Abstract
Purpose
The aim of this study was to evaluate the relationship between semiquantitative parameters on 18F-FDG PET/CT including maximum standardized uptake value (SUVmax), mean standardized uptake value (SUVmean), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) and the expression level of Ki-67 in small-cell lung cancer (SCLC).
Methods
Ninety-four consecutive patients with SCLC were enrolled in this study. They underwent 18F-FDG PET/CT for initial evaluation of SCLC, and we measured SUVmax, avgSUVmean, MTVsum, and TLGtotal on 18F-FDG PET/CT images. The protein expression of Ki-67 was examined by immunohistochemical staining.
Results
Significant correlations were found between the MTVsum and Ki-67 labeling index (r = 0.254, p = 0.014) and the TLGtotal and Ki-67 labeling index (r = 0.239, p = 0.020). No correlation was found between the SUVmax and Ki-67 labeling index (r = 0.116, p = 0.264) and the avgSUVmean and Ki-67 labeling index (r = 0.031, p = 0.770). Dividing the Ki-67 expression level into three categories, it was suggested that increasing Ki-67 expression level caused a stepwise increase in the MTVsum and TLGtotal. (p = 0.028 and 0.039, respectively), but not the SUVmax and avgSUVmean (p = 0.526 and 0.729, respectively).
Conclusion
In conclusion, the volume-based parameters of 18F-FDG PET/CT correlate with immunohistochemical staining of Ki-67 in SCLC. Measurement of the MTVsum and TLGtotal by 18F-FDG PET/CT might be a simple, noninvasive, and useful method to determine the proliferative potential of cancer cells.
Keywords: Small-cell lung carcinoma, 18F-FDG PET/CT, Metabolic tumor volume, Ki-67
Introduction
Lung cancer accounts for 12 % of all new cancer cases and is the second most common cancer in men and women worldwide [1]. Lung cancer is the leading cause of cancer-related death in the USA and the Republic of Korea. Small-cell lung cancer (SCLC) represents 13 % of all newly diagnosed cases of lung cancer worldwide or more than 180,000 cases per year [1, 2]. SCLC generally has a more rapid doubling time, higher growth fraction, and earlier development of widespread metastases compared with those of non-small-cell lung cancer (NSCLC), all of which lead to frequent relapse and a poor prognosis, despite high sensitivity to initial chemotherapy and radiotherapy [1, 3]. Understanding the biological characteristics of this devastating disease could facilitate early diagnosis and individualized treatment and improve the survival rate.
Uncontrolled cell proliferation is a key characteristic of cancer. Some studies have demonstrated that the expression level of Ki-67, an indicator of cell proliferative activity, is an important prognostic factor in various cancers [4–6] including lung cancer [7]. However, molecular markers can only be tested in vitro after tissue has been removed from the patient, and surgical or pathological sampling errors can occur. Therefore, imaging cellular proliferation may be an alternative approach to diagnosing and staging malignancies.
Positron emission tomography/computed tomography (PET/CT) using the tracer 18F-fluorodeoxyglucose (18F-FDG) has emerged as an essential oncological imaging tool for diagnosing, staging, restaging, and determining treatment response [8]. The standardized uptake value (SUV), which is the most popular semiquantitative parameter to objectify PET/CT data, reflects the glucose metabolic rate and is used to characterize tumor metabolism in vivo. Metabolic tumor volume (MTV), defined as the volume of tumor tissue with increased 18F-FDG uptake, and total lesion glycolysis (TLG), defined as the product of MTV and mean SUV (SUVmean), are also important independent prognostic factors in various cancers [9–12]. The degree of 18F-FDG tumor uptake on PET/CT as measured by SUV is correlated with cell proliferative activity as determined by the Ki-67 expression level in patients with NSCLC [13–15]. However, it remains unclear whether macroscopic functional imaging techniques correlate with microscopic predictive molecular markers in patients with SCLC.
