Abstract
Metal-on-metal (MOM) bearing surfaces in hip arthroplasty have distinct advantages that led to the increase in popularity in North America in the early 2000s. However, with their increased use, concerns such as local cytotoxicity and hypersensitivity reactions leading to soft tissue damage and cystic mass formation (known collectively as adverse local tissue reactions (ALTR)) became apparent. The clinical presentation of ALTR is highly variable. The diagnosis of ALTR in MOM articulations in hip arthroplasty can be challenging and a combination of clinical presentation, physical examination, implant track record, component positioning, serum metal ion levels, cross-sectional imaging, histopathologic analysis, and consideration of alternative diagnoses are essential.
Keywords: Total hip arthroplasty (THA), Metal-on-metal (MOM), Adverse local tissue reactions (ALTRs), Pseudotumor, Aseptic lymphocytic vasculitic-associated lesion (ALVAL)
Introduction
Metal-on-metal (MOM) bearing surfaces in hip arthroplasty became increasingly popular in the early 2000s in the USA [1]. In 2006, MOM bearing surfaces were utilized in 35 % of the total hip arthroplasties (THAs) performed in the USA [2]. Advantages of MOM bearing surfaces include significantly lower volumetric wear rates, decreased rates of instability due to the ability to use a large femoral head, and high fracture toughness compared to ceramic-on-ceramic bearing surfaces [1, 3, 4]. However, there have been prominent clinical concerns over unique failure modes over the past decade [1, 5]. Adverse local tissue reactions (ALTRs) have been well described in the literature as potential complications of MOM articulations [3, 5, 6•, 7•]. ALTRs are caused by an inflammatory response to small metal debris particles created by MOM implants [3]. This inflammatory response can lead to metallosis, formation of a bursal soft tissue growth known as a pseudotumor, and generalized synovitis and tissue damage. These reactions can subsequently lead to muscle, capsule, and soft tissue degradation, as well as tendinopathy around the hip joint. This constellation of events is thought to be the origin of pain, instability, and dysfunction in MOM hip arthroplasties with ALTR [1, 8–10]. Histopathological studies have shown that the complex inflammatory response can be both macrophage-induced cytotoxicity stimulated by metal debris and a type IV delayed hypersensitivity reaction to metal particles referred to as aseptic lymphocytic vasculitis-associated lesion (ALVAL) [3, 5, 8, 11•, 12, 13].
While this review will focus on ALTR affecting the hip joint and surrounding soft tissue structures, there may be potential systemic adverse effects of reactions to metal debris [5]. While there has been concern about the carcinogenicity of cobalt and chromium debris, several recent studies show no causal relationship between MOM implants and risk of cancer [14]. However, several reports have shown systemic symptoms of cobalt toxicity including neurologic, renal, and cardiac impairment in patients with high metal ion levels secondary to MOM hip arthroplasties [15].
The goal of this article is to provide an evidence-based review with the most up-to-date literature on the evaluation and diagnosis of ALTR in MOM bearing surfaces.
Clinical presentation and assessment
The diagnosis of ALTR is sometimes obscured by the wide spectrum of clinical presentations [5, 16•]. Patients with large pseudotumors may be asymptomatic, but they may also have high or very minimal wear in the components on implant retrieval analysis [5, 12, 13, 17•]. Asymptomatic patients may also have mildly or even significantly elevated metal ion levels [16•, 18–20] (Fig. 1). Conversely, patients without cross-sectional imaging evidence of ALTR and well-positioned components may have hip pain [16•]. Further, many reports describe these complications in both malpositioned, high-wear components and well-positioned, low-wear components, making radiographic analysis less reliable [6•, 20–22] (Fig. 2). Finally, due to the inflammatory reaction that MOM implant debris causes, the diagnosis of concomitant infection is also challenging [23•].
Fig. 1.
