Abstract
Thermococcus may be an important alternative source of H2 in the hot subseafloor in otherwise low H2 environments such as some hydrothermal vents and oil reservoirs. It may also be useful in industry for rapid agricultural waste treatment and concomitant H2 production. Thermococcus paralvinellae grown at 82°C without sulfur produced up to 5 mmol of H2 L−1 at rates of 5–36 fmol H2 cell−1 h−1 on 0.5% (wt vol−1) maltose, 0.5% (wt vol−1) tryptone, and 0.5% maltose + 0.05% tryptone media. Two potentially inhibiting conditions, the presence of 10 mM acetate and low pH (pH 5) in maltose-only medium, did not significantly affect growth or H2 production. Growth rates, H2 production rates, and cell yields based on H2 production were the same as those for Pyrococcus furiosus grown at 95°C on the same media for comparison. Acetate, butyrate, succinate, isovalerate, and formate were also detected as end products. After 100 h, T. paralvinellae produced up to 5 mmol of H2 L−1 of medium when grown on up to 70% (vol vol−1) waste milk from cows undergoing treatment for mastitis with the bacterial antibiotic Ceftiofur and from untreated cows. The amount of H2 produced by T. paralvinellae increased with increasing waste concentrations, but decreased in P. furiosus cultures supplemented with waste milk above 1% concentration. All mesophilic bacteria from the waste milk that grew on Luria Bertani, Sheep's Blood (selective for Staphylococcus, the typical cause of mastitis), and MacConkey (selective for Gram-negative enteric bacteria) agar plates were killed by heat during incubation at 82°C. Ceftiofur, which is heat labile, was below the detection limit following incubation at 82°C. T. paralvinellae also produced up to 6 mmol of H2 L−1 of medium when grown on 0.1–10% (wt vol−1) spent brewery grain while P. furiosus produced < 1 mmol of H2 L−1. Twelve of 13 enzyme activities in T. paralvinellae showed significant (p < 0.05) differences across six different growth conditions; however, methyl viologen-dependent membrane hydrogenase activity remained constant across all media types. The results demonstrate the potential of at least some Thermococcus species to produce H2 if protein and α-glucosides are present as substrates.
Keywords: hyperthermophile, Thermococcus, hydrogenase, waste remediation, bioenergy
Introduction
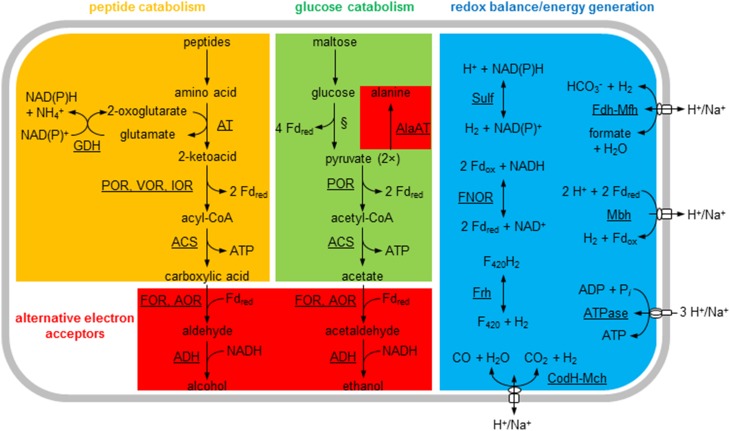
Thermococcus species are hyperthermophilic heterotrophic archaea that catabolize carbohydrates and peptides and produce organic acids and CO2 as metabolites (Figure 1; Adams, 1999). Their growth is often associated with sulfur reduction to H2S, but in the absence of sulfur some species produce H2 as a major end product (Bálint et al., 2005; Kanai et al., 2005). Based on genome sequence analysis, all Thermococcus species have at least one NAD(P)H-dependent cytoplasmic hydrogenase and a ferredoxin (Fd)-dependent membrane hydrogenase that translocates H+ or Na+ across the membrane. Thermococcus onnurineus also produces H2 using a formate-dependent membrane hydrogenase (Kim et al., 2010; Bae et al., 2012) and a CO-dependent membrane hydrogenase (Kim et al., 2013). Thermococcus paralvinellae produced H2 when grown on both maltose and tryptone (0.5% wt vol−1 of each) without sulfur (Hensley et al., 2014). Its complete genome sequence contains seven hydrogenase gene clusters (Jung et al., 2014). Four of these clusters are putatively membrane bound and use ferredoxin, formate and CO as electron donors coupled with H+/Na+ translocation, and three are cytoplasmic and use NAD(P)H or coenzyme F420 as electron pairs (Figure 1). However, nothing is known about H2 production kinetics and enzyme activities in T. paralvinellae when grown on varying substrates or environmental conditions without sulfur.
Figure 1.
General metabolic pathways for Thermococcus paralvinellae. The enzymes are membrane-bound hydrogenases: Fd-dependent (Mbh), formate-dependent (Mfh), and CO-dependent (Mch); cytoplasmic hydrogenases: NADH-dependent (Sulf) and F420-dependent (Frh); NADH:Fd oxidoreductase (FNOR); membrane-bound ATP synthase (ATPase); glutamate dehydrogenase (GDH); alanine aminotransferase (AlaAT); general aminotransferases (AT); pyruvate:Fd oxidoreductase (POR); isovalerate:Fd oxidoreductase (VOR); indolepyruvate:Fd oxidoreductase (IOR); ADP-forming acetyl-CoA/acyl-CoA synthetase (ACS); aldehyde:Fd oxidoreductase (AOR); formaldehyde:Fd oxidoreductase (FOR); and alcohol dehydrogenase (ADH). Fd, electron carrier ferredoxin; F420, electron carrier coenzyme F420; §, modified Embden-Meyerhof pathway. The activities for most of the enzymes listed are shown in Table 4.
Most of what is known about the central metabolism of Thermococcus has been obtained from studying Pyrococcus furiosus (see Adams, 1999; Verhees et al., 2003 for reviews), which like Thermococcus is a member of the Thermococcaceae. Glycolysis in both genera occurs via a modified Embden-Meyerhof pathway that uses ADP-dependent kinases (Kengen et al., 1994) and glyceraldehyde-3-phosphate:Fd oxidoreductase in lieu of glyceraldehyde-3-phosphate dehydrogenase and phosphoglycerate kinase (Mukund and Adams, 1995). Peptide catabolism occurs via peptide transamination and glutamate dehydrogenase to form 2-keto acids and reduction potential in the form of NAD(P)H (Figure 1; Robb et al., 1992). Pyruvate and other 2-keto acids are oxidized to acetate and other carboxylic acids plus CO2, reduced Fd, and ATP (Blamey and Adams, 1993; Schäfer et al., 1993; Heider et al., 1996; Mai and Adams, 1996). Alternatively, pyruvate can be reduced to alanine (Kengen and Stams, 1994), and the acyl-CoA products can be potentially reduced to alcohols (Basen et al., 2014) if alternative terminal electron acceptors are not available. T. paralvinellae produced ethanol and butanol when sulfur was in limited supply suggesting that it can also ferment when necessary (Ma et al., 1995).
Hydrogen-producing Thermococcus support the growth of hydrogenotrophic methanogens in the absence of other H2 sources through syntrophy (Bonch-Osmolovskaya and Stetter, 1991; Canganella and Jones, 1994; Davidova et al., 2012; Ver Eecke et al., 2012) and could be an important alternative source of energy in low-H2 environments. Thermococcus could also be used to consolidate wastewater organic compound remediation and heat treatment for pathogen removal in a single step to decrease costs while producing H2 as an energy product (Angenent et al., 2004; Bougrier et al., 2007; Girguis and Holden, 2012). In the U.S., nearly all food and agricultural waste enters landfills, which is the largest contributor of material entering these sites (Staley and Barlaz, 2009; Levis et al., 2010). It is increasingly being diverted to wastewater treatment facilities for anaerobic digestion to limit landfill growth and eutrophication (Diggelman and Ham, 2003; Lai et al., 2009). The waste contains high concentrations of organic compounds and often harmful chemicals such as antibiotics and hormones (Bougrier et al., 2007; Wang and Wan, 2009; Ntaikou et al., 2010) that foul wastewater treatment equipment leading to increasing restrictions on entering waste content (Lee et al., 2007). Biological pre-treatment of large organic molecules by fermentative organisms lowers the high organic carbon load in waste, lowers wastewater treatment costs, and can produce bioenergy to partially offset costs (Angenent et al., 2004; Wang and Wan, 2009; Ntaikou et al., 2010).
While the catabolic and energy generation pathways of T. paralvinellae are reasonably well established, its physiology, growth, and H2 production kinetics under normal and potentially adverse growth conditions are poorly understood. In this study, the growth and H2 production rates, the production of other metabolites, and the activities of 13 enzymes were measured from T. paralvinellae grown at 82°C on maltose, tryptone, and maltose plus tryptone as well as maltose plus acetate and maltose at low pH (pH 5) as potentially inhibitory conditions. P. furiosus was grown and analyzed in parallel at 95°C for comparison. These two organisms were grown for the first time on waste milk from cows treated for mastitis with Ceftiofur or from untreated cows, and separately on spent brewery grain to examine the feasibility of using them to rapidly remediate agricultural wastes. Ceftiofur is a heat-labile β-lactam that inhibits peptidoglycan synthesis in bacteria (Sunkara et al., 1999). We sought to remove organic compounds during growth and the pathogens and Ceftiofur from the milk by heat treatment during growth of T. paralvinellae with concomitant production of H2.
Materials and methods
Organisms used
T. paralvinellae ES1 (DSM 27261) was from our hyperthermophile culture collection. P. furiosus (DSM 3638) was obtained from the Deutsche Sammlung für Mikroorganismen und Zelkulturen (Braunschweig, Germany).
Growth conditions
The base medium for growth was from Adams et al. (2001). Different carbon sources were added to the base medium solution, which were 0.5% (wt vol−1) maltose plus 0.01% (wt vol−1) yeast extract (enzymatic, Difco); 0.5% (wt vol−1) tryptone (Difco) plus 0.01% yeast extract; and 0.5% maltose, 0.05% tryptone plus 0.01% yeast extract. To examine the effect of acetate on cells, 10 mM acetate was added to 0.5% maltose plus 0.01% yeast extract medium. To examine growth at low pH, 0.5% maltose plus 0.01% yeast extract medium was pH balanced to 5.00 ± 0.05 at room temperature. All other media were pH balanced to 6.80 ± 0.05 at room temperature. For bottle experiments, these media (20 ml) were contained in 60 ml serum bottles with 1 atm of N2 in the headspace. Growth was also tested on 0.5% (wt vol−1) lactose plus 0.01% yeast extract in base medium as a follow-up to the waste milk growth experiments described below.
