Abstract
Q fever is a zoonosis caused by the rickettsial organism Coxiella burnetii. Infection has an acute course, usually with a self-limited febrile illness and the possibility of the evaluation to a chronic course with endocardial involvement. The presence of autoantibodies and various autoimmune disorders have also been associated with C. burnetii infection. We report a case of acute Q fever in which the patient developed large vessel vasculitis. The FDG-PET/CT scan detected inflammation of the thoracic aortic wall, suggesting an unusual immunologic host response to acute Q fever infection.
Keywords: Coxiella burnetii infection, Q fever, Vasculitis, FDG-PET scan
Introduction
Q fever is a zoonosis caused by Coxiella burnetii, a strict intracellular bacterium that lives in host phagocytic cells and exhibits multiple ways of interaction with the innate and adaptive immune system of the host [1], [2].
Q fever can be acute or chronic and may present with a wide spectrum of clinical manifestations, occasionally mimicking various autoimmune diseases, with autoantibodies being frequently present during its course [3], [4], [5].
We report a case of acute Q fever related with imaging and clinical features of Takayasu's arteritis.
Case report
A 60 year old woman was admitted in the Department of Internal Medicine because of a 15 day history of intermittent fever of 38 °C, aching pain over the forehead and fatigue. She reported no rigors, arthralgias, nausea, vomiting rash, postnasal drip, cough, diarrhea, urinary symptoms and any other pain or weight loss.
At the onset of fever, she had been referred to another clinic where she was thought to suffer from some infection. White blood cell count was 11,500/μl, ESR 50 mm/h and CRP 7 mg/dl (normal value <0.5 mg/dl). However, blood and urine cultures were sterile, CT imaging of the brain, sinus, chest and abdomen-pelvis showed no sites of infection and transthoracic echocardiography was normal. Her fever resolved without antibiotics, and after her discharge on the fifth hospital day she stayed afebrile for a further 5 days before her symptoms recurred.
The patient had no other medical history. She mentioned trekking as a hobby and had been on vacation to Cyprus 2 months earlier. She had no known allergies, had never smoked and had no recent exposure to ill persons.
When she presented to our Department, clinical examination confirmed temperature of 38.2 °C while all other vital signs were normal. There was no neck stiffness and the remainder of the neurologic examination was normal. Chest was clear on auscultation but she had a 2/6 systolic murmur at the mitral area which was not present at her previous hospitalization. There were no hepatosplenomegaly, lymphadenopathy, abdominal tenderness or masses. She had no skin lesions and temporal arteries were clinically normal bilaterally.
Laboratory analysis revealed raised ESR (100 mm/h) and CRP (17 mg/dl – normal value <0.5). White cell count was 10,000 mm3 with 72% neutrophils and 21% lymphocytes. Hemoglobin was 12 g/dl and platelet count 200,000/mm3. Electrolytes, total protein, albumin, globulin, uric acid and LDH were normal, as were tests of coagulation, renal and liver function.
Six sets of blood cultures were obtained from different sites at the peak of fever and when she was afebrile, and trans-esophageal echocardiography (TEE) was performed on suspicion of infective endocarditis. Blood cultures were all sterile, however TEE showed a small mitral lesion which resembled vegetation. No abnormality or serious regurgitation of the valve was noticed.
These findings were considered indicative of blood culture negative infective endocarditis. Treatment with intravenous ceftriaxone 2 g was administered focusing on the HACEK group of bacteria. Further diagnostic tests were pursued for fastidious organisms including brucella agglutinin tests (Wright, Wright Coombs), serologic tests for C. burnetii, Bartonella, Chlamydia, Mycoplasma, as well PCR for Tropheryma whippelii. Prolonged incubation of the blood cultures was requested to address the possibilities of HACEK pathogens, and nutritionally deficient streptococci (Abiotrophia defective, Granulicatella). The LightCycler SeptiFast test was also performed to detect, as a multiplex real-time PCR assay, possible bacteria or fungi causing bloodstream infection.
