Abstract
Purpose
Recent studies have proposed central serotonergic dysfunction as a major pathophysiology of migraine. We investigated serotonin transporter (SERT) availability in migraineurs using F-18-N-(3-fluoropropyl)-2β-carbomethoxy-3β-(4-iodophenyl) nortropane ([18F]FP-CIT) positron emission tomography (PET).
Methods
Brain [18F]FP-CIT PET images were obtained in eight women with migraine during headache free phase and 12 healthy adult women, 120 min after injection of 185 MBq. Non-displaceable binding potential (BPND) of [18F]FP-CIT, which is an estimate of SERT availability, was calculated at the brainstem and compared with clinical parameters.
Results
BPND at the brainstem was significantly higher in adult migraineurs (n = 6, 1.15 ± 0.17) than healthy subjects (0.95 ± 0.14) (p = 0.04). Healthy subjects demonstrated negative correlation between brainstem BPND and age (r = −0.64, p = 0.02), whereas this age-related decline pattern was not found in the migraineurs. Severity of migraine attack was significantly correlated with brainstem BPND (r = 0.66, p = 0.02), when age and duration of illness were corrected.
Conclusions
Increased SERT availability in the brainstem of adult migraineurs indicates low serotonin neurotransmission during headache-free phase. Patients who experience more painful headaches have lower serotonin neurotransmission. [18F]FP-CIT PET is a useful in vivo imaging technique for evaluating brainstem SERT availability in migraineurs.
Keywords: Migraine, Serotonin transporter, [18F]FP-CIT, Positron emission tomography, Brainstem
Introduction
Migraine is a common headache disorder which accompanies complex neurologic, gastroenterologic, and autonomic nervous system symptoms [1]. More than two-thirds of migraineurs reported experiencing limitation in their activities of daily life [1, 2]. The serotonergic hypothesis, which argues that serotonin plays an important role in the pathophysiology of the migraine, is widening its ground with a new finding that the brainstem has an integral function in the beginning and the control of migraine [1, 3, 4]. This hypothesis insists that the nociceptive trigeminovascular pathway be activated during the low serotonin concentration state [5–7]. Serotonergic dysfunction in the central nervous system is considered to act as a migraine inducer by increasing a patient’s susceptibility to migraine. The activation of the dorsal raphe nucleus and the locus coeruleus during migraine attack, which are known to be sources of serotonin in the brain, is one of the evidences that support this hypothesis [8]. It also assists the serotonergic hypothesis that intravenous serotonin administration relieves migraine attacks [6].
Serotonergic system in the brain of a living human can be evaluated with F-18-N-(3-fluoropropyl)-2β-carbomethoxy-3β-(4-iodophenyl) nortropane ([18F]FP-CIT) PET. [18F]FP-CIT is basically a dopamine transporter (DAT) radioligand, but has relatively high affinity to serotonin transporter (SERT) and can be used to evaluate serotonergic system [9, 10]. SERT modulates synapses by reuptake and reaccumulation of serotonin in the presynaptic terminal and therefore the function of serotonergic system can be assessed by imaging SERT availability [11]. One advantage of [18F]FP-CIT is that on-site production is not necessary unlike 11C-labeled ligands. We estimated brainstem SERT availability in migraine patients using [18F]FP-CIT PET and investigated if it is correlated with clinical features of migraine.
Materials and Methods
Subjects
Female patients who fulfilled the diagnostic criteria of migraine with aura (MA) or without aura (MO), according to the International Classification of Headache Disorder, 2nd edition [12], were initially considered for the present study. Patients underwent a standardized interview and clinical neurological examinations by a neurologist (K.Y.J.). Interview items included duration of a migraine history, the average number of headaches per month during the previous year, and a visual analogue scale (VAS) to rate the most severe headache experienced during the previous year. Patients were excluded if they had (1) a history of analgesic drug overuse, (2) general, neurological or psychiatric disease, (3) a history of headaches other than migraine, and (4) the possibility of pregnancy. A total of eight female migraineurs (mean age, 29 years; range, 16–38 years) were enrolled. They consisted of five MO and three MA patients. Three out of five MO patients were taking propranolol as preventive medication and one of them was also using topiramate and divalproate. The other two MO patients were taking abortive medication without any preventive medication. MA patients were drug-naive. Table 1 demonstrates each patient’s demographic and headache characteristics. Twelve healthy adult women without history of headache for the previous year (mean age, 32 years; range, 25–39 years) who volunteered in response to an advertisement participated as healthy subjects. They were also interviewed and examined by the same neurologist. All study subjects were informed of the procedure, and informed consent was obtained from each individual in accordance with the guidelines issued by the institutional review board (IRB) of Korea University Anam Hospital. The study protocol was approved by the IRB.