For the waste media experiments, one of three waste types was added as a carbon source to the base medium above. The milk medium contained 0.1, 1, or 10% (vol vol−1) waste milk obtained fresh from a local dairy farm (Barstow Farms, Hadley, MA). For T. paralvinellae, 20, 30, 50, and 70% waste milk were also examined. The milk was from cows with mastitis that had been treated with Ceftiofur within the previous 48 h and from healthy untreated cows. The grain medium contained 0.1, 1, or 10% (wt vol−1) spent brewery grain from a local brewery (Amherst Brewing Company, Amherst, MA). The initial chemical oxygen demand (COD) of 10% waste was 357 ± 6 g l−1 (mean ± standard error) in milk from untreated cows, 363 ± 3 g l−1 in milk from treated cows, and 334 ± 3 g l−1 in spent grain waste as determined from a COD HR test kit (Hach Company). Milk and grain that was not used within 12 h was frozen at −20°C. The waste media (50 ml) were contained in 120 ml serum bottles with 1 atm of N2 in the headspace.
All bottle cultures were incubated in a forced-air oven without stirring at 82°C for T. paralvinellae and 95°C for P. furiosus. The serum bottles were inoculated with a logarithmic growth-phase culture that had been grown and transferred at least three successive times in bottles on the medium used so that cultures were adapted to that medium. For the maltose-only, tryptone-only, maltose-tryptone, maltose-acetate, and low pH maltose media experiments, 12 serum bottles were inoculated concurrently and a pair of bottles was removed at different times during growth until the cultures reached stationary growth phase. For the waste media experiments, triplicate bottles were inoculated and subsampled every 12 h for up to 100 h. Cell concentrations were measured using a Petroff-Hausser counting chamber and phase-contrast light microscopy. The specific growth rate (μ) of the culture was determined by a best-fit curve through the logarithmic portion of the growth data.
Cultures were also grown in duplicate in a 20-L bioreactor for each defined media type and for 1% milk from Ceftiofur-treated cows. The media were flushed with N2:CO2 (80:20%) at a flow rate of 60 ml min−1, stirred at 120–150 rpm, and heated to 82 ± 0.1°C for T. paralvinellae and 95 ± 0.1°C for P. furiosus. CO2 was added to the flushing gas to help offset the CO2 lost from the NaHCO3 in the base medium. With the exception of the low pH condition (pH 5.0 ± 0.1), the pH of the bioreactor media was set at 6.8 at room temperature but rose with temperature to pH 7.2 where it was maintained (±0.1 pH unit) by the automatic addition of 5% (wt vol−1) NaHCO3 at the incubation temperature. Cells from the bioreactor were harvested when they reached late logarithmic growth phase. The medium was drained from the bioreactor through a glass cooling coil bathed in an ice-water-slurry into a carboy. The cells were then concentrated to less than 2 L by ultrafiltration using a hollow fiber cartridge (0.2-μm pore size; Amersham Biosciences) and further concentrated by centrifugation at 10,000 × g for 45 min. The exception was cells grown on the milk due to flocculation in the medium where only centrifugation was used to concentrate the cells. The resulting pellets were resuspended in an anoxic chamber in < 5 ml of degassed 50 mM Tris buffer (pH 8.0) plus 2 mM of sodium dithionite (DT), sealed in a N2-flushed serum bottle, and frozen at −20°C.
Metabolite measurements
Metabolite measurements were only performed on bottle experiments. A subsample (100 μl) of headspace from each bottle at each time point was used to measure the amount of H2 present using a gas chromatograph (Shimadzu GC-8A) equipped with a thermal conductivity detector, a 60/80 Carboxen 1000 column (Supelco), and Ar as the carrier gas. Hydrogen yields per cell (Yp∕x) were determined by plotting the amount of H2 produced against cell concentrations for each set of time points within a growth curve. Specific H2 production rates (q) were calculated from (Yp∕x × μ)/0.693, and theoretical maximum H2 production rates (rmax) were calculated from the product of q times the maximum cell concentration (cellmax). For defined media experiments, soluble metabolites were measured after 18 h of incubation. A 1.5 ml aliquot was transferred into an Eppendorf microfuge tube and spun at 14,000 rpm for 5 min. The supernatant was decanted and filtered through a 0.22 μm pore size filter (13 mm diameter, GVPP, Millipore) and 100 μl of 1 M H2SO4 was added to 1 ml of filtrate. Samples were run through an Aminex HPX-87H ion exclusion column (300 mm × 7.8 mm, Bio-Rad) with guard column (BioRad microguard Cation H) with 5 mM H2SO4 as the mobile phase using an ultra-pressure liquid chromatography (UPLC) system (Shimadzu) equipped with a refractive index detector. The column was kept at 30°C with a 0.6 ml min−1 flow rate and a 30 min sampling time.
Waste remediation
To determine which portion of the milk waste was degraded during growth, 1% waste milk from Ceftiofur treated cows and untreated cows were inoculated separately with each organism and incubated as before along with uninoculated triplicate controls. A 1.5 ml liquid subsample was removed from each serum bottle every 12 h for soluble protein and reducing sugar measurements. For protein measurements, 1 ml of sample was filtered through a 0.22 μm pore size filter (13 mm diameter, GVPP, Millipore) and the protein in the filtrate was measured using a protein assay kit (Bio-Rad). For the reducing sugar measurements, 0.5 ml of sample and 0.5 ml of dinitrosalicylic acid solution (DNS) were heated to 100°C for 5 min, cooled in ice for 5 min, and then absorbance measured at 545 nm with lactose used as standard. The DNS was composed of the following (per liter): 7.06 g of 3,5-dinitrosalicylic acid, 13.2 g of sodium hydroxide, 204 g of Rochelle salt, 5.53 g of sodium metabisulfite, and 5.06 ml phenol (boiled). Uninoculated controls determined if indigenous microbes were affecting waste content or H2 production.
To determine if pathogens were removed from the milk, 1% waste milk from Ceftiofur-treated cows and untreated cows that was less than 48 h old, as well as base medium without an added carbon source, were plated via a dilution-to-extinction method onto Luria Bertani, Sheep's Blood, and MacConkey agar plates both prior to and after 100 h of incubation with T. paralvinellae at 82°C and were incubated up to 2 days at 37°C. Sheep's Blood agar is selective for Gram-positive bacteria, the most common causative agent of bovine mastitis, and MacConkey agar is selective for Gram-negative enteric bacteria, which are another source of mastitis. Technical triplicates of each media, time point, and agar plate type were run. The number of colony forming units per ml of inoculum was determined for all plates. To determine if Ceftiofur was present in waste milk, samples of undiluted waste milk from treated cows and samples of 10% milk media before and after 100 h of incubation at 82°C with and without inoculation with T. paralvinellae were tested using a Ceftiofur ELISA Test Kit (Bioo Scientific) as described by the manufacturer.
Protein fractionation
All sample transfers and manipulations were carried out in an anoxic chamber and buffers were degassed with N2. Stored frozen cells were allowed to warm to room temperature and 2 μg ml−1 of DNase I were added. The cell suspension was mixed for 30 min. The sample vial was then placed in an ice-water slurry and sonicated for 30 min (Fisher Scientific, Sonic Dismembrator 500). Phase-contrast microscopy confirmed cell lysis. The cell suspension was spun in a centrifuge at 100,000 × g for 45 min. The supernatant was removed as the soluble protein fraction and the pellet was resuspended in buffer after being homogenized with a glass tissue grinder. The suspension was spun at 100,000 × g for 45 min as before and resuspended three times to wash the pellet. After the final spin, the pellet was resuspended in 1 ml of buffer. This was used as the insoluble protein fraction. The protein concentrations of the soluble protein fractions were determined spectrophotometrically using a protein assay kit (Bio-Rad). The protein concentrations of the insoluble protein fractions were determined using the DC Protein Assay kit (Bio-Rad). Bovine serum albumin was used as the standard for both procedures. Protein fractions that were not used immediately for enzyme activity assays were flash frozen in liquid N2 and stored at −80°C.
Enzyme assays
Enzyme activities are expressed as units where 1 U is equal to 1 μmol of substrate transformed min−1. The buffer used for all assays was 100 mM EPPS buffer (pH 8.4) and the assay temperature was 80°C, unless otherwise noted. At least three technical replicates were run for each assay. For enzyme assays containing benzyl viologen (BV) or methyl viologen (MV), a trace amount of 2 mM sodium dithionite (DT) was added to slightly reduce the buffer. The amount of all reagents used is given as the final concentration in the reaction vial.
The following anaerobic enzyme activity assays were performed using the insoluble protein fraction and were contained in rubber stopper-sealed serum vials (8 ml volume) that had been degassed and flushed with N2. Membrane-bound hydrogenase (Mbh) (Sapra et al., 2000) activity was determined by following the H2 evolution rate in a discontinuous assay using 3 mM MV reduced with 30 mM DT as the electron donor. H2 was measured by gas chromatography as described above. Membrane-bound ATP synthase (ATPase) (Pisa et al., 2007) activity was determined by following the phosphate evolution rate in a discontinuous assay by adding 2.5 mM sodium ATP after 4 min of initial incubation to 100 mM MES-100 mM Tris buffer (pH 6.0) containing 5 mM MgCl2 and 200 mM KCl. The reaction was stopped after 2, 4, and 6 min by placing the serum vial on ice. The concentration of the inorganic phosphate produced was measured spectrophotometrically as described previously (Heinonen and Lahti, 1981).
The following anaerobic enzyme activity assays were measured spectrophotometrically using the soluble protein fraction and were contained in rubber stopper-sealed glass cuvettes that had been degassed and flushed with N2. Cytoplasmic hydrogenase (Sulf) (Bryant and Adams, 1989; Ma et al., 2000) activity was determined by measuring the reduction of 1 mM BV at 600 nm [ε = 7400 (M•cm)−1] under an H2:CO2 (80:20%) headspace. Pyruvate:Fd oxidoreductase (POR) (Blamey and Adams, 1993) and 2-ketoisovalerate:Fd oxidoreductase (VOR) (Heider et al., 1996) activities were determined by measuring the reduction of 1 mM MV at 600 nm [ε = 12,000 (M•cm)−1] and 1 mM BV, respectively, in an assay mixture that contained 2 mM MgCl2, 0.4 mM thiamine pyrophosphate (TPP), and 0.2 mM coenzyme A (CoA). Pyruvate (10 mM) and 5 mM 2-ketoisovalerate were used as the substrates, respectively. Aldehyde:Fd oxidoreductase (AOR) (Mukund and Adams, 1991) and formaldehyde:Fd oxidoreductase (FOR) (Roy et al., 1999) activities were determined by measuring the reduction of 3 mM BV using 0.5 mM crotonaldehyde and 0.25 mM formaldehyde, respectively, as the substrate. Fd:NAD(P)H oxidoreductase (FNOR) (Ma and Adams, 1994) activity was determined by measuring the reduction of 3 mM BV in 50 mM CAPS buffer (pH 10.3) using 0.4 mM NADPH as the substrate. Alcohol dehydrogenase (ADH) (Ma and Adams, 1999) was determined by measuring the reduction of 0.4 mM NADP+ at 340 nm [ε = 6220 (M•cm)−1] using 150 mM ethanol as the substrate. Formate dehydrogenase (FDH) (Ma et al., 1995) activity was determined by measuring the reduction of 5 mM BV at 600 nm in 100 mM EPPS (pH 8.4) using 10 mM sodium formate as the substrate. Alanine aminotransferase (AlaAT) (Ward et al., 2000) activity was determined by measuring the pyruvate evolution rate in a discontinuous assay by adding sample after 4 min of initial incubation to 100 mM KCl, 20 mM 2-ketoglutarate, 50 μM pyrodixal-5′-phosphate, and 50 mM L-alanine. The reaction was stopped after 2, 4, and 6 min by placing the serum vial on ice. The amount of pyruvate in each vial was determined aerobically at room temperature using a lactate dehydrogenase assay that contained 100 mM potassium phosphate (pH 7.0), 0.4 mM NADPH, and 50 U of LDH from rabbit muscle (Sigma-Aldrich).
