Abstract
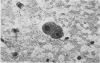
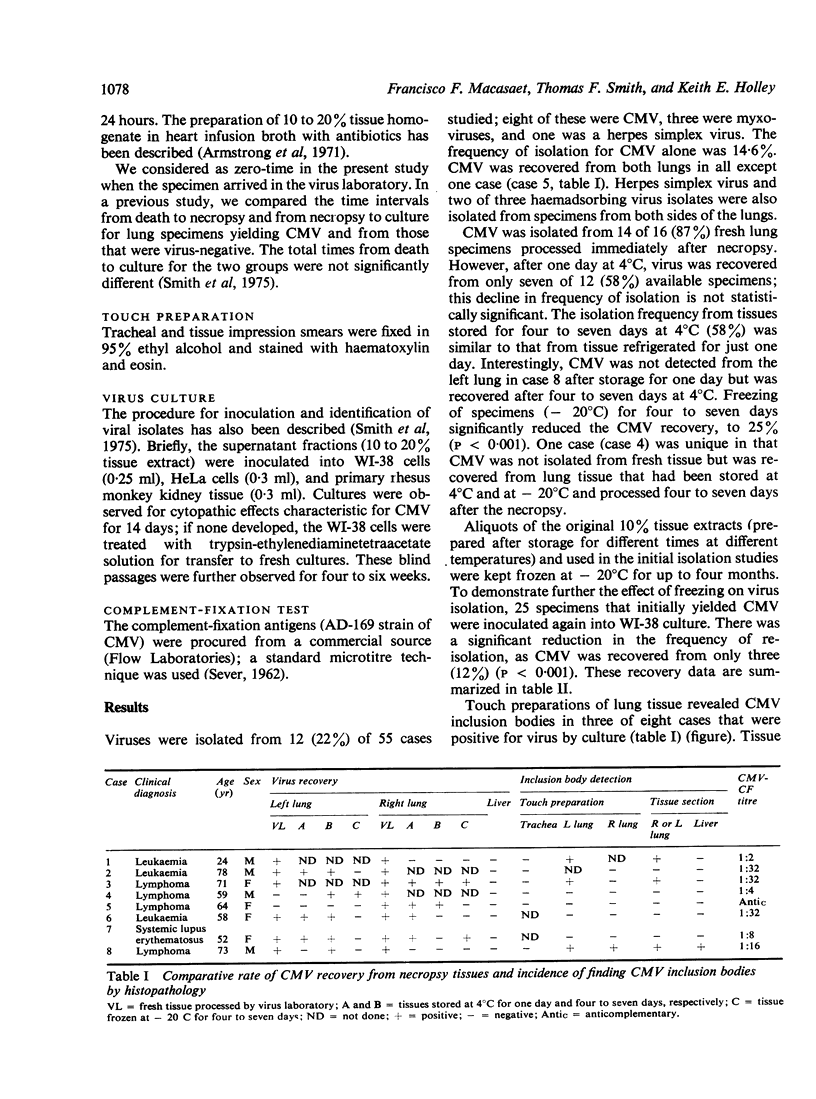
Cytomegalovirus (CMV) was isolated from lung tissue in 8 of 55 (14-6%) necropsies on patients who had received immunosuppressive therapy. Recovery of CMV was best (14 of 16 specimens, 87%) from fresh tissue that had been processed immediately. Storage of specimens at 4 degrees C before inoculation into cell cultures slightly reduced, to 58%, the recovery of CMV. However, conventional freezing of tissue to -- 20 degrees C for four to seven days significantly reduced the recovery of CMV, to 25% (p less than 0-001). In three of eight specimens from which virus was recovered, CMV inclusion bodies were found on tracheal smears. Sixty-five percent of the virus-negative group had measurable complement-fixing antibody in blood taken at necropsy, compared with 86% in the virus-positive group. However, there was no significant differences in antibody levels in the two groups. Our study indicates that specimens submitted for CMV isolation should be sent for virus isolation as rapidly as possible and should not be frozen. The level of antibody in a single serum taken in necropsy does not correlate well with morphological or cultural evidence of active infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D., Ely M., Steger L. Post-transfusion cytomegaloviremia and persistence of cytomegalovirus in blood. Infect Immun. 1971 Jan;3(1):159–163. doi: 10.1128/iai.3.1.159-163.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead J. E. Immunologic response to cytomegalovirus infection in renal allograft recipients. Am J Epidemiol. 1969 Dec;90(6):506–513. doi: 10.1093/oxfordjournals.aje.a121096. [DOI] [PubMed] [Google Scholar]
- Feldman R. A. Cytomegaloviruses in stored urine specimens. A quantitative study. J Pediatr. 1968 Oct;73(4):611–614. doi: 10.1016/s0022-3476(68)80280-x. [DOI] [PubMed] [Google Scholar]
- Hildebrant R. J., Sever J. L., Anderson B. Preservation of infectious cytomegalovirus. Proc Soc Exp Biol Med. 1968 Nov;129(2):504–506. doi: 10.3181/00379727-129-33355. [DOI] [PubMed] [Google Scholar]
- Macasaet F. F., Holley K. E., Smith T. F., Keys T. F. Cytomegalovirus studies of autopsy tissue. II. Incidence of inclusion bodies and related pathologic data. Am J Clin Pathol. 1975 Jun;63(6):859–865. doi: 10.1093/ajcp/63.6.859. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Smith T. F., Holley K. E., Keys T. F., Macasaet F. F. Cytomegalovirus studies of autopsy tissue. I. Virus isolation. Am J Clin Pathol. 1975 Jun;63(6):854–858. doi: 10.1093/ajcp/63.6.854. [DOI] [PubMed] [Google Scholar]
- Vonka V., Benyeshmelnick M. Thermoinactivation of human cytomegalovirus. J Bacteriol. 1966 Jan;91(1):221–226. doi: 10.1128/jb.91.1.221-226.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]