Summary
Background
Haemophilus influenzae type b (Hib) conjugate vaccine, delivered as a three-dose series without a booster, was introduced into the childhood vaccination programme in Kenya in 2001. The duration of protection and need for a booster dose are unknown. We aimed to assess vaccine effectiveness, the impact of the vaccine on nasopharyngeal carriage, and population immunity after introduction of conjugate Hib vaccine in infancy without a booster dose in Kenya.
Methods
This study took place in the Kilifi Health and Demographic Surveillance System (KHDSS), an area of Kenya that has been monitored for vital events and migration every 4 months since 2000. We analysed sterile site cultures for H influenzae type b from children (aged ≤12 years) admitted to the Kilifi County Hospital (KCH) from Jan 1, 2000, through to Dec 31, 2014. We determined the prevalence of nasopharyngeal carriage by undertaking cross-sectional surveys in random samples of KHDSS residents (of all ages) once every year from 2009 to 2012, and measured Hib antibody concentrations in five cross-sectional samples of children (aged ≤12 years) within the KHDSS (in 1998, 2000, 2004–05, 2007, and 2009). We calculated incidence rate ratios between the prevaccine era (2000–01) and the routine-use era (2004–14) and defined vaccine effectiveness as 1 minus the incidence rate ratio, expressed as a percentage.
Findings
40 482 children younger than 13 years resident in KHDSS were admitted to KCH between 2000 and 2014, 38 206 (94%) of whom had their blood cultured. The incidence of invasive H influenzae type b disease in children younger than 5 years declined from 62·6 (95% CI 46·0–83·3) per 100 000 in 2000–01 to 4·5 (2·5–7·5) per 100 000 in 2004–14, giving a vaccine effectiveness of 93% (95% CI 87–96). In the final 5 years of observation (2010–14), only one case of invasive H influenzae type b disease was detected in a child younger than 5 years. Nasopharyngeal H influenzae type b carriage was detected in one (0·2%) of 623 children younger than 5 years between 2009 and 2012. In the 2009 serosurvey, 92 (79%; 95% CI 70–86) of 117 children aged 4–35 months had long-term protective antibody concentrations.
Interpretation
In this region of Kenya, use of a three-dose primary series of Hib vaccine without a booster dose has resulted in a significant and sustained reduction in invasive H influenzae type b disease. The prevalence of nasopharyngeal carriage is low and the profile of Hib antibodies suggests that protection wanes only after the age at greatest risk of disease. Although continued surveillance is important to determine whether effective control persists, these findings suggest that a booster dose is not currently required in Kenya.
Funding
Gavi, the Vaccine Alliance, Wellcome Trust, European Society for Paediatric Infectious Diseases, and National Institute for Health Research.
Introduction
Inclusion of Haemophilus influenzae type b (Hib) conjugate vaccine in the routine infant immunisation programme has led to tremendous reductions in childhood H influenzae type b morbidity and mortality in both developed and developing countries.1, 2 Hib vaccine was introduced into the Kenyan childhood Expanded Program on Immunization (EPI) in November, 2001, as a three-dose series administered at 6, 10, and 14 weeks of age. Within 3 years of introduction, invasive H influenzae type b disease had decreased to 12% of its baseline level.3 A booster dose of Hib vaccine is not included in the Kenyan EPI schedule, nor in the schedules of 72 (92%) of 78 low-income and lower-middle-income countries.4
In the UK, 10 years after the introduction of the Hib primary vaccination, waning levels of antibody to polyribosylribitol phosphate (PRP)—an H influenzae type b polysaccharide capsule component—as well as persistence of H influenzae type b nasopharyngeal colonisation and rising rates of invasive disease, prompted introduction of a booster dose of Hib vaccine for children aged 12–15 months in 2006.5, 6 The Government of Mexico also introduced a booster dose of Hib vaccine 9 years after launching the primary vaccination programme, in part because of waning anti-PRP antibodies in children aged 12–59 months.7 However, persistently low incidence of H influenzae type b meningitis in the western region of The Gambia more than a decade after Hib vaccine introduction shows that the disease can be adequately controlled in the absence of a booster dose.8
Research in context.