The following aerobic enzyme activity assays were performed using the soluble protein fraction and were measured spectrophotometrically in glass cuvettes. Glutamate dehydrogenase (GDH) (Robb et al., 1992) activity was determined by measuring the reduction of 0.4 mM NADP+ at 340 nm [ε = 6220 (M•cm)−1] using 6 mM sodium glutamate as the substrate. ADP-forming acetyl-CoA synthetase (ACS) (Bräsen and Schönheit, 2004) activity was determined by measuring the production of DTNB-CoA by adding sample to 100 mM MOPS buffer (pH 7.0), 0.25 mM DTNB, 5 mM MgCl2, 1 mM ADP, 5 mM K2HPO4, and 0.2 mM acetyl-CoA.
Statistical analyses
Results were subjected to statistical analyses in R (R Core Team, 2013). The growth rates, metabolite production rates, enzyme activities, and colony-forming-unit yields were compared using analysis of variance (ANOVA) and Tukey's Honestly Significant Difference test (α = 0.05). The mean of at least three replicates are reported as means ± standard error (SE).
Results
Growth and metabolite production
Both T. paralvinellae and P. furiosus grew and produced H2 in the various maltose- and tryptone-containing media over 12–24 h (Figure 2), the milk waste media over 100 h (Figure 3) and the spent brewery grain media over 100 h (Figure 4). Neither organism grew on the 0.5% lactose plus 0.01% yeast extract medium. For both organisms, the maltose-tryptone medium was among those that produced the largest cellmax and rmax (Table 1). For T. paralvinellae, this condition was among those that also showed the largest q (specific H2 production rate) (F4 = 330.6, p < 0.001). While H2 was the primary metabolite produced, both organisms also produced acetate, butyrate, succinate, isovalerate, and formate (Table 2). Relative to H2 production, acetate production was highest in tryptone-only and maltose-acetate media for T. paralvinellae and in maltose-only medium for P. furiosus. Assuming four acetate molecules produced per molecule of maltose consumed, less than 0.5 mM maltose was used by either organism. Succinate, isovalerate and butyrate were also produced in various media (Table 2). T. paralvinellae produced formate when grown on maltose-only medium, while P. furiosus produced it in maltose-only and maltose-acetate media (Table 2). Ethanol was not detected in any medium tested for either organism.
Figure 2.
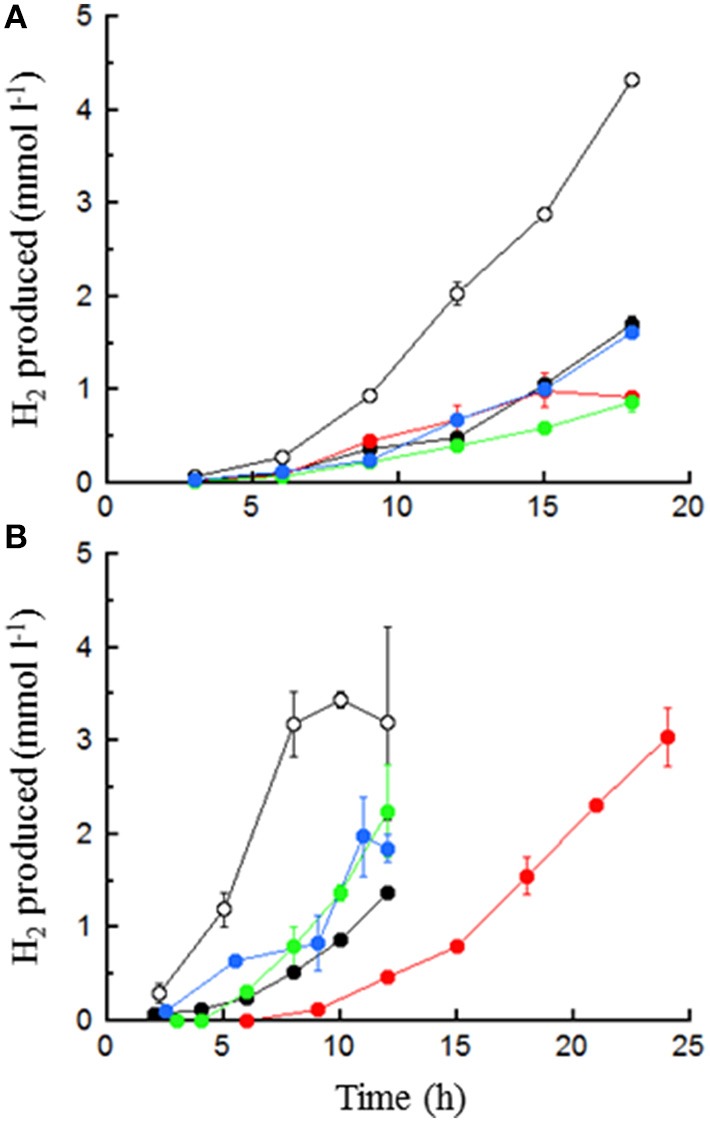
H2 production by T. paralvinellae (A) and P. furiosus (B) in 0.5% maltose (○), 0.5% tryptone ( ), 0.5% maltose + 0.05% tryptone (●), 0.5% maltose + 10 mM acetate (
), 0.5% maltose + 0.05% tryptone (●), 0.5% maltose + 10 mM acetate ( ), and 0.5% maltose at pH 5.0 (
), and 0.5% maltose at pH 5.0 ( ). Error bars represent the standard error.
). Error bars represent the standard error.
Figure 3.
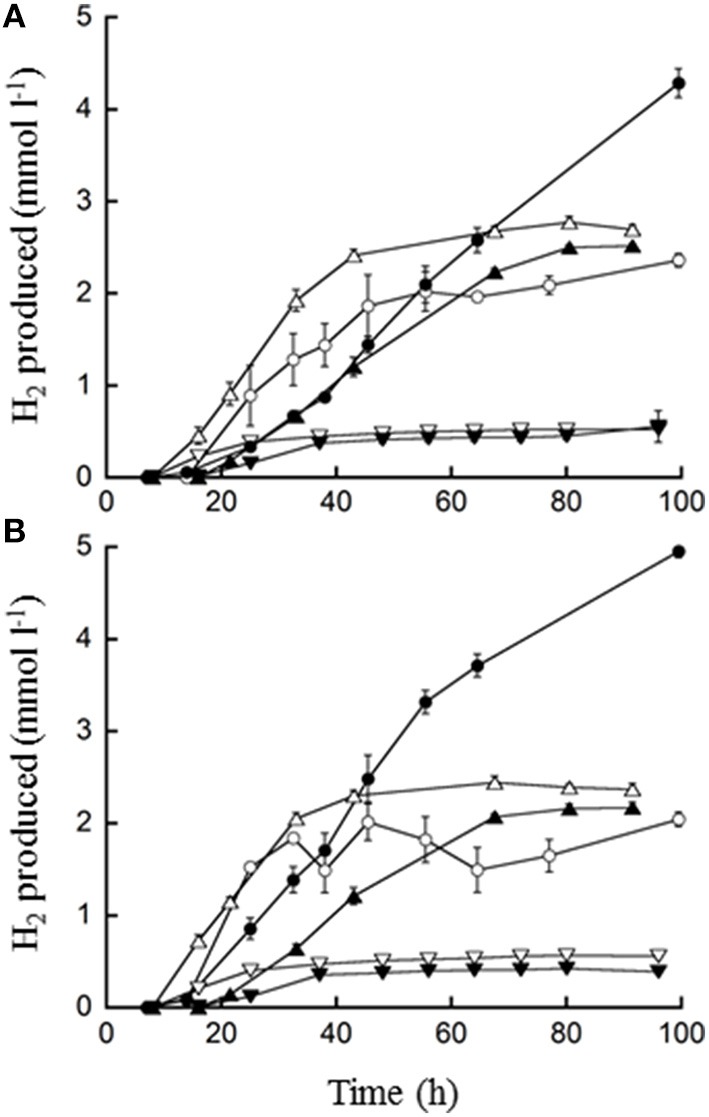
H2 production by T. paralvinellae (filled symbols) and P. furiosus (open symbols) when grown on waste milk from cows treated with Ceftiofur (A) and from untreated cows (B). The concentrations of waste milk used were 10 (○, ●), 1 (Δ, ▲), and 0.1% (▽, ▼). Error bars represent the standard error.
Figure 4.
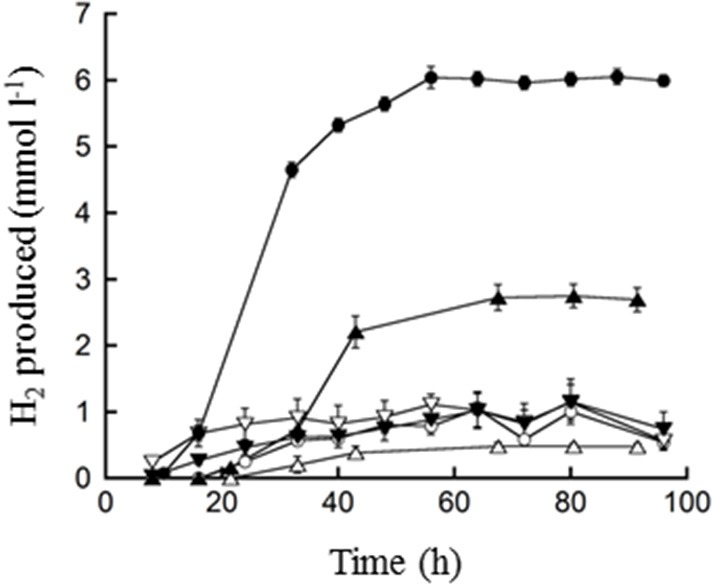
H2 production by T. paralvinellae (filled symbols) and P. furiosus (open symbols) when grown on spent brewery grain. The concentrations spent grain used were 10 (○, ●), 1 (Δ, ▲), and 0.1% (▽, ▼). Error bars represent the standard error.
Table 1.
