Abstract
Aims
The aims of this study were to analyze the causes of stranding of 1,860 loggerhead turtles (Caretta caretta) admitted at the Tafira Wildlife Rehabilitation Center in Gran Canaria Island, Spain, from 1998 to 2014, and to analyze the outcomes of the rehabilitation process to allow meaningful auditing of its quality.
Methods
Primary causes of morbidity were classified into seven categories: entanglement in fishing gear and/or plastics, ingestion of hooks and monofilament lines, trauma, infectious disease, crude oil, other causes, and unknown/undetermined. Final dispositions were calculated as euthanasia (Er), unassisted mortality (Mr), and release (Rr) rates. Time to death (Td) for euthanized and dead turtles, and length of stay for released (Tr) turtles were evaluated.
Results
The most frequent causes of morbidity were entanglement in fishing gear and/or plastics (50.81%), unknown/undetermined (20.37%), and ingestion of hooks (11.88%). The final disposition of the 1,634 loggerhead turtles admitted alive were: Er = 3.37%, Mr = 10.34%, and Rr = 86.29%. Er was significantly higher in the trauma category (18.67%) compared to the other causes of admission. The highest Mr was observed for turtles admitted due to trauma (30.67%). The highest Rr was observed in the crude oil (93.87%) and entanglement (92.38%) categories. The median Tr ranged from 12 days (unknown) to 70 days (trauma).
Conclusions
This survey is the first large-scale epidemiological study on causes of stranding and mortality of Eastern Atlantic loggerheads and demonstrates that at least 71.72% of turtles stranded due to anthropogenic causes. The high Rr (86.29%) emphasizes the importance of marine rehabilitation centers for conservation purposes. The stratified analysis by causes of admission of the three final disposition rates, and the parameters Td and Tr should be included in the outcome research of the rehabilitation process of sea turtles in order to allow comparative studies between marine rehabilitation centers around the world.
Introduction
Two families and seven species of sea turtles are currently recognized [1], all of which are included in the Red List of the World Conservation Union [2]. The family Cheloniidae includes the green turtle (Chelonia mydas), loggerhead (Caretta caretta), hawksbill (Eretmochelys imbricata), Kemp’s ridley (Lepidochelys kempi), olive ridley (Lepidochelys olivacea), and flatback turtle (Natator depressus). The family Dermochelyidae includes only the leatherback (Dermochelys coriacea) [1]. The most common species around the Canary Islands is the loggerhead turtle, mainly coming from the US western Atlantic by the Gulf Stream [3].
There are reports of disease surveys of free-living sea turtles in Australia [4–9], Hawaii [10–16], Florida [17–26], Brazil [27], France [28], Italy [29], and the Canary Islands [30]. However, long-term epidemiological studies of sea turtle diseases covering more than one decade are scarce [14–16,21,29], and only rarely the survival rates have been thoroughly analyzed [14].
The aims of this study were to analyze the causes of stranding in a large population of loggerhead turtles admitted to the Tafira Wildlife Rehabilitation Center (TWRC) in Gran Canaria Island, Spain, from 1998 to 2014 using specific epidemiological data, to compare these results with those obtained in other geographic regions, and to analyze the outcomes of the rehabilitation process to allow meaningful auditing of its quality.
Materials and Methods
Ethics Statement
Sea turtle rehabilitation program at the TWRC was conducted with authorization of the Wildlife Department of the Canary Islands Government (Ms. Guacimara Medina), and the Environment Department of the Cabildo de Gran Canaria (Ms. María del Mar Arévalo). Animal work and all sampling procedures were specifically approved by the TWRC Animal Care Committee and the insular government Cabildo de Gran Canaria, and were consistent with standard vertebrate protocols and veterinary practices. Loggerhead turtles that had to be euthanized for animal welfare reasons were administered barbiturates by intravenous injection.
Animals and study area
A retrospective study was performed using the original medical records of 1,860 loggerhead turtles admitted to the TWRC, Gran Canaria Island, Spain, from 1998 to 2014. The TWRC receives turtles stranded in Gran Canaria and eventually from other islands of the Canary Islands archipelago. Gran Canaria (27°73’-28°18’N and 15°35’-15°83’W) is the third largest island (1,560.1 km2) of the Canary Islands archipelago and has a coastline of 236 km.
Variables analyzed
Straight carapace length (SCL), weight, stranding point, date and primary cause of admission, and final disposition including date of death or release were recorded. Sex was only determined in 248 turtles by gonadal examination at necropsy. No complete records of SCL and weight were found for 565 turtles. Turtles were categorized as pelagic juveniles (SCL <42 cm), juveniles-subadults (42 cm ≤ SCL <70 cm) and adults (SCL ≥70 cm) according to previous studies [31–33]. In order to study the seasonality of the different causes of admission, the year was divided into four seasons: spring (from March to May), summer (June to August), fall (September to November) and winter (December to February).
We defined the primary cause of morbidity as the main condition responsible for the turtle’s need for treatment [30]. When several causes were observed in the same turtle, clinical history and complementary studies were crucial to determine the primary cause of morbidity, and only this primary cause was recorded. Primary causes of morbidity were classified into seven categories: entanglement in derelict fishing gear and/or plastics (synthetic raffia), ingestion of hooks and monofilament lines, trauma (boat strikes), infectious disease, crude oil, other causes, and unknown/undetermined. The infectious disease category was applied when a pathogenic microorganism was confirmed by microbiological or histopathological diagnosis. Other causes were subdivided into: ingestion of plastics, buoyancy disorders, shark attack, malnutrition, and miscellany. The malnutrition category comprised turtles suffering from cachexia in absence of other lesions.
To assign these categories we used different sources: (a) the physical examination performed by the veterinarian at the admission instance; (b) the information from the people that recovered the turtle, (c) the case history; and when possible (d) from complementary studies as radiology, hematology, blood chemistry, cytology, microbiology, parasitology, gross pathology, histopathology, and toxicology.
Three categories were established for studying the final disposition of the loggerheads admitted alive: euthanized turtles (based on poor quality of life and/or prognosis for survival in the wild), dead turtles during the hospitalization period, and released turtles into the wild. Thus, three percentage rates were calculated for the total of loggerheads admitted alive: euthanasia rate (Er), unassisted mortality rate (Mr), and release (survival) rate (Rr). In addition, these percentage rates were also calculated for each cause of admission.
The parameters time until death (Td; difference between the date of admission and the date of the death) for euthanized and dead turtles during the hospitalization period, and length of stay in the center for released turtles (Tr; difference between the date of admission and the release date) were also evaluated for each cause of admission. Percentiles 10 (P10), 50 (P50), 75 (P75) and 90 (P90) for the variables Td and Tr were also calculated.
