Abstract
Hip resurfacing arthroplasty (HRA) is an alternative to conventional, stemmed total hip arthroplasty (THA). The best reported results are young, active patients with good bone stock and a diagnosis of osteoarthritis. Since the 1990s, metal-on-metal (MoM) HRA has achieved excellent outcomes when used in the appropriate patient population. Concerns regarding the metal-on-metal bearing surface including adverse local tissue reaction (ALTR) to metal debris have recently lead to a decline in the use of this construct. The current paper aims to provide an updated review on HRA, including a critical review of the most recent literature on HRA.
Keywords: Hip resurfacing arthroplasty, Metal-on-metal arthroplasty, Arthroplasty
Introduction
Hip resurfacing arthroplasty (HRA) is an alternative to conventional stemmed total hip arthroplasty (THA). HRA was initially made popular in the 1960s, although early implants demonstrated a high rate of wear and loosening secondary to a multitude of factors including flaws in implant design, inconsistent manufacturing, technical errors, and poor initial fixation [1, 2]. Technological advancements resulting in increased manufacturing reliability and the development of designs with low surface roughness, low diametral clearance, and high carbon content have significantly decreased MoM bearing surface wear and improved overall implant dependability [2–4]. As a result of these improvements, HRA regained interest and popularity in the 2000s. The most commonly used contemporary design, the Birmingham Hip Resurfacing (BHR; Smith and Nephew, Memphis, TN USA), was first implanted in 1997 in England and was approved for use in the USA in 2006. The design includes a thin, cementless monoblock acetabular component and a cemented, stemmed femoral component (Fig. 1).
Fig. 1.
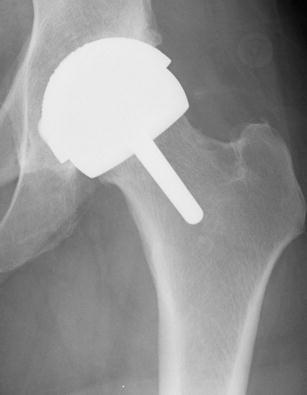
Hip resurfacing arthroplasty
Commonly cited theoretical advantages of HRA over THA include proximal femoral bone stock preservation, return to impact activity, avoidance of stress shielding, a lower dislocation rate, maintenance of normal anatomy, and easier revision surgery [1, 2, 5–7, 8•, 9–26]. However, HRA does not come without potential disadvantages, including early implant failure with specific designs [27–37], femoral neck fracture [30, 38–42], adverse local tissue reaction (ALTR) to metal debris and systemic increase in metal ions [1, 2, 14, 27, 36, 37, 43–50, 51••, 52•, 53], the need for an extended surgical exposure, and increased technical demands for implantation. Recent literature continues to investigate patient selection, improvements in surgical technique, implant-specific outcomes, patient monitoring for local and systemic effects of metal debris, and long-term patient results. The current paper aims to provide an updated critical review on recent literature pertaining to HRA and provide the authors’ insight on current controversies related to the use of HRA.
Patient selection
Several recent investigations have highlighted the importance of patient selection in HRA [2, 7, 9–14, 17–20, 22–24, 29, 38, 46, 54], with current indications limited to male sex, age less than 60 years, and larger patient size. Numerous authors have reported excellent short to mid-term outcomes in this patient population, with similar survivorship to THA, ranging from 96 to 98 % at 5 years and 88 to 99 % at 10 years [7, 9–12, 14, 17–20, 22–24, 26, 29, 46, 55, 56••, 57••]. Alternatively, several risk factors have been shown to contribute to increased rate of HRA wear and failure, including increased acetabular inclination angles, small femoral component sizes, implant design factors, developmental dysplasia of the hip (DDH), female sex, increased patient age, renal disease, and developmental decreased bone mineral density. These recent reports have helped better elucidate which of these factors may be predictive of patient outcomes and are highlighted in the following sections below. In general, we also feel that it is important for patients to either regularly participate in moderate athletic activities or work as a manual laborer to justify the unique risks of the procedure.
Sex
Female sex, which has been reported as an independent risk factor for failure in multiple studies, continues to be a topic of interest in the current literature [9, 17, 19, 22–24, 29, 50, 54]. A systematic review by Haughom et al. demonstrated a higher rate of complications (ALTR, dislocation, aseptic loosening, and revision) in women following MoM HRA [54]. Corroborating this finding, the Canadian Arthroplasty Registry reported female sex as an independent risk factor for early HRA failure [9]. However, Haughom et al. particularly note that a causative relationship between female sex and implant failure cannot be established secondary to various confounding factors including smaller component sizing, gender differences in ligamentous laxity, bone quality, and a higher prevalence of DDH leading to higher combined anteversion [54]. Recent studies have strongly linked smaller femoral component size and cup malpositioning as two of the most significant risk factors for HRA wear, edge loading, and failure; these risk factors are more likely to occur in females given their increased prevalence of DDH and smaller head size [53, 56••, 57••, 58].
It is the authors’ belief that female sex alone is an unlikely direct cause of HRA failure. This idea is supported by a study from Liu and Gross [56••] who reported on a safe zone for acetabular component positioning in hip resurfacing (RAIL: Relative Acetabular Inclination Limit) based on implant size and acetabular inclination angle (AIA). When the AIA fell below the RAIL, there were no adverse wear failures or dislocations. Additionally, the risk of having elevated metal ion levels above 10 μg/L being was <1 % when AIA fell below the RAIL, regardless of sex. Female gender was reported to be a risk for higher metal ion levels, along with increased AIA and smaller femoral component sizes. These findings suggest there is a subset of women who may benefit from HRA without elevated risk, consistent with our experience. High-level evidence pertaining to this topic is scarce, and further investigation is warranted.
Developmental dysplasia of the hip
Patients with arthritis secondary to DDH are more often young and active females. This patient population is known to be at higher risk for complications following THA [59], and similarly, DDH has been identified as an independent risk factor for failure following HRA [12, 54, 59, 60]. Gross et al. [59] reviewed 1216 patients who underwent HRA and reported DDH as the sole risk factor predictive of early failure in their multivariate analysis. Interestingly, women with the primary diagnosis of DDH had a survivorship rate of only 75 % compared to 93 % for the entire group at 8-year follow-up. The authors proposed that the higher incidence of DDH in women is likely the primary contributing factor toward females having an overall higher incidence of failure after undergoing HRA.