In this study, we evaluated the degree of 18F-FDG uptake on PET/CT imaging in combination with immunohistochemical results and found potential correlations among the maximum SUV (SUVmax), SUVmean, MTV, TLG, and Ki-67 expression level in patients with SCLC. We also tried to understand the underlying biological mechanisms of FDG uptake in patients with SCLC.
Materials and Methods
Patients
We retrospectively reviewed 145 patients with histologically or cytologically proven SCLC who underwent a pretreatment 18F-FDG PET/CT scan at our medical center from June 2007 to December 2013. Patients who met the following criteria were excluded: (1) no Ki-67 immunohistochemical staining data; (2) any chemotherapeutic or radiotherapeutic treatments before the 18F-FDG PET/CT scan; (3) brain metastasis; (4) pre- or coexisting primary malignancy; (5) loss of 18F-FDG PET/CT scan imaging data.
18F-FDG PET/CT Image Acquisition
Torso 18F-FDG PET/CT images were obtained on a PET/CT scanner (Gemini TF, Philips Medical Systems, Cleveland, OH, USA). All patients fasted for at least 6 h and had serum glucose levels <140 mg/d before the scan. CT scans were obtained followed by PET scans for 1 min/bed 1 h after an intravenous injection of 370–555 MBq 18F-FDG according to body weight. This PET unit had an 18-cm axial field of view and 4.4-mm spatial resolution. A low-dose CT scan was obtained for attenuation correction and localization on a 16-slice multidetector helical CT unit using the following parameters: 120 kVp; 50 mA; 0.75-s rotation time; 0.75-mm slice collimation; 4-mm scan reconstruction, with a reconstruction index of 4 mm; 50-cm field of view; 512 × 512 matrices. The PET data were reconstructed iteratively following CT using a three-dimensional row action maximum likelihood algorithm with low-dose CT data sets used to correct attenuation. Maximum intensity projection, cross-sectional views, and fusion images were reviewed.
Image Interpretation and Quantitation
All images were interpreted and analyzed by two experienced nuclear medicine physicians on a dedicated workstation (Extended Brilliance Workspace 4.0 with a PET/CT viewer for automated image registration, Philips, Amsterdam, The Netherlands). We used all available clinical information, reviewed it together, and interpreted the PET/CT images by consensus.
We defined a malignant lesion as one that showed relatively increased metabolism compared to the metabolism of adjacent normal tissues and that was not clearly associated with normal structures.
We measured the SUV of malignant lesions by drawing a region of interest (ROI) for the semiquantitative analysis. The ROI was placed over the area of maximum activity within the malignant lesion, and the SUVmax was determined as the highest SUV of the pixels within the ROI.
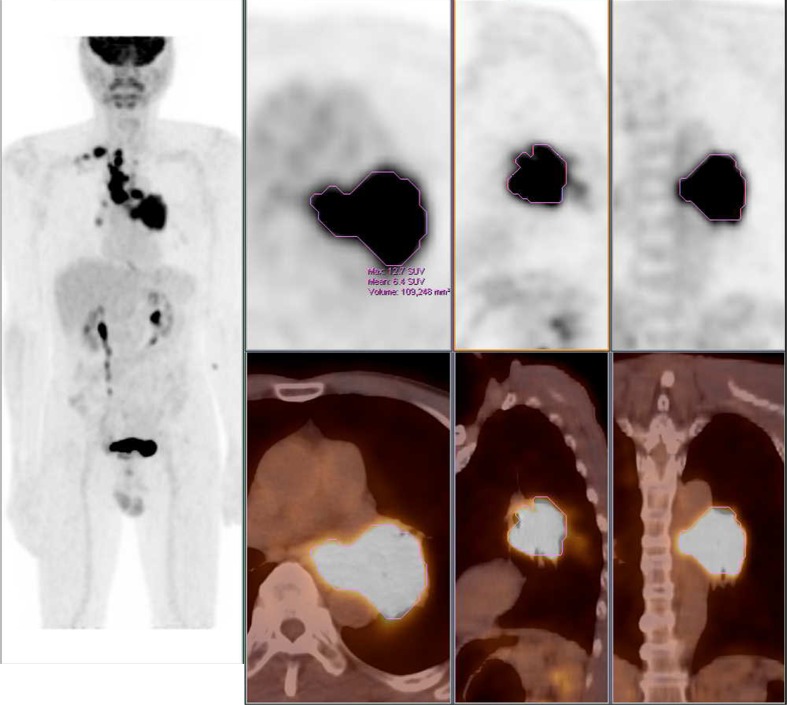
