Abstract
Under twenty-first-century metropolitan conditions, almost all of our vision is mediated by cones and the photopic system, yet cones make up barely 5% of our retinal photoreceptors. This paper looks at reasons why we additionally possess rods and a scotopic system, and asks why rods comprise 95% of our retinal photoreceptors. It considers the ability of rods to reliably signal the arrival of individual photons of light, as well as the ability of the retina to process these single-photon signals, and it discusses the advantages that accrue. Drawbacks in the arrangement, including the very slow dark adaptation of scotopic vision, are also considered. Finally, the timing of the evolution of cone and rod photoreceptors, the retina, and the camera-style eye is summarised.
Contribution of cones and rods to human vision
Under twenty-first-century metropolitan conditions, almost all of our vision is mediated by the cone (photopic) system, yet cones make up barely 5% of our retinal photoreceptors. Rods, on the other hand, contribute to human vision only under quite restricted conditions—namely, after an extended period (often tens of minutes) at very low light levels, of the order of twilight or lower. Why then do we possess rods and a scotopic system? And why do rods make up 95% of our retinal photoreceptors when we so seldom utilise them?
The answers lie in the distant past, because during all but the most recent blip in our evolutionary history, our ancestors lived in near darkness for half their lives. Approximately 400–500 millions of years ago (Mya), a distant piscine ancestor of ours ‘invented' rods to supplement the pre-existing cones, and these new photoreceptors endowed the organism with a major survival advantage at the very lowest light levels, at night and in the ocean depths. That advantage has remained crucial ever since, apart perhaps from the last century or so.
A number of important differences between cones and rods, and between photopic and scotopic vision, are listed in Table 1.
Table 1. Comparison of cones and photopic vision with rods and scotopic vision in humans.
| Cones and photopic vision | Rods and scotopic vision | |
|---|---|---|
| Overall contribution | Cones underlie almost all our vision (under twenty-first century metropolitan light levels) | Rods contribute only under restricted conditions: after a considerable time at very low light levels |
| Spatial acuity | Extremely high (in the fovea) | Very low |
| Speed of response | Very fast (flicker detectable beyond 100 Hz) | Slow |
| Operating range | Enormous (twilight upwards, without limit) | Restricted (twilight downwards several log units) |
| Saturation | Cones do not saturate in steady light, no matter how bright | Rods saturate at roughly twilight levels, and are unresponsive at daytime lighting levels |
| Light adaptation | Cones adapt rapidly, exhibiting Weber Law desensitisation over a huge range of intensities | Rods adapt more slowly and over a narrower range of intensities; at the lowest intensities scotopic adaption follows approximately a square-root law |
| Contrast sensitivity | High (detect contrasts of 0.5%) | Low (need contrasts of ~5%) |
| Recovery of photocurrent after full bleach | Cones recover circulating current within 20 ms | Rods take 20 min to recover circulating current; that is, ~60 000 × slower than cones |
| Dark adaptation of visual system after full bleach | Photopic vision recovers full sensitivity in ~5 min | Scotopic vision takes ~40 min to recover full sensitivity |
| Colour vision | Trichromatic colour vision mediated by comparison of signals from three spectral classes of cone | – |
| Proportion of photoreceptors over entire retina | 5% | 95% |
| Proportion of photoreceptors in foveola | 100% | 0% |
Spatial acuity
Despite the fact that cones comprise only 5% of the total number of photoreceptors in the retina (4.6 million out 92 million), they are crowded at extremely high density into the rod-free foveola, and our very high visual acuity relies on as few as 100 000 cones (or 0.1% of the total number of photoreceptors) packed into this region.1, 2, 3 On the other hand, our spatial acuity in the peripheral field is very low, despite being cone mediated. As shown by Anderson et al,4 this has nothing to do with poor optics, because the off-axis optical quality is remarkably good, and nor indeed is it a direct reflection of the low density of cones in the periphery. Instead, this poor spatial acuity in the periphery results from the pooling by peripheral retinal ganglion cells of photopic signals across extensive retinal areas (see Figure 5 of Anderson et al4). For the scotopic system, the degree of spatial pooling of signals is even more extensive. Although the spatial density of the rods themselves is high, so that the potential for high spatial resolution is present, this potential is sacrificed through very powerful spatial pooling of rod-based signals, as will be described below.