T. paralvinellae and P. furiosus kinetic parameters.
| Kinetic parameter† | Media type | p-value | ||||
|---|---|---|---|---|---|---|
| 0.5% maltose | 0.5% tryptone | 0.5% maltose + 0.05% tryptone | 0.5% maltose + 10 mM acetate | 0.5% maltose (pH 5) | ||
| T. paralvinellae | ||||||
| μ (h−1) | 0.45 ± 0.11 | 0.35 ± 0.21 | 0.34 ± 0.13 | 0.32 ± 0.08 | 0.35 ± 0.11 | NS |
| cellmax (× 107, ml−1) | 13a | 6b | 13a | 8b | 5b | * |
| Yp∕x (fmol H2 cell−1) | 13 ± 3a | 16 ± 5a | 32 ± 7b | 10 ± 1a | 32 ± 8b | *** |
| q (fmol H2 cell−1 h−1) | 8 ± 3a | 8 ± 3a | 16 ± 5b | 5 ± 1a | 16 ± 4b | *** |
| rmax (mmol H2 l−1 h−1) | 1.1 ± 0.5a,b | 0.5 ± 0.3a | 2.0 ± 0.8b | 0.4 ± 0.1a | 0.8 ± 0.4a | ** |
| P. furiosus | ||||||
| μ (h−1) | 0.43 ± 0.08 | 0.31 ± 0.22 | 0.49 ± 0.25 | 0.54 ± 0.13 | 0.39 ± 0.10 | NS |
| cellmax (× 107, ml−1) | 10a,b | 6b | 19a | 4b | 8b | ** |
| Yp∕x (fmol H2 cell−1) | 11 ± 2a | 42 ± 11a,b | 17 ± 3a,b | 46 ± 29b | 17 ± 8a,b | ** |
| q (fmol H2 cell−1 h−1) | 7 ± 1a | 19 ± 6a,b | 12 ± 4a,b | 36 ± 24b | 10 ± 5a,b | ** |
| rmax (mmol H2 l−1 h−1) | 0.7 ± 0.2a | 1.1 ± 0.3a | 2.3 ± 0.8b | 1.4 ± 0.8a,b | 0.7 ± 0.3a | * |
The kinetic parameters are based on the best-fit line through the data (±95% confidence interval). μ, specific growth rate; cellmax, maximum cell concentration; Yp∕x, product yield coefficient per cell; q, specific H2 production rate; rmax, maximum H2 production rate. p-values calculated from one-way ANOVA test comparing results across treatments.
p < 0.05,
p < 0.01, and
p < 0.001.
Tukey post-hoc test indicates groups whose members are not significantly different. NS, no significance.
Table 2.
Mean product formation (mM, ± standard error) by T. paralvinellae and P. furiosus after 18 h of incubation in the defined media relative to uninoculated controls.
| Product | 0.5% maltose | 0.5% tryptone | 0.5% maltose + 0.05% tryptone | 0.5% maltose + 10 mM acetate | 0.5% maltose (pH 5) |
|---|---|---|---|---|---|
| T. paralvinellae | |||||
| H2 | 0.47 ± 0.04 | 0.76 ± 0.02 | 1.18 ± 0.00 | 0.29 ± 0.01 | 0.05 ± 0.01 |
| Acetate | 0.20 ± 0.01 | 1.14 ± 0.09 | 0.30 ± 0.03 | 0.69 ± 0.00 | 0.01 ± 0.00 |
| Butyrate | 0 | 0 | 0 | 0.06 ± 0.00 | 0 |
| Succinate | 0 | 0.24 ± 0.01 | 0 | 0 | 0 |
| Isovalerate | 0 | 0.31 ± 0.00 | 0.02 ± 0.01 | 0 | 0 |
| Formate | 0 | 0.54 ± 0.06 | 0 | 0 | 0 |
| P. furiosus | |||||
| H2 | 2.00 ± 0.00 | 1.71 ± 0.08 | 4.92 ± 0.47 | 5.51 ± 1.18 | 3.71 ± 0.16 |
| Acetate | 0.95 ± 0.05 | 0.06 ± 0.05 | 1.14 ± 0.27 | 1.66 ± 0.28 | 0.79 ± 0.06 |
| Butyrate | 0 | 0 | 0.01 ± 0.00 | 0 | 0 |
| Succinate | 0.14 ± 0.01 | 0.10 ± 0.00 | 0 | 0.22 ± 0.16 | 0 |
| Isovalerate | 0 | 0.46 ± 0.00 | 0.28 ± 0.28 | 0 | 0 |
| Formate | 2.14 ± 0.08 | 0 | 0.01 ± 0.01 | 1.51 ± 0.25 | 0 |
Growth and H2 production in waste media
T. paralvinellae produced increasingly higher amounts of H2 with increasing concentration of waste milk for both milk types (Figure 3). The increase in H2 production continued with increasing milk concentration up to the highest concentration tested (70%; data not shown). For P. furiosus, the amount of H2 produced from 1% waste milk was higher than the amount for both 0.1 and 10% waste milk (Figure 3). T. paralvinellae produced more H2 than P. furiosus in all waste conditions tested except 0.1% milk from Ceftiofur-treated cows and 1% milk from untreated cows. Growth and H2 production did not significantly change for either microorganism between waste milk from cows treated with Ceftiofur and from untreated cows. There was visual clarification by eye of waste milk media following 100 h of incubation at 82°C with T. paralvinellae, but not in the uninoculated control bottle incubated at the same temperature (Figure 5A). When 1% waste milk was incubated with T. paralvinellae, the concentration of soluble protein decreased to undetectable concentrations within 48 h and was significantly lower (p < 0.01) than the soluble protein concentrations in uninoculated bottles (Figure 5B). The amount of reducing sugars in the milk (Figure 5C) and the total COD decreased over time, but the concentrations were not significantly different from those in uninoculated bottles. Cell counts were highly variable when grown on milk due to precipitation and clumping of the milk at high temperatures.
Figure 5.
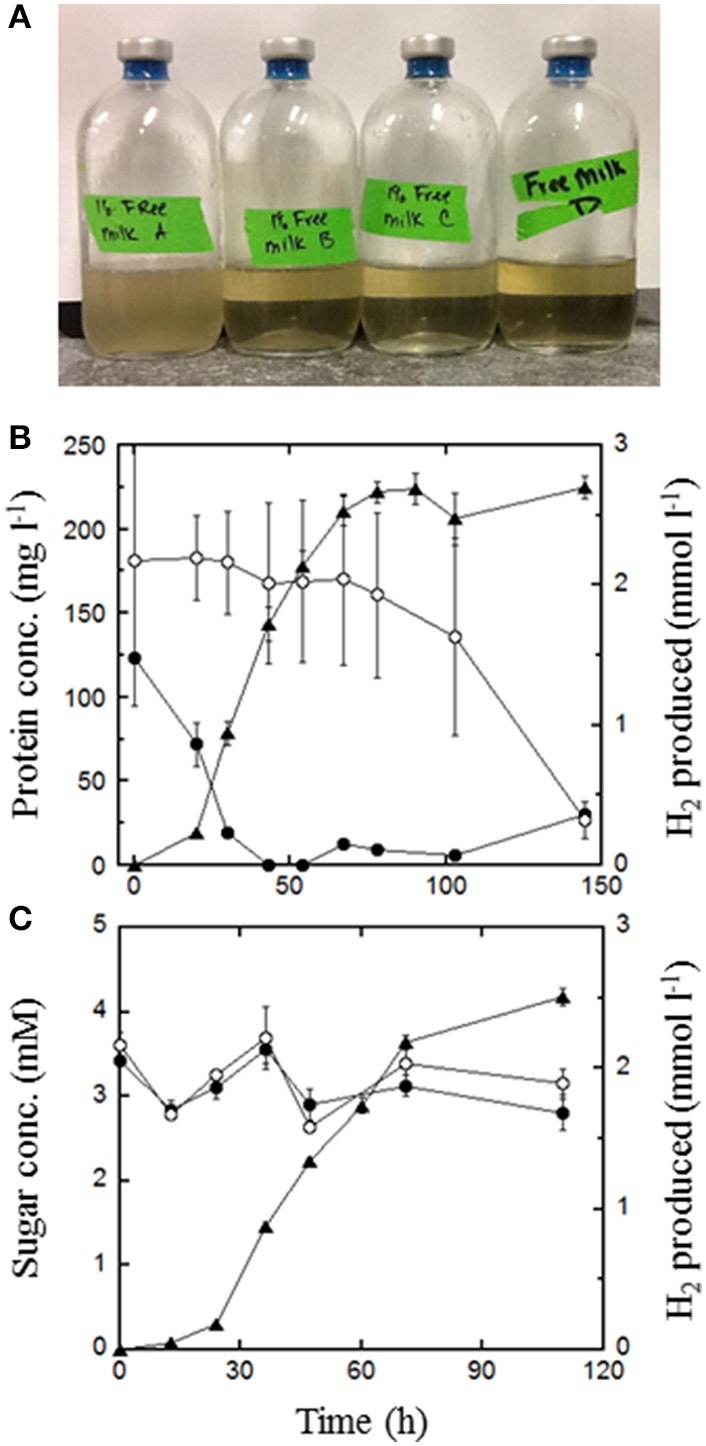
Degradation of waste milk by T. paralvinellae during 100 h of incubation at 82°C. (A) Clarification of media containing 1% waste milk from untreated cows following 100 h incubation. The bottle on the left was an uninoculated control. Concentrations of protein (B) and reducing sugars (C) in 1% waste milk from untreated cows. The data shown are from inoculated bottles (●) and from uninoculated controls (○) as well as H2 produced from inoculated bottles (▲). There was no H2 production in uninoculated bottles. Error bars represent the standard error.
T. paralvinellae produced increasing amounts of H2 (up to 6 mmol l−1) with increasing amounts of spent brewery grain as the feedstock (Figure 4). In contrast, P. furiosus produced less than 1 mmol of H2 l−1 on 0.1, 1, and 10% spent brewery grain (Figure 4). Microscopically, neither T. paralvinellae nor P. furiosus were preferentially associated with the cellulose fibers of the spent brewery grain, but could not be accurately counted due to the presence of the grain.
Remediation of waste milk
Dilution-to-extinction plating of 1% milk showed that milk from Ceftiofur-treated cows and untreated cows contain similar concentrations of bacteria prior to incubation (Table 3). The exception was that there were colony-forming units found on MacConkey agar plates, which is selective for Gram-negative enteric bacteria, from untreated cows that were completely absent from plates from cows treated with Ceftiofur. Also, the concentrations of λ colonies on Sheep's Blood agar plates, which can represent Staphylococcus spp. (the typical cause of mastitis), were higher in cows being treated for mastitis. All plate types showed no colony forming units from medium that had been incubated with T. paralvinellae for 100 h at 82°C (Table 3). The concentration of Ceftiofur in waste milk from a cow treated within 48 h of milking was 25.7 ± 0.2 ng ml−1 (mean ± standard error). With and without inoculation with T. paralvinellae, the amount of Ceftiofur in 10% waste milk medium was below the detection limit within an hour of incubation at 82°C.
Table 3.