We also measured the MTVs of each lesion using a semiautomatic method to delineate the volume of interest over the malignant lesion (Fig. 1). We chose the most intense point on each malignant lesion and drew the lesion boundaries using SUV-based contouring software. A fixed 50 % of local maximum intensity was used as the threshold intensity value, and this was deemed reasonable from previous studies [16–18]. When the automated software included not only malignant lesions but also background activity, we manually removed the background boundaries by reviewing the background CT findings. We calculated the sum of MTVs of all malignant lesions (MTVsum) by adding all MTVs of each lesion. The program also analyzed the SUVmean of each lesion, and we calculated the avgSUVmean by averaging all SUVmeans of each lesion. The TLGtotal value was calculated by multiplying MTVsum by avgSUVmean.
Fig. 1.
Examples of acquiring the volumetric parameters. Standardized uptake value (SUV) thresholds were set to 50 % of SUVmax
Ki-67 Immunohistochemical Staining and Evaluation
Formalin-fixed, paraffin-embedded biopsy specimens were cut 4 μm thick and taken for immunohistochemical staining with the MIB-1 antibody (Dako, Glostrup, Denmark), which was a monoclonal murine antibody specific for human nuclear antigen Ki-67 and used as a primary antibody in a 1:200 dilution.
The percentages of Ki-67-positive cells were evaluated at ×400 magnification, by counting distinctly stained nuclei per 1000 tumor cells in the most representative area, which, in most instances, corresponded to areas with the highest mitotic activity. Then the Ki-67 results were categorized into ten groups at 10 % intervals.
The histopathological slides were examined by an experienced pathologist.
Statistical Analysis
Statistical analyses were performed using SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA). A P-value < 0.05 was considered significant. Two-sided correlations between SUVmax, avgSUVmean, MTVsum, and TLGtotal and the percentages of cells immunostained for Ki-67 were analyzed with Spearman’s rank correlation coefficient. The Ki-67 expression level was divided into three categories of ≤30 % positive tumor cells, 30–60 % positive tumor cells, and >60 % positive tumor cells, based on a previous report [19]. The PET/CT parameters of each category were compared using the Jonckheere-Terpstra test.
Results
Patient Characteristics
Twenty-seven patients were excluded because of the absence of Ki-67 immunohistochemical staining data. Ten patients who were treated with chemotherapy or radiotherapy before the 18F-FDG PET/CT scan, five who had brain metastases, five who had another primary malignancy, and four whose 18F-FDG PET/CT scan imaging data were insufficient were also excluded. Finally, 94 patients were enrolled in this study.
The characteristics of the 94 patients with SCLC are summarized in Table 1. Mean age was 67.17 (±8.7 years). Forty-four patients (46.81 %) had limited disease, and 50 patients (53.19 %) had extensive disease.
Table 1.