Response kinetics
Cones respond to altered illumination very rapidly. Even in dim light (when they are slowest), the time to peak of the cones' response to a superimposed flash is as short as 20 ms.5 As the background intensity rises, the cones' response becomes faster, and in the presence of very bright light the photopic visual system is able to detect flicker in the peripheral retina at a frequency exceeding 100 Hz.6
Operating range and response saturation
Our cone system functions over a huge intensity range, from roughly twilight levels upwards. No matter how bright the background intensity, the cones are able to avoid saturation. (When dark-adapted cones are suddenly exposed to extremely bright illumination they may transiently saturate, but they rapidly return to normal operation.) Our rod system has an effective operating range of just a few log units, from the so-called ‘absolute dark light', corresponding to ∼0.01 photoisomerisations per s per rod, up to roughly twilight levels. Once this intensity level is reached, the rods enter saturation, with their circulating electrical current completely shut off, so that they are unresponsive to any increment in illumination.
Light adaptation
For cones and the photopic system, the dependence of sensitivity on background level conforms closely to Weber's Law, declining inversely with background intensity over many log units of intensity.7 For the scotopic system, the sensitivity also follows Weber's Law over a narrow range, though at the lowest intensities it instead follows an approximately square-root law (reviewed in Barlow8).
Contrast sensitivity
Cone-mediated vision can detect a contrast (either spatial or temporal) as small as 0.5%, whereas rod-mediated vision can at best detect a contrast of 5% at high scotopic intensities; at lower scotopic levels, the contrast required to trigger detection rises even further.
The great advantage of rods: single-photon processing in the retina
Given the poor performance of rods in certain regards and the restricted circumstances under which they contribute to our vision, it is natural to wonder why they evolved and why our modern-day retina is numerically so overwhelming dominated by them. The reason stems from a single property that the ancestral rods developed: the ability to respond reliably to individual photons of light.9, 10, 11 Although the sensitivity of rods is only a little higher than that of cones, and the amplification of the phototransduction cascade is essentially the same in the two classes of cell, rods manage to integrate the photon signal for longer, and also crucially, to reduce the fluctuations (noise) within the cell. In cones, the on-going cellular noise is large, and completely swamps the response to a single photon. However, in rods, the amplitude of the single-photon response substantially exceeds the cellular noise level and is reliably detectable above that noise.
How has this been beneficial to the organism? By permitting the evolution of retinal circuitry that can process these single-photon events, thereby providing scotopic visual sensitivity orders of magnitude better than in the cone system. As a result, a dark-adapted human subject can detect just a handful of photons hitting the retina.9
The synapse from the rod photoreceptor is reliably able to transmit the discrete quantal responses to the rod bipolar cell (RBC), as discovered from measurements of ERG b-wave sensitivity12 and from single-cell recordings.13, 14 The postsynaptic terminal at this synapse utilises a metabotropic mechanism involving a high-gain G protein-coupled cascade (reviewed in Morgans et al15). Remarkably, this cascade is broadly similar to that used in phototransduction and, as in the case of rhabdomeric photoreceptors, the final stage involves a TRP ion channel. This rod synapse appears to operate in a thresholding mode, substantially removing the ongoing photoreceptor dark noise.12, 13, 14 As a result, the response of the RBC comprises discrete single-photon events from each of the rods that synapse onto it, so that it too functions in a ‘photon detecting mode'.
It is instructive to consider what happens in total darkness. If we assume that the rods act as ideal photon detectors, and that the RBCs function as ideal collectors of these quantal signals, then in darkness the RBC signal will reflect the summation of the spontaneous ‘photon-like' events that are known to occur in rods.16, 17 Even in total darkness the rods experience quantal events indistinguishable from real photon hits, that result from spontaneous thermal isomerisations among the vast number of rhodopsin molecules crammed into the outer segment, and that occur in human rods at a rate of roughly one spontaneous event every 160 s. As each RBC collects from ~40 rods, then even in total darkness it will register a random stream of photon-like signals with a mean interval of ~4 s.