Table 1.
Patients’ demographic and headache characteristics
| Current age (years) | Onset age (years) | Duration of illness (years) | Frequency of migraine attack (per month) | Severity of migraine attack (VAS score) | Aura | |
|---|---|---|---|---|---|---|
| Patient 1 | 27 | 22 | 5 | 1 | 7 | No |
| Patient 2 | 37 | 17 | 20 | 3 | 7.5 | No |
| Patient 3 | 17 | 15 | 2 | 3 | 9 | Yes |
| Patient 4 | 38 | 12 | 26 | 3 | 9 | No |
| Patient 5 | 33 | 23 | 10 | 5 | 2.5 | No |
| Patient 6 | 27 | 24 | 3 | 3 | 9 | Yes |
| Patient 7 | 34 | 17 | 17 | 1 | 6 | No |
| Patient 8 | 16 | 15 | 1 | 1 | 5 | Yes |
| mean ± SD | 28.6 ± 8.5 | 18.1 ± 4.4 | 10.5 ± 9.4 | 2.8 ± 1.8 | 7.0 ± 2.2 |
VAS visual analogue scale
Brain MRI
Brain magnetic resonance imaging (MRI) was performed in all patients using a 1.5-T (Magnetom Vision; Siemens, Erlangen, Germany) or 3-T (Magnetom Trio; Siemens, Erlangen, Germany) MR systems to rule out secondary headache. T1-weighted images using a 3D MPRAGE sequence and T2-weighted axial images were acquired. The acquisition parameters for MPRAGE images were; TR = 1,900 ms, TE = 2.8 ms, thickness = 1.2 mm without slice gap, matrix = 230 × 2,350, field of view = 256 × 256. The acquisition parameters for T2-weighted images were; TR = 3,000 ms, TE = 115 ms, thickness = 5.0 mm, slice gap = 6.5 mm, matrix = 512 × 250, field of view = 151 × 230.
Brain [18F]FP-CIT PET
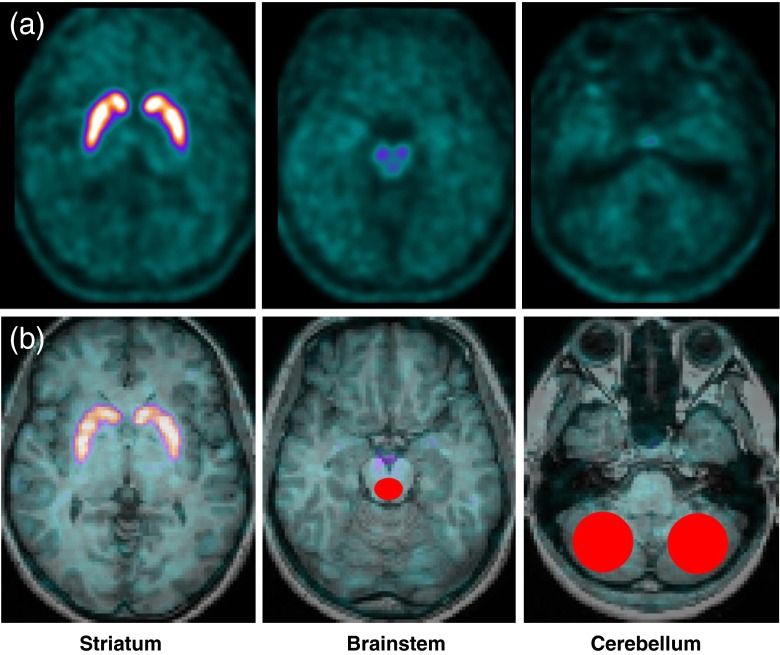
Brain [18F]FP-CIT PET images were obtained when patients were headache-free from −72 to +72 h (the latter was verified by telephone interviews). Imaging started 120 min after the injection of 185 MBq [18F]FP-CIT, using a Gemini TF PET/CT scanner (Philips Medical Systems; Cleveland, OH, USA). [18F]FP-CIT binding to the SERT in the brainstem was analyzed using a region-of-interest (ROI) method. An ellipsoid ROI was created at the posterior part of the brainstem at the midbrain level on the four consecutive transaxial slices of the MRI-coregistered [18F]FP-CIT PET template image which showed the best resolution (Fig. 1b). The volume of the ROI was 0.5 cm3. MRIcro v1.4 (www.mccauslandcenter.sc.edu/mricro/index.html) was used to construct the ROI. In-house PET template was made from 12 healthy subjects. Posterior part of the midbrain was chosen because approximately 80 % of the serotonergic neurons reside within the raphe nuclei [13]. ROIs did not include the anterior part of the midbrain which is abundant in DATs. The standard ROIs were then applied to the spatially normalized images of each subject with no alteration in shape, size and coordinates. SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK) implemented in MATLAB v7.1 (MathWorks, Natick, MA, USA) was used for spatial normalization and coregistration. Mean counts of the ROIs were measured and non-displaceable binding potential (BPND) of [18F]FP-CIT, which is an estimate of SERT availability, was calculated using the cerebellum as a reference region.
