body analysis software (Apex v5.5.2), thought to be a significant improvement from its previous version (Apex v3.2) due to substantial changes to the analysis algorithm. The algorithm was designed to be used on Discovery A, Discovery W, Hologic A, and Hologic W whole body scanners. Here we report %fat in newborns (NB; n=41) and at 1yr follow-up (n=31) as measured by DXA, skinfolds (SF) and PEAPOD (n=28, NB only) as part of a prospective study examining the metabolic influences of the intrauterine environment on infant adiposity. Analysis of DXA scans using the new infant software (DXAv2) were compared to analysis with the previous software version (DXAv1), as well as to SF and PEAPOD. This new software has not been validated using a 4 compartment model or carcass analysis and to our knowledge, this is the first report utilizing this new infant ApexV5.5.2 Hologic software (DXAv2) with simultaneous comparisons to the previous version, PEAPOD and SF at 2wk and 1yr.
Methods
Subject Characteristics
Pregnant women (ages of 18–35yr) were enrolled as part of an NIH-funded prospective study (R01 DK078645) exploring intrauterine and postnatal metabolic determinants of growth. Twenty-five women were normal weight (BMI 20–26 kg/m2) and 16 were obese (BMI 30–38 kg/m2); 4 of the obese were diagnosed with GDM. Women with medical conditions that could increase the risk of growth restriction or preterm birth were excluded. Only term infants (≥ 37 weeks) were included. The Colorado Multiple Institutional Review Board (COMIRB) approved the body composition studies
Dual energy x-ray absorptiometry (DXA)
From 2008–2014, 41 term newborns underwent DXA scanning at Children’s Hospital Colorado Department of Radiology at a mean age of ~2wk on the same instrument using the QDR Discovery fan beam densitometer (Hologic Delphi-W, Hologic Inc, Waltham, MA). Of these, 30 infants also underwent a repeat scan on the same instrument during a 1yr follow-up visit. Calibration of the DXA instrument included daily measurements of a spine phantom, weekly air scans, and tissue bar scans at least once monthly. The DXA scan takes <5 minutes with a radiation dose 1000 times less than the FDA limit for exposure. After a short feeding to facilitate infant sleep, the freshly diapered neonate was bundled in a thin blanket and placed on the scanning bed. At 1yr, gentle swaddling was used to maintain immobilization. Preparation and positioning of the infants were done by the same experienced investigator throughout the study (MR). All NB and 1yr scans were analyzed using QDR infant software (Apex version 3.2) and were analyzed again with the release of the newest software (Apex version 5.5.2). The regions for analysis are automated so that both software packages analyze identical regions.
Air-Displacement Plethysmography (ADP)
On the same day as the DXA visit, body fat was measured by ADP in 28 newborns using PEAPOD™ (Cosmed Inc, Concord, CA), all performed by a single experienced investigator (RMR) at Children’s Hospital Colorado. All infants were calm and/or asleep and in the same fed status as with the DXA since the measures were performed sequentially on the same morning.
Anthropometrics
Infant weight and length were measured in tandem at 2wk and at 1yr. Triceps and subscapular skinfolds (Lange calipers, Beta Technology, Inc., St Albans, U.K., resolution 0.2mm) were measured in triplicate by the same experienced investigator (MR). The sum of the skinfold (SF) measurements and sex of the child were used to estimate percentage body fat according to the Slaughter equation (Males: %fat = 1.21 (tricep + subscapular - 0.008 (tricep + subscapular)2 – 1.7 // Females: %fat = 1.33 (tricep + subscapular - 0.013 (tricep + subscapular)2 – 2.5)16. All measures (DXA, PEAPOD, and SF) were performed sequentially on the same morning under identical conditions.
Statistics
The agreement between %fat measured by the two DXA infant software versions, PEAPOD and SF measures were examined using Bland-Altman analysis, Pearson correlations, and intra-class correlations (ICC). Independent group t-tests were used to examine differences in %fat between the two DXA infant software versions, PEAPOD and SF measures. In the 30 infants who completed a 1yr follow-up visit, %fat changes (delta 2wk to 1yr) were calculated and compared. The alpha level was set at p<0.05 and all statistical analyses were performed in SPSS (v22.0, IBM Corporation). Data are presented as mean±SD unless otherwise specified.