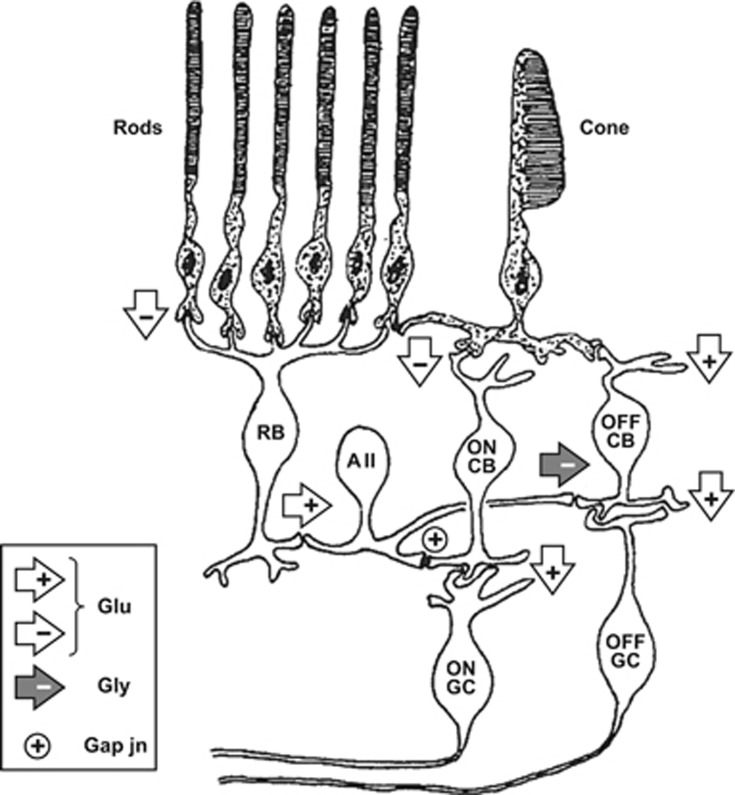
These RBC signals feed into retinal ganglion cells via a circuitous route (Figure 1) that evolution appears to have ‘piggy-backed' onto the classical, and pre-existing, photopic pathway of cone→cone bipolar cell→retinal ganglion cell18 (reviewed recently in Demb and Singer19)(Kolb & Famiglietti, 1975). Thus, the RBCs send their output to a dedicated class of amacrine cells, the AII amacrines, that inject their signals into the cone pathway (shown on the right in Figure 1) at the level of the cone bipolar cells and their synapses onto retinal ganglion cells. The beauty of this arrangement is that it provides a common output pathway for rod and cone signals, yet it avoids introducing synaptic noise into the cone circuitry when the rods are saturated.
Figure 1.
Simplified schematic of cone and rod pathways through the retina. Right-hand side shows the cone pathway and left-hand side shows the main (scotopic) rod pathway that provides input to the cone pathway. Chemical synapses are shown as arrowheads, with white fill denoting glutamate (Glu) synapses and grey fill denoting glycine (Gly) synapses; sign-conserving and sign-inverting synapses are indicated as ‘+' and ‘−' respectively; gap junctions are indicated as ‘⊕'. Cone ON pathway comprises cone photoreceptor to ON cone bipolar cell (ON CB) to ON ganglion cell. Cone OFF pathway comprises cone photoreceptor to OFF cone bipolar cell (OFF CB) to OFF ganglion cell. The scotopic pathway begins as: rod photoreceptor to rod bipolar cell (RB) to AII amacrine cell. The AII amacrine provides sign-conserving input via connexin-36 gap junctions onto ON cone bipolar cell terminals, as well as sign-inverting glycinergic input onto OFF cone bipolar cell terminals, thereby providing push–pull signals to ON and OFF ganglion cells. The sign-inverting glutamate synapses (from cones to cone ON bipolar cells, and from rods to rod bipolar cells) use a metabotropic postsynaptic mechanism involving a G-protein cascade, whereas the other chemical synapses use ionotropic mechanisms. Light hyperpolarises the photoreceptors, so that the sign-inverting synapse generates a depolarising light response in the ON cone bipolar cell and rod bipolar cell. Not shown in this diagram are surround mechanisms and lateral interactions mediated by horizontal cells and other classes of amacrine cell, or rod pathways used at mesopic levels (modified from Robson and Frishman;12 see Demb and Singer19 for recent review).
