Abstract
Over the past two decades there have been significant advances in our understanding of both the anatomy and function of the melanopsin system. It has become clear that rather than acting as a simple irradiance detector the melanopsin system is in fact far more complicated. The range of behavioural systems known to be influenced by melanopsin activity is increasing with time, and it is now clear that melanopsin contributes not only to multiple non-image forming systems but also has a role in visual pathways. How melanopsin is capable of driving so many different behaviours is unclear, but recent evidence suggests that the answer may lie in the diversity of melanopsin light responses and the functional specialisation of photosensitive retinal ganglion cell (pRGC) subtypes. In this review, we shall overview the current understanding of the melanopsin system, and evaluate the evidence for the hypothesis that individual pRGC subtypes not only perform specific roles, but are functionally specialised to do so. We conclude that while, currently, the available data somewhat support this hypothesis, we currently lack the necessary detail to fully understand how the functional diversity of pRGC subtypes correlates with different behavioural responses, and ultimately why such complexity is required within the melanopsin system. What we are lacking is a cohesive understanding of how light responses differ between the pRGC subtypes (based not only on anatomical classification but also based on their site of innervation); how these diverse light responses are generated, and most importantly how these responses relate to the physiological functions they underpin.
The role of melanopsin in multiple non-image forming (NIF) and image forming pathways
Over the last 10–15 years, it has become clear that in addition to rods and cones, the mammalian retina contains a third class of ocular photoreceptor based upon a small number of photosensitive retinal ganglion cells (pRGCs) that express the blue-light sensitive vitamin A-based photopigment melanopsin (OPN4)1, 2, 3 (for reviews see Hankins et al,4 Hughes et al,5 and Do and Yau6). In the years following their identification, it has become clear that melanopsin expressing pRGCs contribute to a wide and diverse range of non-image forming (NIF) responses to light including circadian entrainment,7, 8 the pupillary light response (PLR),9 negative masking of locomotor activity,10 the regulation of sleep–wake states,11, 12, 13, 14 photophobia, and light aversion,15, 16 while also influencing mood-related behaviours and cognitive functions.17 More recently it has become clear that melanopsin contributes not only to a range of NIF pathways but also performs multiple roles in image forming visual pathways. Melanopsin provides visual centres with information regarding overall levels of environmental light, and performs roles in irradiance coding and brightness discrimination,18, 19, 20 contrast detection,21 and adaptation of visual responses.22 Melanopsin also acts as an irradiance detector for the retina,22, 23 providing measures of overall illuminance and facilitating the adaptation of cone photoresponses under bright-light conditions.22, 24 Intraretinal signalling from pRGCs is also known to influence the activity of dopaminergic amacrine cells,25, 26, 27 and displaced AII amacrine cells.28 During development, melanopsin-driven light responses influence the nature of calcium waves that spread across the developing retina and promote the segregation and refinement of retinofugal projections29 (see also, Kirkby and Feller30) and also regulate the formation and patterning of retinal blood vessels that ultimately influence retinal cell numbers.31 Based on this ever growing body of evidence it is now clear that the melanopsin system is far more complex than first realised, providing light information to a wide number of physiological systems and performing a number of different functional roles. How melanopsin signalling is capable of driving so many different behaviours is unclear, but the currently available evidence suggests that the answer may lie in the functional diversity of pRGC subtypes.