Results
Subject characteristics
Forty one mothers and their term infants (18 girls/23 boys) were studied at 2wk of life (15±2 days) and again at 1yr (53±3 weeks; Table 1). Ninety-four percent breast-fed and the majority of women (83%) were still breastfeeding at 6 months.
Table 1.
Maternal and offspring characteristics at birth and at 1yr
| Newborns (n=41) |
1Yr (n=30) |
|
|---|---|---|
| Maternal characteristics | ||
| Age (yrs) | 30±3 | |
| Pre-pregnancy BMI (kg/m2) | 26±5 | |
| Gestational weight gain (kg) | 14.1±5.6 | |
| Gestational age at delivery (wks) | 39.6±1.2 | |
| Girls/Boys | 18/23 | 17/13 |
| Weight (g) | 3318±430 | 9586±1048 |
| Length (cm) | 52.8±1.9 | 73.5±3.6 |
| Ponderal index | 2.42±0.19 | |
| Birth weight >4000g (%) | 10% | |
| Breast feeding (%) | 94% | 21% |
mean±SD;
BMI=body mass index
Newborn body composition
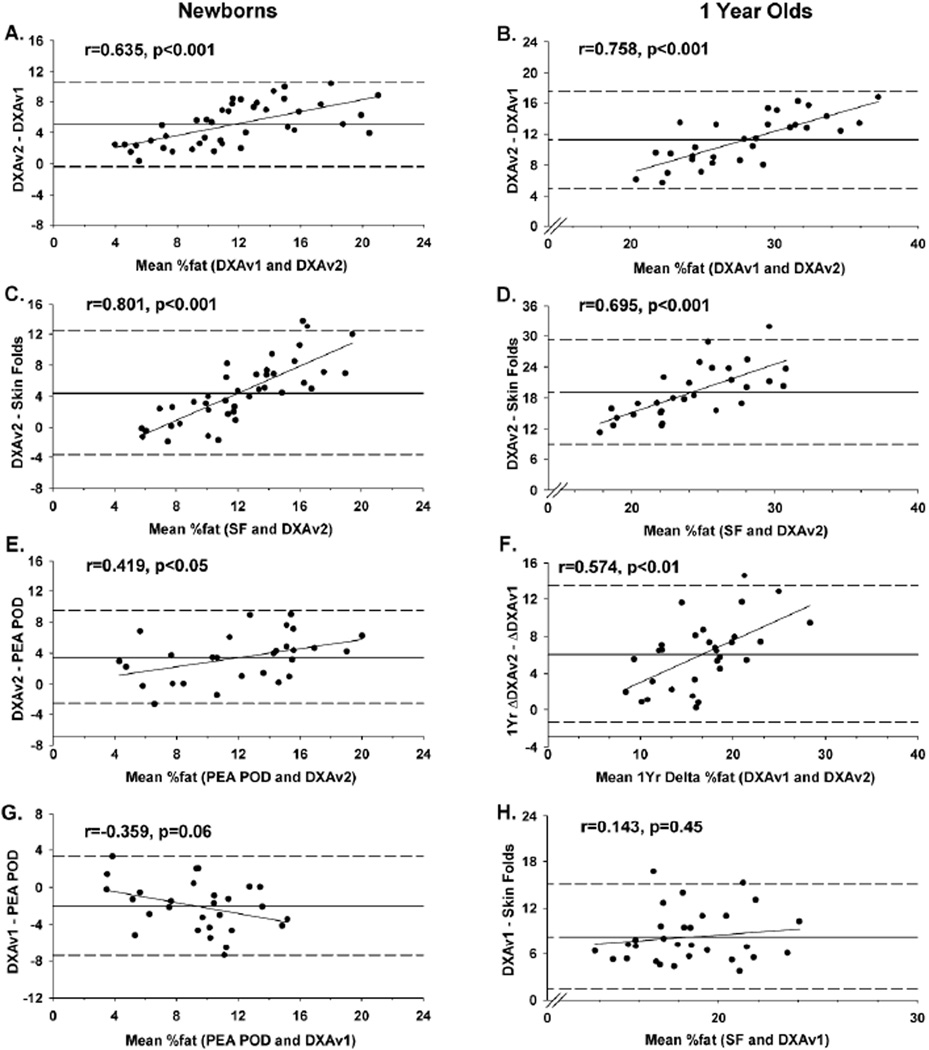
In newborns, FM was 65% higher when the same DXA scans were analyzed using the newer software version 5.5.2 (DXAv2) compared with version 3.2 (DXAv1)(551 vs 333g) (Table 2). The mean DXA %fat by DXAv2 was 55% higher compared to analysis by DXAv1, 45% higher compared to SF, and 36% higher compared to PEAPOD (all p<0.01). When analyzed by DXAv1, %fat was 15% lower than SF and 25% lower than PEAPOD (all p<0.05). Although Pearson correlations were fairly strong among methods of assessing %fat, the ICCs indicated poor agreement (range 0.35–0.65; Table 3). Moreover, Bland-Altman analyses revealed increasing bias in newborn data between methods with increasing adiposity when comparing DXAv2 with: DXAv1 (Figure 1A), SF (Figure 1C), and PEAPOD (Figure 1E). Likewise, there was increasing bias between methods with increasing adiposity when %fat was assessed by DXAv1 compared to PEAPOD, but this did not reach statistical significance (Figure 1G).