What the retinal ganglion cells need to be able to do is to make the best job of detecting additional real photon hits above the on-going ‘chatter' of spontaneous photon-like events in the set of RBCs from which they collect. Electrophysiological recordings made many decades ago showed that under fully dark-adapted conditions, a cat retinal ganglion cell is able to fire additional spikes when just a few photoisomerisations occur within its receptive field.20 Recent recordings from primate retina have likewise shown that ‘parasol' retinal ganglion cells respond with increased firing in ON cells and decreased firing in OFF cells when just a handful of photoisomerisations occur in the cell's receptive field.21
From these considerations we can conclude that the advent of reliable single-photon detection by rod photoreceptors (~400–500 Mya) permitted the evolution of reliable single-photon signalling by rod bipolar cells, together with a dedicated piggy-back mechanism for transmitting these signals into the existing retinal ganglion cell circuitry for photopic signals. Because of these innovations, the dark-adapted scotopic visual system was enabled to operate in what is essentially a photon-detecting mode, thereby permitting the organism's sensitivity for large-area stimuli to be increased by orders of magnitude above what could be achieved using analogue processing in the cone pathway.
We can further conclude that the main reason why rods comprise the vast majority (95%) of our photoreceptors is so that, down at exceedingly low light levels, as many as possible of the scarce photons are captured by rods rather than by cones. If instead there were comparable numbers of cones and rods in the peripheral retina, then (taking account of the larger cross-sectional area of the cone inner segments) more than half of the precious photons would be ‘lost' to cones, and it would therefore not be possible to achieve as high a scotopic sensitivity (ie, as low a threshold) as is actually achieved.
Tradeoffs: the downside of single-photon processing in the retina
The performance of rods and scotopic vision is inferior to that of cones and photopic vision in a variety of ways, as indicated in Table 1. Some of these deficiencies represent consequences of the need for the retina to be able to process individual photon responses at the very lowest intensities. For example, the sluggish response of the rod system is a consequence of the rod's need to integrate the signal for long enough to generate a single-photon response discernible above the noise. Likewise, the very slow dark adaptation of scotopic visual sensitivity following large bleaches has an explanation that involves the exceedingly low final dark-adapted threshold that is achieved by processing single-photon signals.
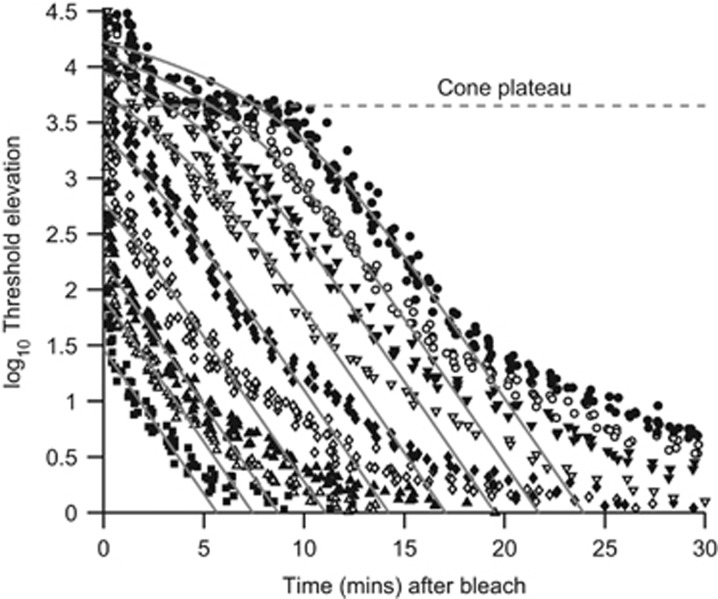
The time course of human dark adaptation is plotted in Figure 2 for recovery after exposures that bleached from 0.5 to 98% of the rhodopsin.22, 23, 24, 25 The photopic system recovers fairly quickly and, following larger bleaches, reaches a steady state indicated by the horizontal dashed line; this ‘cone plateau' occurs because at these times the scotopic system remains more desensitised than the photopic system. At later times the scotopic system achieves greater sensitivity, so that the visual threshold drops below the cone plateau; for a full bleach, the cone/rod break occurs at ∼10–12 min after bleach extinction.