Subtypes of pRGC
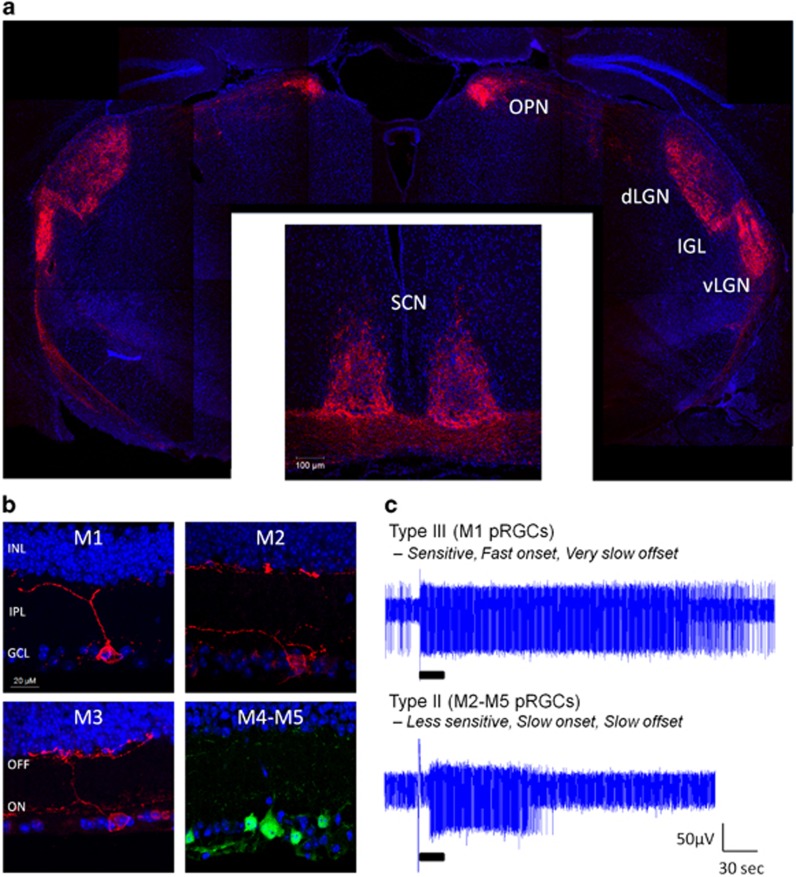
It is now clear that pRGCs are not a uniform homogeneous population, but instead comprises a number of anatomically and functionally distinct cell subtypes (Figure 1a). To date at least five distinct subtypes of pRGC have been identified, termed M1–M5-type pRGCs.1, 2, 32, 33, 34, 35 These cells show differences in morphology and retinal connections, and exhibit light responses with markedly different kinetics and sensitivities (Figure 1b) (for review see Hughes et al5 and Schmidt et al36). Currently available data also indicate that the different pRGC subtypes innervate distinct regions of the brain32, 33, 34, 37, 38, 39 (Figure 1c), and would therefore seem to be tasked with performing different physiological roles (for review see Schmidt et al36, 40). However, the innervations of each class of pRGC are only partially resolved, and the specific roles performed by each class of pRGC are yet to be fully determined.
Figure 1.
(a) Innervations of M1–M5 type pRGCs in the mouse brain as revealed by intravitreal delivery of AAV2 viral vectors containing lox.STOP.lox.mCherry into the retina of Opn4.Cre mice. (b) Images showing the morphology of pRGC subtypes of the mouse retina. M1, M2, and M3-type pRGCs are labelled using anti-melanopsin antibodies. M4 - and M5-type pRGCs are identified by expression of EYFP in Opn4.Cre.EYFP mice and the lack of detectable melanopsin labelling. (c) Examples of types III and II melanopsin responses recorded from the adult mouse retina using multiple electrode arrays, typical of M1 - and non-M1-type pRGCs, respectively. Black bars indicate duration of light pulse (30 s, 480 nm, 15.1 log photons/cm2/s). dLGN, dorsal lateral geniculate nucleus; GCL, ganglion cell layer; IGL, intergeniculate leaflet; INL, inner nuclear layer; IPL, inner plexiform layer; OFF, OFF layer of the inner plexiform layer; ON, ON layer of the inner plexiform layer; OPN, olivary pretectal nucleus; SCN, suprachiasmatic nucleus; vLGN, ventral lateral geniculate nucleus.
The first class of pRGC identified were M1-type pRGCs, characterised by high levels of melanopsin expression and stratification of their dendrites in the OFF sublamina of the inner plexiform layer (IPL).1, 2, 32, 41 M2-type pRGCs express significantly lower levels of melanopsin compared with M1-type pRGCs, and have more complex dendritic fields that stratify exclusively in the ON sublamina of the IPL.32, 34, 35, 42 M3-type pRGCs are rarer than either M1 or M2-type cells, and show a bistratified morphology with dendrites present in both the ON and OFF layers of the IPL,42, 43 and show an intermediary level of melanopsin expression compared to M1 - and M2-type cells.42 The anatomy of M4 - and M5 cells is grossly similar to M2-type cells, having dendrites confined to the ON layer of the IPL, but these cell types are distinguished based on the size and complexity of their dendritic fields, and in the case of M4 cells their large soma size.33 Most characteristically, M4 - and M5-type pRGCs express levels of melanopsin too low to be reliably detected by conventional immunostaining methods,33, 42, 44 and are typically identified by visualisation of fluorescent reporters within Opn4. Cre-based transgenic mouse models33, 44, 45 or following signal amplification techniques.39
Innervations of pRGC subtypes
The projections of M1-type pRGCs have been well documented, using Opn4.tau.LacZ mice that selectively label M1-type pRGCs,2, 32 showing that these cells innervate a number of key brain areas associated with NIF functions, including (but not limited to) the suprachiasmatic nucleus (SCN), intergeniculate leaflet, olivary pretectal nucleus (OPN), ventrolateral preoptic area, ventral lateral geniculate nucleus, medial amygdala, lateral habenula, and the superior colliculus (SC).32 Subsequent studies using Opn4.Cre mice have allowed the combined innervations of M1–M5-type pRGCs to be studied,33, 37 and identified a number of additional brain regions that receive input exclusively from non-M1 pRGCs, including the dorsal lateral geniculate nucleus (dLGN), the core region of the OPN, and additional innervations of the SC.33 However, these Opn4.Cre-based models do not allow the separation of projections from the different pRGC subtypes, as such the regions of the brain innervated specifically by M2, M3, M4, and M5 types pRGCs remain largely undetermined. Some information is available from studies using retrograde labelling from key structures, including the SCN, OPN, and SC, showing that the SCN is innervated primarily by M1-type pRGCs (80%) with lower innervations by M2-type cells (20%),34 the OPN receives input from both M1 (55%, mainly shell region) and M2 cells (45%, mainly core region),34 whereas the SC receives input from M1–M5 cells,46 and the dLGN is innervated primarily by M4-type pRGCs but also receives input from other non-M1 pRGCs but not M1-type pRGCs.39