Table 2.
Body composition by assessment method and time period
| DXAv1 | DXAv2 | Skinfolds† | PEAPOD† | |
|---|---|---|---|---|
| Newborn (n=41) | ||||
| Mass (g) | 3903±550 | 3907±550 | ||
| FM (g) | 369±195* | 572±273 | ||
| FFM (g) | 3534±412* | 3335±371 | ||
| %fat | 9.1±3.8* | 14.2±5.5 | 9.8±2.4* | |
| BMC (g) | 73±12 | 74±13 | ||
| Newborn with PEAPOD (n=28) | ||||
| Mass (g) | 3870±482 | 3876±482 | ||
| FM (g) | 333±152 | 551±251 | 379±171* | |
| FFM (g) | 3538±369 | 3325±322 | 3175±343* | |
| %fat | 8.3±3.1* | 13.8±5.2 | 9.8±2.4* | 10.4±4.0* |
| 1 years old (n=30) | ||||
| Mass (g) | 9586±1048 | 9590±1048 | ||
| FM (g) | 2141±400* | 3221±649 | ||
| FFM (g) | 7445±903* | 6369±938 | ||
| %fat | 22.4±3.4* | 33.6±5.7 | 14.6±2.7* | |
| BMC (g) | 209±28 | 210±29 | ||
| 1Yr Delta | ||||
| FM (g) | 1795±398 | 2667±617 | ||
| FFM (g) | 3906±734* | 3034±750 | ||
| %fat | 13.6±4.0* | 19.7±6.0 | 4.8±2.5* |
p<0.05 different from DXAv2; mean±SD; Delta (change from 2wk to 1yr); BMC=bone mineral content; FFM=fat-free mass; FM=fat mass.
PeaPod FM and FFM are es8mated from Mass (scale weight) and %fat
Table 3.
Intra-class (and Pearson) correlations between assessment methods in newborns (NB) and 1 year old infants (1Yr)
| %fat DXAv2 | %fat SF | %fat PEAPOD | |
|---|---|---|---|
| %fat DXAv1-NB | 0.527 (0.889) | 0.622 (0.694) | 0.620 (0.740) |
| %fat DXAv2-NB | ----- | 0.354 (0.734) | 0.618 (0.812) |
| %fat SF-NB | ----- | ----- | 0.654 (0.745) |
| %fat DXAv1-1Yr | 0.204 (0.888) | 0.109 (0.495) | ----- |
| %fat DXAv2-1Yr | ----- | 0.038 (0.500) | ----- |
Figure 1.