Statistical analysis
Statistical analyses were conducted using SPSS v.22.0 (SPSS Inc., Chicago IL) and R package v.3.1.0 (R Development Core Team 2014, Viena, Austria). Chi-square test (χ2) or Fisher exact tests were used to determine whether there was a significant difference between proportions. Odds Ratio (OR) measure of association was employed for disease comparisons. In order to study differences among years, trend analyses were applied for specific causes.
Results
Descriptive analyses
A total of 1,860 loggerhead turtles were included in this study. Most turtles (87.85%, n = 1,634) were alive when admitted. In the group of turtles whose SCL and weight were measured, the mean ± SD of the SCL and weight were 36.11±11.18 cm (range, 13.00–85.20 cm) and 9.33±8.21 kg (range, 0.27–55.5 kg), respectively. Thus, 69.34% (n = 898) of these turtles were classified as pelagic juveniles, 30.27% (n = 392) as juveniles-subadults, and only 0.39% (n = 5) as adults. 86.66% of turtles (n = 1,612) were classified as undetermined gender, 11.66% (n = 217) were sexed as females and 1.66% (n = 31) as males. In the group of turtles whose sex was determined the sex ratio was female-biased (7/1).
Distribution of causes of morbidity
Number of cases and frequency distribution by causes of admission are shown in Table 1. The most frequent causes of morbidity were entanglement in fishing gear and/or plastics (50.81%, n = 945), unknown/undetermined (20.37%, n = 379), and ingestion of hooks and monofilament lines (11.88%, n = 221). The other primary causes had frequencies below 6%.
Table 1. Number of cases and frequency distribution by causes of admission in loggerhead turtles with different straight carapace length (SCL) stranded during the period 1998–2014.
| Cause of admission | Number of cases | |||||
|---|---|---|---|---|---|---|
| SCL < 42 cm | SCL ≥ 42–70 cm | SCL ≥ 70 cm | Unknown SCL | TOTAL | (%) | |
| Entanglement | 560 | 131 | 0 | 254 | 945 | 50.8 |
| Hooks/monofilament lines | 28 | 141 | 1 | 51 | 221 | 11.9 |
| Trauma (boat strike) | 35 | 35 | 1 | 26 | 97 | 5.2 |
| Infectious disease | 70 | 13 | 0 | 20 | 103 | 5.5 |
| Crude oil | 31 | 4 | 0 | 17 | 52 | 2.8 |
| Other causes | 37 | 10 | 1 | 15 | 63 | 3.4 |
| Ingestion of plastics | 7 | 4 | 0 | 8 | 19 | 1 |
| Buoyancy disorder | 3 | 3 | 0 | 0 | 6 | 0.3 |
| Shark attack | 3 | 1 | 0 | 1 | 5 | 0.3 |
| Malnutrition | 17 | 0 | 0 | 7 | 24 | 1.3 |
| Miscellany | 7 | 2 | 0 | 0 | 9 | 0.5 |
| Unknown/undetermined | 137 | 58 | 2 | 182 | 379 | 20.4 |
| TOTAL | 898 | 392 | 5 | 565 | 1,860 | 100 |
No differences between genders related to any of the analyzed causes were observed. Pelagic juvenile turtles (SCL < 42 cm) had a significant higher risk of entanglement (OR = 3.3; 95%CI: 2.6–4.3; P <0.0001), infectious disease (OR = 2.4; 95%CI: 1.3–4.5; P = 0.02) and crude oil (OR = 3.5; 95%CI: 1.2–10; P = 0.012) compared to juvenile/subadult and adult turtles. Conversely, the ≥42–70 cm SCL group had a significant higher risk of ingestion of hooks and monofilament lines (OR = 16.9; 95%CI: 11.1-25-8; P <0.0001) and trauma (OR = 2.3; 95%CI: 1.4–3.8; P <0.0001) compared to the other groups.
Seasonality
Admissions were distributed as follows: 38.33% (n = 713) in summer, 27.20% (n = 506) in spring, 21.77% (n = 405) in fall, and 12.69% (n = 236) in winter.
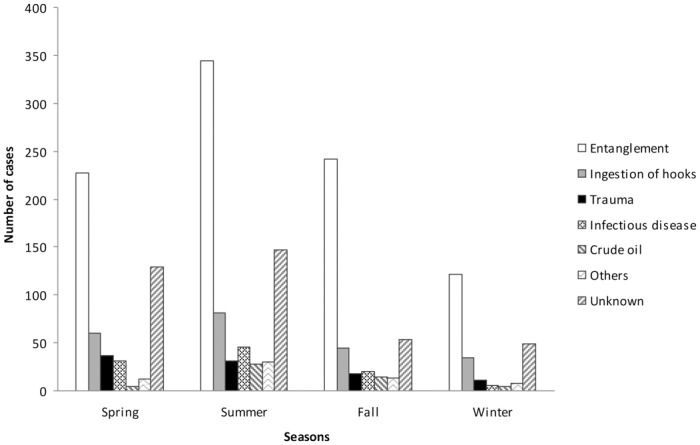
Seasonal variation in causes of admission is shown in Fig 1. Entanglement occurred quite frequently during all seasons (spring, 24.27%, n = 227; summer, 36.79%, n = 344; fall, 25.88%, n = 242; winter, 13.04%, n = 122), but it was significantly more prevalent in summer and fall (χ2 = 105.91, P < 0.0001). A significantly higher number of crude oil cases were observed in summer and fall (χ2 = 27.23, P < 0.0001). Ingestion of hooks, trauma, infectious disease, and unknown causes were more prevalent in spring and summer as compared to fall and winter (χ2 = 21.73, P < 0.0001; χ2 = 17.43, P = 0.001; χ2 = 33.42, P < 0.0001; χ2 = 81.89, P < 0.0001; respectively). We detected significant differences between seasons when other causes category was analyzed (χ2 = 18.07, P = 0.0001), being more prevalent in summer (47.61%, n = 30).
Fig 1. Seasonal variations in causes of admission during 1998–2014.
Annual variation of causes of morbidity
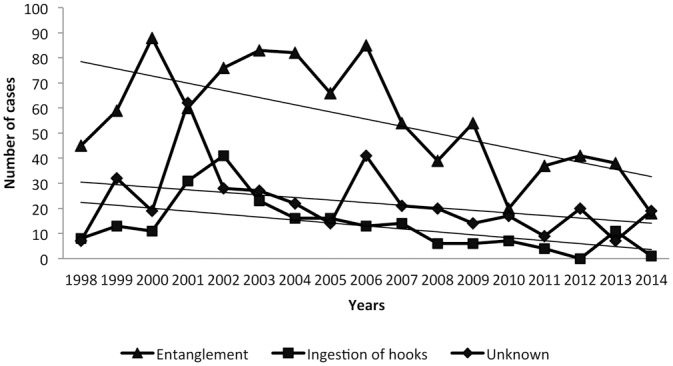
The total number of admissions peaked during 2001–2003 and since then decreased. Annual variation in causes of admission is shown in Fig 2. A significant decrease of cases was detected among the seventeen years of the study in all the stranding categories (Fig 3). Particularly interesting is the annual variation of cases of crude oil: 84.61% (n = 44) of crude oil admissions occurred during 1998–2005, detecting an important decrease since 2006.