Characteristics of the patients
| Characteristics | |
|---|---|
| Age (years) | |
| Mean ± SD | 67.17 ± 8.7 |
| Median | 68 |
| Range | 43 – 88 |
| No. of patients | |
| Sex | |
| Male | 75 (79.79 %) |
| Female | 19 (20.21 %) |
| Disease extent | |
| Limited disease | 44 (46.81 %) |
| Extensive disease | 50 (53.19 %) |
| Total number of patients | 94 |
| mean ± SD | |
| PET/CT parameters | |
| SUVmax | 8.87 ± 3.01 |
| avgSUVmean | 7.20 ± 1.42 |
| MTVsum (cm3) | 186.34 ± 252.57 |
| TLGtotal | 884.96 ± 1129.13 |
Correlations between PET/CT Parameters and Ki-67 Staining
SUVmax, avgSUVmean, MTVsum, and TLGtotal, and the percentages of Ki-67-positive cells in tumor tissues were analyzed (Fig. 2). No correlation was found between SUVmax and Ki-67 expression (Spearman’s r = 0.116, p = 0.264) or between avgSUVmean and Ki-67 expression (r = 0.031, p = 0.770). Significant correlations were found between MTVsum and Ki-67 expression (r = 0.254, p = 0.014) and between TLGtotal and Ki-67 expression (r = 0.239, p = 0.020). In Fig. 2, there are three outliers in the MTV at the high Ki-67 categories (60–100 %). The data were further analyzed, except the three biggiest outliers, and significant correlation was still present between MTVsum and Ki-67 expression (r = 0.208, p = 0.049).
Fig. 2.
Scatter plots of the positron emission tomography/computed tomography (PET/CT) parameters (outliers are indicated in yellow)
Analyses between the PET/CT parameters and Ki-67 expression levels divided into three categories are summarized in Table 2. Increasing Ki-67 expression level increased the MTVsum and TLGtotal stepwise (p = 0.028 and 0.039, respectively, Jonckheere-Terpstra test), but not the SUVmax or avgSUVmean (p = 0.526 and 0.729, respectively).
Table 2.
Analysis between PET/CT parameters and Ki-67 expression level (Jonckheere-Terpstra test)
| n | SUVmax | AvgSUVmean | MTVsum (cm3) | TLGtotal | |
|---|---|---|---|---|---|
| Ki-67 | |||||
| ≤30 % | 7 | 6.73 ± 2.28 | 4.18 ± 1.60 | 69.22 ± 34.85 | 294.07 ± 192.94 |
| 30–60 % | 37 | 9.16 ± 3.03 | 4.96 ± 1.56 | 127.48 ± 133.27 | 681.56 ± 757.85 |
| >60 % | 50 | 9.07 ± 3.11 | 4.74 ± 1.28 | 292.45 ± 446.29 | 1366.49 ± 2421.18 |
| P value | 0.526 | 0.729 | 0.028 | 0.039 | |
Data are mean ± standard deviation
Discussion
Ki-67 is widely considered a cell proliferation marker and is mainly expressed during the active phases of the cell cycle, i.e., G1, S, G2, and mitosis [20]. A high cell proliferation rate is a hallmark of cancer, and high Ki-67 expression predicts poor survival in patients with multiple myeloma, prostate cancer, and breast cancer [4–6]. Some studies have revealed that glioma, bladder cancer, and anal cancer with high Ki-67 positivity are more aggressive and invasive [21–23]. A meta-analysis reported that the Ki-67 labeling index has prognostic significance in patients with NSCLC and that a high index suggests a poor prognosis [24]. A correlation between cell metabolic activity as determined by 18F-FDG PET/CT SUV and cell proliferative activity as determined by the Ki-67 expression level has been suggested by some studies on NSCLC [13–15].
In contrast to studies on other malignancies, there have been few studies about the impact of Ki-67 in SCLC and no studies considering the correlation between the volumetric parameters of 18F-FDG PET/CT and the Ki-67 proliferation index in patients with SCLC. This may be due to the difficulty obtaining sufficient and adequate tumor material. SCLC is not a candidate for surgical resection, and only a small specimen is available to investigate biologic markers in most patients. Information from a single biopsy may not reflect the true picture of the entire tumor. Furthermore, SCLC tissue is often crushed with crushed nuclei; thus, an evaluation of nuclear markers may be difficult. About 20 % of our patients (27 patients) were excluded because no Ki-67 immunohistochemical staining data were available because of inadequate specimens. Consequentially, it is difficult to use the Ki-67 labeling index as a prognostic biomarker to comprehensively characterize an individual tumor before SCLC treatment.
