Abstract
Background
The Affordable Care Act (ACA) eliminated cost-sharing for evidence-based preventive services in an effort to encourage use.
Objective
To evaluate use of colorectal cancer (CRC) screening in a national population-based sample before and after implementation of the ACA.
Design
Repeated cross-sectional analysis of the Medical Expenditure Panel Survey (MEPS) between 2009 and 2012 comparing CRC screening rates before and after implementation of the ACA.
Participants
Adults 50–64 with private health insurance and adults 65–75 with Medicare.
Main Measures
Self-reported receipt of screening colonoscopy, sigmoidoscopy, or fecal occult blood test (FOBT) within the past year among those eligible for screening.
Key Results
Our study included 8617 adults aged 50–64 and 3761 adults aged 65–75. MEPS response rates ranged from 58 to 63%. Among adults aged 50–64, 18.9–20.9% received a colonoscopy in the survey year, 0.59–2.1% received a sigmoidoscopy, and 7.9–10.4% received an FOBT. For adults aged 65–75, 23.6–27.7% received a colonoscopy, 1.3–3.2% a sigmoidoscopy, and 13.5–16.4% an FOBT. In adjusted analyses, among participants aged 50–64, there was no increase in yearly rates of colonoscopy (−0.28 percentage points, 95% CI −2.3 to 1.7, p = 0.78), sigmoidoscopy (−1.1%, 95% CI −1.7 to −0.46, p = <0.001), or FOBT (−1.6%, 95% CI −3.2 to −0.03, p = 0.046) post-ACA. For those aged 65–75, rates of colonoscopy (+2.3%, 95% CI −1.4 to 6.0, p = 0.22), sigmoidoscopy (+0.34%, 95% CI 0.88 to 1.6, p = 0.58) and FOBT (−0.65, 95% CI −4.1 to 2.8, p = 0.72) did not increase. Among those aged 65–75 with Medicare and no additional insurance, the use of colonoscopy rose by 12.0% (95% CI 3.3 to 20.8, p = 0.007). Among participants with Medicare living in poverty, colonoscopy use also increased (+5.7%, 95% CI 0.18 to 11.3, p = 0.043).
Conclusions
Eliminating cost-sharing for CRC screening has not resulted in changes in the use of CRC screening services for many Americans, although use may have increased in the post-ACA period among some Medicare beneficiaries.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-015-3504-2) contains supplementary material, which is available to authorized users.
KEY WORDS: colorectal cancer, health care reform, health insurance, preventive care
INTRODUCTION
The Patient Protection and Affordable Care Act (ACA), landmark health care reform legislation signed into law in March of 2010, included provisions designed to encourage the use of clinical preventive services.1 In particular, the ACA required that private insurers cover preventive services recommended by the United States Preventive Services Task Force (USPSTF) without additional out-of-pocket costs to the beneficiary. This provision took effect on September 23, 2010, and applied to non-grandfathered private insurance plans (i.e., individual plans purchased or new group plans created after March 23, 2010).2 The ACA also eliminated cost-sharing for evidence-based preventive services already covered by Medicare beginning on January 1, 2011.3
Evidence from prior studies suggests that cost-sharing for preventive services may indeed discourage use. Forty years ago, the landmark Rand Health Insurance Experiment demonstrated that cost-sharing for preventive services resulted in lower utilization.4 More recent observational studies of cost-sharing for mammography, colorectal cancer screening, and other preventive services have reached similar conclusions.5–8 Other studies, though, have suggested that copayments, if small, do not affect the use of preventive services.9
The goal of our study was to evaluate whether the ACA’s preventive services provisions have resulted in increased use of colorectal cancer screening services in a national representative sample. Currently, the USPSTF recommends that adults aged 50–75 undergo screening for colorectal cancer. The USPSTF guidelines offer a choice of three screening strategies: annual high-sensitivity fecal occult blood testing (FOBT); sigmoidoscopy every 5 years, with high-sensitivity FOBT every 3 years; or colonoscopy every 10 years.10 The ACA requires that private insurers cover these screening modalities at the intervals defined by the USPSTF.2 The law also explicitly requires that Medicare cover screening FOBT, flexible sigmoidoscopy, and screening colonoscopy for beneficiaries without cost-sharing.3
Prior to this legislation, many private insurers covered some portion of the cost of colorectal cancer screening, though many required some cost-sharing, which often totaled hundreds of dollars for an expensive service such as colonoscopy.11 Those with high deductibles or significant coinsurance might expect to pay even more, given typical payments for colonoscopy. Costs associated with FOBT were generally low, which likely translated into small or no out-of-pocket expenses.12
Medicare has covered colorectal cancer screening since 1998.13 Prior to the ACA, FOBT was covered without a deductible or copayment. Colonoscopy and sigmoidoscopy were covered without a deductible, though beneficiaries were responsible for 20% coinsurance for office-based procedures and 25% coinsurance for procedures performed in hospital outpatient departments or ambulatory surgery centers.14 Some Medicare beneficiaries, however, were protected from these additional costs through Medigap plans, concurrent Medicaid coverage, or Medicare Advantage (MA) plans.15
Given the varied landscape of cost-sharing for colorectal cancer screening, we sought to determine whether the use of colonoscopy, sigmoidoscopy, and FOBT has changed in the post-ACA period.