Bland-Altman plots of the agreement between %fat: 1) analyzed by DXAv2 compared to DXAv1 in newborns (panel A) and 1 year old infants (panels B); 2) analyzed by DXAv2 compared to SF in newborns (panel C) and 1 year old infants (panel D); 3) analyzed by DXAv2 compared to PEAPOD in newborns (panel E); 4) changes over 1 year measured by DXAv1 compared to DXAv2 (panel F); 5) analyzed by DXAv1 compared to PEAPOD (panel G); and 6) analyzed by DXAv1 compared to SF in 1 year old infants (panel H). Reference lines are mean differences (solid) ± 2 standard deviations (dashed) between methods.
One year body composition
In infants at 1yr, FM by DXA was 51% higher when the same DXA scans were analyzed using the newer software v5.5.2 (DXAv2) compared with v3.2 (DXAv1) (Table 2). Mean %fat when analyzed by software v5.5.2 (DXA2) was 51% higher (33.6% vs 22.4%) compared to analysis by v3.2 (DXAv1) and more than twice as high compared to SF estimates (14.6%; all p<0.01). DXA %fat when analyzed by the earlier DXAv1 was 53% higher than SF (p<0.01). Furthermore, correlations between both DXA versions and SF at 1yr were modest and the ICCs were poor (range 0.04–0.20), indicating little agreement between methods (Table 3). As observed in the newborns, Bland-Altman analyses revealed increasing bias between methods as adiposity increased when comparing DXAv2 with DXAv1 (Figure 1B) and SF (Figure 1D) among 1yr infants. Assessment of %fat by DXAv1 was consistently higher than %fat by SF, but the bias between methods was not related to increasing infant adiposity (Figure 1H).
One year changes in body composition
The change over 1yr was 49% higher for FM when DXA scans were analyzed using the newer software v5.5.2 (DXAv2) compared with v3.2 (DXAv1) (Table 2). Change in DXA %fat was 45% higher when analyzed by DXAv2 compared to DXAv1, and more than 4 times higher compared to SF (all p<0.01). Bland-Altman analysis revealed bias between methods, with increasing adiposity when comparing the 1yr changes calculated from DXAv2 with DXAv1 (Figure 1F).
Discussion
This is the first prospective study to compare the newest infant Hologic software (Apex v5.5.2) to the previous version (Apexv3.2) with simultaneous measures (PEAPOD, skinfolds) at 2wk and repeated measures of DXA and skinfolds at 1yr. The compelling findings are that the newer DXA software version (DXAv2), designed to improve air background detection and discriminate soft bone from fat mass, demonstrated a 50–55% increase in %fat at 2wk and 1yr compared to the previous version. Furthermore, the increase in %fat from 2wk to 1yr was 13.6% using the earlier DXA software version compared to 19.7% in the later re-analysis. Both versions supported a ~2.4 fold increase in %fat from 2wk to 1yr (9.1 to 22.4% with DXAv1 vs. 14.2 to 33.6% with DXAv2). Many studies only report correlations between modalities, and although the two DXA software versions are highly correlated at 2wk and 1yr (Pearson r=0.89), the ICC and the Bland-Altman analyses revealed poor agreement between analyses. The ICC was modest between DXAv1 and DXAv2 at 2wk (0.527) and was poor at 1yr (0.204). Moreover, the Bland-Altman analyses demonstrated bias with DXA2 compared to DXA1 that worsened with increasing adiposity (Figure 1).