Evidence before this study
We searched PubMed with the terms “Hib”, “Haemophilus influenzae type b”, “vaccine”, “effectiveness”, “seroepidemiology”, “anti-PRP”, “booster”, “cross reactive”, “carriage”, and “colonization” for articles published in any language before May 31, 2015. To identify additional publications we searched the reference lists of retrieved articles. More than a decade after conjugate Haemophilus influenzae type b (Hib) vaccines became available, only 2% of the global H influenzae type b disease burden was being prevented by vaccination. In 2001, Gavi, the Vaccine Alliance, offered financial support for the introduction of Hib vaccine in developing countries, and Kenya became one of the first African countries to include Hib vaccine in the national immunisation schedule. Like the vast majority of low-income and lower-middle-income countries, Kenya used a three-dose primary series of Hib vaccine, without a booster dose. A three-dose schedule without a booster is highly effective in reducing the burden of H influenzae type b disease in the short term; however, whether a booster dose is required to achieve sustained disease control is unclear. Although data from some countries have prompted the addition of a booster dose, other data show good control of H influenzae type b disease in the absence of a booster. The need for a booster dose of Hib vaccine is probably affected by local epidemiology and factors such as the potential for natural boosting.
Added value of this study
This study provides new data documenting the near elimination of invasive H influenzae type b disease in Kilifi, Kenya, in the 12 years after introduction of vaccine into the routine infant vaccination schedule without a booster dose. The detailed seroepidemiology work before and after vaccine introduction shows that the vaccine has led to improvements in population immunity in the youngest, highest-risk age groups without compromising immunity in older children.
Implications of all the available evidence
This study delivers compelling evidence of the long-term operational impact of a three-dose primary series of Hib vaccine in a low-income country and provides a clear answer to a pertinent policy question in Kenya: a booster dose of vaccine is not currently needed to control H influenzae type b disease.
The long-term effectiveness of a primary series of Hib vaccine in infancy can be inferred from incidence of invasive H influenzae type b disease, nasopharyngeal carriage prevalence, and seroepidemiological data from the general population. Hib vaccination induces serum antibody production and reduces the nasopharyngeal carriage prevalence of H influenzae type b, thereby diminishing the risk of invasive disease. Reductions in carriage also reduce transmission of Hib between individuals. This contributes to herd protection, but also limits the opportunity for intermittent natural boosting of serological immunity. The pattern of H influenzae type b serological immunity in different age groups across time and the persistence of H influenzae type b serological immunity throughout the years of highest risk for H influenzae type b disease are likely to be important determinants of vaccine effectiveness beyond the primary vaccination period.
There is equipoise in the scientific community regarding the need for a booster dose of Hib vaccine to control disease in the long term.9 Herein we report vaccine effectiveness, the impact of the vaccine on nasopharyngeal carriage of H influenzae type b, and population immunity to H influenzae type b in the 13 years after introduction of conjugate Hib vaccine in infancy without a booster dose in Kenya.
Methods
Population
This surveillance study took place in the Kilifi Health and Demographic Surveillance System (KHDSS), a rural community on the Kenyan coast covering an area of 891 km2.10 A census of the KHDSS in 2000 defined the resident population and, since 2000, fieldworkers have been monitoring migration events by visiting every participating household roughly every 4 months. The annual population was 199 732 in 2000, 239 396 in 2007, and 279 877 in 2014. The population is served by several government-funded health centres and by one government hospital, Kilifi County Hospital (KCH). Among women attending antenatal care at KCH, the prevalence of HIV infection ranged between 2·4% and 4·6% during 2005–13, with a general downwards trend. The prevalence of HIV in children in Kenya was estimated in 2012 to be 0·9% nationally.11
On Nov 1, 2001, the Government of Kenya introduced tetanus-toxoid-conjugated Hib vaccine as part of a pentavalent formulation in which lyophilised Hib vaccine (Hiberix; GlaxoSmithKline, Rixensart, Belgium) was resuspended in the diphtheria, tetanus, whole-cell pertussis, hepatitis B vaccine (Tritanrix, GlaxoSmithKline). The first children eligible to receive a 6-week dose of this pentavalent vaccine were born on Sept 20, 2001, and would have been eligible to receive their third dose at the end of December, 2001.
The protocol was approved by the Oxford Tropical Ethical Review Committee (No. 30-10) and the Kenya National Ethical Review Committee (SSC1433). Parents or guardians of all study participants provided written informed consent.