Fig. 1.
Brain [18F]FP-CIT PET images and the standard ROIs for analysis. a [18F]FP-CIT binding to the serotonin transporters at the brainstem is nicely visualized, as well as its binding to the striatal dopamine transporters. b Standard ROIs are demonstrated in red on the coregistered fusion images of spatially normalized PET and MR images
Statistical Analysis
Mann–Whitney U test was used to compare brainstem BPND of migraine patients and healthy subjects. In migraine patients, correlation analysis was made using Spearman’s rho as a measure of linear association between each clinical parameter and brainstem BPND. Semi-partial correlation coefficient was calculated between VAS and brainstem BPND to control for possible confounding effects of age and duration of illness. In healthy subjects, correlation between age and brainstem BPND was assessed using Spearman’s correlation analysis. All results were considered significant where p < 0.05.
Results
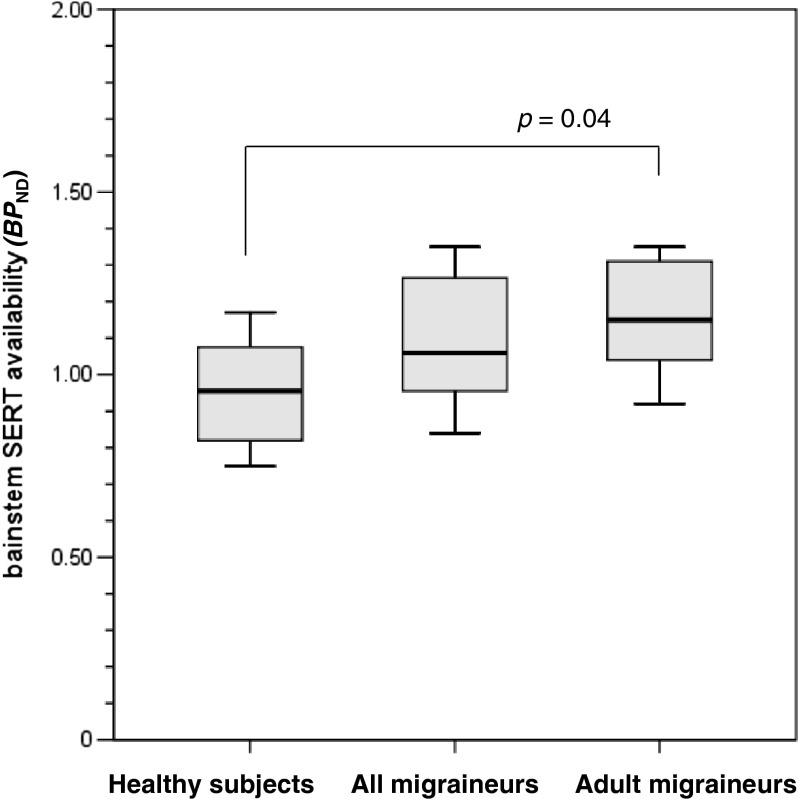
Mean brainstem BPND was 1.09 ± 0.18 in the migraine patients and 0.95 ± 0.14 in the healthy subjects. There was no significant difference in brainstem BPND between the two groups (p = 0.11). However, when two teenager migraine patients were excluded in the analysis, brainstem BPND was significantly higher in the six adult migraine patients (1.15 ± 0.17) than in the healthy subjects (p = 0.04, Fig. 2).