Mean colony forming units (±standard error, n = 3) per ml of fresh (< 2 h old) 1% (vol vol−1) waste milk from Ceftiofur-treated and untreated cows in base medium, and base medium without a carbon source.
| Treatment | Luria Broth (× 103) | Sheep's blood | MacConkey | |||
|---|---|---|---|---|---|---|
| α (× 102) | β (× 102) | λ (× 102) | Fermenter | Non-fermenter | ||
| WITHOUT HEAT TREATMENT | ||||||
| Ceftiofur-treated | 5.7 ± 4.2 | 7.0 ± 3.0 | 2.0 ± 0.3 | 93 ± 77 | 0 | 0 |
| Untreated | 4.4 ± 3.7 | 4.3 ± 3.8 | 4.6 ± 2.8 | 2.6 ± 1.2 | 3 ± 3 | 66 ± 56 |
| Control | 0 | 0 | 0 | 0 | 0 | 0 |
| 100 h at 82°C | ||||||
| Ceftiofur-treated | 0 | 0 | 0 | 0 | 0 | 0 |
| Untreated | 0 | 0 | 0 | 0 | 0 | 0 |
| Control | 0 | 0 | 0 | 0 | 0 | 0 |
Growth in a 20-l bioreactor
The growth rates of T. paralvinellae in the bioreactor were 0.67 ± 0.06 h−1 (mean ± standard error) on maltose only (pH 6.8), 0.59 ± 0.05 h−1 on tryptone only, 0.53 ± 0.14 h−1 on maltose plus tryptone, 0.38 ± 0.05 h−1 on maltose plus acetate, 0.36 ± 0.04 h−1 on maltose only (pH 5.0), and 0.49 ± 0.09 h−1 on milk containing Ceftiofur (Figure 6A). For T. paralvinellae, specific growth rates increased in the bioreactor relative to bottles when the cultures were grown on maltose and maltose-tryptone media. Otherwise, specific growth rates on the other three defined media were unchanged. T. paralvinellae growth rates on maltose at pH 5 or in the presence of acetate were lower in the bioreactor than maltose only in the bioreactor suggesting that pH and acetate can have some inhibitory effect in a bioreactor. Specific growth rates of P. furiosus on each of the media increased significantly in the 20-l bioreactor relative to growth in serum bottles (Figure 6B). The growth rates of P. furiosus in the bioreactor were 0.95 ± 0.08 h−1 (mean ± standard error) on maltose only (pH 6.8), 0.73 ± 0.04 h−1 on tryptone only, 0.73 ± 0.05 h−1 on maltose plus tryptone, 0.67 ± 0.06 h−1 on maltose plus acetate, 0.71 ± 0.02 h−1 on maltose only (pH 5.0), and 0.29 ± 0.03 h−1 on milk with Ceftiofur. As seen in the bottles, the maximum cell concentration of P. furiosus in the bioreactor on peptide medium was much lower than rates on maltose and maltose-tryptone media, while there was no difference in the growth rates in these media with T. paralvinellae.
Figure 6.
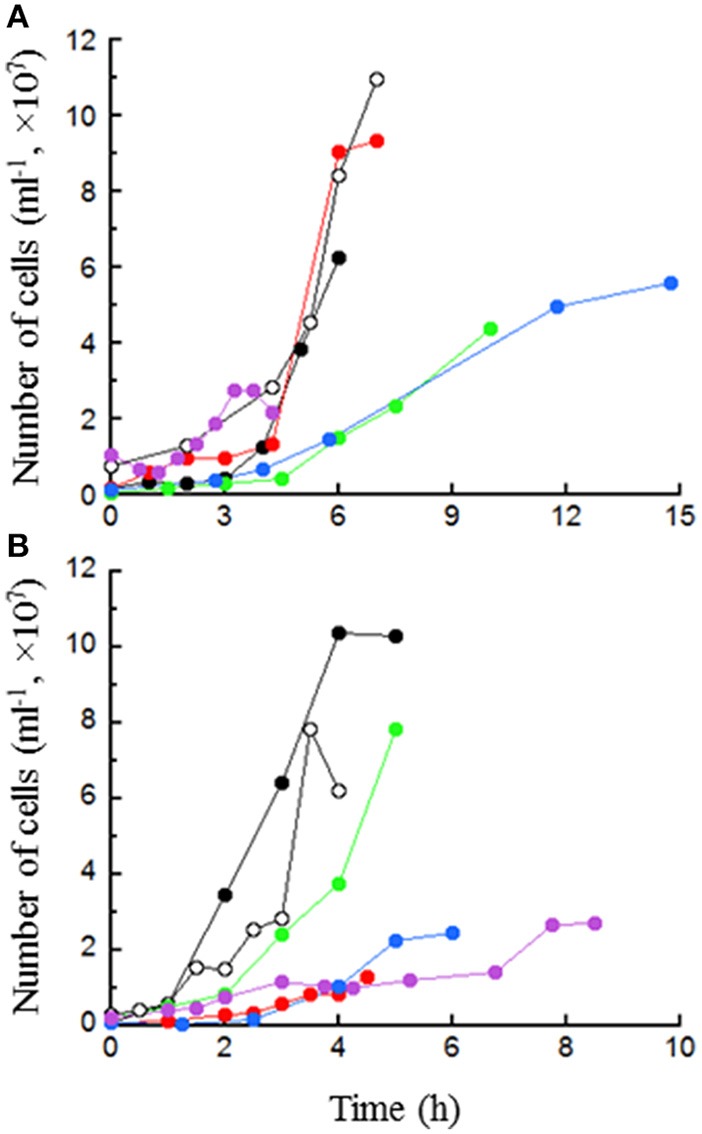
Growth of T. paralvinellae (A) and P. furiosus (B) in the 20-l bioreactor in 0.5% maltose (●), 0.5% tryptone ( ), 0.5% maltose + 0.05% tryptone (○), 0.5% maltose + 10 mM acetate (
), 0.5% maltose + 0.05% tryptone (○), 0.5% maltose + 10 mM acetate ( ), 0.5% maltose at pH 5.0 (
), 0.5% maltose at pH 5.0 ( ), and 0.1% waste milk (
), and 0.1% waste milk ( ). Data are examples from individual bioreactor reactions.
). Data are examples from individual bioreactor reactions.
Enzyme activities
A summary of all specific enzyme activities are provided in Table 4. Overall, enzymatic activities in both organisms changed the most when cells were grown under stressful conditions. T. paralvinellae and P. furiosus showed the largest dissimilarity in enzyme activities when grown on maltose-acetate medium (11 of 13 enzymes for both organisms) and low pH maltose medium (6 of 13 activities for T. paralvinellae and 2 of 13 enzymes for P. furiosus). For T. paralvinellae, membrane-bound ATP synthase, GDH, and AlaAT activities were higher than all other enzyme activities. For P. furiosus, Mbh activities were higher than any other enzyme activity for all defined media except for FNOR activity which was higher when cells were grown on maltose-only medium and GDH activity which was higher when cells were grown on tryptone-only medium.
Table 4.
T. paralvinellae and P. furiosus specific enzyme activities.
| Enzyme† | Media type | p-value | |||||
|---|---|---|---|---|---|---|---|
| 0.5% maltose | 0.5% tryptone |
0.5% maltose 0.05% tryptone |
0.5% maltose + 10 mM acetate |
0.5% maltose (pH 5) | 1% waste milk | ||
| T. paralvinellae | |||||||
| Mbh | 3.34 ± 0.91 | 4.62 ± 2.20 | 1.53 ± 0.31 | 1.36 ± 0.29 | 0.18 ± 0.18 | ND | NS |
| Sulf | 0.21 ± 0.03a | 0.54 ± 0.20a | 1.53 ± 0.15 | 0.06 ± 0.01a | 0.05 ± 0.01a | 0.35 ± 0.14a | *** |
| ATPase | 0.23 ± 0.04a,b | 3.30 ± 0.44d | 0.39 ± 0.29a,c | 4.41 ± 0.03d | 1.33 ± 0.20b,c | ND | *** |
| FNOR | 0.21 ± 0.07a | 3.42 ± 0.16c,d | 3.33 ± 0.60b,c | 9.10 ± 1.02 | 1.84 ± 0.22a,b,d | 1.71 ± 0.22a,b,d | *** |
| GDH | 12.09 ± 0.75a | 9.03 ± 0.56a,c | 3.88 ± 1.26b | 3.89 ± 0.91b | 8.31 ± 0.30c | 0.53 ± 0.14b | *** |
| AlaAT | 1.21 ± 0.47a | 5.80 ± 2.46a,b | 5.08 ± 1.27a,b | 3.01 ± 0.11a,b | 8.00 ± 0.37b | 2.42 ± 0.45a,b | * |
| POR | 2.59 ± 0.13a | 0.46 ± 0.10b | 1.29 ± 0.09 | 0.41 ± 0.05b | 2.16 ± 0.40a | 0.18 ± 0.05b | *** |
| VOR | 0.34 ± 0.07a | 0.11 ± 0.03b | 0.44 ± 0.05a | 0.02 ± 0.01b | 0.11 ± 0.03b | 0.14 ± 0.03b | *** |
| ACS | 0.34 ± 0.07a | 0.52 ± 0.02b | 0.60 ± 0.03b | 0.21 ± 0.02a,c | 0.60 ± 0.02b | 0.13 ± 0.06c | *** |
| AOR | 0.35 ± 0.05a | 0.48 ± 0.09a | 0.29 ± 0.05a | 0.60 ± 0.10a | 1.55 ± 0.43 | 0.10 ± 0.03a | *** |
| FOR | 0.28 ± 0.10a,b | 0.62 ± 0.14b | 0.12 ± 0.03a | 0.34 ± 0.13a,b | 0.46 ± 0.06a,b | 0.16 ± 0.02a | ** |
| ADH | 0.03 ± 0.01a,b | 0.02 ± 0.00a,b | 0.07 ± 0.03a | 0b | 0.02 ± 0.01a,b | 0b | ** |
| FDH | 0.06 ± 0.02a | 0.03 ± 0.00a,b | 0.02 ± 0.00a,b | 0.04 ± 0.01a,b | 0.02 ± 0.00a,b | 0.01 ± 0.01b | * |
| P. furiosus | |||||||
| Mbh | 4.99 ± 0.31a | 9.55 ± 0.50a,b | 12.74 ± 0.24b | 11.42 ± 0.19b | 5.39 ± 0.40a,b | ND | ** |
| Sulf | 1.27 ± 0.11a,a,c | 0.73 ± 0.19a,a,d | 3.44 ± 0.49 | 1.48 ± 0.29a,a,e | 0.15 ± 0.05d,f | 0.52 ± 0.02c,c,f | *** |
| ATPase | 0.05 ± 0.00a | 0.01 ± 0.01a | 0.70 ± 0.22 | 0a | 0a | ND | * |
| FNOR | 6.10 ± 0.60a | 3.90 ± 0.32a,b | 4.59 ± 0.48a | 3.83 ± 0.47a,b | 0.31 ± 0.12b | 3.16 ± 1.59a,b | *** |
| GDH | 1.85 ± 0.36a | 9.90 ± 1.08c | 4.69 ± 0.42b | 0.69 ± 0.08a | 0.91 ± 0.09a | 0.68 ± 0.16a | *** |
| AlaAT | 0.54 ± 0.25 | 3.05 ± 0.61 | 2.59 ± 1.76 | 0.72 ± 0.14 | 0.36 ± 0.05 | 0.54 ± 0.29 | NS |
| POR | 2.27 ± 0.43 | 0.93 ± 0.10a | 0.72 ± 0.03a | 0.85 ± 0.09a | 0.30 ± 0.02a | 0.01 ± 0.01a | *** |
| VOR | 1.88 ± 0.23a | 2.01 ± 0.45a | 0.56 ± 0.12b,c | 0.56 ± 0.06b,d | 1.35 ± 0.05a,a,d | 0.11 ± 0.05c,d | *** |
| ACS | 0.87 ± 0.09a,a,c | 1.30 ± 0.12b | 1.15 ± 0.24a | 0.68 ± 0.09a,c | 0.57 ± 0.04a,c | 0.44 ± 0.19c | *** |
| AOR | 0.36 ± 0.04a | 0.12 ± 0.03b,c,d | 0.24 ± 0.01a,b,d | 0.26 ± 0.07a,c | 0.08 ± 0.02d | 0.06 ± 0.02d | *** |
| FOR | 0.47 ± 0.08 | 0.27 ± 0.06 | 0.36 ± 0.01 | 0.45 ± 0.11 | 0.31 ± 0.09 | 0.47 ± 0.23 | NS |
| ADH | 0 | 0 | 0.01 ± 0.00 | 0 | 0 | 0.01 ± 0.01 | NS |
| FDH | 0.02 ± 0.00 | 0.06 ± 0.02 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.03 ± 0.01 | 0 | NS |
The specific activities (U [mg protein]−1) are the averages of no less than three technical replicates and two biological replicates (±standard error). ND, a specific activity was not determined. p-values calculated from one-way ANOVA test comparing enzymatic activity across treatments.
p < 0.05,
p < 0.01, and
p < 0.001.