Figure 2.
Psychophysical dark adaptation recovery for a normal human subject. The symbols plot measurements of log threshold elevation, following intense exposures that bleached from 0.2% to 98% of the rhodopsin (data from Pugh 22). Horizontal dashed line indicates the cone plateau at 3.6 log units above the absolute scotopic threshold. Grey curves plot the predicted decline of log threshold elevation for a model in which opsin recombines with 11-cis retinal produced by a rate-limited enzymatic reaction (representing, eg, RDH5 or RPE65 activity).25 Exposures ranged from 4.7 to 7.6 log scotopic troland s, and were estimated to have bleached 0.17, 0.5, 1, 3.7, 14, 32, 53, 74, and 98% of the rhodopsin.
Characteristically, the time course of decline of scotopic log threshold follows straight-line kinetics, indicated by the parallel grey curves, over a mid-range of thresholds across all bleaching levels.23 The slope of this ‘S2' component of recovery is ~0.24 log units per min in normal human subjects.23 Ultimately, after more than 40 min have elapsed following a full bleach, the final dark-adapted visual threshold is reached, some 3.6 log units (~4000-fold) below the cone plateau.
Why does recovery take so long? And what mechanism(s) is/are causing the threshold elevation? The first crucial point to note is that, in this regime, the threshold elevation is not remotely caused by the lack of rhodopsin available to absorb photons. Consider, for example, recovery following a 32% bleach, indicated by ∇ in Figure 2. At ~1 min after bleach extinction, the threshold was right around the cone plateau level, indicating that even though ~68% of the rhodopsin was still present, the scotopic threshold was nevertheless elevated by ~4000-fold, out of all proportion to the lack of visual pigment.
Instead, the elevation of threshold is caused by the presence of a product of rhodopsin bleaching.23 This product is ‘free opsin' that is, the protein component after all-trans retinal has detached, and before fresh 11-cis retinal binds to regenerate rhodopsin. Opsin has been shown to activate the phototransduction cascade,26 although with an efficacy ~105 × lower than that of light-activated rhodopsin (R*). As a result, the presence of opsin after a bleach causes photoreceptor activation similar to that caused by light, and the observed elevation of threshold is a consequence of this ‘equivalent light'. As time progresses after a bleach, 11-cis retinal recombines with opsin, so that the quantity of free opsin steadily declines, thereby causing a corresponding decline in equivalent background intensity and scotopic threshold.
Why does the elimination of opsin not occur more rapidly than this? In order to achieve speedier regeneration of rhodopsin, the delivery of 11-cis retinaldehyde would need to be faster, and this would generate a higher concentration of the retinoid. However, this aldehyde is potentially toxic, and a high concentration over the long term would be likely to cause retinal damage. On the other hand, the actual speed of dark adaptation is probably just sufficient to have prevented a survival disadvantage over evolutionary times. Indeed, it appears that the time course of human dark adaptation is matched to the fading of light at dusk on this planet, suggesting that the delivery of 11-cis retinaldehyde has been adjusted to a level sufficient to accomplish this, without creating a concentration so high as to cause toxicity.
The speed of scotopic dark adaptation is potentially an important predictor of the approaching onset of AMD.27, 28 In particular, the slope of the S2 component of recovery (indicated by the parallel curves in Figure 2) is found to be lower than normal in patients with even the earliest stages of age-related maculopathy. It has been proposed24 that the common link between these phenomena is disturbance around Bruch's membrane, with the age-related ‘clogging' of the membrane leading on the one hand to local vitamin-A deficiency in the RPE that manifests as slowed dark adaptation, and on the other hand and more importantly for the patient, leading via other mechanisms to maculopathy. Thus, although there is no causal link between slowed dark adaptation and maculopathy, both are likely to represent consequences of impaired function at Bruch's membrane.
Finally, why does attainment of full dark adaptation takes so much longer for the rod system than for the cone system? Two factors seem relevant. First, the regeneration of visual pigment is a factor of approximately threefold faster in cones,29 possibly because of the ability of cones to access a source of retinoid recycling within the Müller cells in addition to the source from the RPE. However, secondly, the dark-adapted scotopic threshold is more than 3 orders of magnitude lower than the photopic threshold (cone plateau). Hence, the ‘dark light' elicited by the presence of opsin needs to fall at least an additional 3 log units in the scotopic system, so that even if the rate of decline were the same the time to reach full dark adaptation would necessarily be considerably longer for the scotopic system.