DXA directly measures the attenuation of X-rays through tissue and unlike PEAPOD, it can provide global and regional estimates for bone, fat, and lean tissue. The validity of DXA in human infants was mainly based on direct carcass analysis of piglets11. Some have suggested that DXA underestimates bone mass and overestimates fat mass in infants17. In addition, differences in manufacturers, hardware, phantoms, acquisition and analysis techniques, and software algorithms may affect body composition estimates with proportionally greater errors in smaller sizes11, 14. A re-analysis of pediatric scans in children 1.7–17.2 years was reported in 2008 using a newer DXA software version (Hologic Discovery V12.1) compared to a previous version (V11.2). Interestingly, the body composition values for younger, smaller subjects were most affected, girls more than boys, with an upward change in % body fat up to 7.2% in girls weighing 8–12 kg and 6.5% in boys14. The authors comment that children <40 kg were most affected and 14% of the girls and 10% of the boys would have been reclassified from “normal” %BF to “at risk of obesity.” Infant phantom studies should be interpreted with caution due to difficulty in constructing phantoms which accurately simulate the x-ray attenuation characteristics of fat, lean, and bone tissues in vivo. Nevertheless, infant phantoms designed for quality control and cross-calibration have revealed significant differences among DXA instruments. Because lean tissue is the largest compartment of body weight in an infant phantom, any inter-instrument variability in lean mass measurement will cause disproportionally greater variability in fat measurement18. Body mass in human infants and adults is also dominated by lean constituents and therefore errors in lean mass measurement have proportionately greater effects on %fat results.
Fat-free mass changes significantly during the chemical maturation of the body from early infancy to adulthood due to decreases in total body water and increases in bone mineral density. Both Hologic analysis software versions assume a lean mass hydration of 80% (versus 72.3% in adults) which partially compensates for the higher body water found in neonates. This is necessary because water appears slightly fatty to the x-rays with an apparent fat content of ~8.6% on a scale where the leanest soft tissue is 0% and pure fat is 100%. In addition, both analysis algorithms must exclude bone pixels from the body composition analysis because DXA cannot resolve more than two materials simultaneously (i.e., bone, lean and fat). As a result the composition of bone points is estimated from nearby non-bone containing pixels. In a typical adult DXA scan, approximately 40–45% of pixels contain all three constituents and in infants the proportion is even higher. Hologic made further changes to include improved air background detection, rejection of thin artifacts (e.g. blankets) from the air background, and the rejection of super-lean bone pixels from the soft tissue background. The enhancements in air background determination are significant because DXA measures fat and lean mass as attenuation above “air background” (i.e. the zero point above which fat and lean mass are measured). Artificially high air background values may reduce the apparent soft tissue mass and therefore affect the proportion of fat and lean measured. Super-lean bone points are pixels that do not meet the minimum threshold requirements for bone but may contain a small amount of bone mineral. Partially mineralized collagen matrix, common in neonates and at the growth plates of long bones, appears super-lean. These pixels must be excluded from soft tissue analysis because bone appears about 600% lean on the fat and lean scale described above. Even the inclusion of a small number of super-lean pixels may bias soft tissue determinations toward increasingly lean results. In summary, the new algorithms used for analyzing DXA scans were intended to more effectively deal with potential measurement biases, particularly problematic in a growing infant, but have yet to be validated.
ADP (PEAPOD) is a potential alternative to DXA9, 19, 20 and measures total body volume by detecting air pressure differences between the test chamber holding the infant and a reference chamber with controlled air pressures. Body density is computed from body mass and volume using known fat and FFM age- and sex-specific density coefficients to estimate total body FM, FFM and %fat. The largest reliability study was conducted on 36 infants and demonstrated a within-day coefficient of variation (CV) for %fat of 4.9%21. Validation studies using bovine phantoms suggest the %CV increases as bovine fat decreases (18% CV for the <10% fat)22. The impact of crying has not been clearly established for ADP and the largest tray limits infant weight to <10 kg, which is what was used for this study. In our study, the %fat by PEAPOD was intermediate (10.4%) between DXAv1 (8.3%) and DXAv2 (13.8%) at 2wk, with modest agreement (ICC r=0.62) to both DXA versions. Our data in 2wk infants comparing PEAPOD with Hologic DXA are consistent with those described in 6 month infants comparing PEAPOD with Lunar DXA, although better agreement was reported (ICC r=0.925)15. No newborn and 1yr data were obtained.