Fig 2. Annual variation in causes of admission of loggerhead turtles among the period 1998–2014.
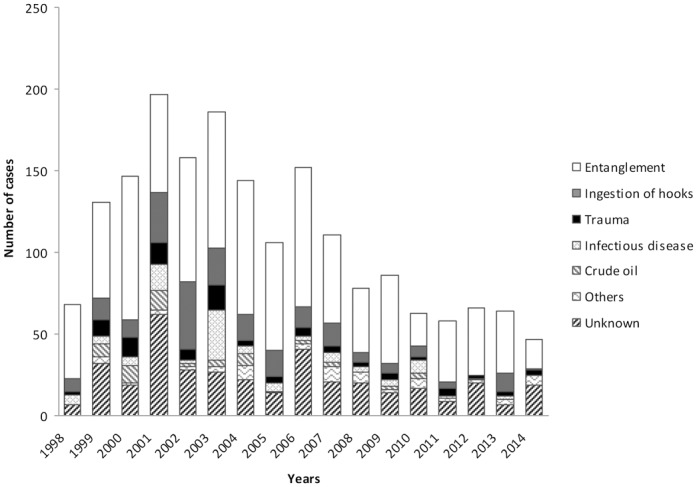
Fig 3. Tendency of the three most frequent causes of admissions during the period 1998–2014.
Final disposition
A total of 226 loggerhead turtles were dead when admitted. In these turtles, the most frequent causes of mortality were unknown/undetermined causes (70.35%, n = 159), entanglement (11.50%, n = 26), trauma (9.37%, n = 22), and ingestion of hooks (6.19%, n = 14).
The final disposition of the 1,634 loggerhead turtles admitted alive showed the following rates: Er = 3.37% (n = 55), Mr = 10.34% (n = 169), Rr = 86.29% (n = 1,410). The final dispositions by causes of admission are shown in Table 2.
Table 2. Final disposition of the loggerhead turtles admitted alive during the period 1998–2014.
| Cause of admission | Number of turtles | Final disposition | |||||
|---|---|---|---|---|---|---|---|
| Euthanized | Dead | Released | |||||
| Number | Er (%) | Number | Mr (%) | Number | Rr (%) | ||
| Entanglement | 919 | 21 | 2.28 | 49 | 5.33 | 849 | 92.38 |
| Hooks/monofilament lines | 207 | 7 | 3.38 | 36 | 17.39 | 164 | 79.22 |
| Trauma (boat strike) | 75 | 14 | 18.67 | 23 | 30.67 | 38 | 50.67 |
| Infectious disease | 102 | 6 | 5.88 | 26 | 25.49 | 70 | 68.62 |
| Crude oil | 49 | 1 | 2.04 | 2 | 4.08 | 46 | 93.87 |
| Other causes | 62 | 2 | 3.22 | 12 | 19.35 | 48 | 77.41 |
| Ingestion of plastics | 18 | 0 | 0 | 2 | 11.11 | 16 | 88.89 |
| Buoyancy disorders | 6 | 0 | 0 | 1 | 16.67 | 5 | 83.33 |
| Shark attack | 5 | 0 | 0 | 1 | 20 | 4 | 80 |
| Malnutrition | 24 | 0 | 0 | 8 | 33.33 | 16 | 66.67 |
| Miscellany | 9 | 2 | 22.22 | 0 | 0 | 7 | 77.78 |
| Unknown/undetermined | 220 | 4 | 1.81 | 21 | 9.54 | 195 | 88.63 |
| TOTAL | 1,634 | 55 | 3.37 | 169 | 10.34 | 1,410 | 86.29 |
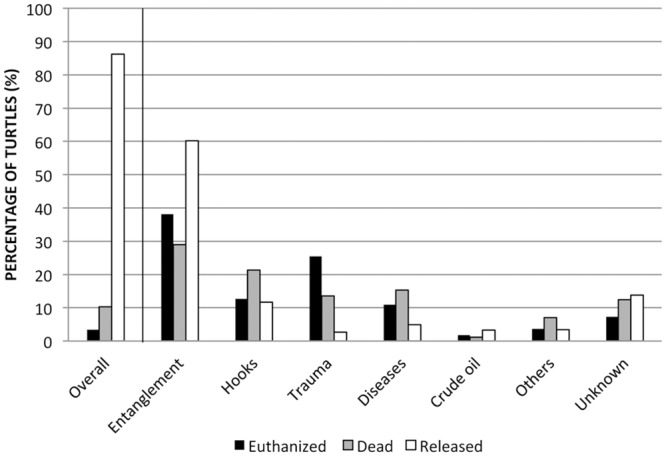
The euthanasia rate was significantly higher in the trauma category (18.67%) compared to the other main causes of admission (Fig 4). Turtles admitted due to trauma and infectious diseases had the highest unassisted mortality rates, 30.67% and 25.49%, respectively. The release rate was significantly higher in the crude oil (93.87%) and entanglement (92.38%) categories compared to the cause with the lowest release rate (trauma, 50.67%). In the subgroup of turtles with SCL known, only a significant difference was detected when release rates of pelagic juveniles and juvenile/subadult turtles for the unknown/undetermined category were compared (P = 0.021).
Fig 4. Resolution rates of euthanized (Er), dead (Mr), and released (Rr) loggerhead turtles relative to the overall population and the main cause of admission.
Time until death and length of stay at the TWRC
Within the group of euthanized turtles the longest median Td was observed for the unknown category (Td = 85 days), whereas the shortest median Td was recorded for the trauma category (Td = 1 day) (Table 3). The median Td in the dead turtles ranged from 1.5 days (crude oil) to 8 days (entanglement, trauma, and other causes). Within the group of released turtles the median time of stay in the TWRC ranged from 12 days (unknown) to 70 days (trauma).