Evolution of rods and single-photon processing
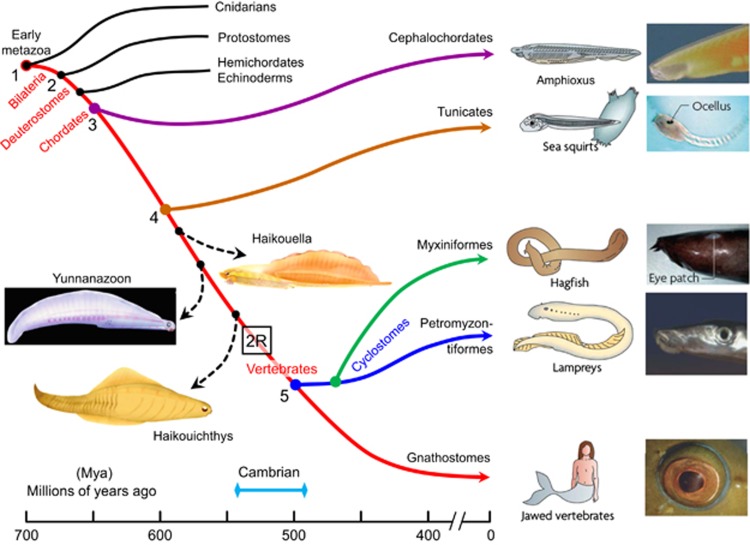
How did the ability to process single-photon signals arise? To examine this, we need to consider the evolution of the vertebrate eye, and indeed the evolution of vertebrates, as summarised schematically in Figure 3.30 The red curve in this diagram traces the descent of our direct ancestors from the time of a very simple metazoan organism at ∼700 Mya to the appearance of jawed vertebrates (gnathostomes) at ∼400 Mya. It shows the divergence at ∼600 Mya of tunicates, whose extant members possess the simplest of eyespots (ocelli), and it also shows the divergence of jawed and jawless vertebrates at ∼500 Mya.
Figure 3.
Evolution of vertebrates and the vertebrate camera-style eye. The origin of vertebrates is shown, over a timescale from roughly 700 to 400 millions of years ago (Mya). The red curve indicates our direct ancestors, beginning with early metazoans; dashed curves indicate extinct taxa of potential interest. By the time the ancestors of lampreys diverged at ∼500 Mya, they and our own ancestors were vertebrates. By ∼420 Mya, our own ancestors had evolved jaws and are referred to as jawed vertebrates or gnathostomes. Crucial events in the evolution of our camera-style eye occurred between the points marked 4 and 5: the divergence of tunicates at ∼600 Mya and the appearance of vertebrates at ∼500 Mya. Our last common ancestor with tunicates is presumed to have had no more than a simple eye-spot (ocellus), whereas our last common ancestor with lampreys is presumed to have had a camera-style eye. ‘2R' denotes the two rounds of whole-genome duplication that occurred before the radiation of vertebrates (from Lamb30).
The anatomy and physiology of retinal photoreceptors, and of the retinal circuitry and camera-style eye, bear extremely close homology across all jawed vertebrates. Furthermore, this remarkable homology extends even to the jawless lampreys. The homologies are so extensive that they lead to the inescapable conclusion that the last common ancestor that we share with lampreys already possessed fundamentally the same camera-style eye that we possess, with homologous (though not identical) photoreceptors.
The lamprey retina has a three-layered structure closely resembling that in gnathostomes,31 and lamprey photoreceptors utilise the same five classes of visual opsin as used by gnathostomes.32 One class of lamprey photoreceptor expresses rhodopsin and exhibits comparable sensitivity to gnathostome rods,33, 34 despite exhibiting cone-like morphology. However, there have not yet been reports to indicate whether the lamprey retina has the ability to operate in a photon-processing mode. On the other hand, it has long been known that the response properties of dogfish retinal bipolar cells are closely comparable to those of mammalian RBCs,35 and that deep-sea fish exhibit extremely high visual sensitivity. Thus, it seems very likely that single-photon processing in the retina had already been achieved by the time that early jawed fish evolved, more than 400 Mya.