During acetabular component positioning in HRA, the cup is ideally placed near the recommended 40° of abduction. However, the vertical, anteverted nature of the dysplastic hip results in significant cup uncoverage at the anterosuperior edge when the cup is placed in this position [57••, 59]. As a result, many surgeons may elect to increase the cup version and abduction angle to obtain better bony coverage, despite this being a known risk factor for increased edge loading, wear, and early HRA failure. Additionally, patients with DDH may have higher femoral anteversion, which is challenging to correct given the nature of femoral resurfacing. This further increases the risk of edge loading and potential failure. Thus, the presence of DDH is a relative contraindication for HRA. However, DDH occurs on a spectrum and each patient should be treated as such. Currently, there do not appear to be evidence-based solutions to this problem, leaving opportunity for new implant designs and techniques to emerge.
Osteonecrosis
Osteonecrosis (ON) of the femoral head is usually progressive and frequently results in collapse of the femoral head requiring subsequent hip arthroplasty. Mixed results have been reported regarding outcomes of HRA in this patient population [12, 61–64]. Proponents of the HRA in the setting of ON argue that adequate removal of necrotic bone during femoral head preparation will provide a strong, healthy scaffold for cementation and prosthesis support [62, 64]. Opponents argue ON may result in aseptic loosening and femoral neck narrowing secondary to ON progression [12, 54, 61, 64]. Additionally, cases of extensive femoral head debridement present further challenges because of reduced surface area for implant fixation and increased failure rates with small femoral components [12, 54, 61, 64].
It is our opinion that young male patients with femoral head ON may be offered HRA as an operative intervention if they meet specific imaging criteria. Magnetic resonance imaging (MRI) should be utilized during surgical planning to assess the extent of signal enhancement encompassing the head and neck. In our practice, patients with ON extending into the neck are not considered suitable candidates for HRA. Patients with limited peripheral ON which does not significantly extend into the neck or areas of implant fixation are treated on a case-by-case basis, extensively outlining the risks and benefits of the HRA versus THA in this setting.
Surgical factors
There are several surgeon-related factors that may contribute to improved long-term outcomes in patients undergoing HRA, which will be reviewed here.
Acetabular component positioning
A growing body of recent evidence attributes higher metal ion levels and wear rates to increased acetabular inclination and higher degrees of combined anteversion [7, 10–12, 39, 43, 46, 49, 53, 54, 56••, 57••, 58, 60]. As previously highlighted, Liu and Gross [56••] reported on RAIL, which outlines a safe zone for acetabular component positioning in HRA based on implant size and AIA. They found that there were no wear-related failures (with the risk of metal ion levels above 10 μg/L being <1 %) when AIA falls below the RAIL. They reported AIA to be an independent risk factor for elevated metal ion levels outside of this acceptable limit.
Amstutz et al. [57••] substantiate the findings of Liu and Gross, reporting 98 and 94.3 % survivorship at 5 and 10 years, respectively, with the only variable associated with revision being lower contact patch to rim distance (CPRD). CPRD is described as the distance between the center of the contact patch (the contact area of the femoral articular surface with the acetabular component during any and all functions) and the acetabular rim, which had previously been used to determine susceptibility to edge loading [65]. Cups with higher AIA result in shortened CPRD, which is thought to increase edge loading, accelerate wear, and lead to elevated ion levels. Using the Conserve Plus (Wright Medical Technology Inc, Arlington, TN, USA) HRA, Amstutz concluded the preferred cup orientation is an abduction angle of approximately 42° ± 10° and anteversion angle of approximately 15° ± 10° [57••]. These results were substantiated by Matthies et al. [58] who also reported CPRD as a predictor of wear edge loading. Yoon et al. [53] subsequently reported on CPRD as an indirect predictor for increased metal ion levels and abnormal wear in HRA. Patients with CPRD less than or equal to 10 mm had a 37-fold increased risk of having elevated serum cobalt levels and a 11-fold increased risk of having elevated serum chromium levels.
These studies provide convincing evidence that acetabular component orientation, specifically elevated AIA above 55° and CPRD less than 10 mm, increase the risk of abnormal component wear, elevated metal ion levels, and early component failure. Although Liu and Gross provide an early model for an acetabular safe zone in HRA, continued research is needed to corroborate these findings. In our experience, excellent results in a young and highly active population have been demonstrated when these parameters are followed, and we believe further investigation is warranted in the form of large, prospective studies.
Femoral preparation
Intra-operative notching of the femoral neck and varus component positioning has been implicated in increasing femoral neck fracture risk [2, 8•, 9, 30, 35, 54, 63]. Notching violates the cortex, creating a stress riser, and thereby increasing the risk of femoral neck fracture during the postoperative period. Varus malposition of the component increases strain experienced by the superior femoral neck, with a resultant increased risk in fracture and loosening. Biomechanical analysis has shown notch depth of just 2 mm weakens the neck by 25 %, with a larger 5-mm notch decreasing the neck strength by nearly 50 % [66]. As even small cortical neck notches and mild varus malpositioning may have potentially devastating construct consequences, surgeons must take care when preparing the femoral resurfacing component.
Smaller femoral head sizes have been shown to be associated with increased rates of wear, ALTR, and component failure in both clinical and biomechanical settings [3, 4, 9, 29, 36, 46, 49, 51••, 56••, 59]. However, these factors are largely patient-dependent and are difficult to control intra-operatively. An early biomechanical study assessing the role of head diameter and clearance demonstrated that head diameters should be as large as possible and diametral clearances as low as practicable [4]. The authors explained that as the head diameter increases, the articulation is more likely to promote fluid film lubrication, subsequently improving wear characteristics. More recently, patients with smaller femoral sizes (38–44 mm) have been targeted in screening for high metal ion concentrations, as this population has been found more likely to have blood metal ion levels above 7 μg/L [49].