Tukey post-hoc test indicates groups whose members are not significantly different. NS, no significance.
Mbh and ATP synthase activities could not be measured from cells grown on milk due to co-precipitation of insoluble cell fractions with heat curdled milk. The Mbh activities in T. paralvinellae did not show any significant change between the defined media. In contrast, Mbh activity in P. furiosus cells grown in maltose-tryptone medium was higher (F4 = 12.05, p < 0.05) than activities in cells from maltose and low pH maltose media, and activity in cells from maltose-acetate medium was higher than that in cells from maltose medium. Mbh activities in P. furiosus were higher than those in T. paralvinellae for cells grown on maltose-tryptone (F1 = 798, p < 0.001), maltose-acetate (F1 = 847, p < 0.005), and low pH maltose (F1 = 142.9, p < 0.01) media. Sulf activities from maltose-tryptone medium were higher in T. paralvinellae and P. furiosus cells than activities in cells from the other four defined media and 1% milk medium (F5 = 23.2, p < 0.001). Sulf activities were higher in P. furiosus than in T. paralvinellae for cells grown on maltose (F1 = 66.7, p < 0.001), maltose-tryptone (F1 = 14.5, p < 0.01), and maltose-acetate (F1 = 20.5, p < 0.01) media. Membrane-bound ATP synthase activity in T. paralvinellae was higher in cells grown on maltose-acetate and tryptone media than on the other three defined media (F4 = 54.2, p < 0.001). In P. furiosus, ATP synthase activity in cells grown on maltose-tryptone medium was higher than activities in cells from the other four defined media (F4 = 10.4, p < 0.02). Membrane-bound ATP synthase activity was higher in T. paralvinellae than in P. furiosus for cells grown on tryptone (F1 = 57.0, p < 0.02), maltose-acetate (F1 = 2.1 × 104, p < 0.001), and low pH maltose (F1 = 44.4, p < 0.05) media.
In T. paralvinellae, AOR and FNOR activities were higher in cells grown on maltose-acetate medium than any of the other four defined media and 1% waste milk medium (F5 = 7.75, p < 0.001 and F5 = 37.72, p < 0.001, respectively). FNOR activity was lower in cells grown on maltose medium than on maltose-tryptone and tryptone media (F5 = 37.73, p < 0.01). VOR activity was higher in cells grown on maltose and maltose-tryptone media than any other media (F5 = 14.45, p < 0.05), POR activity was higher in cells grown on maltose and low pH maltose media than any other media (F5 = 30.32, p < 0.05), and GDH activity was higher (F5 = 28.8, p < 0.02) in cells grown on maltose medium than cells grown on low pH maltose, maltose-acetate, maltose-tryptone, and 1% milk media. In P. furiosus, GDH activity was higher in cells grown on tryptone medium than any of the other four defined media and 1% waste milk (F5 = 50.0, p < 0.001). POR activity was higher in cells grown on maltose medium than any of the other four defined media and 1% waste milk (F5 = 11.28, p < 0.001). The other enzyme activities for both organisms either did not change with growth medium or did not show a consistent pattern in their differences.
Discussion
Growth, H2 production kinetics, and enzyme activities
T. paralvinellae ES1 was isolated from a polychaete worm collected from a deep-sea hydrothermal vent in the northeastern Pacific Ocean and is commonly associated with growth on peptides and sulfur to produce H2S (Pledger and Baross, 1989). However, T. paralvinellae possesses seven hydrogenase gene clusters (Jung et al., 2014) and produces H2 in lieu of H2S when sulfur is omitted from the medium (Hensley et al., 2014). In this study, T. paralvinellae produced H2 when grown without sulfur on either a carbohydrate (maltose) or on peptides and under potentially inhibiting conditions such as high acetate concentrations and at pH 5. The maltose-tryptone medium improved H2 production for T. paralvinellae (Figure 2) rather than have no effect or a negative effect as observed with most mesophilic and thermophilic anaerobes (Kim et al., 2004; Pawar and van Niel, 2013). In many other H2 producing organisms, as the pH decreases H2 production becomes increasingly inhibited due to the effect of low pH on hydrogenases (Dabrock et al., 1992). While pH 5 did decrease the specific activity of many enzymes in T. paralvinellae and P. furiosus, it did not decrease the growth or H2 production rates relative to growth on maltose at pH 6.8. It was previously reported that the addition of 30 and 60 mM acetate did not affect the growth of P. furiosus (Krahe et al., 1996), but this is the first study to measure the impact of acetate on H2 production kinetics and enzyme activities. T. paralvinellae grew and produced H2 at rates and yields that were comparable to those of P. furiosus without sulfur on all media types tested. These data demonstrate that T. paralvinellae is amenable to producing H2 on diverse substrates and conditions.
Although the H2 production yields (Yp∕x) for the defined media were generally similar between T. paralvinellae and P. furiosus, the MV-dependent Mbh activity was lower in T. paralvinellae than in P. furiosus for most media. T. paralvinellae also produced larger amounts of H2 than P. furiosus when grown on high concentrations of wastes. These results may be due to differing H2 production pathways in the two organisms. T. paralvinellae possesses genes for putative CO-, F420-, and formate-dependent hydrogenases that are lacking in P. furiosus, in addition to the Mbh and Sulf in both organisms (Jung et al., 2014). If active, they may ameliorate possible H2 inhibition by having a more diverse electron carrier pool to draw from. For T. paralvinellae, POR, VOR, AOR, and GDH activities were significantly higher and FNOR lower when grown on maltose-containing media relative to the other media. Enzyme activities and metabolite production in P. furiosus were largely as previously described (Adams et al., 2001; Schut et al., 2003). POR activity in P. furiosus was significantly higher in cells grown on maltose and GDH activity was significantly higher in cells grown on peptides. This pattern differs somewhat from that seen in T. paralvinellae suggesting that redox balancing in T. paralvinellae might involve factors not found in P. furiosus.
As expected for both organisms, H2:acetate ratios were approximately two when cultures were grown on maltose-only medium, and isovalerate was only produced in tryptone-containing media. Final acetate concentrations were comparable to those reported previously for Pyrococcus and Thermococcus species grown in batch culture (Schäfer and Schönheit, 1992; Ma et al., 1995; Nohara et al., 2014). Alanine, ethanol, butanol, and formate are other metabolites that have been reported for various Thermococcus and Pyrococcus species when terminal electron acceptors are in limited supply or when the H2 partial pressure is elevated (Kengen and Stams, 1994; Ma et al., 1995; Nohara et al., 2014). Alcohol production and ADH and FDH activities increased in T. paralvinellae when it was grown on low (1 g l−1) sulfur concentrations (Ma et al., 1995). Recently, it was shown that P. furiosus reduces acetate and other carboxylic acids first to an aldehyde by AOR and then to an alcohol when a recombinant adhA gene is introduced into the organism's genome (Basen et al., 2014). The genomes of T. paralvinellae and P. furiosus contain four and two adhA genes, respectively (Robb et al., 2001; Jung et al., 2014), suggesting that they naturally have the ability to produce alcohols from aldehydes. However, alcohols were undetectable in this study and ADH activities were very low to undetectable in both organisms suggesting that alcohol production is not a significant alternative pathway for electron disposal for these organisms even in the presence of high acetate concentrations or low pH. Formate was produced by both organisms, sometimes surpassing acetate production, but FDH activities were low in both organisms and the mechanism for its production is unknown. Formate has not been reported previously for P. furiosus, although this may be for analytical reasons since previous detection methods were not focused on formate. Formate has been reported as a metabolite for Thermococcus kodakarensis (Nohara et al., 2014).
Growth, H2 production kinetics, and enzyme activities
Over the past decade, food waste in the U.S. has risen to account for the largest proportion (21%) of municipal solid waste (MSW; Staley and Barlaz, 2009; United States Environmental Protection Agency (US EPA), 2014). As of 2012, only 3% of food waste in the U.S. was treated with the rest becoming a part of MSW and buried in landfills (United States Environmental Protection Agency (US EPA), 2014). Food waste is produced at every stage of production from farms and food processing facilities as pre-consumer waste to domestic waste (Lin et al., 2013). Although the amount of waste for each sector is fairly evenly spread, waste produced by the agricultural and manufacturing sector is generated in a more concentrated manner and would therefore be easier to collect, process, and treat to keep it from entering landfills and help offset the costs of collection. Therefore, recent developments in waste management technology have focused on pre-consumer waste. Diverting food waste to composting and biological treatment is considered promising to limit landfill growth and produce biofuels (Hermann et al., 2011). Microorganisms that catabolize long-chain carbohydrates and polypeptides and produce an energy product such as H2, CH4, and alcohols are ideal for these processes (Angenent et al., 2004; Alper and Stephanopoulos, 2009). This study showed that T. paralvinellae and P. furiosus grew and produced H2 on waste milk from cows treated with Ceftiofur and from healthy untreated cows and spent brewery grain. T. paralvinellae produced more H2 and grew on higher feedstock concentrations relative to P. furiosus, but generally grew slower than P. furiosus on the wastes.