Physiological roles performed by pRGC subtypes
Although it may be logical to conclude that different pRGC subtypes mediate different responses to light on the basis of their anatomical projections (for review see Schmidt et al36), in most cases the roles performed by specific classes of pRGC remain undetermined. To date only one study has shown a direct link between distinct populations of pRGCs and behavioural responses.38 Chen et al38 show that M1-type pRGCs that innervate the SCN and OPN are two molecularly distinct subtypes of M1-type pRGCs, distinguished by their differential expression of the transcription factor Brn3b. Brn3b-negative M1 pRGCs that project to the SCN are capable of driving circadian entrainment following the genetic ablation of all other pRGCs (Brn3b-positive and including M1–M5 pRGCs).38 The ablation of all Brn3b-positive M1–M5 pRGCs was also shown to disrupt the PLR, yet, given the widespread loss of pRGC subtypes using this approach, it is not possible to conclude from this study which class of pRGC mediates the PLR or other behaviours. However, the timeline with which M1 cells innervate the shell of the OPN coincides with the development of the PLR,37 and the selective ablation of M1-type pRGCs has been shown to severely impair the PLR.47 Therefore it would seem that the PLR is driven by M1-type pRGCs, and thus distinct subsets of M1-type pRGCs are responsible for driving circadian entrainment and the PLR. Unfortunately, the roles performed by other classes of pRGC are less clear.
At present there is little information available regarding the behavioural responses supported by M2-type pRGCs, or indeed M3 or M5-type pRGCs. There is, however, some data concerning the potential roles performed by M4-type pRGCs. The nature and properties of light responses recorded from cells within the dLGN have suggested a role for melanopsin in encoding changes in background illumination and brightness detection18, 19, 20 and the modulation of visual responses, with melanopsin reportedly driving adaptation of visual responses, modulating visual feature selectivity and facilitating a more reliable encoding of complex visual signals.22, 23 Based on retrograde labelling studies, it would seem that M4-type pRGCs project almost exclusively to the dLGN,39 and would, therefore, seem to be predominantly tasked with providing input to visual pathways. In keeping with this hypothesis, M4-type pRGCs have now been identified as a subset of alpha ON ganglion cells,21, 39 a type of retinal ganglion cell known to be involved in the processing of visual information and contrast sensitivity.21 However, the dLGN also receives input from other non-M1-type pRGCs,39 and thus, it has been difficult to determine the specific contribution of each pRGC subtype to these responses.
Diversity of melanopsin light responses
Although available data are incomplete, it does seem likely that distinct subpopulations of pRGCs are responsible for performing distinct physiological roles. But why is such complexity necessary? Why are multiple subtypes of pRGC required? Why can the different target structures not receive information from a uniform class of pRGC? An intriguing hypothesis is that the functional properties of each class of pRGC are specialised for the physiological roles that they perform. The type of light information required to drive different behaviours is likely not the same, and thus the pRGCs that innervate each distinct brain area may exhibit different functional properties in order to meet these demands. From early Ca2+ imaging-based studies, it was clear that multiple types of light response are observed from pRGCs of the mouse retina, including transient, sustained, and repetitive responses.48 Multiple response types were also subsequently identified using multiple electrode array recordings from postnatal mouse retina, termed Type I; sensitive with slow onset and fast offset; Type II, insensitive with slow onset and slow offset; and Type III, sensitive with rapid onset and very slow offset.49 However, only Type II and Type III responses are observed in the adult mouse retina49 (Figure 1c). The subsequent identification of multiple pRGC subtypes has provided an attractive model to explain these multiple response types, yet a decade after these initial observations we are now only just beginning to understand the diversity of pRGC light responses and correlate these responses profiles with specific subtypes of pRGC.