All tests were negative except for the screening test for C. burnetii. An IgG anti-phase II antibody titer of 1/1024 and an IgM anti-phase II antibody titer of 1/64 were detected. Anti-phase I antibodies were IgG 1/256 whereas IgM as well as PCR were found negative (National Reference Center of Parasitology Zoonoses and Geographical Medicine in Heraklion-Crete). These results were strongly indicative of acute Q fever. It is established in the literature that endocarditis is the major clinical manifestation of chronic Q fever. As a result, our first estimation of endocarditis based on the TEE findings and the diagnosis of acute Q fever were contradictory. Considering the febrile course and positive serology, treatment was changed from ceftriaxone to doxycycline 100 mg bid plus hydroxychloroquine 600 mg daily, in case of evolution to chronic Q fever. After 7 days of treatment, the fever persisted. Even more, the patient presented with more frequent and higher peaks of fever up to 38.5 °C which did not respond to regular non-steroidal anti-inflammatory drugs. The patient appeared unwell and laboratory analysis revealed a further rise of inflammatory markers (ESR: 120 mm/h and CRP: 22 mg/ml) and worsening anemia of chronic disease. Renal and liver function remained normal. A second TEE showed the same mitral valve lesion. Therefore, further work-up was performed.
Serum ferritin level was 400 ng/ml (normal range 10–291 ng/ml), not markedly raised to suggest adult-onset Still's disease. PCR assay for CMV was negative as was the serologic test for HIV and the quantiferon test as evidence of latent tuberculosis. The AgK39 for leishmaniasis was also negative. Temporal artery biopsy was normal and bone marrow analysis revealed a reactive marrow with no signs of infiltration or granuloma. Endoscopy of the gastrointestinal tract and biopsies did not suggest inflammatory bowel disease. Investigations for the possibility of a systemic autoimmune disease were requested: serum complement levels were normal; antinuclear antibodies were borderline weak positive at 1/80, anti-dsDNA, ANCA, RF Factor and all other autoantibodies were negative, with the exception of IgM anticardiolipin antibodies and the anti β2 GPI IgM antibodies, which were twice the normal values.
The patient's constitutional symptoms, valvular involvement, raised inflammatory markers and some positive autoantibodies suggested the possibility of a non infectious systemic inflammatory process. FDG-PET/CT scan was requested, which revealed increased uptake of FDG (indicating any kind of inflammation) in the ascending aorta, aortic arch and thoracic descending aorta, strongly suggestive of the presence of a large vessel vasculitis/aortitis (Fig. 1). We concluded that our patient was suffering from acute Q fever, complicated by aortitis. Treatment with intravenous methylprednisolone, 1 g daily for 3 days was initiated. The patient became afebrile within 24 h, inflammatory markers returned to normal and her general status improved markedly and rapidly. Serological follow up, 21 days after the first screening, confirmed the diagnosis of acute Q fever. An IgG anti-phase II antibody titer of 1/512 and an IgM anti-phase II antibody titer of 1/128 were detected, whereas an IgG anti-phase I antibody was only 1/256 (Table 1). The patient recovered and she was discharged from hospital on 15 mg of methotrexate once a week, 32 mg of methylprednisolone per day to be tapered, 200 mg of doxycycline and 600 mg of hydroxychloroquine daily, for at least 1 year.
Fig. 1.