Waste milk from dairy farms and cows being treated for mastitis (a commonly occurring infection and inflammation of utters) with the bacterial antibiotic Ceftiofur is particularly problematic. Cows undergoing treatment need to be milked daily and produce ~37 L of milk per cow per day. While only 0.1% of the initial antibiotic dose is excreted in milk, the milk cannot be added to the food supply for 5 days after treatment (Hornish and Kotarski, 2002). The U.S Food and Drug Administration requires that the concentration of Ceftiofur in waste milk be less than 50 ppb before disposal (Samanidou and Nisyriou, 2008). Because of the antibiotic, waste milk cannot be disposed of with MSW or sewage, nor should it be mixed with manure and spread onto fields as fertilizer due to increasing antibiotic resistance in nature. The waste milk is often pasteurized and fed to calves; however, resistance of calf gut bacteria to antibiotics was shown to increase in calves fed milk with increasing concentrations of antibiotics (Langford et al., 2003). Therefore, an alternative waste disposal mechanism is needed. Ceftiofur is a β-lactam that inhibits peptidoglycan synthesis and thus does not affect Thermococcus and Pyrococcus since they are archaea. In this study, there was no change in either growth rate or H2 production for either T. paralvinellae or P. furiosus when they were grown on milk from Ceftiofur-treated cows relative to milk from untreated cows indicating that the antibiotic was ineffective against them. Furthermore, the half-life of Ceftiofur at 67°C is 6 h, which decreases with increasing temperature (Sunkara et al., 1999). In our study, the concentration of Ceftiofur after 1 h of incubation at 82°C, with and without T. paralvinellae, was below the detection limit (~2 ppb).
Raw bovine milk contains by weight 3% protein (mostly casein), 4% fat, and 5% carbohydrate (mostly lactose; Wong et al., 1988). The solids in spent brewery grain are mostly carbohydrate (starch). In this study, T. paralvinellae and P. furiosus grew fastest and produced the most H2 on media containing a combination of carbohydrate and peptides. Soluble protein was removed from the milk by the organisms, but neither organism grew on lactose or removed it from the milk. They both possess genes for a β-galactosidase (Robb et al., 2001; Jung et al., 2014), for which the recombinant version of the enzyme from P. furiosus cleaved lactose (Dong et al., 2014). They also grow on cellobiose (Oslowski et al., 2011), which chemically is similar to lactose, suggesting that they might be adaptable to growth on lactose as well.
The only previous study to examine agricultural waste degradation linked with H2 production by a hyperthermophile was a two-stage process for keratin degradation. In the first stage, a Bacillus species was used to aerobically degrade feather waste into a keratin hydrolysate, which was then used in the second stage as a feedstock for H2 production by Thermococccus litoralis (Bálint et al., 2005). T. litoralis produced up to 3 mmol L−1 of H2 on that feedstock. Genetically modified T. onnurineus converted the CO in steel industry syngas into H2 three times faster than wild-type cells at rates up to 60 mmol L−1 h−1 demonstrating the potential for enhancing waste conversion rates through genetic engineering (Kim et al., 2013). In this study, T. paralvinellae degraded waste milk and spent brewery grain in a single consolidated processing step. The heat from the incubation eliminated the pathogens present and degraded the heat-labile antibiotic present in the waste milk. T. paralvinellae produced increasing amounts of H2 from both agricultural wastes types with increasing concentration. It also grows over a lower temperature range than P. furiosus, which may give T. paralvinellae a cost advantage over P. furiosus due to lower reactor heating costs. Therefore, it may be feasible to use T. paralvinellae, or a similar hyperthermophilic heterotroph, for rapid, mesoscale treatment of organic wastes to reduce the organic load, generate H2 as an energy byproduct, and remove pathogens.
Author contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Kyunghwa Baek, Dr. Cheon-Seok Park, Marie Kroeger, Begüm Topçuoğlu, Samantha Zelin, Katarina Olsson Gudmundsson, Alexander Basu, Dr. Michael Levine, and the lab of Dr. Jeffrey Blanchard for their assistance with experiments and data analysis; Dr. Kristen DeAngelis and Dr. Caitlyn Butler for their comments on the manuscript; and the Amherst Brewing Company and Barstow Family Farms for providing spent brewery grain and waste milk. This work was supported by grants to JH from the Northeast Sun Grant Institute of Excellence (NE11-26), USDA CSREES (MAS00431), and the Gordon and Betty Moore Foundation (GBMF 3297).
References
- Adams M. W. W. (1999). The biochemical diversity of life near and above 100°C in marine environments. J. Appl. Microbiol. 85, 108S–117S. 10.1111/j.1365-2672.1998.tb05289.x [DOI] [PubMed] [Google Scholar]
- Adams M. W. W., Holden J. F., Menon A. L., Schut G. J., Grunden A. M., Hou C., et al. (2001). Key role for sulfur in peptide metabolism and in regulation of three hydrogenases in the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 183, 716–724. 10.1128/JB.183.2.716-724.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper H., Stephanopoulos G. (2009). Engineering for biofuels: exploiting innate microbial capacity or importing biosynthetic potential? Nat. Rev. Microbiol. 7, 715–723. 10.1038/nrmicro2186 [DOI] [PubMed] [Google Scholar]
- Angenent L. T., Karim K., Al-Dahhan M. H., Wrenn B. A., Domíguez-Espinosa R. (2004). Production of bioenergy and biochemical from industrial and agricultural wastewater. Trends Biotechnol. 22, 477–485. 10.1016/j.tibtech.2004.07.001 [DOI] [PubMed] [Google Scholar]
- Bae S. S., Kim T. W., Lee H. S., Kwon K. K., Kim Y. J., Kim M. S., et al. (2012). H2 production from CO, formate or starch using the hyperthermophilic archaeon, Thermococcus onnurineus. Biotechnol. Lett. 34, 75–79. 10.1007/s10529-011-0732-3 [DOI] [PubMed] [Google Scholar]
- Bálint B., Bagi Z., Tóth A., Rákhely G., Perei K., Kovács K. L. (2005). Utilization of keratin-containing biowaste to produce biohydrogen. Appl. Microbiol. Biotechnol. 69, 404–410. 10.1007/s00253-005-1993-3 [DOI] [PubMed] [Google Scholar]
- Basen M., Schut G. J., Nguyen D. M., Lipscomb G. L., Benn R. A., Prybol C. J., et al. (2014). Single gene insertion drives bioalcohol production by a thermophilic archaeon. Proc. Natl. Acad. Sci. U.S.A. 111, 17618–17623. 10.1073/pnas.1413789111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamey J. M., Adams M. W. W. (1993). Purification and characterization of pyruvate ferredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus. Biochim. Biophys. Acta 1161, 19–27. 10.1016/0167-4838(93)90190-3 [DOI] [PubMed] [Google Scholar]
- Bonch-Osmolovskaya E. A., Stetter K. O. (1991). Interspecies hydrogen transfer in cocultures of thermophilic Archaea. Syst. Appl. Microbiol. 14, 205–208. 10.1016/S0723-2020(11)80369-3 [DOI] [Google Scholar]
- Bougrier C., Battimelli A., Delgenes J. P., Carrere H. (2007). Combined ozone pretreatment and anaerobic digestion for the reduction of biological sludge production in wastewater treatment. Ozone Sci. Eng. 29, 201–206. 10.1080/01919510701296754 [DOI] [Google Scholar]
- Bräsen C., Schönheit P. (2004). Unusual ADP-forming acetyl-coenzyme A synthetases from the mesophilic halophilic euryarchaeon Haloarcula marismortui and from the hyperthermophilic crenarchaeon Pyrobaculum aerophilum. FEBS Lett. 579, 477–482. 10.1016/j.febslet.2004.12.016 [DOI] [PubMed] [Google Scholar]
- Bryant F. O., Adams M. W. W. (1989). Characterization of hydrogenase from the hyperthermophilic archaebacterium, Pyrococcus furiosus. J. Biol. Chem. 264, 5070–5079. [PubMed] [Google Scholar]
- Canganella F., Jones W. J. (1994). Fermentation studies with thermophilic Archaea in pure culture and in syntrophy with a thermophilic methanogen. Curr. Microbiol. 28, 293–298. 10.1007/BF01573209 [DOI] [Google Scholar]
- Dabrock B., Bahl H., Gottschalk G. (1992). Parameters affecting solvent production by Clostridium pasteurium. Appl. Environ. Microbiol. 58, 1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidova I. A., Duncan K. E., Perez-Ibarra B. M., Suflita J. M. (2012). Involvement of thermophilic archaea in the biocorrosion of oil pipelines. Environ. Microbiol. 14, 1762–1771. 10.1111/j.1462-2920.2012.02721.x [DOI] [PubMed] [Google Scholar]
- Diggelman C., Ham R. K. (2003). Household food waste to wastewater or to solid waste? That is the question. Waste Manage. Res. 21, 501–514. 10.1177/0734242X0302100603 [DOI] [PubMed] [Google Scholar]
- Dong Q., Yan X., Zheng M., Yang Z. (2014). Characterization of an extremely thermostable but cold-adaptive β-galactosidase from the hyperthermophilic archaeon Pyrococcus furiosus for use as a recombinant aggregation for batch lactose degradation at high temperature. J. Biosci. Bioeng. 117, 706–710. 10.1016/j.jbiosc.2013.12.002 [DOI] [PubMed] [Google Scholar]
- Girguis P. R., Holden J. F. (2012). On the potential for bioenergy and biofuels from hydrothermal vent microbes. Oceanography 25, 213–217. 10.5670/oceanog.2012.20 [DOI] [Google Scholar]
- Heider J., Mai J., Adams M. W. W. (1996). Characterization of 2-ketoisovalerate ferredoxin oxidoreductase, a new and reversible coenzyme A-dependent enzyme involved in peptide fermentation by hyperthermophilic archaea. J. Bacteriol. 178, 780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen J. E., Lahti R. J. (1981). A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Anal. Biochem. 113, 313–317. 10.1016/0003-2697(81)90082-8 [DOI] [PubMed] [Google Scholar]
- Hensley S. A., Jung J. H., Park C. S., Holden J. F. (2014). Thermococcus paralvinellae sp. nov. and Thermococcus cleftensis sp. nov., new species of hyperthermophilic heterotrophs from deep-sea hydrothermal vents. Int. J. Syst. Evol. Microbiol. 64, 3655–3659. 10.1099/ijs.0.066100-0 [DOI] [PubMed] [Google Scholar]
- Hermann B. G., Debeer L., de Wilde B., Blok K., Patel M. K. (2011). To compost or not to compost: carbon and energy footprints of biodegradable materials' waste treatment. Polym. Degrad. Stab. 96, 1159–1171. 10.1016/j.polymdegradstab.2010.12.02624404593 [DOI] [Google Scholar]
- Hornish R. E., Kotarski S. F. (2002). Cephalosporins in veterinary medicine: ceftiofur use in food animals. Curr. Top. Med. Chem. 2, 717–731. 10.2174/1568026023393679 [DOI] [PubMed] [Google Scholar]
- Jung J. H., Kim Y. T., Jeon E. J., Seo D. H., Hensley S. A., Holden J. F., et al. (2014). Complete genome sequence of hyperthermophilic archaeon Thermococcus sp. ES1. J. Biotechnol. 174, 14–15. 10.1016/j.jbiotec.2014.01.022 [DOI] [PubMed] [Google Scholar]