18F-FDG PET/CT compensates for this to some extent because it noninvasively and reproducibly identifies tumor biological characteristics in vivo. 18F-FDG PET/CT avoids unnecessary diagnostic biopsies of malignant lesions and permits serial assessments during cancer therapy. Therefore, many investigations have evaluated tumor proliferation noninvasively using 18F-FDG PET/CT [13, 14, 15, 25–30]. In addition to its noninvasiveness, 18F-FDG PET/CT imaging permits an evaluation of the entire tumor, overcoming issues of heterogeneity and tumor sampling errors.
A high Ki-67 expression level has been reported to be associated with tumor size in breast cancer and NSCLC [31, 32], indicating that a high proliferation rate is related to tumor volume. Indeed, in the present study, the MTVsum and TLGtotal, which reflect tumor metabolism and volume on 18F-FDG PET/CT, were correlated with cell proliferative activity as determined by the Ki-67 labeling index, and even though the three biggest outliers were omitted, the MTVsum was correlated with cell proliferative activity. It has been difficult to quantify the total body tumor burden directly and systematically. Until now, tumor stage, which is the most important SCLC prognostic factor, and other prognostic factors, such as performance status, weight loss, and serum lactate dehydrogenase level, may simply be used as surrogates for the underlying tumor burden, which is a direct predictor of disease progression and survival [1]. CT-based tumor volume does not represent the actual tumor size or tumor burden because tumors are not always uniformly shaped and could contain a necrotic portion with nonviable tissues. Functional imaging provides metabolic and volumetric information, such as MTV, which represents the number of highly metabolic tumor cells, making it possible to more accurately reflect tumor burden than other prognostic factors. In SCLC, it has been reported that gross tumor volume determined by 18F-FDG PET/CT, as part of radiation treatment planning, and MTV and iSUV (product of MTV and SUVmean) predict prognosis [9, 18, 33].
In contrast, SUVmax and avgSUVmean, which are commonly used objective 18F-FDG PET/CT parameters, were not correlated with cell proliferative activity as determined by Ki-67 immunostaining. Although it seems that increased glucose metabolism produces sufficient energy for proliferation of tumors with high multiplication potential, FDG uptake only reflects part of cell proliferation. FDG uptake may originate from additional mechanisms, such as expression of glucose transporters, hexokinase, and glucose-6-phosphatase, tumor blood flow, viable tumor cell number, or tumor cell density rather than from cell proliferation alone. It is difficult to determine which particular factor plays a critical role in FDG uptake if the influences of other factors are not controlled. Further study will be needed to find the underlying mechanisms of FDG uptake in SCLC.
The glucose metabolism and biological characteristics of SCLC are complex and difficult to understand completely. Nevertheless, it is reasonable to hypothesize that 18F-FDG PET/CT volumetric parameters, such as MTV and TLG, may be more effective for predicting clinicopathological characteristics and stratifying risk groups with the goal of developing individualized therapeutic strategies in patients with SCLC.
There are several limitations including the retrospective nature of the study design and relatively low number and heterogeneity of the patients. We excluded patients with brain metastasis because torso PET/CT scans were performed. However, PET/CT scans tend to be whole-body studies, allowing for a comprehensive assessment of whole-body tumor burden, except the brain. There were three cases of small-cell lung cancer with a low percentage of Ki-67 expression level (below 30 %) in the present study. Even though some studies showed a high Ki-67 percentage in small-cell lung cancers [34, 35], other studies showed small-cell lung cancer cases with a low percentage of Ki-67-positive cells [36, 37]. Therefore, we considered the findings in the present study reliable. Another limitation is that a fixed 50 % of local maximum intensity was used as the threshold intensity value. The consensus for the cutoff value of the delineating tumor boundary is not yet established, and MTV can be affected by various threshold values. Despite these limitations, this report is noteworthy because it is the first study to show correlations between 18F-FDG PET/CT volumetric parameters and the Ki-67 proliferation index in patients with SCLC.