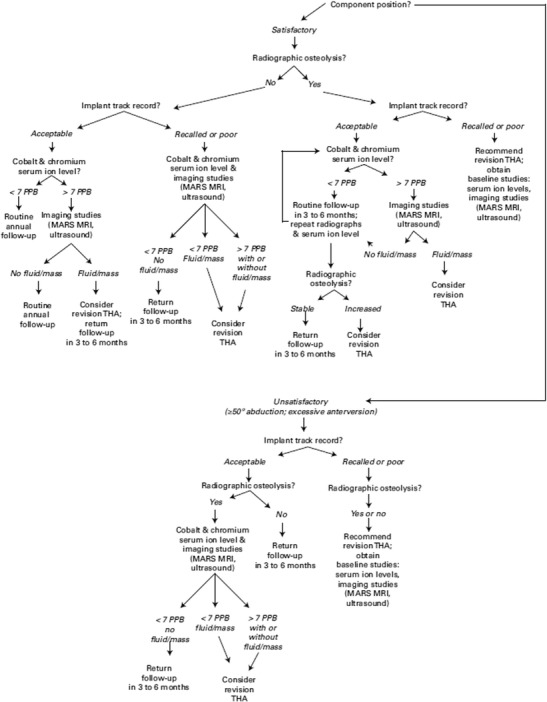
Reproduced with permission and copyright © of the British Editorial Society of Bone and Joint Surgery [14]. An algorithm for diagnosis and treatment of an asymptomatic metal-on-metal total hip arthroplasty
Fig. 2.
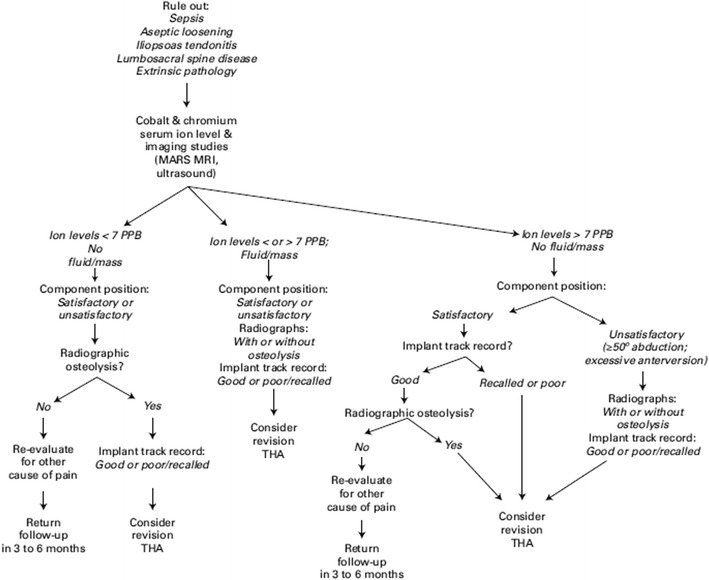
Reproduced with permission and copyright © of the British Editorial Society of Bone and Joint Surgery [14]. An algorithm for diagnosis and treatment of a symptomatic metal-on-metal total hip arthroplasty
The key factors to assess are the following: (1) patient symptomatology, (2) implant track record, (3) positioning of the components, (4) metal ion levels, (5) cross-sectional imaging, (6) histopathologic analysis, and (7) alternative diagnoses, particularly infection [11•, 16] (Table 1). All of these factors are elements in the risk stratification algorithms for the diagnosis and management of ALTR in MOM implants in hip arthroplasty proposed by several recent publications from a consensus statement by the American Academy of Hip and Knee Surgeons (AAHKS), American Academy of Orthopedic Surgeons (AAOS), and Hip Society [16, 11•] (Figs. 1 and 2).
Table 1.
Key factors to assess in diagnosis and management of adverse local tissue reactions in metal-on-metal hip arthroplasty
| Patient symptomatology |
| Implant track record |
| Positioning of the components on imaging |
| Metal ion levels |
| Cross-sectional imaging findings (i.e., pseudotumor) |
| Histopathologic analysis |
| Consideration of alternative diagnosis, particularly infection |
History and physical examination
A thorough history and physical evaluation are essential in evaluating a MOM articulation, regardless of pain. All causes of extrinsic pathologies, such as lumbosacral spine disorders or an inguinal hernia, and intrinsic pathologies, such as infection, aseptic loosening, and iliopsoas tendonitis, after a THA should be considered [5, 16•, 21]. Pain should be evaluated based upon the timing from index arthroplasty, location, duration, and severity. A history of pain since the index arthroplasty or since a sentinel event (e.g., episode of urosepsis), or wound complications, should increase the clinical suspicion of infection. Obtaining original operative reports are also useful in identifying the exact components utilized, and complications or special considerations associated with the index arthroplasty.