pRGC subtypes exhibit light responses with differing kinetics
All pRGC subtypes generate melanopsin based ‘intrinsic' light responses, as well as receiving synaptically driven ‘extrinsic' light responses from the rods and cones of the outer retina.33, 35, 44, 50, 51, 52, 53, 54, 55, 56 In keeping with their high levels of melanopsin expression, M1-type pRGCs exhibit intrinsic light responses with markedly higher sensitivity than the other pRGC subtypes, with the light induced photocurrents of M1 cells showing a lower threshold of activation (~10-fold more sensitive), higher maximal amplitude, and faster onset of response compared with M2-type pRGCs.33, 35, 43, 46 Initial studies indicated that M2 cells receive greater levels of excitatory input from rods and cones, when compared with M1 cells,55 with these findings suggesting that input from the outer retina exerts more influence on the light responses of M2 cells than M1 cells.55 However, subsequent studies have reported that the levels of excitatory input do not differ significantly among pRGC subtypes, including M1–M5-type pRGCs.50 Despite differences in their patterns of stratification, the ON pathway forms the dominant excitatory synaptic input to M1–M5-type pRGCs,35, 46, 52, 55, 57, 58 and includes input from rods and both cone types of the mouse retina.50 M1-type pRGCs also receive low levels of excitatory and inhibitory input from the OFF pathway.46, 52 The initial study of M4- and M5-type cells reported only minimal levels of intrinsic light responses, with both cell types showing photocurrents that were smaller in amplitude and lower in sensitivity than those of M2-type pRGCs. These findings were explained on the basis of the very low levels of melanopsin expressed within M4- and M5-type cells.33 However, subsequent studies have reported that the intrinsic light responses of M4- and M5-type pRGCs are similar in sensitivity to those of M2-type pRGCs46 (and attribute these differences in results to the different methods used to identify fluorescently labelled cells prior to study), although these cell types do show characteristic differences in peak amplitude of response and latency to peak response.46
Correlation of pRGC subtypes with response types
Despite their significantly larger photocurrents, it is worth noting that M1-type pRGCs exhibit maximal action potential spike firing rates that are notably lower than those of M2, M3, M4, and M5-type pRGCs,46, 59 an observation that is potentially explained by the increased propensity of M1 cells to attain a state of depolarisation block during light responses.33, 35, 52 Recent patch clamp studies focusing on the spike firing output elicited by each of the different pRGC subtypes suggest that the light responses recorded from pRGCs of the adult mouse retina can be broadly split into M1- and non-M1-type responses, with the responses of M1 cells being ‘sensitive, small in amplitude, with a fast onset', and the responses of M2, M3, M4, and M5 cells being much more similar, with responses that are ‘less sensitive, large in amplitude, with a slow onset'.46, 59 Studies in the rat have also confirmed the existences of at least five subtypes of pRGCs, with anatomical and biophysical properties similar to murine pRGCs, albeit with a slightly greater diversity of light responses observed between M2- and M5-type cells.59 It is also worth noting that at least two distinct populations of pRGC have also been described in the primate retina with similarities to M1- and non-M1-type pRGCs.51, 60, 61 However, to date, the functional properties of these cell types has not been investigated in detail.
Based on the properties of light responses recorded from defined pRGC subtypes it would seem likely that M1 cells correspond to the originally described Type III responses (sensitive with rapid onset and very slow offset),49 and that M2–M5-type cells combined represent Type II responders (insensitive with slow onset and slow offset).49 Recently it has been reported that M4 cells express much higher levels of melanopsin during postnatal development, and at these time points elicit light responses consistent with the properties of Type I responses.62 The significant down regulation of melanopsin expression within M4 cells during development would, therefore, seem to explain the loss of Type I responses in the adult retina.49, 62 Combined, these observations provide a potential explanation for the variety or light responses observed in the adult and postnatal retina. However, it is clear that this is not the complete story. There is significant heterogeneity observed between light responses of the same subtype, with Type II responders and Type III responders both showing sustained (slow offset) and persistent (very slow offset) responses.62 Significant differences in rates of adaptation and response recovery are also observed between individual pRGCs and pRGC subtypes.63, 64 Most strikingly, it is evident that light responses with markedly different properties can be generated within individual pRGCs depending on levels of dopamine signalling,65 activation of cAMP second messenger systems,66 light history and the wavelength of light.67 Responses of pRGCs may also be spectrally tuned depending on their location in the retina.39, 44 Thus it seems plausible that individual pRGCs are capable of generating responses with different properties under specific conditions, significantly adding to the diversity of signals generated by melanopsin signalling.