Surgical approach
Similar to THA, there is an ongoing debate over the best surgical approach for HRA. The posterior approach has been criticized for compromising femoral head vascularity, as the deep branch of the medial circumflex artery is often violated. This theoretically increases the risk of osteonecrosis, collapse, and subsequent failure [67] Regardless, the posterior approach continues to be the most commonly utilized surgical approach for hip resurfacing because it allows excellent visualization. Modifications of preserving the soft tissue envelope around the neck leading to better preservation of the blood supply have been described, but the long-term benefits of such modifications have yet to be reported [67, 68]. In contrast to the posterior approach, surgical dislocation (via the trochanteric slide) and anterior surgical approaches better preserve the blood supply to the femoral head. Trochanteric nonunion and painful hardware are reported risks of surgical dislocation, while intra-operative fracture and lateral femoral cutaneous nerve injury can occur during the direct anterior approach [68, 69].
A recent Canadian HRA study investigation of nearly 550 consecutive patients reported a 5-year survivorship of 94.5 % with no statistical differences in patient outcomes or reoperation rates between anterior, trochanteric slide, lateral, or posterior approaches [67]. Lorenzen et al. [69] performed a prospective, randomized trial investigating postoperative ischemia following HRA and found the posterior approach resulted in increased postoperative ischemia in the femoral head and neck. However, they also found the anterolateral approach resulted in considerable postoperative femoral head and neck ischemia. The authors convey that other possible explanations, such as intra-operative disruption of the retinacular vessels or altered microcirculation secondary to heating from the cementation process, need to be investigated [69].
Currently, there is no literature to convincingly suggest superiority of one approach over another. The senior author prefers the posterior approach for HRA, as it allows for better visualization and is also the approach most commonly used for his practice. It is our opinion that a surgeon performing HRA use the most familiar surgical approach to that individual, as excellent results have been reported using various approaches.
Implant-specific outcomes
The BHR and Conserve Plus are two of the most commonly utilized and most studied FDA-approved HRA devices in the USA. Both offer mid-long-term outcomes of approximately 88–99 % at 10 years [11, 12, 17–20, 24, 43, 46, 52•, 53, 64, 67, 70]. Concerns about asymptomatic pseudotumors and implant longevity in HRA remain. However, a higher percentage of implant failure in HRA as compared to THA remains to be seen [6, 13, 43, 46, 67, 71].
Two devices have been recalled from the market. In 2010, the Articular Surface Replacement (ASR; DePuy, Warsaw, IN, USA) was recalled secondary to high rates of early failure [28, 29, 35, 36, 72]. These failures were attributed to the acetabular design, which resulted in an unacceptably low clearance, cup deflection, and increased wear [29]. The Durom Acetabular Component (Zimmer Inc., Warsaw, IN, USA) was voluntarily recalled because the company deemed the instructions for use and surgical technique inadequate. In addition, multiple studies revealed higher early failure rates than other designs, with no single design flaw indicated [27, 29, 31, 34].
These implant outcomes must be kept in mind when following a HRA patient, as higher rates of failure are seen with certain implant designs and may provide the surgeon insight on potential etiologies of implant failure.
Patient surveillance
Metal ion levels
Increased systemic levels of chromium (Cr) and cobalt (Co) ions are thought to correlate with bearing surface wear and metal ion levels in synovial fluid [37, 49, 51••, 52•, 58, 65, 70]. Recent attempts have been made to identify patient populations at increased risk of wear and elevated system metal ion levels. During the first 9 to 12 months following HRA, serum metal ion levels appear to reach their peak, followed by a leveling off or a slow decrease in concentrations thereafter [51••, 52•]. Patients with persistently elevated serum metal ion levels have been found to have substantial bearing surface wear with abnormal edge loading [29, 51••, 52•, 57••] Therefore, systemic Cr and Co concentrations are considered surrogate markers of in vivo wear and are routinely followed clinically in patients who have undergone HRA.
A threshold of serum Co and Cr of 7 μg/L was established by the Medicines and Healthcare products Regulatory Agency for monitoring patients who have undergone MoM hip replacement. It is not clear if this threshold accurately identifies a MoM device complication. In an effort to better elucidate a serum metal ion threshold indicative of abnormal wear patterns and possible ALTR, Van Der Straeten et al. [51••] explored whether asymptomatic HRA patients could be differentiated from those with clinical and/or radiographic warning signs based on metal ion levels and sought to determine a threshold for serum metals predictive of the need for clinical intervention. They reported the asymptomatic group’s levels to be lower than the poorly functioning group, with acceptable upper serum levels being Cr 4.6 μg/L and Co 4.0 μg/L for unilateral HRA and Cr 7.4 μg/L, Co 5.0 μg/L for bilateral HRA. The specificity of these levels in predicting poor function was high (95 %) and sensitivity was low (25 %). In an ensuing study, the same author measured metal ion levels at 10 years and found that Co and Cr levels significantly decreased over time in well-functioning implants [52•]. In asymptomatic patients, mean ion levels were Cr 1.95 μg/L and Co 1.62 μg/L for unilateral resurfacings and Cr 3.46 μg/L and Co 2.66 μg/L for bilateral resurfacings. An increase > 2.5 μg/L from baseline measurements was associated with poor function [52•].
It is presently unclear if patients should undergo routine monitoring with serum metal levels. Surveillance may be warranted even in asymptomatic patients in at-risk patient populations, including those with a preoperative diagnosis of DDH, suboptimal component positioning, smaller head size, or decreased CPRD. In asymptomatic HRA patients with elevated metal ion levels, we also recommend close surveillance, as evidence suggests elevated concentrations are associated with early failure secondary to ALTR [72]. Specifically, Co greater than 20 μg/L have been reported to be frequently associated with metal staining of tissues and the development of osteolysis [72]. Revision of asymptomatic patients with elevated metal ions is controversial, as studies have been unable to find a direct correlation between ion levels and the tissue damage observed at the time of revision surgery [45, 73]. In general, we utilize metal artifact reduction sequence (MARS) MRI as an adjunct for monitoring in patients who are symptomatic or who have elevated serum metal levels.