METHODS
We used data from the 2009–2012 Medical Expenditure Panel Survey (MEPS). The MEPS is an annual population-based household survey conducted by the Agency for Healthcare Research and Quality that tracks health care utilization and expenditures among the civilian non-institutionalized U.S. population. Response rates for the MEPS in 2009–2012 ranged from 58 to 63%.16 We specifically examined two main groups of respondents: adults aged 50–64 with private insurance who were eligible for colorectal cancer screening, and adults aged 65–75 with Medicare and who were eligible for screening. We also included adults aged 40–49 with private insurance as a comparison group not directly affected by this policy. Participants were included in these cohorts if they reported having private insurance or Medicare at any time during the survey year. Participants aged 50–75 who reported more than one insurance source (e.g., private insurance and Medicare) were grouped according to age. Those 40–49 who reported private insurance and Medicare were excluded from analyses of colonoscopy, since Medicare covers screening colonoscopy in full even for those under the of age 50. Among those with Medicare, we excluded participants who did not have Part B or MA for any part of the interview year.
We defined participants as eligible for colorectal cancer screening if they had not had a colonoscopy within the past 10 years or a sigmoidoscopy with FOBT within the past 5 years. Those who reported prior screening using only FOBT were still screen-eligible during the survey year, since FOBT is an annual test. By definition, we included respondents who reported a screening exam during the survey year as screen-eligible. We excluded participants who reported a prior history of colorectal cancer or who could not report having received a colorectal cancer screening exam. We also excluded participants who were out of scope (typically due to active military service or institutionalization) at the time of the survey.
The primary outcome was self-reported receipt of a screening colonoscopy, sigmoidoscopy, or FOBT within the past year. Although cost-sharing for FOBT had not changed among Medicare beneficiaries, we included FOBT because changes in the cost of one screening modality may change the use of another. We did not examine CT colonography, as it is not recommended by the USPSTF and is not covered by the preventive services provisions of the ACA.
Our main predictor was implementation of the ACA. We divided the survey years into a pre-ACA period (2009–2010) and a post-ACA period (2011–2012). Although the ACA was passed in March of 2010, provisions affecting cost-sharing for preventive services did not begin to go into effect until September 23, 2010, for private insurers and January 1, 2011, for Medicare. Because cost-sharing continued for most or all of 2010, we included 2010 as a “pre-ACA” year. We also performed a sensitivity analysis omitting 2010 for the privately insured.
Our descriptive analyses detail demographic characteristics and yearly rates of colorectal cancer screening by age/insurance group, accounting for survey weights and design. We used logistic regression with predicted probabilities to describe rates of colonoscopy, sigmoidoscopy, and FOBT use in pre- and post-ACA periods. Models were adjusted for age, sex, family income, and race/ethnicity, as these demographic factors may affect screening rates.17 Standard error estimates accounted for the complex survey design of the MEPS. We performed two main subgroup analyses. First, we examined the effect of the ACA on colonoscopy rates among respondents who reported incomes near or below the poverty level, as cost-sharing may disproportionately affect the poorest.18 For this analysis, we classified respondents based on whether they reported family income less than or greater than 125% of the federal poverty level. Second, we examined the effect of the ACA on rates of colonoscopy according to race/ethnicity. We limited these subgroup analyses to colonoscopy, since it is a relatively expensive procedure, and we hypothesized that any effect of cost-sharing would be most pronounced with this outcome.