Conclusion
Whether 18F-FDG PET/CT can be combined with established predictive factors, such as clinicopathological characteristics, to assess SCLC tumor characteristics is an essential and practical issue worthy of investigation. Our results suggest that 18F-FDG PET/CT MTVsum and TLGtotal are well correlated with the Ki-67 proliferation index in patients with SCLC. Further study will be performed to compare the volumetric PET parameters by various thresholds and evaluate their clinical significance in SCLC.
Acknowledgments
This study was funded by a Korea University Special Research grant, Korea University, Seoul, South Korea (no. K1507831).
Compliance with ethical standards
ᅟ
Conflict of Interest
Soyeon Park, Eunsub Lee, Seunghong Rhee, Jaehyuk Cho, Sunju Choi, Sinae Lee, Jae Seon Eo, Kisoo Pahk, Jae Gol Choe, and Sungeun Kim declare that they have no conflict of interest.
Ethical Statement
This study protocol was approved by the International Review Board for Research on Human Subjects at our hospital. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was waived.
The manuscript has not been published before and is not under consideration for publication anywhere else and has been approved by all coauthors.
References
- 1.van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378:1741–1755. doi: 10.1016/S0140-6736(11)60165-7. [DOI] [PubMed] [Google Scholar]
- 2.Korea National Cancer Information Center. Annual report of cancer statistics in Korea in 2012. http://www.cancer.go.kr/. Accessed 15 Jun 2015.
- 3.Johnson BE, Janne PA. Basic treatment considerations using chemotherapy for patients with small cell lung cancer. Hematol Oncol Clin N Am. 2004;18:309–322. doi: 10.1016/j.hoc.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Alexandrakis MG, Passam FH, Kyriakou DS, Dambaki K, Niniraki M, Stathopoulos E. Ki-67 proliferation index: correlation with prognostic parameters and outcome in multiple myeloma. Am J Clin Oncol. 2004;27:8–13. doi: 10.1097/01.coc.0000045810.91816.41. [DOI] [PubMed] [Google Scholar]
- 5.Li R, Heydon K, Hammond ME, Grignon DJ, Roach M, 3rd, Wolkov HB, et al. Ki-67 staining index predicts distant metastasis and survival in locally advanced prostate cancer treated with radiotherapy: an analysis of patients in radiation therapy oncology group protocol 86–10. Clin Cancer Res. 2004;10:4118–4124. doi: 10.1158/1078-0432.CCR-1052-03. [DOI] [PubMed] [Google Scholar]
- 6.Stuart-Harris R, Caldas C, Pinder SE, Pharoah P. Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast. 2008;17:323–334. doi: 10.1016/j.breast.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Martin B, Paesmans M, Mascaux C, Berghmans T, Lothaire P, Meert AP, et al. Ki-67 expression and patient’s survival in lung cancer: systematic review of the literature with meta-analysis. Br J Cancer. 2004;91:2018–2025. doi: 10.1038/sj.bjc.6602233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology. 2004;231:305–332. doi: 10.1148/radiol.2312021185. [DOI] [PubMed] [Google Scholar]
- 9.Zhu D, Ma T, Niu Z, Zheng J, Han A, Zhao S, et al. Prognostic significance of metabolic parameters measured by 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with small cell lung cancer. Lung Cancer. 2011;73:332–337. doi: 10.1016/j.lungcan.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Lee HY, Hyun SH, Lee KS, Kim BT, Kim J, Shim YM, et al. Volume-based parameter of 18F-FDG PET/CT in malignant pleural mesothelioma: prediction of therapeutic response and prognostic implications. Ann Surg Oncol. 2010;17:2787–2794. doi: 10.1245/s10434-010-1107-z. [DOI] [PubMed] [Google Scholar]