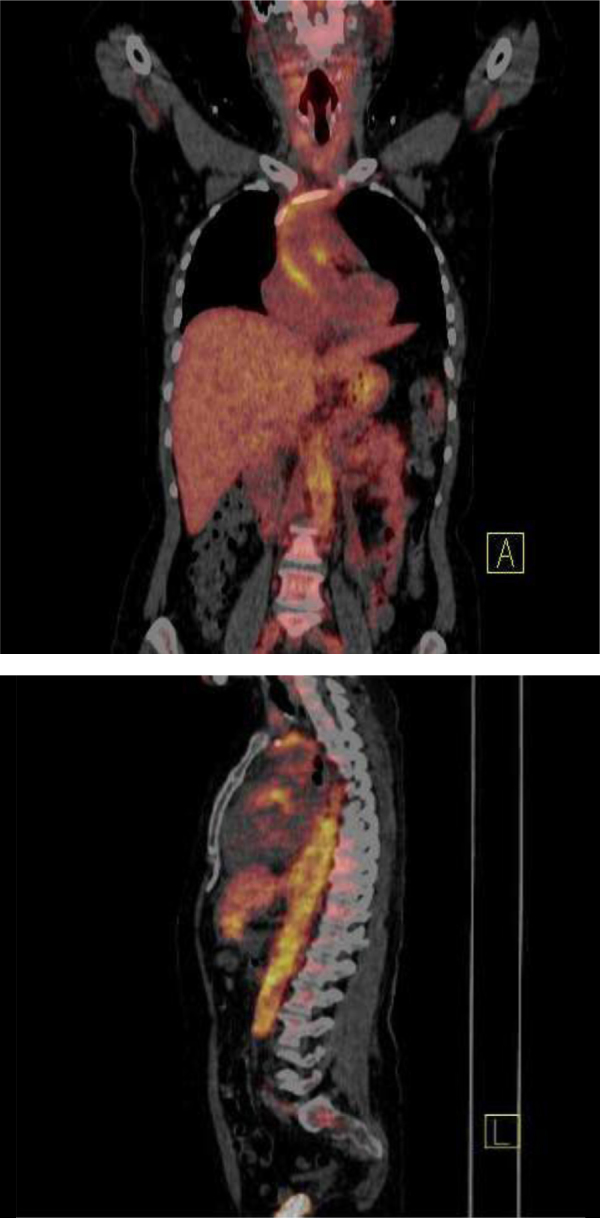
Increased uptake of FDG in the ascending aorta, aortic arch and thoracic descending aorta.
Table 1.
Coxiella burnetii antibodies of the patient.
|
Coxiella burnetii antibodies | ||||
|---|---|---|---|---|
| Phase I |
Phase II |
|||
| IgG | IgM | IgG | IgM | |
| 27/2/2013 | 1/256 | Negative | 1/1024 | 1/64 |
| 19/3/2013 | 1/256 | 1/32 | 1/512 | 1/128 |
| 21/9/2013 | 1/512 | 1/16 | 1/1024 | 1/16 |
| 30/1/2014 | 1/64 | Negative | 1/256 | Negative |
Discussion
Two aspects of our case warrant discussion. First, the probable relation between an infection and a non-infectious inflammatory disease. Second, the treatment strategy and follow-up of the patient.
At the onset of the disease, we were confronted with an unusual combination of serologic diagnosis of acute Q fever and a subtle mitral valve lesion. The phase II IgM and IgG antibodies were 1/64 and 1/1024 indicating recent Q fever infection. However, according to the modified Duke criteria, endocarditis is associated with antiphase I IgG antibody titer >1/800 [6].
It is well accepted that patients with symptomatic acute Q fever usually present with flu-like symptoms and fever which is accompanied by severe headaches. Moreover one quarter of acute Q fever patients experience a biphasic fever, as was noticed in this case [1]. Atypical pneumonia, hepatitis and meningoencephalitis are further major forms of acute Q fever [2]. Cardiac involvement has been infrequently reported in acute Q fever patients and involves myocarditis and pericarditis [1]. Endocarditis is the main clinical manifestation of chronic Q fever, whereas infections of aneurysms and vascular grafts have also been described. Recently FDG-PET/CT has been utilized for estimation of vascular infections due to chronic Q fever [1], [3], [7].