To account for possible secular trends, we performed a difference-in-differences analysis. Here, we compared rates of colonoscopy, sigmoidoscopy, and FOBT among privately insured adults aged 50–64 to those among privately insured adults aged 40–49. The USPSTF does not provide explicit recommendations regarding screening in this younger age group, though some higher-risk individuals may be screened. Adults 40–49 with private insurance who undergo screening, however, may be subject to cost-sharing, depending on their insurers’ policies.19
In addition to our main analyses, we performed a number of sensitivity analyses. First, we examined rates of colorectal cancer screening among all respondents regardless of screening history. Next, we examined rates of colonoscopy, sigmoidoscopy, and FOBT use for any reason (diagnostic or screening) across the entire study population. By examining both kinds of tests together, we included exams that were indeed used for screening but were misclassified by respondents. This analysis also allowed us to look at an extended baseline period. Prior to 2009, the MEPS did not distinguish between screening and diagnostic exams. Thus, expanding our outcomes to include diagnostic and screening tests allowed us to include a longer period extending back to 2007.
Next, we performed a series of exploratory subgroup analyses that more carefully examined the role of respondents’ insurance. First, we repeated our main analyses, looking at respondents aged 50–64 with private insurance but not Medicare or Medicaid. Next, we repeated our analyses among those aged 65–75 with Medicare but not private insurance (including Medigap), Medicaid, or MA. Lastly, we examined the group of respondents aged 50–64 with private insurance who reported high out-of-pocket costs—a group that might have had less generous insurance coverage and may have had cost-sharing for preventive services before the ACA. Here, we examined those who reported out-of-pocket expenses in the top quartile, stratified by family income. Family income was classified as <200%, 200–400%, or ≥400% of the FPL.
All analyses used Stata version 12.1 software (StataCorp LP, College Station, TX, USA). This study was deemed exempt for review by the Stanford University Institutional Review Board.
RESULTS