Table 3. Statistical descriptive of time that loggerheads spent in the TWRC until the final disposition.
| Time (days) from admission to final disposition | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Euthanasia | Unassisted mortality | Release | |||||||||||||
| CAUSE OF ADMISSION | P10 | P25 | P50 | P75 | P90 | P10 | P25 | P50 | P75 | P90 | P10 | P25 | P50 | P75 | P90 |
| Entanglement | 0 | 0 | 2 | 16.5 | 45.6 | 1 | 3 | 8 | 24 | 70 | 3 | 11 | 26 | 52 | 93 |
| Hooks | 0 | 0 | 3 | 61 | 70 | 0.6 | 1 | 3 | 13 | 30 | 7 | 19 | 38 | 67 | 151.1 |
| Trauma | 0 | 0 | 1 | 4.25 | 83 | 1 | 2 | 8 | 30 | 155.2 | 17.1 | 39.5 | 70 | 132 | 331.1 |
| Infectious disease | 2 | 4.25 | 26.5 | 44.7 | 47 | 0 | 1 | 4 | 16 | 32.7 | 12 | 19 | 43 | 63 | 132 |
| Crude oil | N/A | N/A | N/A | N/A | N/A | 1 | 1 | 1.5 | 2 | 2 | 2.8 | 9.5 | 16 | 29 | 52.4 |
| Other causes | 1 | 1 | 32.5 | 64 | 64 | 0.6 | 2 | 8 | 28.5 | 41.8 | 10.7 | 18.5 | 40 | 70.7 | 127 |
| Ingestion of plastics | - | - | - | - | 17 | 17 | 24.5 | 32 | 32 | 10 | 13 | 21.5 | 44 | 76.6 | |
| Buoyancy disorder | - | - | - | - | N/A | N/A | N/A | N/A | N/A | 2 | 8 | 36 | 92 | 127 | |
| Shark attack | - | - | - | - | N/A | N/A | N/A | N/A | N/A | 39 | 41.2 | 53.5 | 102.5 | 117 | |
| Malnutrition | - | - | - | - | 0 | 2 | 2.5 | 18.7 | 46 | 18 | 35.2 | 43 | 112.7 | 154 | |
| Miscellany | 1 | 1 | 32.5 | 64 | 64 | - | - | - | - | 2 | 14 | 56 | 118 | 270 | |
| Unknown/ undetermined | 39 | 39 | 85 | 428 | 428 | 1 | 1 | 4 | 9.5 | 40 | 1 | 5 | 12 | 29 | 57 |
P10, P25, P50, P75, P90: percentiles 10, 25, 50 (median), 75 and 90; N/A: not applicable (only one case)
Discussion
Health status and anthropogenic threats of free-living sea turtle species are usually evaluated using as an important source of information the epidemiological studies of the causes of morbidity and mortality of turtles admitted to wildlife rehabilitation centers [9,14,21,26]. However, studies of the causes of morbidity of mortality of sea turtles covering more than one decade are scarce [14–16,21,29]. The present retrospective study included data of a long period (17 years), allowing a more accurate analysis of the annual variations and trends of the different causes of admission.
In our survey, the mean SCL of the stranded loggerheads was similar to that described for loggerheads from Madeira [34]. However, loggerheads from Azores have been reported to be smaller than those from Madeira, probably because they arrive first to Azores [35]. We found that the sex ratio in the group of turtles whose sex was determined was strongly female-biased (7/1). A 3.9/1 female-biased sex ratio was reported in a study conducted on 89 loggerheads stranded in Florida [26]. Loggerheads in the Canary Islands mainly come from the US western Atlantic by the Gulf Stream [3]. It has been described that three subpopulations (NE Florida, SE Florida, and Yucatán) produce jointly an estimated 75% female hatchlings [35]. However, laparoscopies as well as hormonal essays performed on loggerheads in Madeira indicated a female-biased sex ratio of 2/1 [35].
In our study, entanglement in fishing gear and/or plastics (synthetic raffia) was the main cause of admission (50.81%). Although entanglement has been reported as a common cause of morbidity and mortality in sea turtles [14,29,36,37], the prevalence of entanglement in the present study was higher than that reported in other surveys. Whereas the prevalence of entanglement in a retrospective study conducted on 3,861 sea turtles in Hawaii over a 22-year period was 5% [14], evidence of lesions caused by fishing gear was observed in 11.28% of the 5,938 turtles stranded in Italy during 1980–2008 [29]. The low prevalence of entanglement in Hawaii was also consequence of the high prevalence of fibropapillomatosis (28%) in the region [14]; however, no cases of fibropapillomatosis were reported in Italy [29]. Interactions with the activity of the canarian artisanal fishery are not very common; however, potential interactions between loggerheads and fisheries can take place in waters off Canary Islands mainly when “trasmallos” (a fishing net) are used to catch a wide range of fish species, from sharks and skates to many sparids, striped red mullet (Mullus surmuletus), etc., especially at the end of the summer [38]. Sometimes it is difficult to assess whether entanglement is the consequence of capture in fishing gear or of floating discarded fishing gear; in addition, entanglement in floating material like plastics is also an increasing problem [29]. In this way, it is remarkable than the use of synthetic raffia in the Canary Islands, particularly in the islands devoted to intensive agriculture, is very common. Ruminal disorders in goats due to ingestion of synthetic raffia have been reported in Gran Canaria Island [39]. Legal actions including the compulsory use of biodegradable raffia must be implemented in order to minimize its impact on sea turtle stranding in the Canary Islands.
Unknown/undetermined origin was the second category of stranding (20.37%) in our study. This prevalence was lower than the 49% reported for green turtle strandings in Hawaii [14]. Financial and time constraints usually make difficult to establish an accurate diagnosis in many stranded sea turtles [29]. In addition, rapid development of autolysis can impede more detailed examinations of the carcasses and the histological evaluation of the specimens [14,30]. There are also examples in which, although huge efforts were devoted to identify the cause of stranding, the specific cause could not be satisfactorily identified [23].
Ingestion of hooks and monofilament lines was the third cause of stranding (11.88%) in our survey. This prevalence was higher than that reported in green turtles stranded in Hawaii during 1982–2003 (7%) [14]. However, in a study on non-fibropapilloma causes of mortality in green turtles from Hawaii and the insular Pacifico, foreign body ingestion, including hooks and fishing lines, was observed in 12% of the turtles [16]. Estimated total take of sea turtles in the international waters in the North of the Canary Islands for 1983 to 1991 was 3,000 turtles [40]. However, more recently, impacts of fishing effort have been reported to be decreasing in the Canary Current because fish stocks are depleted throughout the region and management authorities are striving to reduce the fishing pressure [41]. Longline in waters off Canary Islands is deployed to catch benthopelagic to bathipelagic fish species, such as the european hake (Merluccius merluccius), the common mora (Mora moro) and even the meagre (Argyrosomus regius). All these species and other similar are caught with hooks, often baited [42]. In addition, other fishing fleets work in waters off Canary Islands using large longlines to catch sword fish (Xiphias gladius) [43]. Although many of the turtles are released by the fishermen, it has been estimated that approximately 15–50% of the turtles die due to the severe lesions induced by the fishing hooks [44]. Lesions induced by hooks and monofilament lines have been deeply described [15,16,30,45].