How is diversity achieved—the current model of melanopsin signalling fails to account for the diversity of light responses
Based on these observations it is clear that melanopsin signalling is a diverse and dynamic phenomenon, resulting in cellular light responses (and outputs) with markedly different kinetics. However, to date the cellular basis for generating these different response profiles and their physiological functions remain undetermined. The mechanisms of phototransduction in melanopsin expressing pRGCs are known to be markedly different from that of rod and cone photoreceptors. The stimulation of melanopsin leads to the activation of a membrane bound signalling cascade involving Gq/11 type G-proteins, activation of PLCβ4 and ultimately the opening of downstream TRP type ion channels, TRPC6 and TRPC7.49, 68, 69, 70, 71, 72, 73 However, this model describes only the basic core components of what is likely to be a far more complicated signalling pathway and fails to account for the multiple types of photoresponse observed from individual pRGCs. It is currently unclear to what extent the precise mechanisms of melanopsin phototransduction are conserved between different pRGC subtypes, with the majority of detailed analysis performed to date conducted on M1-type pRGCs.68, 69, 71
A role for OPN4L and OPN4S in generating functional diversity?
There is clearly potential for significant diversity in the melanopsin phototransduction cascade. Two distinct isoforms of melanopsin have been identified, generated by alternative splicing of the melanopsin gene.74 The resulting short (OPN4S) and a long (OPN4L) isoforms of melanopsin differ only in their C-terminal tails, with bioinformatic analysis indicating that the longer tail of OPN4L contains additional phosphorylation sites.74 These sites may confer functional differences to OPN4L and OPN4S, most likely influencing rates of adaptation, de-activation, and recovery as has been shown for other G protein coupled receptors,75, 76 including rhodopsin.77 Significantly, the OPN4S and OPN4L isoforms are differentially expressed in subtypes of pRGC and may provide a mechanism for generating different response profiles within these cell types. M1- and M3-type pRGCs express both OPN4S and OPN4L, whereas only OPN4L is detected within M2-type cells.74, 78 Unfortunately, because of the low levels of melanopsin expressed with M4- and M5-type cells, it has not been possible to determine the isoforms of melanopsin expressed within these cells.
Recent studies have shown that silencing of OPN4L and or OPN4S expression in vivo exerts differential effects on a range of NIF responses.79 Silencing of OPN4S alone is sufficient to disrupt the PLR, whereas silencing of both OPN4S and OPN4L is necessary to attenuate the phase-shifting of locomotor activity and the induction of sleep. By contrast, negative masking was attenuated by silencing of only OPN4L, with no apparent dependence on OPN4S. The differential expression of OPN4S and OPN4L isoforms within pRGC subtypes offers a potential mechanism for this effect, and would therefore indicate that OPN4S expressing M1-type cells are responsible for driving phase shifts, circadian entrainment and pupil constriction, whereas M2-type pRGCs (and potentially other classes of non-M1-type pRGC) that lack expression of OPN4S likely mediates negative masking. However, the PLR and circadian entrainment are both known to be driven by M1-type pRGCs,38 yet disruption of phase shifts and entrainment required silencing of both OPN4S and OPN4L, whereas the PLR was inhibited by silencing of only OPN4S, with no apparent additive effect of also silencing OPN4L.79 Based on these observations it is possible that OPN4S and OPN4L are capable of driving different biochemical signals within pRGCs, influencing cellular output, and ultimately contributing to specific behavioural responses.