Adverse local tissue reaction
ALTR is defined as abnormal fluid collections, solid or semisolid pseudotumors, or muscle or bone damage secondary to metal debris [44]. Early diagnosis of ALTR is essential, as delayed diagnosis may result in irreparable soft tissue damage and complicate reconstructive options. Warning signs for early HRA failure include pain, weakness or limp and mechanical symptoms, as well as those with a recalled implant [44]. Recently, imaging studies such as ultrasonography (US), metal artifact reduction sequence magnetic resonance imaging (MARS MRI), and slice encoding for metal artifact correction (SEMAC MRI) have also been advocated to identify patients with ALTRs [44, 74•, 75, 76].
ALTRs have been reported in both symptomatic and asymptomatic MoM patients, with a prevalence of 5–68 % in asymptomatic individuals [44, 75–77]. Although little debate exists on whether revision surgery may be indicated in symptomatic MoM patients with evidence of ALTR, clinical decisions regarding asymptomatic patients with ALTR are not well established. Even selection of the correct imaging modality is debated, evidenced by a recent Level I investigation by Garbuz et al. that prospectively compared US to SEMAC MRI for pseudotumor detection in an asymptomatic cohort of patients with MOM implants [74•]. The authors found an overall pseudotumor incidence of 31 % with US having a sensitivity of 100 % and specificity of 96 % while MRI reporting a sensitivity of 92 % and specificity of 100 %. The authors concluded a negative US reliably rules out pseudotumor in asymptomatic patients, and given its lower cost, they recommend ultrasound as the initial screening tool for pseudotumors. Although more cost effective, the availability of a local ultrasound technician trained in detecting pseudotumors is variable and ultrasonography does not provide as much information regarding the bone or soft tissue as does MRI. Therefore, both US and MRI are highly sensitive and specific tools that may be used for detection of ALTR in HRA patients. Van der Weegan et al. recently proposed guidelines on treatment of ALTR following HRA, suggesting a conservative approach for mild to moderate pseudotumors that are asymptomatic and have normal metal ion levels while recommending surgical intervention in those with severe pseudotumors and elevated ion levels [77].
As we continue to investigate the natural history of ALTR in MoM arthroplasty, it is our opinion that clinicians must continue to treat their patients on a case-by-case basis, using the available evidence-based literature to guide their decision-making process. Surgeons should integrate findings from the history and physical examination, plain radiographs, metal ion levels, advanced imaging studies, implant design, and implant positioning to direct their decision-making.
Return to activity
Debate exists over appropriate timing for return to high impact activity following HRA. Studies reporting on proximal femur bone mineral density (BMD) have shown return of normal BMD as early as 6 months, with maintenance through 5 years [8•, 42, 78–80]. In our practice, HRA patients are allowed to begin jogging and return to heavy labor at 6 months. This is based off a prospective study by Bedigrew et al. [8•] that reported no difference in proximal femoral BMD between 6 months and 1 year. Similarly, we discontinue posterior hip precautions and allow full hip range of motion exercises at 6 weeks following HRA. No dislocations following HRA have been reported in our practice in doing so.
Outcomes and modes of failure
Excellent short to mid-term outcomes have been reported in HRA, with survivorship as high as 96–100 % at 5 years and 93–99 % at 10 years [7, 9–12, 14, 17–20, 22–24, 26, 29, 46, 55, 56••, 57••]. Major factors affecting long-term success have been previously highlighted, including component size, implant design, preoperative diagnosis, patient sex, age, and surgical technique. In the following section, we concentrate on the two most common modes of failure in FDA-approved devices.
Femoral neck fracture
Femoral neck fracture is a common cause of early HRA failure, accounting for up to 35 % of revisions [9, 40, 41]. Poor bone density, older age, and smaller femoral components have been associated with increased risk [9, 40, 41]. A cadaveric study reported that gender, low preoperative DEXA scan, and neck width measurements were risk factors for femoral neck fractures and should be used together to assess fracture risk in patients being considered for HRA [40]. Varus positioning of the femoral component and large regions of osteonecrosis resulting in varus collapse of the implant have also been implicated in femoral component loosening and femoral neck fracture [63]. Femoral neck notching, as discussed above, has also been implicated in increasing fracture risk [8•, 9, 30]. Patients who have experienced an intra-operative notch should be followed closely and be given delayed clearance for high impact activities [8•]. Immediate postoperative protected weight bearing is controversial and should be treated on a case-by-case basis.
Aseptic loosening
Aseptic loosening of either femoral or acetabular components is another common cause of failure in HRA [54, 57••, 60]. Failure of the femoral component was previously reported as the most common cause of aseptic loosening; however, new literature suggests that malpositioning of the acetabular component may result in a higher rate wear and acetabular failure [53, 54, 57••, 60]. Furthermore, recent investigations on BMD surrounding the femoral neck and shaft have reported BMD preservation or improvement over time in patients who have undergone HRA, making femoral neck fracture less likely after 6 months [8•, 42, 78–80]. In light of these new reports, acetabular aseptic loosening and wear may prove to be the most common long-term causes of HRA failure.
Conclusion
Exceptional long-term outcomes following HRA may be achieved with careful patient selection and meticulous surgical technique. HRA remains an excellent choice for surgical intervention in young, active patients who desire return to impact activities and may place a higher demand on their hip reconstruction. Close clinical follow-up and patient surveillance is necessary to quickly identify and address implant failures. Further investigation is necessary to improve the patient selection process, component positioning, component design, and postoperative monitoring.
Compliance with ethics guidelines
Conflict of interest
Robert A. Sershon and Rishi Balkissoon declare that they have no conflict of interest.
Craig J. Della Valle reports grants and personal fees from CD Diagnostics, personal fees from Smith & Nephew, grants from Stryker, personal fees from DePuy, nd personal fees from Zimmer-Biomet, outside the submitted work.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Hip: Metal-on-Metal
Contributor Information
Robert Sershon, Phone: 773-426-9523, Email: robert_sershon@rush.edu.
Rishi Balkissoon, Phone: 301-651-8128, Email: rishibalkissoon@gmail.com.