Fig. 2.
Comparative brainstem SERT availability. The mean value of brainstem SERT availability (BP ND) was significantly higher in adult migraineurs than in healthy subjects
In migraineurs, no significant correlation was found between brainstem BPND and the clinical parameters including age (r = 0.31, p = 0.45), duration of illness (r = 0.01, p = 0.99), frequency (r = 0.11, p = 0.80) and severity (r = 0.46, p = 0.25) of migraine attack. With semi-partial correlation analysis adjusting the confounding effects of age and duration of illness, severity of migraine headache measured by VAS was significantly correlated with brainstem BPND in migraineurs (r = 0.66, p = 0.02), but frequency of migraine attack was not (r = −0.12, p = 0.77).
In the healthy subjects, there was a negative correlation between brainstem BPND and age (r = −0.64, p = 0.02). Unlike healthy subjects, this age-related decline pattern of brainstem SERT availability was not observed in the migraineurs (r = 0.31, p = 0.45) (Fig. 3).
Fig. 3.
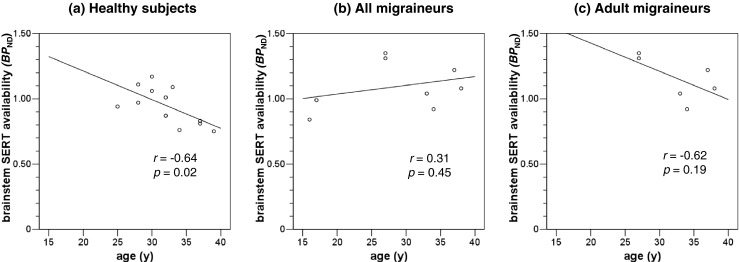
Correlation of age and brainstem SERT availability. a Brainstem SERT availability was negatively correlated with age in healthy subjects. b The age-related decline pattern of brainstem SERT availability was not observed in migraineurs, and c somewhat dampened in adult migraineurs
Discussion
Brainstem SERT availability, estimated by BPND of [18F]FP-CIT, was significantly higher in adult migraine patients than in healthy subjects. Higher brainstem SERT availability in migraine patients during headache-free phase supports the notion that migraine is a disorder of low serotonin neurotransmission. The central serotonergic system in the brainstem at the midbrain level that contains the dorsal raphe nuclei is involved in antinociception via its multiple projections to the thalamus and cortices. SERT modulates serotonin neurotransmission by removing serotonin from the synaptic cleft back to the presynaptic neuron. An imaging study that used a more SERT-selective radioligand [123I]ADAM also demonstrated significantly increased brainstem SERT availability in migraineurs than in healthy subjects [13]. Increased brainstem SERT availability together with low serotonin synthesis rate [14] and increased serotonin turnover [15, 16] during headache-free phase reflect low synaptic serotonin concentration. Serotonin is an inhibitory neurotransmitter in the brain. Low synaptic neurotransmission depicted by increased SERT availability in this study is considered either a cause of brain hyperexcitability and vulnerability to migraine attack or a consequence of chronic adaptation to repetitive headache attacks, or both.
We observed significant positive correlation between migraine severity (VAS score) and the SERT availability which was corrected for the influence of age and duration of illness. This finding suggests that migraine patients who experience more painful headache attacks have lower serotonin transmission. Frequency of migraine attack, however, did not show significant correlation with brainstem SERT availability. Central serotonin system might be most related with the severity of headache attack, while clinical severity of migraine is a complex of degree, quality, frequency and duration of headache or other neuropsychological symptoms, duration of illness, etc.
Brainstem SERT availability of healthy subjects decreased significantly as the age increased in this study. This result is consistent with the findings of previous studies that reported age-related decline of SERT availability of 3.1–4.2 % per decade in the SERT-rich brain regions in healthy adults [17, 18]. So far no in vivo imaging study has been published regarding SERT availability in healthy pediatric/adolescent population. One study investigated SERT availability in depressive drug-naïve children and adolescents using [123I]β-CIT, but adults were not included in the study and no information is available in comparison to adults [19]. It cannot be excluded that SERT availability in the pediatric/adolescent population is different from that of adults, at least in migraine patients, considering different clinical characteristics of pediatric/adolescent migraine from adult migraine. In general, childhood/adolescent migraine has lower incidence of migraine with aura, presents with bilateral headache rather than unilateral, and responds better to placebo treatment [20].