Inspection of the wound and skin, palpation for soft tissue masses, and an assessment of the stability and range of motion of the hip joint are the key features of the physical examination. Abductor muscle strength should be assessed in all patients, as abductor muscle damage and subsequent weakness are common in severe cases of ALTR (Fig. 3). Due to the large femoral heads and large acetabular components of many MOM implants, impingement of the iliopsoas tendon during resisted hip flexion can be a source of pain [24].
Fig. 3.
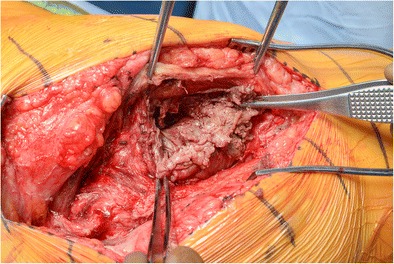
An intraoperative photograph of a 55-year-old male with an adverse local tissue reaction from a metal-on-metal articulation in a total hip arthroplasty causing pseudotumor formation and soft tissue destruction of his hip abductors
Laboratory studies
Metal ion levels
Measurements of blood metal ion levels, specifically cobalt and chromium levels, are useful in diagnosing and treating ALTR. Numerous studies have reported the relationship with increased cobalt and chromium metal ion levels in the serum of MOM hip arthroplasties compared to metal-on-polyethylene (MOP) THAs [25, 26, 27•]. We routinely obtain both levels in all MOM hips for diagnostic and surveillance purposes. While there are no studies directly defining the exact correlation of elevated metal ion levels and increased risk ALTR, several reports have shown that elevated metal ion levels are associated with increased wear rates and failed MOM prostheses [20, 16•, 11, 19]. Several studies have shown that cobalt levels above 7 parts-per-billion (ppb) [16•, 20, 11] are a reasonable threshold for increased concern and closer clinical follow-up, while other studies suggest cobalt levels of 4.5 ppb [18, 19]. Therefore, the results of metal ion levels are best used as another data point in the context of a risk stratification model, rather than absolute numbers [11•, 16•, 18]. Utilizing metal ion levels in conjunction with other data and as trends over time in surveillance of MOM hip arthroplasty is best practice. These levels can aid in the diagnosis of and/or raise clinical suspicion of an underlying ALTR for further investigation.
Several factors may place patients at higher risk for having elevated ion levels. There is a suggestion, but no overwhelming evidence, that young, active patients tend to have higher levels of metal ions in their blood compared to older, less active patients. As discussed below, component malposition has been shown to be a risk factor for higher metal ion levels [16•, 18, 22]. Increased abduction angle of >50°, a small component size, and/or a combined anteversion of >40° have all been shown to be correlated with increased serum metal ion levels [22]. Several studies have shown that metal ion levels increase over time [25, 27•]. Further, there is some evidence that larger femoral heads lead to increased metal ion levels over time compared to smaller femoral heads [25].
Cobalt to chromium ratio was once thought to be useful in predicting wear and ALTR [28]. However, a recent study reported no correlation with cobalt to chromium ratios and the degree of soft tissue damage as determined intraoperatively [28].
Finally, as mentioned previously, there have been reports of systemic toxicity to metal ion levels, particular cobalt [5, 15]. Extremely high cobalt serum ion levels, typically >20 ppb, are of particular concern.
Serum inflammatory markers
Deep periprosthetic joint infection (PJI) should always be considered when evaluating a painful hip arthroplasty, including those with MOM articulations. The evaluation for infection in metal-on-polyethylene articulations includes evaluation of inflammatory markers, namely an erythrocyte sedimentation rate (ESR) and a C-reactive protein (CRP) [29–31]. However, the diagnosis of infection is challenging in patients with MOM articulations as the inflammatory reaction provoked by metal debris can mimic infection serologically [23•, 32–34].
Wyles et al. [18] have shown that CRP and ESR have a poor predictive value in diagnosing PJI in failed MOM hip arthroplasty. They reported a 75 % sensitivity and 67 % specificity in diagnosing PJI if both the CRP was >8.0 mg/L and ESR was >22 mm/h [23•]. There have been other case reports of failed MOM articulations masking as infection in the literature as well [32, 34].