Craig J. Della Valle, Email: craig.dellavalle@rushortho.com.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance, •• Of major importance
- 1.Mont MA, Ragland PS, Etienne G, Seyler TM, Schmalzried TP. Hip resurfacing arthroplasty. J Am Acad Orthop Surg. 2006;14(8):454–63. doi: 10.5435/00124635-200608000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Mont MA, Schmalzried TP. Modern metal-on-metal hip resurfacing: important observations from the first ten years. J Bone Joint Surg Am. 2008;90(Suppl 3):3–11. doi: 10.2106/JBJS.H.00750. [DOI] [PubMed] [Google Scholar]
- 3.Dowson D, Hardaker C, Flett M, Isaac GH. A hip joint simulator study of the performance of metal-on-metal joints: Part I: the role of materials. J Arthroplasty. 2004;19(8 Suppl 3):118–23. doi: 10.1016/j.arth.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Dowson D, Hardaker C, Flett M, Isaac GH. A hip joint simulator study of the performance of metal-on-metal joints: Part II: design. J Arthroplasty. 2004;19(8 Suppl 3):124–30. doi: 10.1016/j.arth.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Gross TP, Liu F. Current status of modern fully porous coated metal-on-metal hip resurfacing arthroplasty. J Arthroplasty. 2014;29(1):181–5. doi: 10.1016/j.arth.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Fink Barnes LA, Johnson SH, Patrick DA, Jr, Macaulay W. Metal-on-metal hip resurfacing compared with total hip arthroplasty: two to five year outcomes in men younger than sixty five years. Int Orthop. 2014;38(12):2435–40. doi: 10.1007/s00264-014-2506-8. [DOI] [PubMed] [Google Scholar]
- 7.Aulakh TS, Jayasekera N, Singh R, Patel A, Roulahamin N, Kuiper JH, et al. Efficacy of hip resurfacing arthroplasty: 6 year results from an international multisurgeon prospective cohort study. Acta Orthop Belg. 2015;81(2):197–208. [PubMed] [Google Scholar]
- 8.•.Bedigrew KM, Ruh EL, Zhang Q, Clohisy JC, Barrack RL, Nunley RM. 2011 Marshall Urist Young Investigator Award: when to release patients to high-impact activities after hip resurfacing. Clin Orthop Relat Res. 2012;470(1):299–306. doi: 10.1007/s11999-011-2131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Society CA The Canadian Arthroplasty Society’s experience with hip resurfacing arthroplasty. An analysis of 2773 hips. Bone Joint J. 2013;95-b(8):1045–51. doi: 10.1302/0301-620X.95B8.31811. [DOI] [PubMed] [Google Scholar]
- 10.Bornert S, Lutzner J, Beyer F, Gunther KP, Hartmann A. Revision rate and patient-reported outcome after Hip resurfacing arthroplasty: a concise follow-Up of 1064 cases. J Arthroplasty. 2015 doi: 10.1016/j.arth.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 11.Coulter G, Young DA, Dalziel RE, Shimmin AJ. Birmingham hip resurfacing at a mean of ten years: results from an independent centre. J Bone Joint Surg (Br) 2012;94(3):315–21. doi: 10.1302/0301-620X.94B3.28185. [DOI] [PubMed] [Google Scholar]
- 12.Daniel J, Pradhan C, Ziaee H, Pynsent PB, McMinn DJ. Results of Birmingham hip resurfacing at 12 to 15 years: a single-surgeon series. Bone Joint J. 2014;96-b(10):1298–306. doi: 10.1302/0301-620X.96B10.33695. [DOI] [PubMed] [Google Scholar]
- 13.Haddad FS, Konan S, Tahmassebi J. A prospective comparative study of cementless total hip arthroplasty and hip resurfacing in patients under the age of 55 years: a ten-year follow-up. Bone Joint J. 2015;97-b(5):617–22. doi: 10.1302/0301-620X.97B5.34537. [DOI] [PubMed] [Google Scholar]
- 14.Hartmann A, Lutzner J, Kirschner S, Witzleb WC, Gunther KP. Do survival rate and serum ion concentrations 10 years after metal-on-metal hip resurfacing provide evidence for continued use? Clin Orthop Relat Res. 2012;470(11):3118–26. doi: 10.1007/s11999-012-2329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koutras C, Antoniou SA, Talias MA, Heep H. Impact of total Hip resurfacing arthroplasty on health-related quality of life measures: a systematic review and meta-analysis. J Arthroplasty. 2015 doi: 10.1016/j.arth.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Le Duff MJ, Takamura KB, Amstutz HC. Metal-on-metal hip resurfacing in patients aged 65 or older. Hip Int. 2012;22(6):648–54. doi: 10.5301/HIP.2012.10350. [DOI] [PubMed] [Google Scholar]
- 17.Matharu GS, McBryde CW, Pynsent WB, Pynsent PB, Treacy RB. The outcome of the Birmingham Hip Resurfacing in patients aged < 50 years up to 14 years post-operatively. Bone Joint J. 2013;95-b(9):1172–7. doi: 10.1302/0301-620X.95B9.31711. [DOI] [PubMed] [Google Scholar]
- 18.Mehra A, Berryman F, Matharu GS, Pynsent PB, Isbister ES. Birmingham hip resurfacing: a single surgeon series reported at a minimum of 10 years follow-up. J Arthroplasty. 2015;30(7):1160–6. doi: 10.1016/j.arth.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 19.Murray DW, Grammatopoulos G, Pandit H, Gundle R, Gill HS, McLardy-Smith P. The ten-year survival of the Birmingham hip resurfacing: an independent series. J Bone Joint Surg (Br) 2012;94(9):1180–6. doi: 10.1302/0301-620X.94B9.29462. [DOI] [PubMed] [Google Scholar]
- 20.Nam D, Nunley RM, Ruh EL, Engh CA, Jr, Rogerson JS, Brooks PJ, et al. Short-term results of Birmingham hip resurfacing in the United States. Orthopedics. 2015;38(8):e715–21. doi: 10.3928/01477447-20150804-60. [DOI] [PubMed] [Google Scholar]