The patient presented a strong inflammatory reaction which resulted in positive ACL and anti β2 GPI IgM antibodies. In this new setting, the atypical valvular vegetation could be attributed to non-bacterial thrombotic endocarditis of the Libman-Sacks type. Moreover the FDG-PET/CT scan detected inflammation of the thoracic aortic wall. The image was strongly indicative of an aortitis, like Takayasu's arteritis [8], and was not classified as aneurysm or probable localization of infection. A variety of autoantibodies have been described in acute Q fever including antibodies to phospholipids, antimitochondrial antibodies, anti smooth muscle, antinuclear and antiSm antibodies [4], [5], [9]. Moreover, dysregulation of cytokines and different host responses in acute Q fever patients have been documented [10], [11]. Recently, seven cases of Q fever were reported, mimicking vasculitis, systemic inflammatory disease or autoimmune disorder. Q fever was acute in 4 of the 7 patients, and the initial presentations suggested Crohn's disease, Good-pasture syndrome, polymyalgia rheumatica and adult-onset Still's disease, polyarteritis nodosa, giant-cell arteritis and essential type II cryoglobulinemia [4].
In particular antibodies to phospholipids are a common immunologic event in the setting of Q fever. Their activity is usually b2GP1-independent (infectious type a PL) and rarely associated with thrombotic events [4], [12]. Furthermore, it has been reported that chronic Q fever may mimic large or medium sized vessel vasculitis [12]. However, in this case it seems that acute Q fever triggered an intense immunologic process which resulted in the damage of the valve tissue and the inflammation of the thoracic aortic wall, with an image from the FDG-PET/CT similar to that of Takayasu's arteritis. The clinical presentation of this disease may range from non specific constitutional symptoms to carotidynia and total cerebrovascular events with aneurysms and aortic rupture [13]. Its pathogenesis remains unknown and both immunologic mechanisms and genetic predisposition seem to be involved. In this case, it is interesting to speculate that the host response to acute Q fever associated with the early expression of the Takayasu's-type vasculitis. It is recommended that in such cases, patients may benefit from steroid treatment [4], [9], [10], with high dosage and a long course of steroids often required [10], [11]. Therefore, we assumed that our patient was suffering from acute Q fever and also developed an immunologically-mediated valvular defect and inflammatory involvement of the aorta. There is great concern regarding the management of such patients. Following primary infection, there should be high clinical suspicion of the patient developing chronic Q fever. The chronic evolution of acute state and reactivation of Q fever in this case is challenging, regarding the pre-existing valvular and vascular defect. Moreover the long-term immunomodulatory therapy for the vasculitis may increase the risk for the patient to develop an insidious, chronic infection [9].
It is estimated that the risk of evolution from acute Q fever to chronic Q fever with endocarditis is ∼40% in patients with underline valvulopathy [14]. In the later case, combination of doxycycline plus hydroxychloroquine is preferred for preventing the development of endocarditis than doxycycline monotherapy. Therefore, we decided that our patient should be treated with doxycycline plus hydroxychloroquine for at least 12 months, while receiving methotrexate and a tapering course of steroids for continuing control of her aortitis. Methotrexate was considered necessary as a steroid sparing treatment for the suppression of the inflammation and preservation of vascular competence of the ascending and thoracic aorta. In addition, we decided serological follow-up every 2 months (as recommended for methotrexate treatment monitoring) and a TEE every 3 months for at least 2 years should be performed [13], [15]. The patient was advised at discharge to be monitored clinically, initially every 3 months, then every 6 months for 2–3 years and then yearly for life [16], [17]. As far as the vasculitis of aorta is concerned, clinical monitoring, inflammatory markers and the FDG-PET/CT imaging were suggested for monitoring disease activity and response to treatment [13].
Eighteen months later, the patient has discontinued doxycycline, hydroxychloroquine and methylprednisolone as well, whereas she is ongoing maintenance therapy with 15 mg methotrexate in order to suppress the immunological reaction of the vasculitis. She remains well with no clinical or laboratory evidences of ongoing inflammatory disease activity. The TEE has not been changed as far as it concerns the mitral valve lesion, whereas a recent FDG-PET/CT revealed no uptake at all, without evidence of seroconversion to chronic Q fever.
Funding
No funding sources.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Competing interests
None declared.