Aspiration of the hip joint
Aspiration of the hip joint prior to surgery is recommended in the evaluation of a painful hip arthroplasty and before revision surgery to rule out infection [29–31]. However, MOM articulations can falsely elevate both the white blood cell (WBC) count and neutrophil percentage. Metal particles, degenerating cells, and foreign debris can falsely elevate the WBC count [32]. One study showed that while automated WBC count of >3000 cells/uL was 100 % sensitive for diagnosing PJI, it was only 57 % specific [23•]. The WBC count in synovial fluid of MOM articulations can be elevated, complicating the diagnosis of infection [35]. Therefore, multiple studies recommend obtaining a manual WBC count from the synovial fluid aspirate to alert the technician that the sample may be inaccurate due to confounding variables that may artificially raise the WBC count [23•, 32]. Once these inaccurate samples are excluded by a manual cell count, the sensitivity and specificity have been shown to be 100 and 94 %, respectively, if a WBC count of 4350 cells/uL was utilized [32].
A manual differential count, rather than an automated differential count, to assess the synovial neutrophil percentage has been shown to be highly accurate for diagnosing infection in an underlying MOM articulation [23•, 32, 35]. One study showed that a neutrophil percentage of >80 % was 100 % sensitive and 97 % specific for infection [23•]. Another study reported an 82 % sensitivity and 87 % sensitivity in diagnosing PJI [32]. Another study showed that a predominance of monocytes is typical for an ALTR [16•].
We consider CRP, ESR, synovial fluid WBC count, and synovial fluid WBC differential and culture result collectively to determine whether or not a painful hip arthroplasty with a MOM articulation has a concomitant infection.
Imaging modalities
Radiographs
The first imaging modality utilized in the evaluation of MOM hip arthroplasties should be plain radiographs. We routinely obtain an AP pelvis, an AP of the hip, and a cross-table lateral of the hip. Careful assessment of radiographs should focus on implant type, other potential causes of pain, and component position. It is important to note that implant type as particular implants, especially those with modular components, have variable track records [5, 11•, 16•]. The track record of a particular implant is another component of the most commonly utilized diagnostic and treatment algorithm proposed in both asymptomatic (Fig. 1) and symptomatic (Fig. 2) patients [11•, 16•]. Comparison of serial radiographs to immediate postoperative radiographs is important in the assessment of osteolytic defects, signs of component loosening, or signs of prosthesis impingement [5, 11•, 16•]. Not only can component malposition cause a host of potential complications, such as iliopsoas impingement and instability, but studies have shown that it has an effect on the metal ion levels generated by a MOM prosthesis. Increased acetabular abduction angle of >50°, combined anteversion of >40°, and/or a small component have all been correlated with increased blood metal ion levels and potentially increase ALTR when compared to less abduction and anteversion [16•, 22]. However, it should be noted that in most cases of ALTR, the plain radiographs appear normal without major component malpositioning [5, 36]. Improper component position should raise clinical suspicion that the patient may have or develop an ALTR.
Ultrasonography
Ultrasonography (US) is one imaging modality that can be utilized as a screening and diagnostic tool in MOM hip arthroplasties. It is particularly useful to evaluate fluid collections and pseudotumors. Advantages of US include the ability to obtain images without metal artifacts, an absence of ionizing radiation, its relatively low cost compared to other imaging modalities, and its availability at most institutions. The major disadvantages are that ultrasonography is operator-dependent and is not as sensitive as metal artifact reduction sequencing magnetic resonance imaging (MARS MRI) [37•, 38–41]. Still, several studies have demonstrated excellent sensitivity and specificity of detecting pseudotumors associated with MOM implants [38–40]. One study reported a US sensitivity of 92 % and specificity of 84 % in diagnosing pseudotumors [38]. The ability of US to detect more subtle ALTR with lack of a major fluid mass or pseudotumor is unclear. Currently, in our practice, US is utilized more in patients that have other contraindications to MRI, such as a cardiac pacemaker [37•].
Magnetic resonance imaging
MARS MRI is the main cross-sectional imaging modality utilized in the diagnosis and characterization of suspected ALTR. First, MRI can assist in detection of other intrinsic and extrinsic causes of pain, such as iliopsoas tendonitis, nerve irritation, and intrapelvic or lumbosacral pathology, among other entities. Furthermore, MARS is a recently developed technique that allows soft tissue visualization, reducing image distortion from surrounding metal implants [9, 36]. Studies have shown that MRI better predicts the severity and degree of soft tissue damage than computed tomography (CT) or US [37•]. In fact, several grading systems have been developed to quantify the degree of soft tissue damage [42]. ALTR as seen on MRI can include regional muscle tendinosis, edema, and atrophy, synovitis in the form of synovial thickness and synovial volume, fluid-filled cysts, and solid masses [9, 10, 36, 37•, 42]. A recent study reported that an MRI predictive model resulted in a sensitivity of 94 % and a specificity of 87 % for detecting ALVAL and a 90 % sensitivity for quantifying intraoperative tissue damage [43]. This MARS MRI technology is also being applied to diagnosis of ALTR secondary to modular taper corrosion rather than a MOM bearing surface [36].