Our study sample included 11,016 adults aged 40–49 with private health insurance, 15,563 adults aged 50–64 with private health insurance, and 9001 adults aged 65–75 with Medicare. Of these, 8617 adults aged 50–65 (approximately 55%, adjusted for survey design) and 3761 adults (43%) aged 65–75 were screen-eligible. Among those aged 40–49, 9350 (87%) had not recently received screening. Demographic characteristics are detailed in Table 1. The mean age of the study population was 53 years, 48% of participants were men, and 75% were white.
Table 1.
Demographic Characteristics
| Demographic Characteristic* | Overall (n = 21,728) | Age 40–49 (n = 9350) | Age 50–64 (n = 8617) | Age 65–75 (n = 3761) |
|---|---|---|---|---|
| Mean age (SE) | 53.0 (0.14) | 44.5 (0.06) | 55.6 (0.73) | 69.4 (0.78) |
| Male, % (SE) | 48% (0.42) | 49% (0.63) | 48% (0.67) | 44% (1.0) |
| Race (SE) | ||||
| White/other | 75% (0.87) | 72% (1.1) | 78% (0.90) | 76% (1.3) |
| Hispanic | 9.8% (0.59) | 11% (0.71) | 8.3% (0.59) | 10.0% (0.92) |
| African American | 9.8% (0.50) | 11% (0.68) | 9.0% (0.49) | 9.8% (0.71) |
| Asian | 5.1% (0.51) | 5.8% (0.59) | 4.7% (0.52) | 4.0% (0.62) |
| Poverty, % (SE) | 6.6% (0.25) | 4.9% (0.31) | 4.6% (0.32) | 16.4 (0.80) |
*Estimates account for survey design and weight
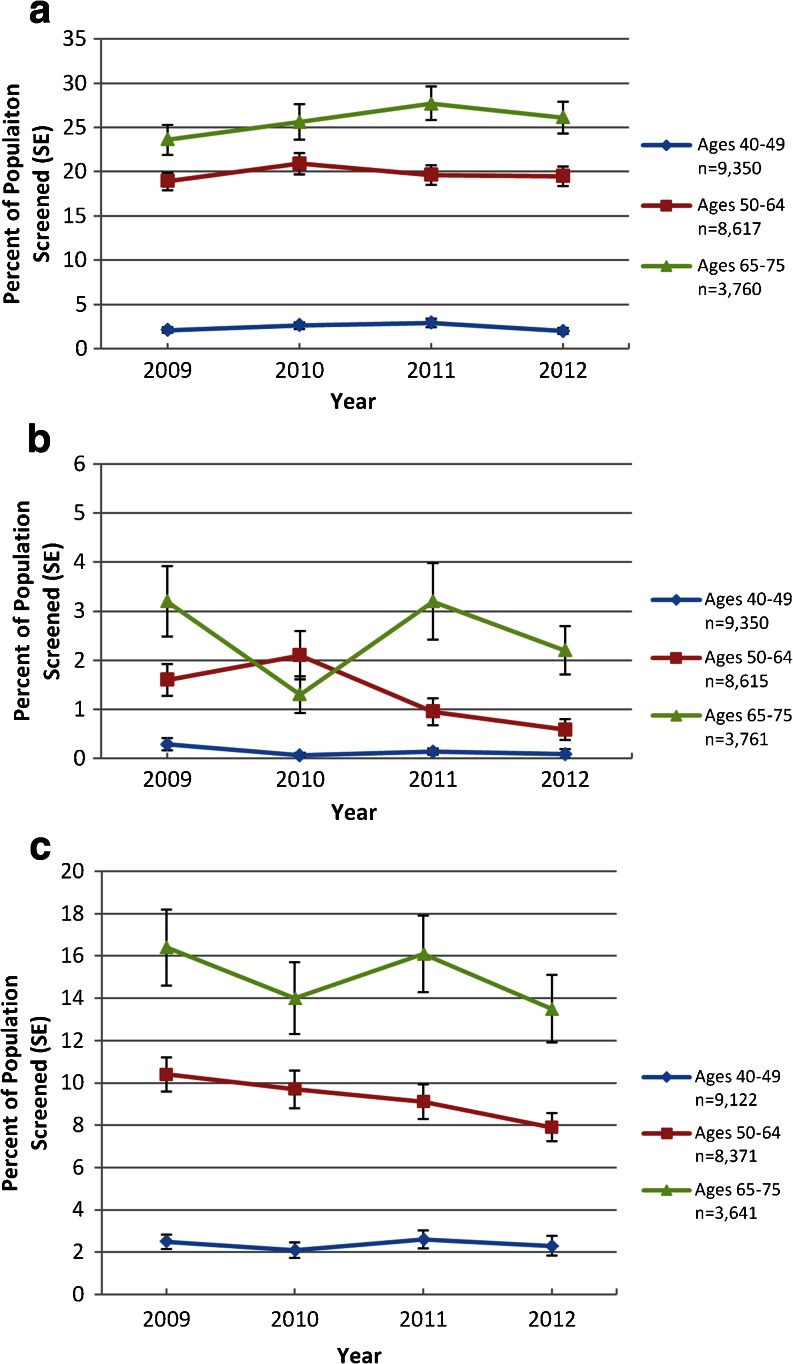
Among respondents eligible for screening, unadjusted rates of colonoscopy ranged from 18.9 to 20.9% per year among those aged 50–64 with private insurance and 23.6 to 27.7% among Medicare beneficiaries. Sigmoidoscopy rates ranged from 0.59 to 2.1% per year among privately insured 50–64-year-olds and 1.3 to 3.2% among those with Medicare. FOBT rates ranged from 7.9 to 10.4% among privately insured 50–64-year-olds and from 13.5 to 16.4% among those with Medicare (Fig. 1).
Fig. 1.
Yearly rates of colorectal cancer screening among study participants. Panels depict annual rates of a colonoscopy, b sigmoidoscopy, and c FOBT as reported by participants. Rates reflect survey weights and design.
The post-ACA period was not associated with a change in colonoscopy rates for participants aged 50–64 with private insurance (−0.28 percentage points, 95% CI −2.3 to 1.7, p = 0.78) or those aged 65–75 with Medicare (+2.3%, 95% CI −1.4 to 6.0, p = 0.22). Sigmoidoscopy use was similarly unchanged among those with Medicare (+0.34%, 95% CI −0.88 to 1.6, p = 0.58) and decreased among those aged 50–64 with private insurance (−1.1, 95% CI −1.7 to −0.46, p = 0.007). Participants aged 50–64 with private insurance were less likely to use FOBT in the post-ACA period (−1.6%, 95% CI −3.2 to −0.03, p = 0.046). Those with Medicare saw no change in FOBT use (−0.65%, 95% CI −4.1 to 2.8, p = 0.72) (Table 2).
Table 2.