- 11.Xie P, Yue JB, Zhao HX, Sun XD, Kong L, Fu Z, et al. Prognostic value of 18F-FDG PET-CT metabolic index for nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2010;136:883–889. doi: 10.1007/s00432-009-0729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li YM, Lin Q, Zhao L, Wang LC, Sun L, Dai MM, et al. Pre-treatment metabolic tumor volume and total lesion glycolysis are useful prognostic factors for esophageal squamous cell cancer patients. Asian Pac J Cancer Prev. 2014;15:1369–1373. doi: 10.7314/APJCP.2014.15.3.1369. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto Y, Nishiyama Y, Ishikawa S, Nakano J, Chang SS, Bandoh S, et al. Correlation of 18F-FLT and 18F-FDG uptake on PET with Ki-67 immunohistochemistry in non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2007;34:1610–1616. doi: 10.1007/s00259-007-0449-7. [DOI] [PubMed] [Google Scholar]
- 14.Vesselle H, Salskov A, Turcotte E, Wiens L, Schmidt R, Jordan CD, et al. Relationship between non-small cell lung cancer FDG uptake at PET, tumor histology, and Ki-67 proliferation index. J Thorac Oncol. 2008;3:971–978. doi: 10.1097/JTO.0b013e31818307a7. [DOI] [PubMed] [Google Scholar]
- 15.Han B, Lin S, Yu LJ, Wang RZ, Wang YY. Correlation of 18F-FDG PET activity with expressions of survivin, Ki67, and CD34 in non-small-cell lung cancer. Nucl Med Commun. 2009;30:831–837. doi: 10.1097/MNM.0b013e32832dcfc4. [DOI] [PubMed] [Google Scholar]
- 16.Huang W, Zhou T, Ma L, Sun H, Gong H, Wang J, et al. Standard uptake value and metabolic tumor volume of 18F-FDG PET/CT predict short-term outcome early in the course of chemoradiotherapy in advanced non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2011;38:1628–1635. doi: 10.1007/s00259-011-1838-5. [DOI] [PubMed] [Google Scholar]
- 17.Ciernik IF, Dizendorf E, Baumert BG, Reiner B, Burger C, Davis JB, et al. Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): a feasibility study. Int J Radiat Oncol Biol Phys. 2003;57:853–863. doi: 10.1016/S0360-3016(03)00346-8. [DOI] [PubMed] [Google Scholar]
- 18.Lee P, Weerasuriya DK, Lavori PW, Quon A, Hara W, Maxim PG, et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int J Radiat Oncol Biol Phys. 2007;69:328–333. doi: 10.1016/j.ijrobp.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 19.Dingemans AM, Witlox MA, Stallaert RA, van der Valk P, Postmus PE, Giaccone G. Expression of DNA topoisomerase IIalpha and topoisomerase IIbeta genes predicts survival and response to chemotherapy in patients with small cell lung cancer. Clin Cancer Res. 1999;5:2048–2058. [PubMed] [Google Scholar]
- 20.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 21.Cummings TJ, Provenzale JM, Hunter SB, Friedman AH, Klintworth GK, Bigner SH, et al. Gliomas of the optic nerve: histological, immunohistochemical (MIB-1 and p53), and MRI analysis. Acta Neuropathol. 2000;99:563–570. doi: 10.1007/s004010051161. [DOI] [PubMed] [Google Scholar]
- 22.Zlotta AR, Schulman CC. Biological markers in superficial bladder tumors and their prognostic significance. Urol Clin N Am. 2000;27:179–189. doi: 10.1016/S0094-0143(05)70246-9. [DOI] [PubMed] [Google Scholar]
- 23.Indinnimeo M, Cicchini C, Stazi A, Limiti MR, Ghini C, Mingazzini P, et al. Immunohistochemical assessment of Ki-67 as prognostic cellular proliferation marker in anal canal carcinoma. J Exp Clin Cancer Res. 2000;19:471–475. [PubMed] [Google Scholar]