However, studies have shown that synovitis and pseudotumors were detected in nearly equal proportions in painful hips and painless, well-functioning hips [33, 44]. As discussed above, the clinical presentation of ALTR is widely variable and may be asymptomatic even in large detected lesions. Cross-sectional imaging should account for one factor in the treatment algorithm of ALTR.
Disadvantages to this modality are the high cost associated with its use and that these specialized MRI scanners may not be universally available [37•]. Utilizing other imaging modalities along with a careful history and physical examination is crucial in these instances.
Histopathology
The histopathologic classification of the inflammatory responses and pseudotumors that occur in ALTR-associated MOM implants has been of interest in understanding the pathogenesis of such reactions. A wide spectrum of histological findings has been reported in multiple studies over the last decade. Tissue necrosis and a prominent macrophage response are noted in the majority of cases. Stimulation of an inflammatory reaction by metal debris leading to cytotoxicity is thought to be the mechanism of tissue destruction [3•, 12, 13]. However, there is also a lymphocytic response known as ALVAL, determined to be a type IV hypersensitivity reaction, which varies in prominence [8, 12]. Two histologic scoring systems have been developed to better characterize the prominence of the lymphocytic infiltrate, termed the ALVAL score [12, 13].
Current data suggests that both of these inflammatory responses play a differential role in ALTR and pseudotumor formation depending in part on the degree of component wear [5, 8, 12, 13]. Both studies by Campbell et al. [13] and Grammatopoulos et al. [12] show that tissues revised in patients with high wear components had a lower ALVAL score and a predominant macrophage and metal particle histology. Conversely, tissues and pseudotumors from patients revised with low wear components showed higher ALVAL scores. This suggests that while both mechanisms likely contribute to ALTR in all patients, the etiology of ALTR in patients with low wear prostheses is likely more due to a type IV hypersensitivity while cytotoxicity from metal debris and macrophage response is the more likely mechanism of tissue destruction in patients with high wear prosthesis [12, 13]. This theory, however, has not been shown in other studies. The specific inflammatory response is likely just one aspect of the etiology of pseudotumors that likely involves the implant, specific patient factors, and surgical considerations [5].
Conclusion
Diagnosis of pain from ALTR after a MOM hip arthroplasty can challenge the clinician. A host of factors must be considered. Understanding the variability in clinical presentation of patients with ALTR from MOM articulations is the first step. A thorough clinical history including obtaining operative reports and implant specifics, physical examination, and evaluation of serial plain radiographs is essential in evaluation for both ALTR and alternative intra-articular or extra-articular etiologies for pain after a MOM hip arthroplasty. Metal ion levels can be utilized in conjunction with cross-sectional imaging to further hone the diagnosis of ALTR once alternative etiologies are excluded. The diagnosis and evaluation of a PJI in a MOM implant with wear and an ALTR can be convoluted. Use of a manual WBC count that is uncontaminated by metal debris and a manual WBC cell differential has been shown highly sensitive and specific for diagnosing infection. Finally, histopathologic analysis at the time of revision surgery can also assist in the diagnosis of ALTR. We consider patient demographics and symptomatology, implant track record, positioning of the components, metal ion levels, cross-sectional imaging studies, histopathologic analysis, and alternative diagnoses in diagnosing and managing all individuals with MOM articulations in hip arthroplasty.
Compliance with ethical standards
Conflict of interest
Brian P. Chalmers, Kevin I. Perry, Tad M. Mabry, and Matthew P. Abdel declare that they have no conflict of interest.