Skinfolds are a frequently used due to lower cost and convenience and usually performed by a combination of sites, most commonly biceps, triceps, subscapular, suprailiac and quadriceps thicknesses. Equations including that by Slaughter to estimate %fat from SF are based on the theoretical assumption that subcutaneous fat is a uniform layer enveloping the trunk and limbs, each having a measurable length and circumference16. However, total body fat is likely underestimated due to exclusion of fat in the intra-abdominal region or within the FFM compartment. Reproducibility is also low, particularly when more than one person performs the measures. Furthermore, %fat by SF was weakly correlated with estimates by isotope dilution or magnetic resonance and may be only marginally better than simply measuring weight and length11. When compared to DXA (Hologic QDR 1500, software v5.67), %fat by SF at birth and 4 months estimated by 5 different equations, including Slaughter, has been reported to be 10–23% lower, with more systematic error as %fat increases23. Correlations were not tight enough to allow individual predictions of FM from SF measures; ICCs were not reported. Although the Slaughter equation has been used in large cohorts including infants23, 24, it was originally validated in prepubescent children so its accuracy in infants remains unclear. Consistent with these findings, our gender-specific estimates of %fat by SF correlated reasonably with PEAPOD, DXAv1, and DXAv2 at 2 weeks (0.69–0.74) but only modestly agreed using ICC with DXA and PEAPOD (ICC 0.35–0.65) despite being performed by one investigator. Moreover, SF markedly underestimated %fat at 1yr. Even more striking was the lower estimate of the %fat change from 2wk to 1yr using SF compared to DXAv2 (4.8% vs. 19.7%). Bland-Altman analyses showed a pronounced bias of %fat by SF compared to DXA2.
One limitation of our study was that PEAPOD was not available at our institution until ~2 years into the study, so two-thirds of the NBs had this measure (28/41). For the SF estimates of adiposity, we measured tricep and subscapular SF thicknesses using the Slaughter equation16. It is possible that additional SF measures and a different equation could improve the agreement between DXA and ADP. Although our infants born to normal weight and obese women provided a wide range of infant adiposity, we do not know if our results are generalizable to preterm/growth-restricted infants or other ethnicities.
The implications of our findings are concerning. Birth weight and childhood BMI percentile are thought to be poorer predictors of childhood obesity and metabolic syndrome compared to fat mass8. Studies attempting to discern the developmental determinants in in-utero and postnatal life that predict risk for later childhood metabolic disease often rely on estimates of adiposity at birth and during the first two years of life2, 25, 26. This study raises numerous questions, including: 1) whether previous estimates of %fat at birth and at 1yr have been underestimated; 2) whether the newer DXA software version overestimates %fat; and 3) whether DXA or PEAPOD can be considered accurate measures of %fat. New DXA software versions should be validated against a 4-compartment model or carcass analysis before conclusions about accuracy can be made. These data also strongly suggest that although our skinfold %fat estimates were correlated at birth, they markedly underestimated adiposity at 1yr. Skinfold %fat estimates in infants may require serial validation with DXA or PEAPOD frequently over time. Our findings underscore challenges in using different modalities to assess body composition or to define norms of FM and FFM in infants. Furthermore, they underscore the necessity of utilizing the same method longitudinally to determine changes in body composition. The data also clearly show that to advance our understanding of how early life exposures affect obesity and metabolic risk, improvement in the accuracy and standardization of technologies to measure infant body composition are greatly needed.
What is already known about this subject
Adiposity at birth and throughout the first 2 yrs of life is an important predictor of childhood obesity and metabolic syndrome.
DXA and PEAPOD are often used to estimate infant adiposity and longitudinal fat mass increases but there is no consensus over the best measure; PEAPOD cannot be used in infants weighing >10 kg.
Different DXA scanners and infant software packages are utilized across studies and agreement between DXA and PEAPOD measures has been investigated in a limited manner.