Colonoscopy, Sigmoidoscopy, and FOBT Use Pre- and Post-ACA
| Model | Adjusted percentage screened pre-ACA (95% CI) | Adjusted percentage screened post-ACA (95% CI) | Percentage point change (95% CI) | P value |
|---|---|---|---|---|
| Colonoscopy | ||||
| Age 40–49 (n = 9350) | 2.4 (1.9 to 2.9) | 2.4 (1.8 to 3.0) | 0.02 (−0.08 to 0.8) | 0.96 |
| Age 50–64 (n = 8617) | 19.9 (18.3 to 21.5) | 19.6 (18.0 to 21.2) | −0.28 (−2.3 to 1.7) | 0.78 |
| Age 65–75 (n = 3760) | 24.8 (22.2 to 27.3) | 27.1 (24.3 to 29.9) | 2.3 (−1.4 to 6.0) | 0.22 |
| Sigmoidoscopy | ||||
| Age 40–49 (n = 8450) | 1.9 (0.5 to 3.3) | 1.3 (0.05 to 2.5) | −0.06 (−0.02 to 0.01) | 0.50 |
| Age 50–64 (n = 8615) | 1.9 (1.3 to 2.5) | 0.77 (0.45 to 1.1) | −1.1 (−1.7 to −0.46) | 0.0007 |
| Age 65–75 (n = 3761) | 2.3 (1.5 to 3.1) | 2.7 (1.8 to 3.5) | 0.34 (−0.88 to 1.6) | 0.58 |
| FOBT | ||||
| Age 40–49 (n = 9122) | 2.4 (1.8 to 2.9) | 2.4 (1.8 to 3.1) | 0.07 (−0.70 to 0.84) | 0.87 |
| Age 50–64 (n = 8371) | 10.1 (8.9 to 11.3) | 8.5 (7.4 to 9.6) | −1.6 (−3.2 to −0.03) | 0.046 |
| Age 65–75 (n = 3641) | 15.3 (12.6 to 18.0) | 14.7 (12.3 to 17.0) | −0.65 (−4.1 to 2.8) | 0.72 |
| Colonoscopy Subgroup Analyses | ||||
| Poverty | ||||
| Age 50–64, income ≥125% FPL (n = 8100) |
19.8 (18.0 to 21.5) | 19.9 (18.2 to 21.5) | 0.12 (−2.0 to 2.2) | 0.91 |
| Age 50–64, income <125% FPL (n = 517) |
21.4 (14.2 to 28.5) | 15.1 (8.7 to 21.6) | −6.2 (−1.6 to 3.3) | 0.20 |
| Age 65–75, income ≥125% FPL (n = 2802) |
26.0 (23.2 to 29.3) | 28.6 (25.4 to 31.8) | 2.3 (−2.0 to 6.7) | 0.30 |
| Age 65–75, income <125% FPL (n = 958) |
15.3 (11.7 to 19.0) | 21.3 (16.5 to 25.7) | 5.7 (0.18 to 11.3) | 0.043 |
| Ethnicity (n = 12,377) | ||||
| White/other (n = 7131) |
21.4 (19.7 to 23.1) | 21.4 (19.7 to 23.1) | 0.02 (−0.22 to 2.1) | 0.98 |
| Hispanic (n = 2042) |
16.7 (13.7 to 19.8) | 18.4 (15.4 to 21.5) | 1.7 (−2.1 to 5.5) | 0.38 |
| African American (n = 2229) |
28.4 (24.5 to 32.0) | 31.6 (27.9 to 35.4) | 3.2 (−2.0 to 8.4) | 0.22 |
| Asian (n = 975) |
13.8 (10.1 to 17.5) | 12.6 (9.7 to 15.5) | −1.2 (−5.8 to 3.4) | 0.61 |
FOBT fecal occult blood test, FPL federal poverty level
Results were similar when we repeated our analyses for the entire study population, not just those we classified as screen-eligible (Online Appendix Table 1). Extending our baseline period to 2007 and including both diagnostic and screening exams also produced similar results (Online Appendix Table 2). Excluding the year 2010 did not substantively alter our findings (Online Appendix Table 3).
Next, we examined whether eliminating cost-sharing had income-specific effects. Among privately insured adults, poverty status did not affect the relationship between the ACA and receipt of colonoscopy. Among participants with Medicare, those who lived in poverty had higher rates of colonoscopy use after the ACA (+5.7%, 95% CI 0.18–11.3, p = 0.043) (Table 2). Rates of colonoscopy use varied by ethnicity, though no group saw a definitive increase in colonoscopy use after passage of the ACA. (Table 2).