In our study, all the other primary causes had frequencies below 6%. As previously reported, it is remarkable the absence of fibropapillomatosis in the loggerheads stranded in the Canary Islands because sea turtle fibropapillomatosis is a disease of global distribution [30]. The prevalence of fibropapillomatosis in a retrospective study conducted on 3,861 stranded green turtles in Hawaii over a 22-year period was 28% [14]. Similarly, the prevalence of fibropapillomatosis in a retrospective survey conducted on 3,016 stranded green turtles in Florida during the period extending from 1980 to 1998 was 22.6% [21]. However, fibropapillomatosis was not present in other Pacific islands examined by Work et al. [16], and no cases of fibropapillomatosis were reported in a retrospective study conducted on 5,938 stranded loggerheads in Italy during 1980–2008 [29].
As previously reported in 2005 [30] no cases of spirorchiid infection were observed among loggerheads admitted to the TWRC during 1998–2014. Spirorchiid trematodes are implicated as an important cause of stranding and mortality in sea turtles worldwide [7,9,13,23]. In a retrospective study conducted on 100 stranded green turtles in Australia over a 4-year period, spirorchiidiasis was found to be the most frequently cause of mortality (41.8%) [9]. However, although high prevalence of spirorchiid infection was observed in a survey conducted on 148 sea turtles in Florida, most infections were regarded as incidental to the cause of death [26]. A high percentage of green turtles with fibropapillomatosis in Hawaii are also infected with spirorchiid trematodes [10,13]. Detection of infection with spirorchiids in turtles usually is done at necropsy, when adult worms or eggs are observed either grossly or at microscopy [13]. None of the necropsied loggerheads included in our study had intravascular adult flukes or trematode eggs in tissues. Antemortem detection of infection is also possible using enzyme-linked immunosorbent assays [13,46,47]. However, no antemortem diagnosis using serology was attempted on the loggerheads included in our study due to financial constraint. Work et al. [13] hypothesized that immature green turtles become infected with spirorchiids shortly after recruiting into coastal foraging pastures from the pelagic environment. All necropsied loggerheads included in our survey were juvenile and subadult specimens but different alimentary habits of both species can explain the absence of spirorchiid fluke infection. Sea turtles acquire the flukes by ingesting unknown cercaria-rich intermediate hosts. Although preliminary results on detection of spirorchiids in gastropod tissues by polymerase chain detection have been reported [48], the only life cycles that have been established in this group were for species in the freshwater genera Enterohaemototrema, Spirorchis, and Vasotrema [49].
Because the Canary Islands are included throughout the year in a thermal gradient centered on the 21°C isotherm [50], no cases of cold-stunning were observed among loggerheads included in our study. However, hypothermic stunning events have been described affecting high number of sea turtles in Florida [20,24].
In our survey, seasonal analysis of the strandings showed that these were more frequent in summer, probably reflecting loggerheads are more abundant around the Canary Islands in this season. It has also been reported that loggerheads are more abundant in Madeiran waters during the summer months [35]. In addition, the total number of admissions included in our study peaked during 2001–2003 and since then decreased. Several studies have indicated that the abundance of loggerhead nests along the Atlantic coast and in southwestern Florida is declining [51,52]. Because loggerheads in the Canary Islands mainly come from the US western Atlantic, decline in nest abundance on those beaches could have a negative impact on the juvenile and subadult loggerhead population around the Canary Islands. Skeletochronology of mostly loggerheads from Madeira showed the duration of the oceanic stage to be equal or longer than 7 years [53]. No studies on determination of the age of the loggerheads around the Canary Islands have been published. Assuming this age is similar to that reported for Madeiran loggerheads, the decrease observed in our study, particularly since 2003, could be consequence of the decrease of nests on the US western Atlantic coast since mid 90’s. Estimated declines in nest abundance on the Atlantic and southwestern coasts of Florida ranged from 29% to 37% between 1989 and 2006 [52].
It is remarkable that admissions due to crude oil decreased significantly since 2006. In a previous study on crude oil as stranding cause among loggerheads in the Canary Islands, authors concluded that the designation of the Canary Islands as a Particularly Sensitive Sea Area (PSSA) in 2005 by the International Maritime Organization (IMO) was associated with positive effects on the reduction of sea turtle strandings caused by crude oil [54]. A PSSA is an area of the marine environment that needs special protection through action by the IMO because of its significance for recognized ecological, socio-economic, or scientific attributes where such attributes may be vulnerable to damage by international shipping activities [55]. The associated protective measures after the international recognition of the waters of the Canary Islands as PSSA were adopted in 2006, and included: traffic separation systems (recommended routes), areas to be avoided, and mandatory ship reporting system [55].
Wildlife clinical practice guidelines dealing with welfare rehabilitation standards and pre-release health screening protocols have been published [56,57], but no quality indicators of the rehabilitation process of injured sea turtles have been defined. In our study, we analyzed the outcomes of the rehabilitation of free-living loggerhead turtles at the TWRC, adopting the three categories of the final disposition, the time until death and the length of stay, as indicators of the quality audit of the rehabilitation process before release into the wild.
According to our data, 86.29% of loggerheads admitted alive to the TWRC were successfully released, and only 13.71% of turtle admissions resulted in euthanasia or unassisted mortality. References on the final dispositions of sea turtle rehabilitation are scarce because they usually have been focused on the causes of mortality [14].
Based on animal welfare, euthanasia is a final option in all wildlife species rehabilitation [58]. In our survey, the overall rate of euthanasia was 3.37%, and the highest value was found in the trauma (boat strikes) category (18.67%). Turtles with boat strike injuries usually have severe fractures of the carapace/plastron, and severe traumatic lesions mainly penetrating into the lungs and kidneys [30]. Affection of these vital organs, because the anatomical location, dorsally attached to the carapace, explain the generally poor prognosis for turtles with severe traumatic injuries in the carapace [30].
Unassisted mortality rate has been used as a quality indicator parameter in rehabilitation of birds of prey [59]; however, no reports on quality auditing of the rehabilitation process in free-living sea turtles admitted at marine rehabilitation centers have been published. In our study, the overall rate of unassisted mortality was 10.34%, and the highest value was found in the trauma (boat strikes) category (30.67%). The reasons for the poor prognosis for these turtles have been explained above. A high Mr (25.49%) was also found in the infectious disease category. According to the necropsy findings and microbiological studies, septicemia was diagnosed in the majority of those cases [30].