What this study adds
This is the first study to directly compare the new DXA Hologic infant software (DXAv2) to the previous version (DXAv1) at both 2 weeks of age and 1 year. Percent fat estimates are ~50% higher at both time points using the newer version.
Compared to PEAPOD and skinfolds, the new Hologic software, which utilizes a new analysis algorithm, results in significantly higher newborn %fat estimates, poor agreement between methods, and bias with increasing adiposity.
This study raises questions as to the accuracy of the newer compared to the older software and underscores the problems using different methodologies and software programs longitudinally to estimate fat mass development.
Acknowledgements
We are grateful for our funding sources which include the NIH (DK078645, DK088105) and a CTRC grant (CCTSI is supported in part by Colorado CTSA Grant UL1 TR001082 from NCATS/NIH). We greatly appreciate the support of Drs. William Hay and Jill Davies in the oversight of this study and the data management expertise of Laura Kay Moss.
NCT#: NCT00826904
Given the study was investigator-initiated by Dr. Linda Barbour and the study sponsor is the NIH (DK078645, DK088105), and it was NOT sponsored by industry. The only author who has any relationship with Hologic is Thomas Kelly who is a Senior Scientist at Hologic and an employee and shareholder. Mr. Kelly had no role analyzing or interpreting the data and his expertise was limited to contributing his scientific and technical knowledge of DXA and its software. Linda Barbour wrote the first draft of the manuscript and there was no honorarium, grant, or other form of payment given to anyone on this manuscript.
Footnotes
Conflicts of Interests:
There are no conflicts of interest. All other authors who had roles in the design, execution, analysis, interpretation or the writing of this manuscript had no conflict of interests.
References
- 1.Symonds ME, Mendez MA, Meltzer HM, et al. Early life nutritional programming of obesity: mother-child cohort studies. Annals of nutrition & metabolism. 2013;62:137–145. doi: 10.1159/000345598. [DOI] [PubMed] [Google Scholar]
- 2.Chandler-Laney PC, Gower BA, Fields DA. Gestational and early life influences on infant body composition at 1 year. Obesity (Silver Spring) 2013;21:144–148. doi: 10.1002/oby.20236. [DOI] [PubMed] [Google Scholar]
- 3.Barbour LA. Changing perspectives in pre-existing diabetes and obesity in pregnancy: maternal and infant short- and long-term outcomes. Current opinion in endocrinology, diabetes, and obesity. 2014;21:257–263. doi: 10.1097/MED.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. The New England journal of medicine. 2014;370:1660–1661. doi: 10.1056/NEJMc1402397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catalano PM, McIntyre HD, Cruickshank JK, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes care. 2012;35:780–786. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114:e29–e36. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- 7.Black MH, Sacks DA, Xiang AH, Lawrence JM. The relative contribution of prepregnancy overweight and obesity, gestational weight gain, and IADPSG-defined gestational diabetes mellitus to fetal overgrowth. Diabetes care. 2013;36:56–62. doi: 10.2337/dc12-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalano PM, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. The American journal of clinical nutrition. 2009;90:1303–1313. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andres A, Shankar K, Badger TM. Body fat mass of exclusively breastfed infants born to overweight mothers. Journal of the Academy of Nutrition and Dietetics. 2012;112:991–995. doi: 10.1016/j.jand.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 10.Katzmarzyk PT, Shen W, Baxter-Jones A, et al. Adiposity in children and adolescents: correlates and clinical consequences of fat stored in specific body depots. Pediatric obesity. 2012;7:e42–e61. doi: 10.1111/j.2047-6310.2012.00073.x. [DOI] [PubMed] [Google Scholar]