Our difference-in-differences analysis compared the use of colonoscopy, sigmoidoscopy, and FOBT among privately insured adults aged 50–64 to those in a control group: privately insured adults aged 40–49. The difference-in-differences estimate for colonoscopy was −0.25% (95% CI −2.1 to 1.7, p = 0.80), for sigmoidoscopy was −0.60% (95% CI −1.1 to −0.07, p = 0. 028), and for FOBT was −1.4% (95% CI −3.0 to 2.7%, p = 0.10) (Table 3).
Table 3.
Difference in Differences
| Difference in Differences: | Percentage point change age 50–64 | Percentage point change age 40–49 | Difference in differences | p |
|---|---|---|---|---|
| Colonoscopy (n = 17,788) | −0.23 (−1.8 to 1.4) | 0.02 (−1.2 to 1.2) | −0.25 (−2.1 to 1.7) | 0.80 |
| Sigmoidoscopy (n = 17,965) | −0.73 (−1.1 to 0.29) | −0.12 (−0.46 to 0.21) | −0.60 (−1.1 to −0.07) | 0.028 |
| FOBT (n = 24,902) | −1.2 (−2.3 to −0.03) | 0.17 (−1.1 to 1.4) | −1.4 (−3.0 to 0.27) | 0.10 |
Lastly, we explored whether participants who were likely to have had more cost-sharing obligations prior to the ACA saw a change in the use of colorectal cancer screening services post-ACA. Among participants with Medicare but not private insurance/Medigap, Medicaid, or MA, we found that the use of colonoscopy increased in the post-ACA period (+12.0%, 95% CI 3.3–20.8, p = 0.007) (Online Appendix Table 4). We found no increased likelihood of using colorectal cancer screening services post-ACA among the privately insured, those without additional public insurance, or those with higher out-of-pocket costs (Online Appendix, Tables 5 and 6).
DISCUSSION
Our study examined yearly rates of colorectal cancer screening before and after implementation of the preventive services provisions of the ACA. We found that, in general, there has been no large increase in the use of colorectal cancer screening services in the post-ACA period. Our results do, however, suggest that there may be specific populations in which the policy has had an effect. In particular, colonoscopy use rose among those with Medicare who live in poverty and those with only original Medicare coverage in the post-ACA period. While our findings suggest that these populations may have benefitted, we also believe that these results should be interpreted with some caution, as we examined multiple subgroups, and there is considerable uncertainty around our effect size estimates.
Our results generally resonate with recent published literature on the use of colorectal cancer screening after the ACA took effect. Chung and colleagues evaluated Medicare beneficiaries in a large multispecialty practice and found that colorectal cancer screening rates had declined slightly, though perhaps less than expected, among Medicare fee-for-service beneficiaries.20 A series of reports published by the Department of Health and Human Services concluded that the absolute number of Medicare beneficiaries using preventive services has increased.21,22 Most recently, Fedewa et al. examined colorectal cancer screening use before and after passage of the ACA and found a modest increase in use, particularly among the poor and elderly.23
Though our results suggest that some populations may have benefited from this policy, the broader effect thus far seems limited. Why might this be the case? First, awareness of this new policy remains low. A recent poll by the Kaiser Family Foundation found that, in 2014, only 43% of Americans were aware of these benefits.24 Even among those aware of the new benefits, eliminating cost-sharing may simply not be sufficient incentive to persuade patients to undergo screening. Other factors, including beliefs about screening, perception of risk, inconvenience, access to screening, and trust in the ordering provider may all play a role in decisions whether to undertake screening.17
In addition, the law did not actually cost-sharing for some. Some individuals with private insurance probably already had first-dollar coverage for screening, even before the ACA, as certain insurers had already expanded benefits. Others had grandfathered plans and were not required to eliminate cost-sharing for preventive services. In 2012, an estimated 48% of plans still fell into this category.25 Medicare beneficiaries were still subject to cost-sharing if part of the screening test were billed as a diagnostic service, a scenario that might arise when a patient had a biopsy or polypectomy.26
Given the relatively modest effects of the policy thus far, what can be done to improve its reach? First, patients must be aware of the new benefits available to them. Educating providers may also help, since providers are involved in the decision-making process at the point of care. Lastly, maintaining cost-sharing for diagnostic components that are integral to the screening process may dampen the policy's effect by leaving patients with unexpected and unwelcome bills. Policymakers could consider removing cost-sharing for testing that is considered part of the screening continuum.