- 21.Rahman WA, Greidanus NV, Siegmeth A, Masri BA, Duncan CP, Garbuz DS. Patients report improvement in quality of life and satisfaction after hip resurfacing arthroplasty. Clin Orthop Relat Res. 2013;471(2):444–53. doi: 10.1007/s11999-012-2645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribas M, Cardenas C, Astarita E, Moya E, Bellotti V. Hip resurfacing arthroplasty: mid-term results in 486 cases and current indication in our institution. Hip Int. 2014;24(Suppl 10):S19–24. doi: 10.5301/hipint.5000172. [DOI] [PubMed] [Google Scholar]
- 23.Sandiford NA, Ahmed S, Doctor C, East DJ, Miles K, Apthorp HD. Patient satisfaction and clinical results at a mean eight years following BHR arthroplasty: results from a district general hospital. Hip Int. 2014;24(3):249–55. doi: 10.5301/hipint.5000126. [DOI] [PubMed] [Google Scholar]
- 24.Su EP, Housman LR, Masonis JL, Noble JW, Jr, Engh CA. Five year results of the first US FDA-approved hip resurfacing device. J Arthroplasty. 2014;29(8):1571–5. doi: 10.1016/j.arth.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Tan TL, Le Duff MJ, Takamura KM, Amstutz HC. Do clinical and quality of life scores change over time after hip resurfacing? Hip Int. 2015;25(2):146–51. doi: 10.5301/hipint.5000208. [DOI] [PubMed] [Google Scholar]
- 26.Woon RP, Johnson AJ, Amstutz HC. The results of metal-on-metal hip resurfacing in patients under 30 years of age. J Arthroplasty. 2013;28(6):1010–4. doi: 10.1016/j.arth.2012.07.043. [DOI] [PubMed] [Google Scholar]
- 27.Althuizen MN, VHooff ML, v d Berg-v Erp SH, VLimbeek J, Nijhof MW. Early failures in large head metal-on-metal total hip arthroplasty. Hip Int. 2012;22(6):641–7. doi: 10.5301/HIP.2012.10340. [DOI] [PubMed] [Google Scholar]
- 28.Borgwardt A, Borgwardt L, Borgwardt L, Zerahn B, Fabricius SD, Ribel-Madsen S. Clinical performance of the ASR and ReCap resurfacing implants-7 years follow-up. J Arthroplasty. 2015;30(6):993–7. doi: 10.1016/j.arth.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 29.Jameson SS, Baker PN, Mason J, Porter ML, Deehan DJ, Reed MR. Independent predictors of revision following metal-on-metal hip resurfacing: a retrospective cohort study using National Joint Registry data. J Bone Joint Surg (Br) 2012;94(6):746–54. doi: 10.1302/0301-620X.94B6.29239. [DOI] [PubMed] [Google Scholar]
- 30.Kohan L, Field CJ, Kerr DR. Early complications of hip resurfacing. J Arthroplasty. 2012;27(6):997–1002. doi: 10.1016/j.arth.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 31.Leclercq S, Lavigne M, Girard J, Chiron P, Vendittoli PA. Durom hip resurfacing system: retrospective study of 644 cases with an average follow-up of 34 months. Orthop Traumatol Surg Res. 2013;99(3):273–9. doi: 10.1016/j.otsr.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Li J, He C, Li D, Zheng W, Liu D, Xu W. Early failure of the Durom prosthesis in metal-on-metal hip resurfacing in Chinese patients. J Arthroplasty. 2013;28(10):1816–21. doi: 10.1016/j.arth.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 33.Matharu GS, Daniel J, Ziaee H, McMinn DJ. Failure of a novel ceramic-on-ceramic hip resurfacing prosthesis. J Arthroplasty. 2015;30(3):416–8. doi: 10.1016/j.arth.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Naal FD, Pilz R, Munzinger U, Hersche O, Leunig M. High revision rate at 5 years after hip resurfacing with the Durom implant. Clin Orthop Relat Res. 2011;469(9):2598–604. doi: 10.1007/s11999-011-1792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penny JO, Ding M, Varmarken JE, Ovesen O, Overgaard S. Early micromovement of the Articular Surface Replacement (ASR) femoral component: two-year radiostereometry results. J Bone Joint Surg (Br) 2012;94(10):1344–50. doi: 10.1302/0301-620X.94B10.29030. [DOI] [PubMed] [Google Scholar]
- 36.Reito A, Puolakka T, Elo P, Pajamaki J, Eskelinen A. High prevalence of adverse reactions to metal debris in small-headed ASR hips. Clin Orthop Relat Res. 2013;471(9):2954–61. doi: 10.1007/s11999-013-3023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson PG, Wilkinson AJ, Meek RM. Metal ion levels and revision rates in metal-on-metal hip resurfacing arthroplasty: a comparative study. Hip Int. 2014;24(2):123–8. doi: 10.5301/hipint.5000113. [DOI] [PubMed] [Google Scholar]
- 38.Asaad A, Hart A, Khoo MM, Ilo K, Schaller G, Black JD, et al. Frequent femoral neck osteolysis with Birmingham mid-head resection resurfacing arthroplasty in young patients. Clin Orthop Relat Res. 2015 doi: 10.1007/s11999-015-4348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cadossi M, Tedesco G, Sambri A, Mazzotti A, Giannini S. Hip resurfacing implants. Orthopedics. 2015;38(8):504–9. doi: 10.3928/01477447-20150804-07. [DOI] [PubMed] [Google Scholar]
- 40.Davis ET, Olsen M, Zdero R, Smith GM, Waddell JP, Schemitsch EH. Predictors of femoral neck fracture following hip resurfacing: a cadaveric study. J Arthroplasty. 2013;28(1):110–6. doi: 10.1016/j.arth.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Matharu GS, McBryde CW, Revell MP, Pynsent PB. Femoral neck fracture after Birmingham hip resurfacing arthroplasty: prevalence, time to fracture, and outcome after revision. J Arthroplasty. 2013;28(1):147–53. doi: 10.1016/j.arth.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 42.Penny JO, Brixen K, Varmarken JE, Ovesen O, Overgaard S. Changes in bone mineral density of the acetabulum, femoral neck and femoral shaft, after hip resurfacing and total hip replacement: two-year results from a randomised study. J Bone Joint Surg (Br) 2012;94(8):1036–44. doi: 10.1302/0301-620X.94B8.28222. [DOI] [PubMed] [Google Scholar]