- Kanai T., Imanaka H., Nakajima A., Uwamori K., Omori Y., Fukui T., et al. (2005). Continuous hydrogen production by the hyperthermophilic archaeon, Thermococcus kodakaraensis KOD1. J. Biotechnol. 116, 271–282. 10.1016/j.jbiotec.2004.11.002 [DOI] [PubMed] [Google Scholar]
- Kengen S. W., de Bok F. A., van Loo N. D., Dijkema C., Stams A. J., de Vos W. M. (1994). Evidence for the operation of a novel Embden-Meyerhof pathway that involves ADP-dependent kinases during sugar fermentation by Pyrococcus furiosus. J. Biol. Chem. 269, 17537–17541. [PubMed] [Google Scholar]
- Kengen S. W. M., Stams A. J. M. (1994). Formation of l-alanine as a reduced end product in carbohydrate fermentation by the hyperthermophilic archaeon Pyrococcus furiosus. Arch. Microbiol. 161, 168–175. 10.1007/BF00276479 [DOI] [Google Scholar]
- Kim M. S., Bae S. S., Kim Y. J., Kim T. W., Lim J. K., Lee S. H., et al. (2013). CO-dependent H2 production by genetically engineered Thermococcus onnurineus NA1. Appl. Environ. Microbiol. 9, 2048–2053. 10.1128/AEM.03298-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Han S. K., Shin H. S. (2004). Feasibility of biohydrogen production by anaerobic co-digestion of food waste and sewage sludge. Int. J. Hydrogen Energy 29, 1607–1216. 10.1016/j.ijhydene.2004.02.018 [DOI] [Google Scholar]
- Kim Y. J., Lee H. S., Kim E. S., Bae S. S., Lim J. K., Matsumi R., et al. (2010). Formate-driven growth coupled with H2 production. Nature 467, 352–355. 10.1038/nature09375 [DOI] [PubMed] [Google Scholar]
- Krahe M., Antranikian G., Märkl H. (1996). Fermentation of extremophilic microorganisms. FEMS Microbiol. Rev. 18, 271–285. 10.1111/j.1574-6976.1996.tb00243.x [DOI] [Google Scholar]
- Lai C. M., Ke G. R., Chung M. Y. (2009). Potentials of food wastes for power generation and energy conservation in Taiwan. Renew. Energy 34, 1913–1915. 10.1016/j.renene.2008.12.007 [DOI] [Google Scholar]
- Langford F. M., Weary D. M., Fisher L. (2003). Antibiotic resistance in gut bacteria from dairy calves: a dose response to the level of antibiotics fed in milk. J. Dairy Sci. 86, 3963–3966. 10.3168/jds.S0022-0302(03)74006-5 [DOI] [PubMed] [Google Scholar]
- Lee S. H., Choi K. I., Osako M., Dong J. I. (2007). Evaluation of environmental burdens caused by changes of food waste management systems in Seoul, Korea. Sci. Total Environ. 387, 42–53. 10.1016/j.scitotenv.2007.06.037 [DOI] [PubMed] [Google Scholar]
- Levis J. W., Barlaz M. A., Themelis N. J., Ulloa P. (2010). Assessment of the state of food waste treatment in the United States and Canada. Waste Manag. 30, 1486–1494. 10.1016/j.wasman.2010.01.031 [DOI] [PubMed] [Google Scholar]
- Lin C. S. K., Pfaltzgraff L. A., Herrero-Davila L., Mudofu E. B., Abderrahim S., Clark J. H., et al. (2013). Food waste as a valuable resource for the production of chemicals, materials and fuels: current situation and global perspective. Energy Environ. Sci. 6, 426–464. 10.1039/c2ee23440h [DOI] [Google Scholar]
- Ma K., Adams M. W. W. (1994). Sulfide dehydrogenase from the hyperthermophilic archaeon Pyrococcus furiosus: a new multifunctional enzyme involved in the reduction of elemental sulfur. J. Bacteriol. 176, 6509–6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K., Adams M. W. W. (1999). An unusual oxygen-sensitive, iron- and zinc-containing alcohol dehydrogenase from the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 181, 1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K., Loessner H., Heider J., Johnson M. K., Adams M. W. W. (1995). Effects of elemental sulfur on the metabolism of the deep-sea hyperthermophilic archaeon Thermococcus strain ES-1: characterization of a sulfur-regulated, non-heme iron alcohol dehydrogenase. J. Bacteriol. 177, 4748–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K., Weiss R., Adams M. W. W. (2000). Characterization of hydrogenase II from the hyperthermophilic archaeon Pyrococcus furiosus and assessment of its role in sulfur reduction. J. Bacteriol. 182, 1864–1871. 10.1128/JB.182.7.1864-1871.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai X., Adams M. W. W. (1996). Purification and characterization of two reversible acyl-CoA synthetases (ADP-forming) from the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 178, 5897–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukund S., Adams M. W. W. (1991). The novel tungsten-iron-sulfur protein of the hyperthermophilic archaebacterium, Pyrococcus furiosus, is an aldehyde ferredoxin oxidoreductase. J. Biol. Chem. 266, 14208–14216. [PubMed] [Google Scholar]
- Mukund S., Adams M. W. W. (1995). Glyceraldehyde-3-phosphate ferredoxin oxidoreductase, a novel tungsten-containing enzyme with a potential glycolytic role in the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 270, 8389–8392. 10.1074/jbc.270.15.8389 [DOI] [PubMed] [Google Scholar]
- Nohara K., Orita I., Nakamura S., Imanaka T., Fukui T. (2014). Genetic examination and mass balance analysis of pyruvate/amino acid oxidation pathways in the hyperthermophilic archaeon Thermococcus kodakarensis. J. Bacteriol. 196, 3831–3839. 10.1128/JB.02021-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntaikou I., Antonopoulou G., Lyberatos G. (2010). Biohydrogen production from biomass and wastes via dark fermentation: a review. Waste Biomass Valorization 1, 21–39. 10.1007/s12649-009-9001-2 [DOI] [Google Scholar]
- Oslowski D. M., Jung J. H., Seo D. H., Park C. S., Holden J. F. (2011). Production of hydrogen from α-1,4- and β-1,4-linked saccharides by marine hyperthermophilic archaea. Appl. Environ. Microbiol. 77, 3169–3173. 10.1128/AEM.01366-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar S. S., van Niel E. W. J. (2013). Thermophilic biohydrogen production: how far are we? Appl. Microbiol. Biotechnol. 97, 7999–8009. 10.1007/s00253-013-5141-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisa K. Y., Huber H., Thomm M., Müller V. (2007). A sodium ion-dependent A1A0 ATP synthase from the hyperthermophilic archaeon Pyrococcus furiosus. FEBS J. 274, 3928–3938. 10.1111/j.1742-4658.2007.05925.x [DOI] [PubMed] [Google Scholar]
- Pledger R. J., Baross J. A. (1989). Characterization of an extremely thermophilic archaebacterium isolated from a black smoker polychaete (Paralvinella sp.) at the Juan de Fuca Ridge. Syst. Appl. Microbiol. 12, 249–256. 10.1016/S0723-2020(89)80070-0 [DOI] [Google Scholar]
- R Core Team (2013). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; ISBN 3-900051-07-0. Available online at: http://www.R-project.org. [Google Scholar]
- Robb F. T., Maeder D. L., Brown J. R., DiRuggiero J., Stump M. D., Yeh R. K., et al. (2001). Genomic sequence of hyperthermophile, Pyrococcus furiosus: implications for physiology and enzymology. Methods Enzymol. 330, 134–157. 10.1016/S0076-6879(01)30372-5 [DOI] [PubMed] [Google Scholar]
- Robb F. T., Park J. B., Adams M. W. W. (1992). Characterization of an extremely thermostable glutamate dehydrogenase: a key enzyme in the primary metabolism of the hyperthermophilic archaebacterium, Pyrococcus furiosus. Biochim. Biophys. Acta 1120, 267–272. 10.1016/0167-4838(92)90247-B [DOI] [PubMed] [Google Scholar]
- Roy R., Mukund S., Schut G. J., Dunn D. M., Weiss R., Adams M. W. W. (1999). Purification and molecular characterization of the tungsten-containing formaldehyde ferredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus: the third of a putative five-member tungstoenzyme family. J. Bacteriol. 181, 1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanidou V., Nisyriou S. (2008). Multi-residue methods for confirmatory determination of antibiotics in milk. J. Sep. Sci. 31, 2068–2090. 10.1002/jssc.200700647 [DOI] [PubMed] [Google Scholar]
- Sapra R., Verhagen M. F. J. M., Adams M. W. W. (2000). Purification and characterization of a membrane-bound hydrogenase from the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 182, 3423–3428. 10.1128/JB.182.12.3423-3428.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer T., Schönheit P. (1992). Maltose fermentation to acetate, CO2 and H2 in the anaerobic hyperthermophilic archaeon Pyrococcus furiosus: evidence for the operation of a novel sugar fermentation pathway. Arch. Microbiol. 158, 188–202. 10.1007/BF00290815 [DOI] [Google Scholar]
- Schäfer T., Selig M., Schönheit P. (1993). Acetyl-CoA synthetase (ADP-forming) in archaea, a novel enzyme involved in acetate formation and ATP synthesis. Arch. Microbiol. 159, 72–83. 10.1007/BF00244267 [DOI] [Google Scholar]
- Schut G. J., Brehm S. D., Datta S., Adams M. W. W. (2003). Whole-genome DNA microarray analysis of a hyperthermophile and an archaeon: Pyrococcus furiosus grown on carbohydrates or peptides. J. Bacteriol. 185, 3935–3947. 10.1128/JB.185.13.3935-3947.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley B. F., Barlaz M. A. (2009). Composition of municipal solid waste in the US and implications for carbon sequestration and methane yield. J. Environ. Eng. 135, 901–909. 10.1061/(ASCE)EE.1943-7870.0000032 [DOI] [Google Scholar]
- Sunkara G., Navarre C. B., Kompella U. B. (1999). Influence of pH and temperature on kinetics of Ceftiofur degradation in aqueous solutions. J. Pharm. Pharmacol. 51, 249–255. 10.1211/0022357991772411 [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency (US EPA) (2014). Municipal Solid Waste Generation, Recycling, and Disposal in the United States: Facts and Figures for 2012. EPA-530-F-14-001, Washington, DC. [Google Scholar]
- Ver Eecke H. C., Butterfield D. A., Huber J. A., Lilley M. D., Olson E. J., Roe K. K., et al. (2012). Hydrogen-limited growth of hyperthermophilic methanogens at deep-sea hydrothermal vents. Proc. Natl. Acad. Sci. U.S.A. 109, 13674–13679. 10.1073/pnas.1206632109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhees C. H., Kengen S. W. M., Tuininga J. E., Schut G. J., Adams M. W. W., de Vos W. M., et al. (2003). The unique features of glycolytic pathways in Archaea. Biochem. J. 375, 231–246. 10.1042/bj20021472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wan W. (2009). Experimental design methods for fermentative hydrogen production: a review. Int. J. Hydrogen Energy 34, 235–244. 10.1016/j.ijhydene.2008.10.008 [DOI] [Google Scholar]
- Ward D. E., Kengen S. W. M., van der Oost J., de Vos W. M. (2000). Purification and characterization of the alanine aminotransferase from the hyperthermophilic archaeon Pyrococcus furiosus and its role in alanine production. J. Bacteriol. 182, 2559–2566. 10.1128/JB.182.9.2559-2566.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong N. P., Jenness R., Keeney M., Marth E. H. (1988). Fundamentals of Dairy Chemistry, 3rd Edn. New York, NY: Van Nostrand Reinhold. [Google Scholar]