Michael J. Taunton reports personal fees from DJO Global, outside of the submitted work.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Hip: Metal-on-Metal
Contributor Information
Brian P. Chalmers, Phone: (507) 284-2884, Email: chalmers.brian@mayo.edu
Kevin I. Perry, Phone: (507) 284-2884, Email: perry.kevin@mayo.edu
Michael J. Taunton, Phone: (507) 284-2884, Email: taunton.michael@mayo.edu
Tad M. Mabry, Phone: (507) 284-2884, Email: mabry.tad@mayo.edu
Matthew P. Abdel, Phone: (507) 284-2884, Email: abdel.matthew@mayo.edu
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Fehring KA, Fehring TK. Modes of failure in metal-on-metal total hip arthroplasty. Orthop Clin North Am. 2015;46:185–92. doi: 10.1016/j.ocl.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Bozic KJ, Kurtz S, Lau E, Ong K, Chiu V, Vail TP, et al. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:1614–20. doi: 10.2106/JBJS.H.01220. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs JJ, Urban RM, Hallab NJ, Skipor AK, Fischer A, Wimmer MA. Metal-on-metal bearing surfaces. J. Am. Acad. Orthop Surg. 2009;17:69–76. doi: 10.5435/00124635-200902000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Anissian HL, Stark A, Good V, Dahlstrand H, Clarke IC. The wear pattern in metal-on-metal hip prostheses. J Biomed Mater Res. 2001;58:673–8. doi: 10.1002/jbm.1068. [DOI] [PubMed] [Google Scholar]
- 5.Kwon Y-M, Jacobs JJ, MacDonald SJ, Potter HG, Fehring TK, Lombardi AV. Evidence-based understanding of management perils for metal-on-metal hip arthroplasty patients. J Arthroplasty. 2012;27:20–5. doi: 10.1016/j.arth.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 6.•.Nawabi DH, Nassif NA, Do HT, Stoner K, Elpers M, Su EP, et al. What causes unexplained pain in patients with metal-on metal hip devices? A retrieval, histologic, and imaging analysis. Clin Orthop Relat Res. 2014;472:543–54. doi: 10.1007/s11999-013-3199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.•.Berry DJ, Abdel MP, Callaghan JJ. What are the current clinical issues in wear and tribocorrosion? Clin Orthop Relat Res. 2014;3659–64. This article presents an excellent review of current diagnostic and treatment strategies for wear and corrosion. More importantly, it also highlights critical areas for which improvement in wear properties, diagnosis, management, and treatment can be made. [DOI] [PMC free article] [PubMed]
- 8.Natu S, Sidaginamale RP, Gandhi J, Langton DJ, Nargola VF. Adverse reactions to metal debris: histopathological features of periprosthetic soft tissue reactions seen in association with failed metal on metal hip arthroplasties. J Clin Pathol. 2012;65:409–18. doi: 10.1136/jclinpath-2011-200398. [DOI] [PubMed] [Google Scholar]
- 9.Hayter CL, Gold SL, Koff MF, Perino G, Nawabi DH, Miller TT, et al. MRI findings in painful metal-on-metal hip arthroplasty. Am J Roentgenol. 2012;199:884–93. doi: 10.2214/AJR.11.8203. [DOI] [PubMed] [Google Scholar]
- 10.Hauptfleisch J, Pandit H, Grammatopoulos G, Gill HS, Murray DW, Ostlere S. A MRI classification of periprosthetic soft tissue masses (pseudotumours) associated with metal-on-metal resurfacing hip arthroplasty. Skelet Radiol. 2012;41:149–55. doi: 10.1007/s00256-011-1329-6. [DOI] [PubMed] [Google Scholar]
- 11.•.Kwon Y, Lombardi AV, Jacobs JJ, Fehring TK, Lewis CG, Cabanela ME. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am. 2014;96(1):e4. doi:10.2106/JBJS.M.00160. This study outlines the consensus statement by the Hip Society, AAOS, and AAHKS on the diagnostic algorithm in symptomatic and asymptomatic patients with MOM articulations in THA. It incorporates all of the diagnostic factors considered in this review. [DOI] [PubMed]
- 12.Grammatopoulos G, Pandit H, Orth F, Kamali A, Maggiani F, Athanasou N. The correlation of wear with histological features. J Bone Joint Surg Am. 2013;81:1–10. doi: 10.2106/JBJS.L.00775. [DOI] [PubMed] [Google Scholar]
