Abstract
Common causes of pulmonary-renal syndrome include anti-glomerular basement membrane (anti-GBM) disease anti-neutrophil cytoplasmic antibody (ANCA) positive vasculitis, and systemic lupus erythematosus. We describe a case of life-threatening pulmonary hemorrhage associated with Campylobacter hemolytic uremic syndrome (HUS), which we believe is a new disease entity. We hypothesize that the cause of this pulmonary-renal syndrome was an immunological reaction to Campylobacter; and that the initiation of high-dose steroids was responsible for the rapid reversal of the patient’s pulmonary and renal impairment. The aim of this article is to raise awareness of this unusual cause of a pulmonary-renal syndrome, guiding physicians to recognize it as a potential complication, and to consider high-dose steroids in managing the condition.
KEY WORDS: Campylobacter jejuni, hemolytic uremic syndrome, pulmonary hemorrhage
CASE REPORT
A 22-year-old previously well Caucasian woman was transferred to our renal unit with a 6 day history of watery, non-bloody diarrhea and vomiting. She also complained of a frontal headache that began a few days after the diarrhea, which was moderate in severity and described as ‘pressure around the head’. She had eaten a carry-out steak kebab 24 h before the onset of her symptoms.
Past medical history included polycystic ovarian syndrome and mild endometriosis. Her only regular medication was the oral contraceptive pill co-cyprindiol. She had taken diclofenac for her headache. She denied any illicit drug use and was a lifelong non-smoker. She had no history of recent travel and worked as a beauty therapist.
Vital signs on transfer revealed a blood pressure of 103/58 mmHg, heart rate of 92 beats per minute, respiratory rate of 18 breaths per minute and pulse oximetry saturation of 99 % on room air. She was pale and found to have orthostatic hypotension by a measured postural drop in systolic blood pressure and an accompanied rise in heart rate upon standing. Furthermore, she was oliguric (315 mls urine output in the preceding 24 h), with no evidence of peripheral edema and a non-visible jugular venous pressure (JVP). She had no focal neurological symptoms, neck stiffness or photophobia. Her abdomen was soft with normal bowel sounds. She had no rash or skin findings.
Laboratory findings revealed anemia, thrombocytopenia, and acute kidney injury with a metabolic acidosis (creatinine 4.98 mg/dL, bicarbonate 14 mmol/L); see Table 1. A blood smear showed red cell fragments. Acute kidney injury (AKI) screens, including anti-glomerular basement membrane antibodies (anti-GBM), anti-neutrophil cytoplasmic antibodies (ANCA) and anti-nuclear antibodies (ANA), were negative. Urine dipstick was positive for 3+ blood and protein. Doppler ultrasound revealed 10.5 cm kidneys with no evidence of renal artery stenosis.
Table 1.
Summary of Laboratory Values at Initial Presentation, Discharge and at Re-presentation
| Investigation (units) | Presentation | Discharge | Re-presentation |
|---|---|---|---|
| Hemoglobin (g/dL) | 7.2 | 12.6 | 7.1 |
| Platelets (x109/L) | 25 | 311 | 208 |
| Creatinine (mg/dL) | 4.98 | 2.87 | 3.94 |
| Urea (mg/dL) | 97.5 | 30.2 | 58.8 |
| Bicarbonate (mmol/L) | 14 | 22 | 18 |
| Bilirubin (mg/dL) | 1.9 | 1.3 | 2.5 |
| LDH (iu/L) | 873 | 255 | 665 |
| Albumin (g/dL) | 3.2 | 3.1 | 2.8 |
| CRP (mg/L) | 60 | <2 | 223 |
CRP C-reactive protein, LDH lactate dehydrogenase
A diagnosis of diarrhea-associated hemolytic uremic syndrome (D+HUS) was made. Management was supportive with intravenous fluid resuscitation with sodium bicarbonate. However, her urine output fell, and she developed peripheral edema and acute hemodialysis was commenced. Stool culture was positive for Campylobacter jejuni. ADAMTS13 (von Willebrand factor-cleaving protease) activity level was inconsistent with a diagnosis of thrombotic thrombocytopenic purpura (TTP), with an activity level of 13 %. The decision was made to avoid antimicrobial treatment; given that this has not been proven to be of any benefit in the treatment of Shiga-toxin–associated HUS.1 Over the next 10 days, she improved clinically, and was discharged dialysis-independent with a creatinine of 2.87 mg/dL.