It is apparent from Figure 3 that the interval of 100 million years from 600 to 500 Mya was a crucial period for the evolution of many of the features that characterise the vertebrate eye, retina, and photoreceptors. In particular, the occurrence of two rounds of whole-genome duplication (2R WGD, denoted ‘2R' in Figure 3) was absolutely critical to the advent of these features, in addition to its importance in the emergence of numerous other features that enabled the highly successful radiation of vertebrate species. However, it is not yet clear whether single-photon processing in the retina had appeared by 500 Mya or whether that was a gnathostome invention.
Synthesis
A distant ancestor of ours, a jawless proto-vertebrate that lived more than 500 Mya, had already evolved paired lateral camera-style eyes that utilised photoreceptors resembling modern-day cones. Two classes of those cones existed, expressing either a short- or long-wavelength-sensitive (SWS or LWS) opsin. The retina was three layered and processed signals in broadly the same way as is done in the photopic division of the modern vertebrate retina, providing dichromatic colour vision in daylight lighting levels. A descendant of this creature underwent genome quadruplication through two rounds of WGD, and it was this quadruplication of genes that provided the flexibility that enabled the massive radiation of vertebrate species. In the retina, this quadruplication led to the advent of four classes of cone opsin (3 SWS and 1 LWS), with individual spectra covering the whole of the visible region. In addition, the photoreceptor expressing the fourth of the quadruplicated SWS opsins (Rh1) became specialised for operation at very low intensities (night-time and in the deep ocean), and eventually achieved the ability to reliably detect individual photons of light: this cell became the ancestral rod photoreceptor. Presented with these quantal signals from rods, the retina at some stage evolved the ability to process them as discrete signals, rather than as analogue signals, and thereby achieved a huge advantage in extending the visual threshold down to exceedingly low levels. The circuitry that evolved to accomplish this discrete signalling utilised rod bipolar cells and AII amacrine cells that were piggy-backed onto the pre-existing photopic retinal signalling pathway. That duplex system, which was in place at least 400 Mya, proved highly advantageous to the organism, and has remained substantially unchanged ever since.
Acknowledgments
I thank Professor Paul R Martin for comments on a draft.
The author declares no conflict of interest.
References
- 1Østerberg G. Topography of the layer of rods and cones in the human retina. Acta Ophthalmol 1935; 13(S6): 1–103. [Google Scholar]
- 2Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol 1990; 292: 497–523. [DOI] [PubMed] [Google Scholar]
- 3Curcio CA, Hendrickson AE. Organization and development of the primate photoreceptor mosaic. Prog Retinal Res 1991; 10: 89–120. [Google Scholar]
- 4Anderson SJ, Mullen KT, Hess RF. Human peripheral spatial resolution for achromatic and chromatic stimuli: limits imposed by optical and retinal factors. J Physiol 1991; 442: 47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5van Hateren JH, Lamb TD. The photocurrent response of human cones is fast and monophasic. BMC Neurosci 2006; 7: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Tyler CW, Hamer RD. Analysis of visual modulation sensitivity. IV. Validity of the Ferry-Porter law. J Opt Soc Am A 1990; 7: 743–758. [DOI] [PubMed] [Google Scholar]
- 7Burkhardt DA. Light adaptation and photopigment bleaching in cone photoreceptors in situ in the retina of the turtle. J Neurosci 1994; 14: 1091–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Barlow HB. Dark and light adaptation: psychophysics. In: Jameson D, Hurvich LM (eds). Handbook of Sensory Physiology, Vol. VII-4, Visual Psychophysics. Springer-Verlag: Berlin, 1972. pp 1–28. [Google Scholar]
- 9Hecht S, Shlaer S, Pirenne M. Energy, quanta, and vision. J Gen Physiol 1942; 25: 819–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Yau K-W, Lamb TD, Baylor DA. Light-induced fluctuations in the membrane current of single toad rod outer segments. Nature 1977; 269: 78–80. [DOI] [PubMed] [Google Scholar]