Conclusions
It is now clear that the melanopsin system is far more complex than first envisaged. Multiple subtypes of pRGC are now known to exist, with these cell types capable of generating light responses with differing properties, and seemingly tasked with performing distinct physiological roles. However, despite major advances in our understanding of the anatomy, physiology, and function of the melanopsin photoreceptor system, key issues remain unresolved. The nature of inputs received by different target structures is incomplete, with the innervations of each class of pRGC only partially resolved. We also lack the complete details of how light responses differ between pRGC subtypes, not just based on their morphological subtype but also based on their sites of innervation, and thus we know relatively little about the specific nature of light signals that are projected to different target structures. For example, it is unclear to what extent the properties of light responses vary between the distinct subclasses of M1-type pRGCs that project to the SCN and the OPN, and how these responses may be tailored to meet the different physiological roles performed by these cells. Indeed it should be noted that in total, pRGC innervations are detected for as many as 32 distinct brain regions,32, 33, 80 and in the majority of these cases the subtypes of pRGCs innervating these areas, and the specific properties of light responses transmitted to these regions has received little attention. There are also significant gaps in our understanding of the melanopsin phototransduction signalling pathway. The current model is clearly incomplete and fails to account for the diversity of light responses observed from individual pRGCs. Defining the nature of pRGC innervations to specific regions of the brain, and understanding the nature of light information transmitted to these target structures is essential to better understand the roles of melanopsin in NIF behaviours, visual pathways and human physiology. If this extraordinary light detecting system of the eye is to be understood at any meaningful level, then we must move beyond generalisations to a precise mechanistic understanding.
The authors declare no conflict of interest.
References
- 1Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002; 295: 1070–1073. [DOI] [PubMed] [Google Scholar]
- 2Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 2002; 295: 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci 2000; 20: 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Hankins MW, Peirson SN, Foster RG. Melanopsin: an exciting photopigment. Trends Neurosci 2008; 31: 27–36. [DOI] [PubMed] [Google Scholar]
- 5Hughes S, Hankins MW, Foster RG, Peirson SN. Melanopsin phototransduction: slowly emerging from the dark. Prog Brain Res 2012; 199: 19–40. [DOI] [PubMed] [Google Scholar]
- 6Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev 2010; 90: 1547–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 2002; 298: 2213–2216. [DOI] [PubMed] [Google Scholar]
- 8Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z et al. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science 1999; 284: 502–504. [DOI] [PubMed] [Google Scholar]
- 9Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science 2003; 299: 245–247. [DOI] [PubMed] [Google Scholar]
- 10Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int 2003; 20: 989–999. [DOI] [PubMed] [Google Scholar]
- 11Hubbard J, Ruppert E, Gropp CM, Bourgin P. Non-circadian direct effects of light on sleep and alertness: lessons from transgenic mouse models. Sleep Med Rev 2013; 17: 445–452. [DOI] [PubMed] [Google Scholar]
- 12Altimus CM, Guler AD, Villa KL, McNeill DS, Legates TA, Hattar S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci USA 2008; 105: 19998–20003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, Ruby NF et al. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(-/-) mice. PLoS Biol 2009; 7: e1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Lupi D, Oster H, Thompson S, Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci 2008; 11: 1068–1073. [DOI] [PubMed] [Google Scholar]
- 15Semo M, Gias C, Ahmado A, Sugano E, Allen AE, Lawrence JM et al. Dissecting a role for melanopsin in behavioural light aversion reveals a response independent of conventional photoreception. PloS One 2010; 5: e15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Johnson J, Wu V, Donovan M, Majumdar S, Renteria RC, Porco T et al. Melanopsin-dependent light avoidance in neonatal mice. Proc Natl Acad Sci USA 2010; 107: 17374–17378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H et al. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature 2012; 491: 594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Brown TM, Tsujimura S, Allen AE, Wynne J, Bedford R, Vickery G et al. Melanopsin-based brightness discrimination in mice and humans. Curr Biol 2012; 22: 1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ et al. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol 2010; 8: e1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Davis KE, Eleftheriou CG, Allen AE, Procyk CA, Lucas RJ. Melanopsin-derived visual responses under light adapted conditions in the mouse dLGN. PLoS One 2015; 10: e0123424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Schmidt TM, Alam NM, Chen S, Kofuji P, Li W, Prusky GT et al. A role for melanopsin in alpha retinal ganglion cells and contrast detection. Neuron 2014; 82: 781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Allen AE, Storchi R, Martial FP, Petersen RS, Montemurro MA, Brown TM et al. Melanopsin-driven light adaptation in mouse vision. Curr Biol 2014; 24(21): 2481–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Hankins MW, Hughes S. Vision: melanopsin as a novel irradiance detector at the heart of vision. Curr Biol 2014; 24: R1055–R1057. [DOI] [PubMed] [Google Scholar]