Four days later, she re-presented with breathlessness and hemoptysis. She was treated for pneumonia on the basis of clinical findings and a chest X-ray showing diffuse bilateral patchy shadowing. She was promptly transferred back to the renal unit and was found to be fluid overloaded and hypertensive. Laboratory investigations showed a raised C-reactive protein (CRP), anemia and worsening renal function (creatinine 3.94 mg/dL); see Table 1. Antimicrobial treatment was escalated to intravenous piperacillin/tazobactam and clarithromycin. An attempt was made to diurese her with intravenous furosemide and glyceryl trinitrate, with an initial response that was not sustained. She was transferred to the intensive care unit for continuous positive airway pressure (CPAP) non-invasive ventilation and hemofiltration. Despite several days of negative fluid balance and the addition of an anti-fungal agent (liposomal amphotericin; as empirical treatment for fungal pneumonia) she required intubation.
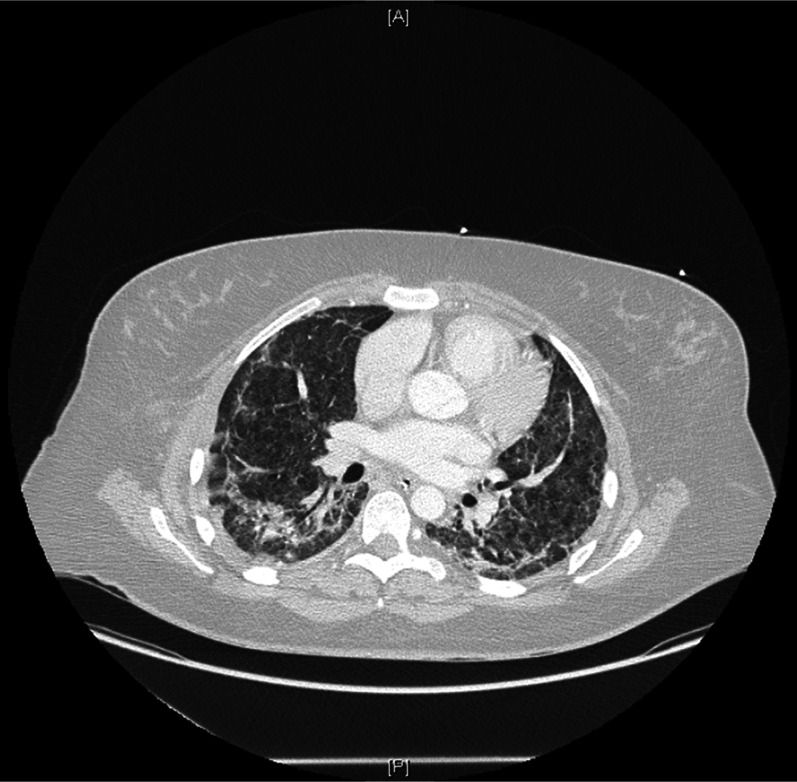
A computed tomography (CT) pulmonary angiogram was performed to exclude a pulmonary embolus, given that she was taking an oral contraceptive and recently hospitalized. This showed extensive bilateral ground glass opacities and peri-bronchovascular consolidation consistent with pulmonary hemorrhage (Fig. 1). Bronchoscopy showed mucosal injury (generalised mucosal edema and hyperemia), but failed to identify an active bleeding site. Broncho-alveolar lavage (saline washings infused into the distal broncho-alveolar tree and subsequently suctioned out to obtain cells) failed to culture any microorganisms. Atypical pneumonia screening, repeat ANCA, anti-GBM and viral serology were negative. Blood smears showed no evidence of hemolysis. The patient ultimately required increasing ventilatory pressures, which resulted in a pneumothorax. At this time, she was considered for extracorporeal membrane oxygenation (ECMO).