This study has a number of important limitations. First, as mentioned, our follow-up period was limited, with data only through 2012. Over the next few years, as newer nationally representative data become available, we will be able to more fully assess the policy’s effect. Second, our main outcome measure relied on self-report. Indeed, prior studies suggest that patients consistently underreport the use of health care services.27 Nonetheless, we would expect under-reporting to be consistent from year to year, and thus should not confound our findings. Similarly, we were unable to control for some factors that may be related to decisions about colorectal cancer screening, including provider recommendation and patient values.17 These factors, however, are also unlikely to have changed over the short time period covered in our study, and thus are likely not significant confounders. Third, because MEPS does not provide detailed data about insurance benefits, out-of-pocket costs for colorectal cancer screening services, or grandfathered plan status, we were unable to definitely identify participants who experienced changes in cost-sharing. Even so, we were able to define subgroups of participants for whom changes in cost-sharing were plausible. Fourth, the ACA enacts policy for the entire country, making it difficult to establish a true control group. In our difference-in-differences analysis, we chose adults aged 40–49 as a comparator. Patients in this group who undergo screening, however, may be at higher risk than those in the 50–64 age group, and may respond differently to cost-sharing. Given these limitations of our comparison group, it is difficult to conclusively predict what might have happened to colorectal cancer screening rates absent the ACA. Lastly, in a number of cases, we present null findings, raising the possibility that we did not have sufficient power to detect a change in the use of screening services. Nonetheless, among our main results, many of the point estimates for changes in screening service use were near zero, suggesting that even with a larger sample, we might draw similar conclusions. One main finding stood out as having a larger effect size: we observed that among those with Medicare, the point estimate for a change in the use of colonoscopy was 2.3%. For this analysis, we had reasonable power, about 78%, to reject the null hypothesis. Furthermore, this point estimate is probably driven by a specific subgroup, namely those with original Medicare only and no additional insurance.
In conclusion, our study suggests that in the first 2 years after the passage of the ACA, the use of colorectal cancer screening has not changed substantially for most screen-eligible Americans, though some Medicare beneficiaries may have experienced an increase in the use of colonoscopy. In the coming years, further study will help inform whether this policy has met its intended objective.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 16 kb)
(DOCX 17 kb)
(DOCX 17 kb)
(DOCX 16 kb)
(DOCX 16 kb)
(DOCX 18 kb)
Acknowledgments
Funding/Support
Drs. Richman, Owens, and Asch are supported by the U.S. Department of Veterans Affairs.
Prior presentations
A version of this work was presented at the Society of General Internal Medicine Annual Meeting in Toronto, Ontario on April 24, 2015.
Disclaimer
Dr. Owens is a member of the United States Preventive Services Task Force (USPSTF). This article does not necessarily represent the views and policies of the USPSTF.
Conflict of Interest
Dr. Owens is a member of the United States Preventive Services Task Force. Drs. Richman, Asch, and Bhattacharya report no conflicts of interest.
References
- 1.Koh HK, Sebelius KG. Promoting prevention through the Affordable Care Act. N Engl J Med. 2010;363(14):1296–9. doi: 10.1056/NEJMp1008560. [DOI] [PubMed] [Google Scholar]
- 2.Interim final rules for group health plans and health insurance issuers relating to coverage of preventive services under the Patient Protection and Affordable Care Act. Interim final rules with request for comments. Fed Regist. 2010;75(137):41726–60. [PubMed]
- 3.Medicare program; payment policies under the physician fee schedule and other revisions to Part B for CY 2011. Final rule with comment period. Fed Regist. 2010;75(228):73169–860. [PubMed]
- 4.Lillard LA, Manning WG, Peterson CE, Lurie N, Goldberg GA, Phelps CE. Preventive Medical Care: Standards, Usage, and Efficiency. Santa Monica, CA: Rand Corporation; 1986. [Google Scholar]