- 24Barnard AR, Hattar S, Hankins MW, Lucas RJ. Melanopsin regulates visual processing in the mouse retina. Curr Biol 2006; 16: 389–395. [DOI] [PubMed] [Google Scholar]
- 25Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci USA 2008; 105: 14181–14186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Atkinson CL, Feng J, Zhang DQ. Functional integrity and modification of retinal dopaminergic neurons in the rd1 mutant mouse: roles of melanopsin and GABA. J Neurophysiol 2013; 109: 1589–1599. [DOI] [PubMed] [Google Scholar]
- 27Zhang DQ, Belenky MA, Sollars PJ, Pickard GE, McMahon DG. Melanopsin mediates retrograde visual signaling in the retina. PLoS One 2012; 7: e42647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Reifler AN, Chervenak AP, Dolikian ME, Benenati BA, Li BY, Wachter RD et al. All spiking, sustained ON displaced amacrine cells receive gap-junction input from melanopsin ganglion cells. Curr Biol 2015; 25(21): 2763–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Renna JM, Weng S, Berson DM. Light acts through melanopsin to alter retinal waves and segregation of retinogeniculate afferents. Nat Neurosci 2011; 14: 827–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Kirkby LA, Feller MB. Intrinsically photosensitive ganglion cells contribute to plasticity in retinal wave circuits. Proc Natl Acad Sci USA 2013; 110: 12090–12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Rao S, Chun C, Fan J, Kofron JM, Yang MB, Hegde RS et al. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature 2013; 494(7436): 243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol 2006; 497: 326–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T et al. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron 2010; 67: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Baver SB, Pickard GE, Sollars PJ. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci 2008; 27: 1763–1770. [DOI] [PubMed] [Google Scholar]
- 35Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci 2009; 29: 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci 2011; 34: 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37McNeill DS, Sheely CJ, Ecker JL, Badea TC, Morhardt D, Guido W et al. Development of melanopsin-based irradiance detecting circuitry. Neural Dev 2011; 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature 2011; 476: 92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Estevez ME, Fogerson PM, Ilardi MC, Borghuis BG, Chan E, Weng S et al. Form and function of the M4 cell, an intrinsically photosensitive retinal ganglion cell type contributing to geniculocortical vision. J Neurosci 2012; 32: 13608–13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Schmidt TM, Do MT, Dacey D, Lucas R, Hattar S, Matynia A. Melanopsin-positive intrinsically photosensitive retinal ganglion cells: from form to function. J Neurosci 2011; 31: 16094–16101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature 2002; 415: 493. [DOI] [PubMed] [Google Scholar]
- 42Berson DM, Castrucci AM, Provencio I. Morphology and mosaics of melanopsin-expressing retinal ganglion cell types in mice. J Comp Neurol 2010; 518: 2405–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Schmidt TM, Kofuji P. Structure and function of bistratified intrinsically photosensitive retinal ganglion cells in the mouse. J Comp Neurol 2011; 519: 1492–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44Hughes S, Watson TS, Foster RG, Peirson SN, Hankins MW. Nonuniform distribution and spectral tuning of photosensitive retinal ganglion cells of the mouse retina. Curr Biol 2013; 23: 1696–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Buch T et al. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PloS One 2008; 3: e2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46Zhao X, Stafford BK, Godin AL, King WM, Wong KY. Photoresponse diversity among the five types of intrinsically photosensitive retinal ganglion cells. J Physiol 2014; 592: 1619–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature 2008; 453: 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48Sekaran S, Foster RG, Lucas RJ, Hankins MW. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr Biol 2003; 13: 1290–1298. [DOI] [PubMed] [Google Scholar]
- 49Tu DC, Zhang D, Demas J, Slutsky EB, Provencio I, Holy TE et al. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron 2005; 48: 987–999. [DOI] [PubMed] [Google Scholar]
- 50Weng S, Estevez ME, Berson DM. Mouse ganglion-cell photoreceptors are driven by the most sensitive rod pathway and by both types of cones. PLoS One 2013; 8: e66480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 2005; 433: 749–754. [DOI] [PubMed] [Google Scholar]
- 52Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol 2007; 582: 279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol 2003; 460: 380–393. [DOI] [PubMed] [Google Scholar]