- 43.Bisschop R, Boomsma MF, Van Raay JJ, Tiebosch AT, Maas M, Gerritsma CL. High prevalence of pseudotumors in patients with a Birmingham Hip Resurfacing prosthesis: a prospective cohort study of one hundred and twenty-nine patients. J Bone Joint Surg Am. 2013;95(17):1554–60. doi: 10.2106/JBJS.L.00716. [DOI] [PubMed] [Google Scholar]
- 44.Fehring TK, Odum S, Sproul R, Weathersbee J. High frequency of adverse local tissue reactions in asymptomatic patients with metal-on-metal THA. Clin Orthop Relat Res. 2014;472(2):517–22. doi: 10.1007/s11999-013-3222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hart AJ, Sabah SA, Sampson B, Skinner JA, Powell JJ, Palla L, et al. Surveillance of patients with metal-on-metal Hip resurfacing and total Hip prostheses: a prospective cohort study to investigate the relationship between blood metal Ion levels and implant failure. J Bone Joint Surg Am. 2014;96(13):1091–9. doi: 10.2106/JBJS.M.00957. [DOI] [PubMed] [Google Scholar]
- 46.Holland JP, Langton DJ, Hashmi M. Ten-year clinical, radiological and metal ion analysis of the Birmingham Hip Resurfacing: from a single, non-designer surgeon. J Bone Joint Surg (Br) 2012;94(4):471–6. doi: 10.1302/0301-620X.94B4.27895. [DOI] [PubMed] [Google Scholar]
- 47.Johnson AJ, Le Duff MJ, Yoon JP, Al-Hamad M, Amstutz HC. Metal ion levels in total hip arthroplasty versus hip resurfacing. J Arthroplasty. 2013;28(7):1235–7. doi: 10.1016/j.arth.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 48.Junnila M, Seppanen M, Mokka J, Virolainen P, Polonen T, Vahlberg T, et al. Adverse reaction to metal debris after Birmingham hip resurfacing arthroplasty. Acta Orthop. 2015;86(3):345–50. doi: 10.3109/17453674.2014.1004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matharu GS, Berryman F, Brash L, Pynsent PB, Treacy RB, Dunlop DJ. Predicting high blood metal ion concentrations following hip resurfacing. Hip Int. 2015 doi: 10.5301/hipint.5000258. [DOI] [PubMed] [Google Scholar]
- 50.Savarino L, Cadossi M, Chiarello E, Baldini N, Giannini S. Do ion levels in metal-on-metal hip resurfacing differ from those in metal-on-metal THA at long-term followup? Clin Orthop Relat Res. 2013;471(9):2964–71. doi: 10.1007/s11999-013-2981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.••.Van Der Straeten C, Grammatopoulos G, Gill HS, Calistri A, Campbell P, De Smet KA. The 2012 Otto Aufranc award: the interpretation of metal ion levels in unilateral and bilateral hip resurfacing. Clin Orthop Relat Res. 2013;471(2):377–85. doi: 10.1007/s11999-012-2526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.•.Van Der Straeten C, Van Quickenborne D, De Roest B, Calistri A, Victor J, De Smet K. Metal ion levels from well-functioning Birmingham hip resurfacings decline significantly at ten years. Bone Joint J. 2013;95-b(10):1332–8. doi: 10.1302/0301-620X.95B10.32022. [DOI] [PubMed] [Google Scholar]
- 53.Yoon JP, Le Duff MJ, Johnson AJ, Takamura KM, Ebramzadeh E, Amstutz HC. Contact patch to rim distance predicts metal ion levels in hip resurfacing. Clin Orthop Relat Res. 2013;471(5):1615–21. doi: 10.1007/s11999-012-2711-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haughom BD, Erickson BJ, Hellman MD, Jacobs JJ. Do complication rates differ by gender after metal-on-metal Hip resurfacing arthroplasty? A systematic review. Clin Orthop Relat Res. 2015;473(8):2521–9. doi: 10.1007/s11999-015-4227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Hamad M, Le Duff MJ, Takamura KM, Amstutz HC. Acetabular component thickness does not affect mid-term clinical results in hip resurfacing. Clin Orthop Relat Res. 2014;472(5):1528–34. doi: 10.1007/s11999-014-3468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.••.Liu F, Gross TP. A safe zone for acetabular component position in metal-on-metal hip resurfacing arthroplasty: winner of the 2012 HAP PAUL award. J Arthroplasty. 2013;28(7):1224–30. doi: 10.1016/j.arth.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 57.••.Amstutz HC, Le Duff MJ, Johnson AJ. Socket position determines hip resurfacing 10-year survivorship. Clin Orthop Relat Res. 2012;470(11):3127–33. doi: 10.1007/s11999-012-2347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matthies AK, Henckel J, Cro S, Suarez A, Noble PC, Skinner J, et al. Predicting wear and blood metal ion levels in metal-on-metal hip resurfacing. J Orthop Res. 2014;32(1):167–74. doi: 10.1002/jor.22459. [DOI] [PubMed] [Google Scholar]
- 59.Gross TP, Liu F. Prevalence of dysplasia as the source of worse outcome in young female patients after hip resurfacing arthroplasty. Int Orthop. 2012;36(1):27–34. doi: 10.1007/s00264-011-1290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amstutz HC, Le Duff MJ. Aseptic loosening of cobalt chromium monoblock sockets after hip resurfacing. Hip Int. 2015 doi: 10.5301/hipint.5000251. [DOI] [PubMed] [Google Scholar]
- 61.Gross TP, Liu F. Comparative study between patients with osteonecrosis and osteoarthritis after hip resurfacing arthroplasty. Acta Orthop Belg. 2012;78(6):735–44. [PubMed] [Google Scholar]