Figure 1.
CT pulmonary angiogram showing bilateral ground glass opacities throughout both lungs and peri-bronchovascular consolidation mainly within the lower lobes bilaterally; consistent with pulmonary hemorrhage.
A multi-professional team meeting was held and a decision was made to treat her with intravenous pulsed methylprednisolone, 500 mg on 3 consecutive days. Her respiratory function improved rapidly and she was extubated 4 days later. Oral steroids were continued for 2 weeks. Also, her kidneys improved (creatinine of 1.97 mg/dL) allowing her to be dialysis independent..
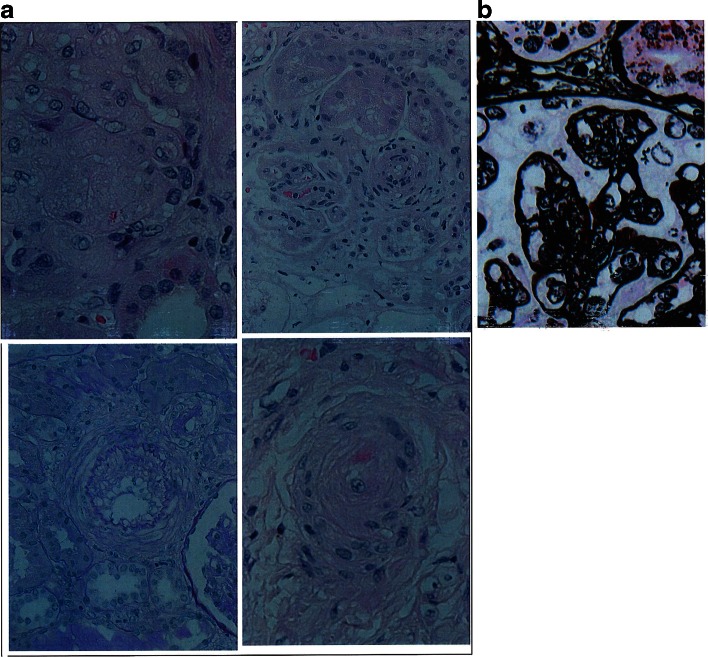
However, her creatinine level remained elevated 6 weeks later (although she did not require any renal replacement therapy). Given that her renal function had not returned to baseline, a renal biopsy was performed. This showed 28 pale glomeruli with mesangiolytic changes. Mesangial proliferation and double contours resembling a membrano-proliferative architecture were seen. There were marked vascular changes with virtual obliteration of some arterioles. Immunostaining revealed some trapping of immunoglobulins and complement components, consistent with a chronic thrombotic microangiopathy, such as seen in HUS (Fig. 2a and b).
Figure 2.
a: Renal biopsy (haematoxylin and eosin stain) photomicrographs showing mesangiolytic changes and marked vascular changes with virtual obliteration of some arterioles. b: Silver stain showing double contours of the glomerular basement membrane, resembling a membrano-proliferative architecture.