FP-CIT is available for SPECT imaging with I-123 radiolabeling. [123I]FP-CIT has been developed earlier than [18F]FP-CIT and is the most widely used radiotracer for DAT imaging. [123I]FP-CIT and [18F]FP-CIT have same binding affinity for DAT as well as SERT, because they have same molecular structure except for the location and type of radiolabeling. [18F]FP-CIT has advantages over [123I]FP-CIT, such as faster kinetics, higher spatial resolution and detector sensitivity. High resolution is especially crucial to accurately estimate bindings in small regions such as the dorsal raphe.
Our study has some potential limitations. [18F]FP-CIT is good to measure SERT availability in the posterior part of the midbrain where approximately 80 % of serotonergic neurons reside within the raphe nucleus. However, it is not adequate to evaluate cortical SERT availability due to its relatively lower selectivity to cortical SERT compared with [11C]DASB or [123I]ADAM. [18F]FP-CIT also binds to DAT in the substantia nigra, which is located close to the raphe nucleus. We did our best to delineate the raphe ROI, but spillover from the substantia nigra into the raphe region cannot be excluded. Thalamic SERT availability could not be estimated, even if it is also of interest in migraine. The thalamus is known to have 20 times more SERT than DAT binding sites [21]. However, the signal-to-noise ratio in the thalamic region was very low and that made it unreliable to evaluate thalamic binding. The number of patients was relatively small and hindered further comparison between migraine with aura and without aura. We included only female participants in the study to avoid possible confounding effect of sex, but did not consider menstruation cycle. The timing of scanning spanned from winter to summer and this can be a potential confounding factor. Seasonal fluctuation in SERT availability has been reported [22].
Conclusion
Increased SERT availability in the brainstem of adult migraineurs indicates that migraine is a disorder of low serotonin neurotransmission. Patients who experience more painful headaches have lower serotonin neurotransmission. [18F]FP-CIT PET is a useful in vivo imaging technique for evaluating brainstem SERT availability in migraineurs.
Acknowledgments
We thank Mi-Sun Ahn and Sun Young Oh for assistance in data acquisition; Jae Sung Lee for providing FMItool; Mi-Ok Kim, Jae-Hoon Baek, Ji-Han Kim and the rest of the staff at the Korea University Anam Hospital PET center for excellent technical assistance. This work was supported by Korea University Grants [K0931131, K0932081].
Conflict of Interest
Eunkyung Park, Yu Mi Hwang, Min Kyung Chu, and Ki-Young Jung declare that they have no conflicts of interest.
Ethical statement
The current study was approved by an institutional review board and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects in the study gave written informed consent.
References
- 1.Lee KS. The diagnosis and most-updated therapy of migraine. J Korean Med Assoc. 2009;52:500–6. doi: 10.5124/jkma.2009.52.5.500. [DOI] [Google Scholar]
- 2.Roh JK, Kim JS, Ahn YO. Epidemiologic and clinical characteristics of migraine and tension-type headache in Korea. Headache. 1998;38:356–65. doi: 10.1046/j.1526-4610.1998.3805356.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferrari MD. Migraine. Lancet. 1998;351:1043–51. doi: 10.1016/S0140-6736(97)11370-8. [DOI] [PubMed] [Google Scholar]
- 4.Tfelt-Hansen PC, Koehler PJ. History of the use of ergotamine and dihydroergotamine in migraine from 1906 and onward. Cephalalgia. 2008;28:877–86. doi: 10.1111/j.1468-2982.2008.01578.x. [DOI] [PubMed] [Google Scholar]
- 5.Chung JM. Preventive medications for migraine. Korean J Headache. 2010;11:42–9. [Google Scholar]
- 6.Ferrari MD, Saxena PR. On serotonin and migraine: a clinical and pharmacological review. Cephalalgia. 1993;13:151–65. doi: 10.1046/j.1468-2982.1993.1303151.x. [DOI] [PubMed] [Google Scholar]