- 62.Nakasone S, Takao M, Sakai T, Nishii T, Sugano N. Does the extent of osteonecrosis affect the survival of hip resurfacing? Clin Orthop Relat Res. 2013;471(6):1926–34. doi: 10.1007/s11999-013-2833-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tai CL, Chen YC, Hsieh PH. The effects of necrotic lesion size and orientation of the femoral component on stress alterations in the proximal femur in hip resurfacing—a finite element simulation. BMC Musculoskelet Disord. 2014;15:262. doi: 10.1186/1471-2474-15-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woon RP, Johnson AJ, Amstutz HC. Results of Conserve Plus(R) metal-on-metal hip resurfacing for post-traumatic arthritis and osteonecrosis. Hip Int. 2012;22(2):195–202. doi: 10.5301/HIP.2012.9226. [DOI] [PubMed] [Google Scholar]
- 65.Langton DJ, Sprowson AP, Joyce TJ, Reed M, Carluke I, Partington P, et al. Blood metal ion concentrations after hip resurfacing arthroplasty: a comparative study of articular surface replacement and Birmingham Hip Resurfacing arthroplasties. J Bone Joint Surg (Br) 2009;91(10):1287–95. doi: 10.1302/0301-620X.91B10.22308. [DOI] [PubMed] [Google Scholar]
- 66.Davis ET, Olsen M, Zdero R, Papini M, Waddell JP, Schemitsch EH. A biomechanical and finite element analysis of femoral neck notching during hip resurfacing. J Biomech Eng. 2009;131(4):041002. doi: 10.1115/1.3072889. [DOI] [PubMed] [Google Scholar]
- 67.Zylberberg AD, Nishiwaki T, Kim PR, Beaule PE. Clinical results of the conserve plus metal on metal hip resurfacing: an independent series. J Arthroplasty. 2015;30(1):68–73. doi: 10.1016/j.arth.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 68.Steffen RT, De Smet KA, Murray DW, Gill HS. A modified posterior approach preserves femoral head oxgenation during hip resurfacing. J Arthroplasty. 2011;26(3):404–8. doi: 10.1016/j.arth.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 69.Lorenzen ND, Stilling M, Ulrich-Vinther M, Trolle-Andersen N, Pryno T, Soballe K, et al. Increased post-operative ischemia in the femoral head found by microdialysis by the posterior surgical approach: a randomized clinical trial comparing surgical approaches in hip resurfacing arthroplasty. Arch Orthop Trauma Surg. 2013;133(12):1735–45. doi: 10.1007/s00402-013-1851-1. [DOI] [PubMed] [Google Scholar]
- 70.Amstutz HC, Campbell PA, Dorey FJ, Johnson AJ, Skipor AK, Jacobs JJ. Do ion concentrations after metal-on-metal hip resurfacing increase over time? A prospective study. J Arthroplasty. 2013;28(4):695–700. doi: 10.1016/j.arth.2012.07.040. [DOI] [PubMed] [Google Scholar]
- 71.Brown NM, Foran JR, Della Valle CJ. Hip resurfacing and conventional THA: comparison of acetabular bone stock removal, leg length, and offset. Orthopedics. 2013;36(5):e637–41. doi: 10.3928/01477447-20130426-28. [DOI] [PubMed] [Google Scholar]
- 72.Langton DJ, Sidaginamale RP, Joyce TJ, Natu S, Blain P, Jefferson RD et al. The clinical implications of elevated blood metal ion concentrations in asymptomatic patients with MoM hip resurfacings: a cohort study. BMJ Open. 2013;3(3). doi:10.1136/bmjopen-2012-001541. [DOI] [PMC free article] [PubMed]
- 73.Griffin WL, Fehring TK, Kudrna JC, Schmidt RH, Christie MJ, Odum SM, et al. Are metal ion levels a useful trigger for surgical intervention? J Arthroplasty. 2012;27(8 Suppl):32–6. doi: 10.1016/j.arth.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 74.•.Garbuz DS, Hargreaves BA, Duncan CP, Masri BA, Wilson DR, Forster BB. The John Charnley Award: diagnostic accuracy of MRI versus ultrasound for detecting pseudotumors in asymptomatic metal-on-metal THA. Clin Orthop Relat Res. 2014;472(2):417–23. doi: 10.1007/s11999-013-3181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hart AJ, Satchithananda K, Liddle AD, Sabah SA, McRobbie D, Henckel J, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case–control study using three-dimensional computed tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94(4):317–25. doi: 10.2106/JBJS.J.01508. [DOI] [PubMed] [Google Scholar]
- 76.Nawabi DH, Hayter CL, Su EP, Koff MF, Perino G, Gold SL, et al. Magnetic resonance imaging findings in symptomatic versus asymptomatic subjects following metal-on-metal hip resurfacing arthroplasty. J Bone Joint Surg Am. 2013;95(10):895–902. doi: 10.2106/JBJS.K.01476. [DOI] [PubMed] [Google Scholar]
- 77.van der Weegen W, Sijbesma T, Hoekstra HJ, Brakel K, Pilot P, Nelissen RG. Treatment of pseudotumors after metal-on-metal hip resurfacing based on magnetic resonance imaging, metal ion levels and symptoms. J Arthroplasty. 2014;29(2):416–21. doi: 10.1016/j.arth.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 78.Huang Q, Shen B, Yang J, Zhou ZK, Kang PD, Pei FX. Changes in bone mineral density of the acetabulum and proximal femur after total hip resurfacing arthroplasty. J Arthroplasty. 2013;28(10):1811–5. doi: 10.1016/j.arth.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 79.Malviya A, Ng L, Hashmi M, Rawlings D, Holland JP. Patterns of changes in femoral bone mineral density up to five years after hip resurfacing. J Arthroplasty. 2013;28(6):1025–30. doi: 10.1016/j.arth.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 80.Gerhardt DM, Smolders JM, Rijnders TA, Hol A, van Susante JL. Changes in bone mineral density and femoral neck narrowing in the proximal femur three to five years after hip resurfacing versus conventional total hip arthroplasty. J Arthroplasty. 2015;30(2):308–14. doi: 10.1016/j.arth.2014.09.007. [DOI] [PubMed] [Google Scholar]