- 11.Demerath EW, Fields DA. Body composition assessment in the infant. American journal of human biology : the official journal of the Human Biology Council. 2014;26:291–304. doi: 10.1002/ajhb.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brumbaugh DE, Tearse P, Cree-Green M, et al. Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. The Journal of pediatrics. 2013;162:930 e1–936 e1. doi: 10.1016/j.jpeds.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butte NF, Hopkinson JM, Wong WW, Smith EO, Ellis KJ. Body composition during the first 2 years of life: an updated reference. Pediatr Res. 2000;47:578–585. doi: 10.1203/00006450-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Shypailo RJ, Butte NF, Ellis KJ. DXA: can it be used as a criterion reference for body fat measurements in children? Obesity (Silver Spring) 2008;16:457–462. doi: 10.1038/oby.2007.81. [DOI] [PubMed] [Google Scholar]
- 15.Fields DA, Demerath EW, Pietrobelli A, Chandler-Laney PC. Body composition at 6 months of life: comparison of air displacement plethysmography and dual-energy X-ray absorptiometry. Obesity (Silver Spring) 2012;20:2302–2306. doi: 10.1038/oby.2012.102. [DOI] [PubMed] [Google Scholar]
- 16.Slaughter MH, Lohman TG, Boileau RA, et al. Skinfold equations for estimation of body fatness in children and youth. Human biology. 1988;60:709–723. [PubMed] [Google Scholar]
- 17.Agency IAE, editor. Human Health Series: IAEA. 2013. Body Composition Assessment from Birth to Two Years of Age; p. 62. [Google Scholar]
- 18.Shypailo RJ, Ellis KJ. Solid anthropomorphic infant whole-body DXA phantom: design, evaluation, and multisite testing. Pediatric research. 2013;74:486–493. doi: 10.1038/pr.2013.148. [DOI] [PubMed] [Google Scholar]
- 19.Ellis KJ, Yao M, Shypailo RJ, Urlando A, Wong WW, Heird WC. Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. The American journal of clinical nutrition. 2007;85:90–95. doi: 10.1093/ajcn/85.1.90. [DOI] [PubMed] [Google Scholar]
- 20.Fields DA, Gilchrist JM, Catalano PM, Gianni ML, Roggero PM, Mosca F. Longitudinal body composition data in exclusively breast-fed infants: a multicenter study. Obesity (Silver Spring) 2011;19:1887–1891. doi: 10.1038/oby.2011.11. [DOI] [PubMed] [Google Scholar]
- 21.Ma G, Yao M, Liu Y, et al. Validation of a new pediatric air-displacement plethysmograph for assessing body composition in infants. The American journal of clinical nutrition. 2004;79:653–660. doi: 10.1093/ajcn/79.4.653. [DOI] [PubMed] [Google Scholar]
- 22.Sainz RD, Urlando A. Evaluation of a new pediatric air-displacement plethysmograph for body-composition assessment by means of chemical analysis of bovine tissue phantoms. The American journal of clinical nutrition. 2003;77:364–370. doi: 10.1093/ajcn/77.2.364. [DOI] [PubMed] [Google Scholar]
- 23.Schmelzle HR, Fusch C. Body fat in neonates and young infants: validation of skinfold thickness versus dual-energy X-ray absorptiometry. The American journal of clinical nutrition. 2002;76:1096–1100. doi: 10.1093/ajcn/76.5.1096. [DOI] [PubMed] [Google Scholar]
- 24.Wohlfahrt-Veje C, Tinggaard J, Winther K, et al. Body fat throughout childhood in 2647 healthy Danish children: agreement of BMI, waist circumference, skinfolds with dual X-ray absorptiometry. European journal of clinical nutrition. 2014;68:664–670. doi: 10.1038/ejcn.2013.282. [DOI] [PubMed] [Google Scholar]
- 25.Stettler N, Iotova V. Early growth patterns and long-term obesity risk. Current opinion in clinical nutrition and metabolic care. 2010;13:294–299. doi: 10.1097/MCO.0b013e328337d7b9. [DOI] [PubMed] [Google Scholar]
- 26.Goran MI, Ventura EE. International journal of pediatric obesity: year in review 2010. International journal of pediatric obesity : IJPO : an official journal of the International Association for the Study of Obesity. 2011;6:163–168. doi: 10.3109/17477166.2011.590205. [DOI] [PubMed] [Google Scholar]