DISCUSSION
Hemolytic uremic syndrome (HUS) is a triad of microangiopathic hemolytic anemia, thrombocytopenia, and acute kidney injury.2. It typically affects children, with 90 % of cases following gastroenteritis secondary to Shiga toxin (Stx)-producing Escherichia coli3. Stx-producing Shigella dysenteriae and Campylobacter jejuni have also been linked to diarrhea-associated HUS (D+HUS).2,4 D+HUS is much more common in children than adults, due to high expression of Stx receptors in their renal glomeruli.5 Therefore, adult cases tend to be sporadic or in association with outbreaks of E.coli infection, such as that reported in Germany in 2011.6 Adult D+HUS is thought to have the same etiology and pathogenesis as childhood D+HUS, but is more likely to have severe clinical sequelae; such as chronic proteinuria, hypertension, end-stage renal failure and higher mortality rates.7,8
Campylobacter jejuni infection is usually a self-limiting gastroenteritis, but in rare cases it can cause post-infectious complications including D+ HUS, uveitis and endocarditis. Most notable is its association with Guillian-Barré Syndrome (GBS); it is a well-described trigger for GBS, with 30 % of cases being preceded by an infection with the pathogen. The immunogenicity of C. jejuni is further apparent in individuals with HLA-B27 antigen who are pre-disposed to a reactive arthritis in the weeks following infection. Given that both conditions are observed after an interval of a few weeks from initial infection, it is hypothesized that immunologic cross-reactivity such as molecular mimicry is involved.4,9 In our patient, there was an interval between initial infection with Campylobacter jejuni and subsequent development of pulmonary hemorrhage. We hypothesize that the pulmonary-renal syndrome described in our case report was driven by an immunological reaction to Campylobacter.
Our case is unique as it is the first describing pulmonary hemorrhage in association with D+HUS in an adult. There is one previous report of HUS presenting with pulmonary hemorrhage in an adolescent man in Spain that proved fatal.10 Piastra et al. described a case of pulmonary hemorrhage in association with typical HUS in a 20-month-old child in 2004. The patient required peritoneal dialysis, invasive ventilation for acute respiratory distress syndrome (ARDS) and was treated with high dose methylprednisolone. Despite pulmonary complications, the HUS showed a benign course following steroid treatment with no residual renal impairment.11 Pulmonary hemorrhage in association with atypical HUS secondary to mitomycin C chemotherapy in two adult women has been reported by Torra et al.12. This is thought to be due to angiomatoid vascular changes within the lungs and is likely to represent a pathological process different from that observed in our patient with typical D+ HUS13.
Pulmonary-renal syndrome occurs in anti-GBM disease, ANCA positive vasculitis and systemic lupus erythematosus. In our patient, ANCA, ANA and anti-GBM antibodies were negative at both initial presentation and at re-presentation. Of note, complement levels were normal, excluding factor H deficiency as a cause. Factor H has been identified as a key plasma protein involved in activation of the complement cascade via the alternative pathway. Deficiency in factor H is known to result in recurrent infections, membranoproliferative glomerulonephritis type 2 and HUS. Factor H gene mutations are associated with HUS in 20 % of familial cases and 8 % of sporadic cases. It is vital to exclude factor H deficiency as a cause of HUS because risk of recurrence after kidney transplantation (resulting in subsequent graft failure) approaches 80 %.14,15 It is well accepted that HUS results in loss of capillary integrity in the kidney; but it remains unclear as to why this rarely manifests clinically in the lungs. Given the rarity of pulmonary hemorrhage as a complication of D+ HUS, it is difficult to draw conclusions regarding pathogenesis. However, given that this is the second case of typical D+ HUS with pulmonary hemorrhage that has responded to steroid treatment, an immunological mechanism is quite likely.
It is clearly impossible to prove the link between the isolated Campylobacter jejuni and the subsequent development of pulmonary hemorrhage, but in the absence of any other cause and the strong temporal relationship in an otherwise completely healthy young woman makes this plausible. It is also impossible to prove that the high-dose steroid therapy reversed this disease process, but the almost immediate improvement in a patient who was showing life-threatening and worsening respiratory compromise for several days is very persuasive. In conclusion, we feel that if pulmonary hemorrhage complicates a case of Campylobacter-induced HUS, then high-dose intravenous steroids should be urgently administered, and may be life saving.
Acknowledgements
Contributors
No other contributors other than the listed authors.