- 13.Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468:2321–7. doi: 10.1007/s11999-010-1372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tharani R, Dorey FJ, Schmalzried TP. The risk of cancer following total hip or knee arthroplasty. J Bone Joint Surg Am. 2001;83-A:774–80. doi: 10.2106/00004623-200105000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92:2847–51. [DOI] [PubMed]
- 16.•.Lombardi AV, Jr, Barrack RL, Berend KR, Cuckler JM, Jacobs JJ, Mont MA, et al. The Hip Society: algorithmic approach to diagnosis and management of metal-on-metal arthroplasty. J Bone Joint Surg Br. 2012;94-B:14–8. doi: 10.1302/0301-620X.94B11.30680. [DOI] [PubMed] [Google Scholar]
- 17.•.Kwon YM, Ostlere SJ, McLardy-Smith P, Athanasou NA, Gill HS, Murray DW. “Asymptomatic” pseudotumors after metal-on-metal hip resurfacing arthroplasty. Prevalence and metal ion study. J Arthroplasty. 2011;26:511–8. doi: 10.1016/j.arth.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 18.Griffin WL. Metal ion levels: how can they help us? J Arthroplasty. 2014;29:659–60. doi: 10.1016/j.arth.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Hart AJ, Sabah SA, Bandi AS, Maggiore P, Tarassoli P, Sampson B, et al. Sensitivity and specificity of blood cobalt and chromium metal ions for predicting failure of metal-on-metal hip replacement. J Bone Joint Surg Br. 2011;93-B:1308–13. doi: 10.1302/0301-620X.93B10.26249. [DOI] [PubMed] [Google Scholar]
- 20.Pajam KJJ. Surveillance of patients with metal-on-metal hip resurfacing and total hip prostheses. J Bone Join Surg Am. 2014;96(13):1091–9. doi: 10.2106/JBJS.M.00957. [DOI] [PubMed] [Google Scholar]
- 21.Bartelt RB, Yuan BJ, Trousdale RT, Sierra RJ. The prevalence of groin pain after metal-on-metal total hip arthroplasty and total hip resurfacing. Clin Orthop Relat Res. 2010;468:2346–56. doi: 10.1007/s11999-010-1356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Haan R, Pattyn C, Gill HS, Murray DW, Campbell PA, De Smet K. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J. Bone Joint Surg Br. 2008;90:1291–7. doi: 10.1302/0301-620X.90B10.20533. [DOI] [PubMed] [Google Scholar]
- 23.•.Wyles CC, Larson DR, Houdek MT, Sierra RJ, Trousdale RT. Utility of synovial fluid aspirations in failed metal-on-metal total hip arthroplasty. J Arthroplasty. 2013;28:818–23. doi: 10.1016/j.arth.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Cobb JP, Davda K, Ahmad A, Harris SJ, Masjedi M, Hart AJ. Why large-head metal-on-metal hip replacements are painful: the anatomical basis of psoas impingement on the femoral head-neck junction. J. Bone Joint Surg Br. 2011;93:881–5. doi: 10.1302/0301-620X.93B7.26054. [DOI] [PubMed] [Google Scholar]
- 25.Engh CA, Macdonald SJ, Sritulanondha S, Korczak A, Naudie D. Metal ion levels after metal-on-metal total hip arthroplasty: a five-year, prospective randomized trial. J Bone Joint Surg Am. 2014;96:448–55. doi: 10.2106/JBJS.M.00164. [DOI] [PubMed] [Google Scholar]
- 26.Engh CA, MacDonald SJ, Sritulanondha S, Thompson A, Naudie D, Engh CA. 2008 John Charnley award: metal ion levels after metal-on-metal total hip arthroplasty: a randomized trial. Clin Orthop Relat Res. 2009;467:101–11. doi: 10.1007/s11999-008-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.•.Levine BR, Hsu AR, Skipor AK, Hallab NJ, Paprosky WG, Galante JO, et al. Ten-year outcome of serum metal ion levels after primary total hip arthroplasty: a concise follow-up of a previous report*. J Bone Joint Surg Am. 2013;95:512–8. doi: 10.2106/JBJS.L.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fehring TK, Carter JL, Fehring KA, Odum SM, Griffin WL. Cobalt to chromium ratio is not a key marker for altr in metal on metal hips. J Arthroplasty. 2015;30(9 Suppl):107–9. doi: 10.1016/j.arth.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 29.Della Valle C, Parvizi J, Bauer TW, DiCesare PE, Evans RP, Segreti J, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: the diagnosis of periprosthetic joint infections of the hip and knee. J Bone Joint Surg Am. 2011;93:1355–7. doi: 10.2106/JBJS.9314ebo. [DOI] [PubMed] [Google Scholar]