The release rate in our survey was higher in the crude oil (93.87%) and entanglement (92.38%) categories compared to the cause with the lowest release rate (trauma, 50.67%). Ingestion of crude oil and subsequent internal lesions can threaten sea turtle survival, but lesions in the skin, carapace, and plastron are not fatal in the majority of cases [53]. Entanglement in fishing gear and/or plastics can result in severe ulcerative dermatitis, and amputation of flippers [30]; however, sea turtles cope with amputations well, regardless of whether the amputation is front or rear [60].
The parameter time to death provides direct insight into the initial assessment and prognostication, the overall rehabilitation process, and the validity of veterinary protocols [59]. In our study, the shortest median time to euthanasia was recorded for the trauma category (1 day) meaning that the decision is made very soon based on the poor prognosis of these cases as was discussed above. Within the group of dead turtles during the rehabilitation process, the median time to death ranged from 1.5 days (crude oil) to 8 days (entanglement, trauma, and other causes). This fact suggests that first week of stay at the rehabilitation center is critical, and intensive cares should be performed on all turtles during the first week, despite their apparently less severe appearance.
The parameter length of stay must be as short as possible to reduce the risk of captive-related complications, infectious diseases, and behavioral disorders [61]. In our study, the median time of stay in the TWRC ranged from 12 days (unknown) to 70 days (trauma). This fact suggests that especially turtles admitted due to trauma represent an important consume of time and efforts.
In conclusion, this survey is the first large-scale epidemiological study on causes of stranding and mortality of Eastern Atlantic loggerhead sea turtles, providing useful information for the conservation of these reptiles. In absence of diseases commonly reported in other regions, such as fibropapillomatosis [14,21] and spirorchiidiasis [7,9,13,23,26], at least 71.72% (n = 1,334) of loggerheads included in our study stranded due to anthropogenic causes, what deserves critical reflection, especially taking into account that stranded loggerhead turtles may represent as little as 7% of the at-sea mortality of sea turtles [62]. In addition, the high survival rate for stranded loggerheads (86.29%) achieved at the TWRC emphasizes the importance of marine rehabilitation centers for the conservation of sea turtles. Finally, we propose that at least the stratified analysis by causes of admission of the three final disposition rates (Er, Mr, and Rr), and the parameters time until death (Td) and length of stay at the center (Tr) should be included in the outcome research of the rehabilitation process of free-living sea turtles in order to allow comparative studies between marine rehabilitation centers around the world.
Supporting Information
(XLSX)
Acknowledgments
We thank all the staff of the Tafira Wildlife Rehabilitation Center (Cabildo Insular de Gran Canaria). We are grateful to J. Rocha and A. Santana from the Department of Mathematics of the University of Las Palmas de Gran Canaria for the technical advice on statistical methods. We also thank V. Hernández-García (University of Las Palmas de Gran Canaria) for providing us with proper information about the current activity of the canarian artisanal fishery and the fishing activity carried out in waters off Canary Islands.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Pritchard PCH. Evolution, phylogeny, and current status In: Lutz PL, Musick JA, editors. The biology of sea turtles. Boca Raton, FL: CRC Press; 1997. pp. 1–28. [Google Scholar]
- 2.IUCN/SSC website. The IUCN red list of threatened species. 2015. Available: http://www.iucnredlist.org
- 3.Monzón-Argüello C, Rico C, Carreras C, Calabuig P, Marco A, López-Jurado LF. Variation in spatial distribution of juvenile loggerhead turtles in the eastern Atlantic and western Mediterranean Sea. J Exp Mar Biol Ecol. 2009;373: 79–86. [Google Scholar]
- 4.Glazebrook JS, Campbell RS, Blai D. Studies on cardiovascular fluke (Digenea: Spirorchiidae) infections in sea turtles from the Great Barrier Reef, Queensland, Australia. J Comp Pathol. 1989;101: 231–250. [DOI] [PubMed] [Google Scholar]
- 5.Glazebrook JS, Campbell RSF. A survey of the diseases of marine turtles in northern Australia II. Oceanarium-reared and wild turtles. Dis Aquat Org.1990;9: 97–104. [Google Scholar]
- 6.Gordon AN, Kelly WR, Lester RJ. Epizootic mortality of free-living green turtles, Chelonia mydas, due to coccidiosis. J Wildl Dis. 1993;29: 490–494. [DOI] [PubMed] [Google Scholar]
- 7.Gordon AN, Kelly WR, Cribb TH. Lesions caused by cardiovascular flukes (Digenea: Spirorchidae) in stranded green turtles (Chelonia mydas). Vet Pathol. 1998;35: 21–30. [DOI] [PubMed] [Google Scholar]
- 8.Raidal SR, Ohara M, Hobbs RP, Prince RI. Gram-negative bacterial infections and cardiovascular parasitism in green sea turtles (Chelonia mydas). Aust Vet J. 1998;76: 415–417. [DOI] [PubMed] [Google Scholar]
- 9.Flint M, Patterson-Kane JC, Limpus CJ, Mills PC. Health surveillance of stranded green turtles in southern Queensland, Australia (2006–2009): An epidemiological analysis of causes of disease and mortality. EcoHealth. 2010;7: 135–145. 10.1007/s10393-010-0300-7 [DOI] [PubMed] [Google Scholar]
- 10.Aguirre AA, Spraker TR, Balazs GH, Zimmerman B. Spirorchidiasis and fibropapillomatosis in green turtles from the Hawaiian Islands. J Wildl Dis. 1998;34: 91–98. [DOI] [PubMed] [Google Scholar]
- 11.Work TM, Balazs GH, Wolcott M, Morris R. Bacteraemia in free-ranging Hawaiian green turtles Chelonia mydas with fibropapillomatosis. Dis Aquat Org. 2003;53: 41–46. [DOI] [PubMed] [Google Scholar]
- 12.Work TM, Balazs GH, Rameyer RA, Morris RA. Retrospective pathology survey of green turtles Chelonia mydas with fibropapillomas in the Hawaiian Islands, 1993–2003. Dis Aquat Org. 2004;62: 163–176. [DOI] [PubMed] [Google Scholar]
- 13.Work TM, Balazs GH, Schumacher JL, Amarisa M. Epizootiology of spirorchiid infection in green turtles (Chelonia mydas) in Hawaii. J Parasitol. 2005;91: 871–876. [DOI] [PubMed] [Google Scholar]