- 24.Jakobsen JN, Sorensen JB. Clinical impact of ki-67 labeling index in non-small cell lung cancer. Lung Cancer. 2013;79:1–7. doi: 10.1016/j.lungcan.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Yap CS, Czernin J, Fishbein MC, Cameron RB, Schiepers C, Phelps ME, et al. Evaluation of thoracic tumors with 18F-fluorothymidine and 18F-fluorodeoxyglucose-positron emission tomography. Chest. 2006;129:393–401. doi: 10.1378/chest.129.2.393. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe K, Nomori H, Ohtsuka T, Naruke T, Ebihara A, Orikasa H, et al. [F-18]fluorodeoxyglucose positron emission tomography can predict pathological tumor stage and proliferative activity determined by Ki-67 in clinical stage IA lung adenocarcinomas. Jpn J Clin Oncol. 2006;36:403–409. doi: 10.1093/jjco/hyl043. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen XC, Lee WW, Chung JH, Park SY, Sung SW, Kim YK, et al. FDG uptake, glucose transporter type 1, and Ki-67 expressions in non-small-cell lung cancer: correlations and prognostic values. Eur J Radiol. 2007;62:214–219. doi: 10.1016/j.ejrad.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Yang W, Zhang Y, Fu Z, Yu J, Sun X, Mu D, et al. Imaging of proliferation with 18F-FLT PET/CT versus 18F-FDG PET/CT in non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2010;37:1291–1299. doi: 10.1007/s00259-010-1412-6. [DOI] [PubMed] [Google Scholar]
- 29.Buck AK, Halter G, Schirrmeister H, Kotzerke J, Wurziger I, Glatting G, et al. Imaging proliferation in lung tumors with PET: 18F-FLT versus 18F-FDG. J Nucl Med. 2003;44:1426–1431. [PubMed] [Google Scholar]
- 30.Kaira K, Serizawa M, Koh Y, Takahashi T, Hanaoka H, Oriuchi N, et al. Relationship between 18F-FDG uptake on positron emission tomography and molecular biology in malignant pleural mesothelioma. Eur J Cancer. 2012;48:1244–1254. doi: 10.1016/j.ejca.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Inwald EC, Klinkhammer-Schalke M, Hofstadter F, Zeman F, Koller M, Gerstenhauer M, et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139:539–552. doi: 10.1007/s10549-013-2560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabata K, Tanaka T, Hayashi T, Hori T, Nunomura S, Yonezawa S, et al. Ki-67 is a strong prognostic marker of non-small cell lung cancer when tissue heterogeneity is considered. BMC Clin Pathol. 2014;14:23. doi: 10.1186/1472-6890-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reymen B, Van Loon J, van Baardwijk A, Wanders R, Borger J, Dingemans AM, et al. Total gross tumor volume is an independent prognostic factor in patients treated with selective nodal irradiation for stage I to III small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;85:1319–1324. doi: 10.1016/j.ijrobp.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Pelosi G, Rodriguez J, Viale G, Rosai J. Typical and atypical pulmonary carcinoid tumor overdiagnosed as small-cell carcinoma on biopsy specispecimens: a major pitfall in the management of lung cancer patients. Am J Surg Pathol. 2005;29:179–187. doi: 10.1097/01.pas.0000149690.75462.29. [DOI] [PubMed] [Google Scholar]
- 35.Righi L, Volante M, Tavaglione V, Bille A, Daniele L, Angusti T, et al. Somatostatin receptor tissue distribution in lung neuroendocrine tumours: a clinicopathologic and immunohistochemical study of 218 ‘clinically aggressive’ cases. Ann Oncol. 2010;21:548–555. doi: 10.1093/annonc/mdp334. [DOI] [PubMed] [Google Scholar]
- 36.Bohm J, Koch S, Gais P, Jutting U, Prauer H, Hofler H. Prognostic value of MIB-1 in neuroendocrine tumours of the lung. J Pathol. 1996;178:402–409. doi: 10.1002/(SICI)1096-9896(199604)178:4<402::AID-PATH498>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Arbiser Z, Arbiser J, Cohen C, Gal A. Neuroendocrine lung tumors: grade correlates with proliferation but not angiogenesis. Mod Pathol. 2001;14:1195–1199. doi: 10.1038/modpathol.3880459. [DOI] [PubMed] [Google Scholar]