References
- 1.Maurin M., Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelakis E., Raoult D. Q fever. Vet Microbiol. 2010;140:297–309. doi: 10.1016/j.vetmic.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Ordi-Ros J., Selva-O’Callaghan A., Monegal-Ferran F., Monasterio-Aspiri Y., Juste-Sanchez C., Vilardell-Tarres M. Prevalence, significance, and specificity of antibodies to phospholipids in Q fever. Clin Infect Dis. 1994;18:213–218. doi: 10.1093/clinids/18.2.213. [DOI] [PubMed] [Google Scholar]
- 4.Lefebvre M., Grossi O., Agard C., Perret C., Le Pape P., Raoult D. Systemic immune presentations of Coxiella burnetii infection (Q Fever) Semin Arthritis Rheum. 2010;39:405–409. doi: 10.1016/j.semarthrit.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Ohgunchi H., Hirabayashi Y., Kodera T., Ishii T., Munakata Y., Sasaki T. Q fever with clinical features resembling systemic lupus erythematosus. Intern Med. 2006;45(5):323–326. doi: 10.2169/internalmedicine.45.1382. [DOI] [PubMed] [Google Scholar]
- 6.Li J.S., Sexton D.J., Mick N., Nettles R., Fowler V.G., Jr., Ryan T. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;13(4):633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 7.Barten D., Delsing C., Keijimel S., Sprong T., Timmermans J., Oyen W.J.G. Localising chronic Q fever: a challenging query. BMC Infect Dis. 2013;13:413. doi: 10.1186/1471-2334-13-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arend W.P., Michel B.A., Bloch D.A., Hunder G.G., Calabrese L.H., Erdworthy S.M. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33(8):1129–1134. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]
- 9.Levy P., Raoult D., Razongles J.J. Q-fever and autoimmunity. Eur J Epidemiol. 1989;5:447–453. doi: 10.1007/BF00140139. [DOI] [PubMed] [Google Scholar]
- 10.Lai C.H., Lin J.N., Chang L.L., Chen Y.H., Lin H.H. Circulating cytokines and procalcitonin in acute Q fever granulomatous hepatitis with poor response to antibiotic and short-course steroid therapy: a case report. BMC Infect Dis. 2010;10:193. doi: 10.1186/1471-2334-10-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honstettre A., Imbert G., Ghigo E., Gouriet F., Capo C., Raoult D. Dysregulation of cytokines in acute Q fever: role of interleukin-10 and tumor necrosis factor in chronic evolution of Q fever. J Infect Dis. 2003;187:956–962. doi: 10.1086/368129. [DOI] [PubMed] [Google Scholar]
- 12.Fournier P.E., Casalta J.P., Piquet P., Tournigand P., Branchereau A., Raoult D. Coxiella burnetii infection of aneurysms or vascular grafts: report of seven cases and review. Clin Infect Dis. 1998;26:116–121. doi: 10.1086/516255. [DOI] [PubMed] [Google Scholar]
- 13.Mavrogeni S., Dimitroulas T., Chatziioannou S., Kitas G.D. The role of multimodality imaging in the evaluation of Takayasu arteritis. Vasculitis. 2013;42(4):401–412. doi: 10.1016/j.semarthrit.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Landais C., Fenollar F., Thuny F., Raoult D. From acute Q fever to endocarditis: serological follow-up strategy. Clin Infect Dis. 2007;44:1337–1340. doi: 10.1086/515401. [DOI] [PubMed] [Google Scholar]
- 15.Raoult D. Host factors in the severity of Q fever. Ann N Y Acad Sci. 1990;590:33–38. doi: 10.1111/j.1749-6632.1990.tb42204.x. [DOI] [PubMed] [Google Scholar]
- 16.Fenollar F., Fournier P.E., Carrieri M.P., Habib G., Messana T., Raoult D. Risks factors and prevention of Q fever endocarditis. Clin Infect Dis. 2001;33:312–316. doi: 10.1086/321889. [DOI] [PubMed] [Google Scholar]
- 17.Million M., Thuny F., Richet H., Raoult D. Long-term outcome of Q fever endocarditis: a 26-year personal survey. Lancet Infect Dis. 2010;10:527–535. doi: 10.1016/S1473-3099(10)70135-3. [DOI] [PubMed] [Google Scholar]