- 14.Chaloupka M, Work TM, Balazs GH, Murakawa S, Morris R. Cause-specific temporal and spatial trends in green sea turtle strandings in the Hawaiian Archipelago (1982–2003). Mar Biol. 2008;154: 887–898. [Google Scholar]
- 15.Work TM, Balazs GH. Pathology and distribution of sea turtles landed as bycatch in the Hawaii-based North Pacific pelagic longline fishery. J Wildl Dis. 2010;46: 422–432. [DOI] [PubMed] [Google Scholar]
- 16.Work TM, Balazs GH, Summers TM, Hapdei JR, Tagarino AP. Causes of mortality in green turtles from Hawaii and the insular Pacific exclusive of fibropapillomatosis. Dis Aquat Org. 2015;115: 103–110. 10.3354/dao02890 [DOI] [PubMed] [Google Scholar]
- 17.Smith GM, Coates CW. Fibro-epithelial growths of the skin in large marine turtles, Chelonia mydas (Linnaeus). Zool. 1938;23: 93–98. [Google Scholar]
- 18.Wolke RE, Brooks DR, George A. Spirorchidiasis in loggerhead sea turtles (Caretta caretta): pathology. J Wildl Dis. 1982;18: 175–185. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson ER, Mansell JL, Sundberg JP, Hajjar L, Reichmann ME, Ehrhart LM et al. Cutaneous fibropapillomas of green turtles (Chelonia mydas). J Comp Pathol. 1989;101: 39–52. [DOI] [PubMed] [Google Scholar]
- 20.Witherington BE, Ehrhart LE. Hypothermic stunning and mortality of marine turtles in the Indian River Lagoon System, Florida. Copeia. 1989;1989: 696–703. [Google Scholar]
- 21.Foley AM, Schroeder BA, Redlow AE, Fick-Child KJ, Teas WG. Fibropapillomatosis in stranded green turtles (Chelonia mydas) from the eastern United States (1980–98): trends and associations with environmental factors. J Wildl Dis. 2005;41: 29–41. [DOI] [PubMed] [Google Scholar]
- 22.Greenblatt RJ, Work TM, Dutton P, Sutton CA, Spraker TR, Casey RN et al. Geographic variation in marine turtle fibropapillomatosis. J Zoo Wildl Med. 2005;36: 527–530. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson ER, Homer BL, Stacy BA, Greiner EC, Szabo NJ, Chrisman CL et al. Neurological disease in wild loggerhead sea turtles Caretta caretta. Dis Aquat Org. 2006;70: 139–154. [DOI] [PubMed] [Google Scholar]
- 24.Foley AM, Singel KE, Dutton PH, Summers TM, Redlow AE, Lessman J. Characteristics of a green turtle (Chelonia mydas) assemblage in northwestern Florida determined during a hypothermic stunning event. Gul Mex Sci. 2007;25: 131–143. [Google Scholar]
- 25.Stacy BA, Wellehan JF, Foley AM, Coberley SS, Herbst LH, Manire CA et al. Two herpesviruses associated with disease in wild Atlantic loggerhead sea turtles (Caretta caretta). Vet Microbiol. 2008;126: 63–73. [DOI] [PubMed] [Google Scholar]
- 26.Stacy BA, Foley AM, Greiner EC, Herbst LH, Bolten AB, Klein PA et al. Spirorchiidiasis in stranded loggerhead Caretta caretta and green turtles Chelonia mydas in Florida (USA): Host pathology and significance. Dis Aquat Org. 2010;89: 237–259. 10.3354/dao02195 [DOI] [PubMed] [Google Scholar]
- 27.Deus Santos MR, Silva Martins A, Baptistotte C, Work TM. Health condition of juvenile Chelonia mydas related to fibropapillomatosis in southeast Brazil. Dis Aquat. Org. 2015;115: 193–201. 10.3354/dao02883 [DOI] [PubMed] [Google Scholar]
- 28.Duguy R, Morinière P, Le Milinaire C. Facteurs de mortalité observés chez les tortues marines dans le golfe de Gascogne. Oceanol Acta. 1998;21: 383–388. [Google Scholar]
- 29.Casale P, Affronte M, Insacco G, Freggi D, Vallini C, d’Astore PP et al. Sea turtle strandings reveal high anthropogenic mortality in Italian waters. Aquatic Conserv: Mar Freshw Ecosyst. 2010;20: 611–620. [Google Scholar]
- 30.Orós J, Torrent A, Calabuig P, Déniz S. Diseases and causes of mortality among sea turtles stranded in the Canary Islands, Spain (1998–2001). Dis Aquat Org. 2005;63: 13–24. [DOI] [PubMed] [Google Scholar]
- 31.Bjorndal KA, Bolten AB, Martins HR. Somatic growth model of juvenile loggerhead sea turtles Caretta caretta: duration of pelagic stage. Mar Ecol Prog Ser. 2000;202: 265–272. [Google Scholar]
- 32.Seminoff JA, Resendiz A, Resendiz B, Nichols WJ. Occurrence of loggerhead sea turtles (Caretta caretta) in the Gulf of California, México: evidence of life-history variation in the Pacific ocean. Herpetol Rev. 2004;35: 24–27. [Google Scholar]
- 33.Casal AB, Camacho M, López-Jurado LF, Juste C, Orós J. Comparative study of hematologic and plasma biochemical variables in Eastern Atlantic juvenile and adult nesting loggerhead sea turtles (Caretta caretta). Vet Clin Pathol. 2009;38: 213–218. 10.1111/j.1939-165X.2008.00106.x [DOI] [PubMed] [Google Scholar]
- 34.Bolten AB, Martins HR, Bjorndal KA, Gordon J. Size distribution of pelagic-stage loggerhead sea turtles (Caretta caretta) in the waters around the Azores and Madeira. Arquipélago. 1993;11: 49–54. [Google Scholar]
- 35.Dellinger T. Behavioural ecology and conservation of oceanic-stage sea turtles: the Madeira Island loggerhead sea turtle project In: López-Jurado LF, Loza AA, editors. Marine turtles: recovery of extinct populations. Las Palmas de Gran Canaria: Instituto Canario de Ciencias Marinas; 2007. pp. 97–109. [Google Scholar]
- 36.George RH. Health problems and diseases of sea turtles In: Lutz PL, Musick JA, editors. The biology of sea turtles. Boca Raton, FL: CRC Press; 1997. pp. 363–385. [Google Scholar]
- 37.Lutcavage ME, Plotkin P, Witherington B, Lutz PL. Human impacts on sea turtle survival In: Lutz PL, Musick JA, editors. The biology of sea turtles. Boca Raton, FL: CRC Press; 1997. pp. 387–409. [Google Scholar]
- 38.Castro JJ. Artes y embarcaciones de pesca In: Bas C, Castro JJ, Hernández-García V, Lorenzo JM, Moreno T, Pajuelo JG et al. , editors. La pesca en Canarias y áreas de influencia. Las Palmas de Gran Canaria: Ediciones del Cabildo de Gran Canaria; 1995. pp. 153–214. [Google Scholar]
- 39.Gutiérrez C, Corbera JA, Juste MC, Morales M, Montoya JA. La ruminotomía en los pequeños ruminates: estudio de 34 casos In: Beltrán de Heredia J, Urarte E, editors. II Jornadas Internacionales de la SEOC. Vitoria-Gasteiz: Diputación Foral de Álava; 1998. pp. 349–350. [Google Scholar]
- 40.Blanco JC, González JL. Libro rojo de los vertebrados españoles. Madrid: Ministerio de Agricultura, Pesca y Alimentación. Colección Técnica ICONA; 1992. 704 p. [Google Scholar]
- 41.Conant TA, Dutton PH, Eguchi T, Epperly SP, Fahy CC, Godfrey MH et al. Loggerhead sea turtle (Caretta caretta) 2009 status review under the U. S. Endangered Species Act. Report of the Loggerhead Biological Review team to the National Marine Fisheries Service. 2009. 222 p.