- 7.Hamel E. Serotonin and migraine: biology and clinical implications. Cephalalgia. 2007;27:1293–300. doi: 10.1111/j.1468-2982.2007.01476.x. [DOI] [PubMed] [Google Scholar]
- 8.Weiller C, May A, Limmroth V, Juptner M, Kaube H, Schayck RV, et al. Brain stem activation in spontaneous human migraine attacks. Nat Med. 1995;1:658–60. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
- 9.Kim JS. Practical approach for the clinical use of dopamine transporter imaging. Nucl Med Mol Imaging. 2008;42:425–34. [Google Scholar]
- 10.Booij J, de Jong J, de Bruin K, Knol R, de Win MM, van Eck-Smit BL. Quantification of striatal dopamine transporters with 123I-FP-CIT SPECT is influenced by the selective serotonin reuptake inhibitor paroxetine: a double-blind, placebo-controlled, crossover study in healthy control subjects. J Nucl Med. 2007;48:359–66. [PubMed] [Google Scholar]
- 11.Lesch KP, Balling U, Gross J, Strauss K, Wolozin BL, Murphy DL, et al. Organization of the human serotonin transporter gene. J Neural Transm Gen Sect. 1994;95:157–62. doi: 10.1007/BF01276434. [DOI] [PubMed] [Google Scholar]
- 12.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24:9–160. [DOI] [PubMed]
- 13.Schuh-Hofer S, Richter M, Geworski L, Villringer A, Israel H, Wenzel R, et al. Increased serotonin transporter availability in the brainstem of migraineurs. J Neurol. 2007;254:789–96. doi: 10.1007/s00415-006-0444-0. [DOI] [PubMed] [Google Scholar]
- 14.Sakai Y, Dobson C, Diksic M, Aube M, Hamel E. Sumatriptan normalizes the migraine attack-related increase in brain serotonin synthesis. Neurology. 2008;70:431–9. doi: 10.1212/01.wnl.0000299095.65331.6f. [DOI] [PubMed] [Google Scholar]
- 15.Chugani DC, Niimura K, Chaturvedi S, Muzik O, Fakhouri M, Lee ML, et al. Increased brain serotonin synthesis in migraine. Neurology. 1999;53:1473–9. doi: 10.1212/WNL.53.7.1473. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari MD, Odink J, Tapparelli C, Van Kempen GM, Pennings EJ, Bruyn GW. Serotonin metabolism in migraine. Neurology. 1989;39:1239–42. doi: 10.1212/WNL.39.9.1239. [DOI] [PubMed] [Google Scholar]
- 17.Pirker W, Asenbaum S, Hauk M, Kandlhofer S, Tauscher J, Willeit M, et al. Imaging serotonin and dopamine transporters with 123I-beta-CIT SPECT: binding kinetics and effects of normal aging. J Nucl Med. 2000;41:36–44. [PubMed] [Google Scholar]
- 18.van Dyck CH, Malison RT, Seibyl JP, Laruelle M, Klumpp H, Zoghbi SS, et al. Age-related decline in central serotonin transporter availability with [(123)I]beta-CIT SPECT. Neurobiol Aging. 2000;21:497–501. doi: 10.1016/S0197-4580(00)00152-4. [DOI] [PubMed] [Google Scholar]
- 19.Dahlstrom M, Ahonen A, Ebeling H, Torniainen P, Heikkila J, Moilanen I. Elevated hypothalamic/midbrain serotonin (monoamine) transporter availability in depressive drug-naive children and adolescents. Mol Psychiatry. 2000;5:514–22. doi: 10.1038/sj.mp.4000766. [DOI] [PubMed] [Google Scholar]
- 20.Maas HJ, Danhof M, Della Pasqua OE. Analysis of the relationship between age and treatment response in migraine. Cephalalgia. 2009;29:772–80. doi: 10.1111/j.1468-2982.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- 21.Koch W, Unterrainer M, Xiong G, Bartenstein P, Diemling M, Varrone A, et al. Extrastriatal binding of [(1)(2)(3)I]FP-CIT in the thalamus and pons: gender and age dependencies assessed in a European multicentre database of healthy controls. Eur J Nucl Med Mol Imaging. 2014;41:1938–46. doi: 10.1007/s00259-014-2785-8. [DOI] [PubMed] [Google Scholar]
- 22.Ruhe HG, Booij J, Reitsma JB, Schene AH. Serotonin transporter binding with [123I]beta-CIT SPECT in major depressive disorder versus controls: effect of season and gender. Eur J Nucl Med Mol Imaging. 2009;36:841–9. doi: 10.1007/s00259-008-1057-x. [DOI] [PubMed] [Google Scholar]