- 5.Trivedi AN, Rakowski W, Ayanian JZ. Effect of cost sharing on screening mammography in Medicare health plans. N Engl J Med. 2008;358(4):375–83. doi: 10.1056/NEJMsa070929. [DOI] [PubMed] [Google Scholar]
- 6.Wharam JF, Galbraith AA, Kleinman KP, Soumerai SB, Ross-Degnan D, Landon BE. Cancer screening before and after switching to a high-deductible health plan. Ann Intern Med. 2008;148(9):647–55. doi: 10.7326/0003-4819-148-9-200805060-00004. [DOI] [PubMed] [Google Scholar]
- 7.Solanki G, Schauffler HH, Miller LS. The direct and indirect effects of cost-sharing on the use of preventive services. Health Serv Res. 2000;34(6):1331–50. [PMC free article] [PubMed] [Google Scholar]
- 8.Blustein J. Medicare coverage, supplemental insurance, and the use of mammography by older women. N Engl J Med. 1995;332(17):1138–43. doi: 10.1056/NEJM199504273321706. [DOI] [PubMed] [Google Scholar]
- 9.Cherkin DC, Grothaus L, Wagner EH. The effect of office visit copayments on preventive care services in an HMO. Inquiry: J Med Care Org Provision Financ. 1990;27(1):24–38. [PubMed] [Google Scholar]
- 10.Calonge N, Petitti DB, DeWitt TG, Dietrich AJ, Gregory KD, Harris R, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2008;149(9):627. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 11.Pyenson B, Scammell C, Broulette J. Costs and repeat rates associated with colonoscopy observed in medical claims for commercial and Medicare populations. BMC Health Serv Res. 2014;14:92. doi: 10.1186/1472-6963-14-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ladabaum U, Levin Z, Mannalithara A, Brill JV, Bundorf MK. Colorectal testing utilization and payments in a large cohort of commercially insured US adults. Am J Gastroenterol. 2014;109(10):1513–25. doi: 10.1038/ajg.2014.64. [DOI] [PubMed] [Google Scholar]
- 13.Colorectal Cancer: Preventable, Treatable, and Beatable-Medicaer Coverage and Billing for Colorectal Cancer Screening: Centers for Medicare and Medicaid 2012 Contract No.: 7/20/2015.
- 14.CMS Manual System. Centers for Medicare and Medicaid. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R1160CP.pdf. Accessed 7/6/15.
- 15.Gold M, Cupples HM. Medicare Advantage Benefit Design: What Does It Provide, What Doesn’t It Provide, and Should Standards Apply? Washington, DC: AARP; 2009. [Google Scholar]
- 16.MEPS-HC Response Rates by Panel. http://meps.ahrq.gov/survey_comp/hc_response_rate.jsp. Accessed 3/16/2015.
- 17.Subramanian S, Klosterman M, Amonkar MM, Hunt TL. Adherence with colorectal cancer screening guidelines: a review. Prev Med. 2004;38(5):536–50. doi: 10.1016/j.ypmed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Swartz K. Cost Sharing: Effects on spending and outcome: Robert Wood Johnson Foundation. 2010.
- 19.Pollitz K, Lucia K, Keith K, Smith R, Doroshenk M, Wolf H, et al. Coverage of Colonoscopies under the Affordable Care Act’s Prevention Benefit: Kaiser Family Foundation, American Cancer Society, National Colorectal Cancer Roundtable. 2012.
- 20.Chung S, Lesser LI, Lauderdale DS, Johns NE, Palaniappan LP, Luft HS. Medicare annual preventive care visits: use increased among fee-for-service patients, but many do not participate. Health Aff. 2015;34(1):11–20. doi: 10.1377/hlthaff.2014.0483. [DOI] [PubMed] [Google Scholar]
- 21.Beneficiaries Utilizing Free Preventive Services by State, YTD 2014: US Department of Health and Human Services. 2015.
- 22.Beneficiaries Utilizing Free Preventive Services by State, YTD 2013: US Department of Health and Human Services 2014 Contract No.: 2/25/2015.
- 23.Fedewa SA, Goodman M, Flanders WD, Han X, Smith RA EMW, et al. Elimination of cost-sharing and receipt of screening for colorectal and breast cancer. Cancer. 2015 doi: 10.1002/cncr.29494. [DOI] [PubMed] [Google Scholar]
- 24.Hamel L, Firth J, Brodie M. Kaiser Health Tracking Poll: March 2014. 2014. http://kff.org/health-reform/poll-finding/kaiser-health-tracking-poll-march-2014/. Accessed 2/24/2015 2015.
- 25.Employer Health Benefits 2013 Annual Survey: Kaiser Family Foundation and Health Research and Educational Trust. 2013.
- 26.Green BB, Coronado GD, Devoe JE, Allison J. Navigating the murky waters of colorectal cancer screening and health reform. Am J Public Health. 2014;104(6):982–6. doi: 10.2105/AJPH.2014.301877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhandari A, Wagner T. Self-reported utilization of health care services: improving measurement and accuracy. Med Care Res Rev: MCRR. 2006;63(2):217–35. doi: 10.1177/1077558705285298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 16 kb)
(DOCX 17 kb)
(DOCX 17 kb)
(DOCX 16 kb)
(DOCX 16 kb)
(DOCX 18 kb)