- 42.Bas C, Castro JJ, Hernández-García V, Lorenzo JM, Moreno T, Pajuelo JG et al. Las pesca en Canarias y áreas de influencia. Las Palmas de Gran Canaria: Ediciones del Cabildo de Gran Canaria; 1995. 331 p. [Google Scholar]
- 43.Hernández-García V. The diet of the swordfish Xiphias gladius Linnaeus, 1758, in the central east Atlantic with an emphasis on the role of cephalopods. Fish Bull. 1995;93: 403–411. [Google Scholar]
- 44.Lizana M, Barbadillo LJ. Legislación, protección y estado de conservación de los anfibios y reptiles españoles In: Pleguezuelos JM, editor. Distribución y biogeografía de los anfibios y reptiles en España y Portugal. Granada: Universidad de Granada Editorial; 1997. pp. 477–516. [Google Scholar]
- 45.Orós J, Calabuig P, Déniz S. Digestive pathology of sea turtles stranded in the Canary Islands between 1993 and 2001. Vet Rec. 2004;155: 169–174. [DOI] [PubMed] [Google Scholar]
- 46.Graczyk TK, Aguirre AA, Balazs GH. Detection by ELISA of circulating anti-blood flukes (Carettacola, Hapalotrema, and Laeradius) immunoglobulins in Hawaiian green turtles (Chelonia mydas). J Parasitol. 1995;81: 416–421. [PubMed] [Google Scholar]
- 47.Herbst LH, Greiner EC, Ehrhart LM, Bagley DA, Klein PA. Serological association between spirorchidiiasis, herpesvirus infection, and fibropapillomatosis in green turtles from Florida. J Wildl Dis. 1998;34: 496–507. [DOI] [PubMed] [Google Scholar]
- 48.Stacy BA, Frankovich T, Greiner EC, Alleman AR, Herbst LH, Klein PA et al. Detection of spirorchiid trematodes in gastropod tissues by polymerase chain reaction: preliminary identification of an intermediate host of Learedius learedi. J. Parasitol. 2010;96: 752–757. 10.1645/GE-2382.1 [DOI] [PubMed] [Google Scholar]
- 49.Smith JW. The blood flukes (Digenea: Sanguinicolidae and Spirorchidae) of cold-blooded vertebrates: Part 1. A review of the literature published since 1971, and bibliography. Heminthol Abstr. 1997;66: 255–294. [Google Scholar]
- 50.Llinás O, Rueda MJ, Pérez-Marrero J, Villagarcía M, Barrera C, Cianca A et al. Oceanographic conditions of the Macaronesian marine space. Relationships with the distribution and behavior of the sea turtle Caretta caretta In: López-Jurado LF, Loza AA, editors. Marine turtles: recovery of extinct populations. Las Palmas de Gran Canaria: Instituto Canario de Ciencias Marinas; 2007. pp. 35–49. [Google Scholar]
- 51.TEWG (Turtle Expert Working Group). An assessment of the loggerhead turtle population in the western North Atlantic. NOAA Technical Memorandum NMFS-SEFSC-575. 2009. 131 p.
- 52.Witherington BE, Kubilis P, Brost B, Meylan A. Decreasing annual nest counts in a globally important loggerhead sea turtle population. Ecol Appl. 2009;19:30–54. [DOI] [PubMed] [Google Scholar]
- 53.Bjorndal KA, Bolten AB, Dellinger T, Delgado C, Martins HR. Compensatory growth in oceanic loggerhead sea turtles: response to a stochastic environment. Ecol. 2003;84: 1237–1249. [Google Scholar]
- 54.Camacho M, Calabuig P, Luzardo OP, Boada LD, Zumbado M, Orós J. Crude oil as a stranding cause among loggerhead sea turtles (Caretta caretta) in the Canary Islands, Spain (1998–2011). J Wildl Dis. 2013;49: 637–640. 10.7589/2012-03-093 [DOI] [PubMed] [Google Scholar]
- 55.International Maritime Organization (IMO). PSSA Particularly Sensitive Sea Areas. London, UK: IMO Publication; 2007. 144 pp. [Google Scholar]
- 56.Woodford MH. Quarantine and health screening protocols for wildlife prior to translocation and release into the wild. In: Woodford MH, editor. Gland: IUCN Species Survival Commissions’s Veterinary Specialist Group; 2000. 87 p. [Google Scholar]
- 57.Miller EA. Minimum standards for wildlife rehabilitation. 4th Edition St. Cloud: NWRA & IWRC; 2012. 116 p. [Google Scholar]
- 58.Sleeman JM. Use of wildlife rehabilitation centres as monitors of ecosystem health In: Fowler ME, Miller RE, editors. Zoo and wild animal medicine. Saint Louis: Elsevier-Saunders; 2008. pp. 97–104. [Google Scholar]
- 59.Molina-López RA, Casal J, Darwich L. Final disposition and quality auditing of the rehabilitation process in wild raptors admitted to a wildlife rehabilitation centre in Catalonia, Spain, during a twelve year period (1995–2007). PLoS One. 2013;8: e60242 10.1371/journal.pone.0060242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wyneken J, Mader DR, Webber ES III, Merigo C. Medical care of sea turtles In: Mader DR, editor. Reptile medicine and surgery 2nd edition St. Louis: Saunders-Elsevier; 2006. pp. 972–1007. [Google Scholar]
- 61.Cooper JE, Cooper ME. Ethical and legal implications of treating casualty wild animals. In Practice. 2006;28: 2–6. [Google Scholar]
- 62.Epperly SP, Braun J, Chester AJ, Cross FA, Merriner JV, Tester PA et al. Beach strandings as an indicator of at-sea mortality of sea turtles. Bull Mar Sci. 1996;59: 289–297. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