- 54Pickard GE, Baver SB, Ogilvie MD, Sollars PJ. Light-induced fos expression in intrinsically photosensitive retinal ganglion cells in melanopsin knockout (opn4) mice. PloS One 2009; 4: e4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55Schmidt TM, Kofuji P. Differential cone pathway influence on intrinsically photosensitive retinal ganglion cell subtypes. J Neurosci 2010; 30: 16262–16271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56Wong KY. A retinal ganglion cell that can signal irradiance continuously for 10 hours. J Neurosci 2012; 32: 11478–11485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57Perez-Leon JA, Warren EJ, Allen CN, Robinson DW, Brown RL. Synaptic inputs to retinal ganglion cells that set the circadian clock. Eur J Neurosci 2006; 24: 1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58Schmidt TM, Taniguchi K, Kofuji P. Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development. J Neurophysiol 2008; 100: 371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59Reifler AN, Chervenak AP, Dolikian ME, Benenati BA, Meyers BS, Demertzis ZD et al. The rat retina has five types of ganglion-cell photoreceptors. Exp Eye Res 2015; 130: 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60Grunert U, Jusuf PR, Lee SC, Nguyen DT. Bipolar input to melanopsin containing ganglion cells in primate retina. Vis Neurosci 2011; 28: 39–50. [DOI] [PubMed] [Google Scholar]
- 61Jusuf PR, Lee SC, Hannibal J, Grunert U. Characterization and synaptic connectivity of melanopsin-containing ganglion cells in the primate retina. Eur J Neurosci 2007; 26: 2906–2921. [DOI] [PubMed] [Google Scholar]
- 62Sexton TJ, Bleckert A, Turner MH, Van Gelder RN. Type I intrinsically photosensitive retinal ganglion cells of early post-natal development correspond to the M4 subtype. Neural Dev 2015; 10: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63Do MT, Yau KW. Adaptation to steady light by intrinsically photosensitive retinal ganglion cells. Proc Natl Acad Sci USA 2013; 110: 7470–7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64Wong KY, Dunn FA, Berson DM. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron 2005; 48: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 65Van Hook MJ, Wong KY, Berson DM. Dopaminergic modulation of ganglion-cell photoreceptors in rat. Eur J Neurosci 2012; 35: 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66Sodhi P, Hartwick AT. Adenosine modulates light responses of rat retinal ganglion cell photoreceptors througha cAMP-mediated pathway. J Physiol 2014; 592: 4201–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67Emanuel AJ, Do MT. Melanopsin tristability for sustained and broadband phototransduction. Neuron 2015; 85: 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68Warren EJ, Allen CN, Brown RL, Robinson DW. The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells. Eur J Neurosci 2006; 23: 2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69Hartwick AT, Bramley JR, Yu J, Stevens KT, Allen CN, Baldridge WH et al. Light-evoked calcium responses of isolated melanopsin-expressing retinal ganglion cells. J Neurosci 2007; 27: 13468–13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70Sekaran S, Lall GS, Ralphs KL, Wolstenholme AJ, Lucas RJ, Foster RG et al. 2-Aminoethoxydiphenylborane is an acute inhibitor of directly photosensitive retinal ganglion cell activity in vitro and in vivo. J Neurosci 2007; 27: 3981–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71Graham DM, Wong KY, Shapiro P, Frederick C, Pattabiraman K, Berson DM. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J Neurophysiol 2008; 99: 2522–2532. [DOI] [PubMed] [Google Scholar]
- 72Xue T, Do MT, Riccio A, Jiang Z, Hsieh J, Wang HC et al. Melanopsin signalling in mammalian iris and retina. Nature 2011; 479: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73Hughes S, Jagannath A, Hickey D, Gatti S, Wood M, Peirson SN et al. Using siRNA to define functional interactions between melanopsin and multiple G Protein partners. Cell Mol Life Sci 2015; 72: 165–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74Pires SS, Hughes S, Turton M, Melyan Z, Peirson SN, Zheng L et al. Differential expression of two distinct functional isoforms of melanopsin (Opn4) in the mammalian retina. J Neurosci 2009; 29: 12332–12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol 1998; 38: 289–319. [DOI] [PubMed] [Google Scholar]
- 76Ritter SL, Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Biol 2009; 10: 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77Vishnivetskiy SA, Raman D, Wei J, Kennedy MJ, Hurley JB, Gurevich VV. Regulation of arrestin binding by rhodopsin phosphorylation level. J Biol Chem 2007; 282: 32075–32083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78Hughes S, Welsh L, Katti C, Gonzalez-Menendez I, Turton M, Halford S et al. Differential expression of melanopsin isoforms Opn4L and Opn4S during postnatal development of the mouse retina. PLoS One 2012; 7: e34531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79Jagannath A, Hughes S, Abdelgany A, Pothecary CA, Di Pretoro S, Pires SS et al. Isoforms of melanopsin mediate different behavioral responses to light. Curr Biol 2015; 25: 2430–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80Morin LP, Studholme KM. Retinofugal projections in the mouse. J Comp Neurol 2014; 522: 3733–3753. [DOI] [PMC free article] [PubMed] [Google Scholar]