- 11Baylor DA, Lamb TD, Yau K-W. Responses of retinal rods to single photons. J Physiol 1979; 288: 613–634. [PMC free article] [PubMed] [Google Scholar]
- 12Robson JG, Frishman LJ. Dissecting the dark-adapted electroretinogram. Doc Ophthalmol 1998–1999; 95: 187–215. [DOI] [PubMed] [Google Scholar]
- 13Field GD, Rieke F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron 2002; 34: 773–785. [DOI] [PubMed] [Google Scholar]
- 14Berntson A, Smith RG, Taylor WR. Transmission of single photon signals through a binary synapse in the mammalian retina. Vis Neurosci 2003; 20: 621–626. [DOI] [PubMed] [Google Scholar]
- 15Morgans CW, Brown RL, Duvoisin RM. TRPM1: the endpoint of the mGluR6 signal transduction cascade in retinal ON-bipolar cells. Bioessays 2010; 32: 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Baylor DA, Matthews GG, Yau K-W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol 1980; 309: 591–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol 1984; 357: 575–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Kolb H, Famiglietti EV. Rod and cone pathways in retina of cat. Invest Ophthalmol 1976; 15: 935–946. [Google Scholar]
- 19Demb JB, Singer JH. Intrinsic properties and functional circuitry of the AII amacrine cell. Vis Neurosci 2012; 29: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Barlow HB, Levick WR, Yoon M. Responses to single quanta of light in retinal ganglion cells of the cat. Vision Res 1971; (Suppl 3): 87–101. [DOI] [PubMed]
- 21Ala-Laurila P, Rieke F. Coincidence detection of single-photon responses in the inner retina at the sensitivity limit of vision. Curr Biol 2014; 24: 2888–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Pugh EN. Rushton's paradox: rod dark adaptation after flash photolysis. J Physiol 1975; 248: 413–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Lamb TD. The involvement of rod photoreceptors in dark adaptation. Vision Res 1981; 21: 1773–1782. [DOI] [PubMed] [Google Scholar]
- 24Lamb TD, Pugh EN. Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res 2004; 23: 307–380. [DOI] [PubMed] [Google Scholar]
- 25Lamb TD, Corless RM, Pananos D. The kinetics of regeneration of rhodopsin under enzyme-limited availability of 11-cis retinoid. Vision Res 2015; 110: 23–33. [DOI] [PubMed] [Google Scholar]
- 26Cornwall MC, Fain GL. Bleached pigment activates transduction in isolated rods of the salamander retina. J Physiol 1994; 480: 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Jackson GR, Owsley C, McGwin G. Aging and dark adaptation. Vision Res 1999; 39: 3975–3982. [DOI] [PubMed] [Google Scholar]
- 28Owsley C, Jackson GR, White M, Feist R, Edwards D. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology 2001; 108: 1196–1202. [DOI] [PubMed] [Google Scholar]
- 29Mahroo OAR, Lamb TD. Recovery of the human photopic electroretinogram after bleaching exposures: estimation of pigment regeneration kinetics. J Physiol 2004; 554: 417–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Lamb TD. Evolution of phototransduction, vertebrate photoreceptors and retina. Prog Retin Eye Res 2013; 36: 52–119. [DOI] [PubMed] [Google Scholar]
- 31Fritzsch B, Collin SP. Dendritic distribution of two populations of ganglion cells and the retinopetal fibres in the retina of the silver lamprey (Ichthyomyzon unicuspis. Vis Neurosci 1990; 4: 533–545. [DOI] [PubMed] [Google Scholar]
- 32Collin SP, Knight MA, Davies WL, Potter IC, Hunt DM, Trezise AEO. Ancient colour vision: multiple opsin genes in the ancestral vertebrates. Curr Biol 2003; 13: R864–R865. [DOI] [PubMed] [Google Scholar]
- 33Asteriti S, Grillner S, Cangiano L. A Cambrian origin for vertebrate rods. Elife 2015; 4: e07166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Morshedian A, Fain GL. Single-photon sensitivity of lamprey rods with cone-like outer segments. Curr Biol 2015; 25: 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Ashmore JF, Falk G. Responses of rod bipolar cells in the dark-adapted retina of the dogfish, Scyliorhinus canicula. J Physiol 1980; 300: 115–150. [DOI] [PMC free article] [PubMed] [Google Scholar]