Funding
No financial support has been received for the work represented in this manuscript.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
REFERENCES
- 1.McGannon CM, Fuller CA, Weiss AA. Different classes of antibiotics differentially influence Shiga toxin production. Antimicrob Agents Chemother. 2010;54(9):3790–8. doi: 10.1128/AAC.01783-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noris M, Remuzzi G. Hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16(4):1035–50. doi: 10.1681/ASN.2004100861. [DOI] [PubMed] [Google Scholar]
- 3.Garg AX, Suri RS, Barrowman N, et al. Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: a systematic review, meta-analysis, and meta-regression. JAMA. 2003;290(10):1360–70. doi: 10.1001/jama.290.10.1360. [DOI] [PubMed] [Google Scholar]
- 4.Acheson D. Campylobacter jejuni Infections: update on emerging issues and trends. Clin Infect Dis. 2001;32(8):1201–6. doi: 10.1086/319760. [DOI] [PubMed] [Google Scholar]
- 5.Ray PE, Liu XH. Pathogenesis of Shiga toxin-induced hemolytic uremic syndrome. Pediatr Nephrol. 2001;16:823–39. doi: 10.1007/s004670100660. [DOI] [PubMed] [Google Scholar]
- 6.Rasko DA, Webster DR, Sahl JW, et al. Origins of the E. coli strain causing an outbreak of hemolytic–uremic syndrome in germany. N Engl J Med. 2011;365:709–17. doi: 10.1056/NEJMoa1106920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piero R, Marina N, Giuseppe R. Thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura. Kidney Int. 2001;60:831–46. doi: 10.1046/j.1523-1755.2001.060003831.x. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan BS, Meyers KE, Schulman SL. The pathogenesis and treatment of hemolytic uremic syndrome. J Am Soc Nephrol. 1998;9:1126–33. doi: 10.1681/ASN.V961126. [DOI] [PubMed] [Google Scholar]
- 9.Rees JH, Soudain SE, Gregson NA, Hughes RAC. Campylobacter Infection and Guillain-Barré Syndrome. NEJM. 1995;333:1374–9. doi: 10.1056/NEJM199511233332102. [DOI] [PubMed] [Google Scholar]
- 10.Garnacho Montero J, Marmesat Ríos I, Leal Noval SR, González Fernández FJ, Goñi Belzunegui MV, Camacho LP. The Haemolytic-uraemic syndrome: a report of a case which started as a massive pulmonary haemorrhage. An Med Interna. 1990;7:416–8. [PubMed] [Google Scholar]
- 11.Piastra M, et al. Pulmonary hemorrhage complicating a typical haemolytic-uremic syndrome. Respiration. 2004;71:537–41. doi: 10.1159/000080643. [DOI] [PubMed] [Google Scholar]
- 12.Torra R, Poch E, Torras A, Bombi JA, Revert L. Pulmonary haemorrhage as a clinical manifestation of haemolytic-uraemic syndrome associated with mitomycin C therapy. Chemotherapy. 1993;39:453–6. doi: 10.1159/000238992. [DOI] [PubMed] [Google Scholar]
- 13.Chang-Poon VY, Hwang WS, Wong A, Berry J, Klassen J, Poon MC. Pulmonary angiomatoid vascular changes in mitomycin C-associated haemolytic-uraemic syndrome. Arch Pathol Lab Med. 1985;109:877–8. [PubMed] [Google Scholar]
- 14.Blackall DP, Marques MB. Hemolytic uremic syndrome revisited: shiga toxin, factor H, and fibrin generation. Am J Clin Pathol. 2004;121(Suppl 1):S81–8. doi: 10.1309/06W402EHNGVVB24C. [DOI] [PubMed] [Google Scholar]
- 15.Besbas N, Karpman D, Landau D, Loirat C, Proesmans W, Remuzzi G, Rizzoni G, Taylor CM, Van de Kar N, Zimmerhack LB. A classification of hemolytic uremic syndrome and thrombotic thrombocytopenic purpura and related disorders. Kidney Int. 2006;70:423–31. doi: 10.1038/sj.ki.5001581. [DOI] [PubMed] [Google Scholar]