- 30.Berbari E, Mabry T, Tsaras G, Spangehl M, Erwin PJ, Murad MH, et al. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2010;92:2102–9. doi: 10.2106/JBJS.I.01199. [DOI] [PubMed] [Google Scholar]
- 31.Spangehl MJ, Masri BA, O’Connell JX. Duncan CP prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81:672–83. doi: 10.2106/00004623-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Yi PH, Cross MB, Moric M, Levine BR, Sporer SM, Paprosky WG, et al. Do serologic and synovial tests help diagnose infection in revision hip arthroplasty with metal-on-metal bearings or corrosion? Clin Orthop Relat Res. 2014;473:498–505. doi: 10.1007/s11999-014-3902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liddle AD, Sabah SA, Mcrobbie D, Henckel J, Cobb JP, Skinner JA, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses. J Bone Joint Surg Am. 2012;94:317–25. doi: 10.2106/JBJS.J.01508. [DOI] [PubMed] [Google Scholar]
- 34.Mikhael MM, Hanssen AD, Sierra RJ. Failure of metal-on-metal total hip arthroplasty mimicking hip infection. A report of two cases. J. Bone Joint Surg Am. 2009;91:443–6. doi: 10.2106/JBJS.H.00603. [DOI] [PubMed] [Google Scholar]
- 35.Wyles CC, Van Demark RE, Sierra RJ, Trousdale RT. High rate of infection after aseptic revision of failed metal-on-metal total hip arthroplasty. Clin Orthop Relat Res. 2014;472:509–16. doi: 10.1007/s11999-013-3157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon YM. Cross-sectional imaging in evaluation of soft tissue reactions secondary to metal debris. J Arthroplasty. 2014;29:653–6. doi: 10.1016/j.arth.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 37.•.Nam D, Barrack RL, Potter HG. What are the advantages and disadvantages of imaging modalities to diagnose wear-related corrosion problems? Clin Orthop Relat Res. 2014;3665–73. This article provides an excellent overview of cross-sectional imaging modalities utilized to screen and diagnose ALTR in MOM articulations in THA. It is an excellent discussion with up-to-date research referenced that analyzes the advantages and disadvantages of US, CT, and MRI. [DOI] [PMC free article] [PubMed]
- 38.Nishii T, Sakai T, Takao M, Yoshikawa H, Sugano N. Is ultrasound screening reliable for adverse local tissue reaction after hip arthroplasty? J Arthroplasty. 2014;29:2239–44. doi: 10.1016/j.arth.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 39.Nishii T, Sakai T, Takao M, Yoshikawa H, Sugano N. Ultrasound screening of periarticular soft tissue abnormality around metal-on-metal bearings. J Arthroplasty. 2012;27:895–900. doi: 10.1016/j.arth.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Muraoka K, Naito M, Nakamura Y, Hagio T, Takano K. Usefulness of ultrasonography for detection of pseudotumors after metal-on-metal total hip arthroplasty. J Arthroplasty. 2014;30:879–84. doi: 10.1016/j.arth.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Siddiqui IA, Sabah SA, Satchithananda K, Lim AK, Cro S, Henckel J, et al. A comparison of the diagnostic accuracy of MARS MRI and ultrasound of the painful metal-on-metal hip arthroplasty. Acta Orthop. 2014;85:1–8. doi: 10.3109/17453674.2014.908345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson H, Toms AP, Cahir JG, Goodwin RW, Wimhurst J, Nolan JF. Grading the severity of soft tissue changes associated with metal-on-metal hip replacements: reliability of an MR grading system. Skelet Radiol. 2011;40:303–7. doi: 10.1007/s00256-010-1000-7. [DOI] [PubMed] [Google Scholar]
- 43.Nawabi DH, Gold S, Lyman S, Fields K, Padgett DE, Potter HG. MRI predicts ALVAL and tissue damage in metal-on-metal hip arthroplasty. Clin Orthop Relat Res. 2014;472:471–81. doi: 10.1007/s11999-013-2788-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibanez HE, Grand MG, Meredith TA, Wippold FJ. Magnetic resonance imaging findings in Vogt-Koyanagi-Harada syndrome. Retina. 1994;14:164–8. doi: 10.1097/00006982-199414020-00010. [DOI] [PubMed] [Google Scholar]