Assessment of vaccine effectiveness
To assess vaccine effectiveness, we determined the prevalence of invasive H influenzae type b disease in children aged 12 years or younger admitted to KCH between Jan 1, 2000, and Dec 31, 2014. Blood samples are routinely taken for culture at the time of admission (except for trauma patients or patients admitted for elective surgery). Blood was cultured using an automated BACTEC instrument (BD Diagnostics, Franklin Lakes, NJ, USA). From 1998 to 2014, with the exception of a brief change in practice in 2004–05, the clinical indications for lumbar puncture were impaired consciousness or meningism in children younger than 5 years, prostration in children younger than 3 years, seizures (other than febrile seizures) in children younger than 2 years, and suspicion of sepsis in children younger than 60 days. Cerebrospinal fluid (CSF) was cultured on horse blood and chocolate agar. Beginning in 2003, HIV testing was done on the blood of children admitted to KCH according to the Kenya national policy for paediatric hospital admissions, using two rapid antibody tests. Treatment for all disorders was according to WHO guidelines at the time of admission.
Isolates of H influenzae from sterile-site cultures were identified by colony morphology, Gram stain, and X and V factor dependence at the KEMRI-Wellcome Trust laboratory, located adjacent to KCH. Capsular type was identified by PCR using either the cap locus (done by the Haemophilus Reference Unit/WHO Collaborating Centre for Haemophilus influenzae, Respiratory and Systemic Infection Laboratory, Health Protection Agency Centre for Infections, London, UK, for isolates collected in 2000–04) or the bexA locus (done by the KEMRI-Wellcome Trust laboratory in Kilifi, Kenya, for isolates collected in 2005–13).12 We defined a case of invasive H influenzae type b disease as isolation of type b H influenzae from a sterile-site culture in a child aged 12 years or younger admitted to KCH.
Assessment of nasopharyngeal carriage
We investigated nasopharyngeal carriage of H influenzae type b by undertaking annual cross-sectional surveys of a sample of KHDSS residents of all ages, selected at random from the KHDSS population register once every year from 2009 to 2012, as described elsewhere.13 Isolates of H influenzae type b from nasopharyngeal swabs were identified in the same way as for sterile-site samples.
Assessment of serological immunity
We assessed serological immunity to H influenzae type b in five cross-sectional samples of children aged 12 years or younger within the study area, consisting of four convenience samples from the Junju, Ngerenya, and Chonyi locations in Kilifi County during 1998, 2000, 2004–05, and 2007,14, 15 and an age-stratified sample (50 children in each of ten age strata: 0 years, 1 year, 2 years, 3 years, 4 years, 5 years, 6 years, 7 years, 8–9 years, and 10–14 years) selected at random using Stata (version 10.1) from each age strata from the population register of the KHDSS in 2009.
Serum samples were stored at −70°C until they were tested using an ELISA for antibodies to PRP. ELISA was done at the WHO Pneumococcal Reference Laboratory, Institute of Child Health, London, UK. Methods were as documented elsewhere,16 but with the following alteration: HbOHA antigen (National Institute for Biological Standards and Controls, Hertfordshire, UK) was used at 3 mg/mL. Test, control, and reference (lot 1983; US Food and Drug Administration) serum samples were incubated at 37°C for 1 h. The antibody-binding reaction was monitored by absorbance readings at 410 nm and 630 nm. We determined anti-PRP concentrations by referring to a standard curve generated from the reference wells using four-parameter sigmoid curve fitting. Median values were reported for test serum samples displaying non-parallelism to this curve. Values below the lower limit of quantitation (0·09 mg/mL) were reported as 0·05 mg/mL.
Statistical analysis
For population-based analyses, we designated Jan 1, 2000, through to Dec 31, 2001, as the prevaccine era and Jan 1, 2004, through to Dec 31, 2014, as the routine-use era, to allow time for sufficient vaccine uptake, given that the Hib vaccine was introduced without a catch-up campaign. We calculated the incidence of invasive H influenzae type b disease as the number of KHDSS residents admitted to KCH and confirmed by sterile-site culture to have H influenzae type b infection, divided by the resident population at the midpoint of each observation period. We calculated the incidence of H influenzae type b meningitis as the number of KHDSS residents admitted to KCH with culture-confirmed H influenzae type b (from any sterile site) who met a definition of probable meningitis (CSF white cell count ≥50 × 106 cells/L or a ratio of CSF glucose to plasma glucose of <0·1), divided by the resident population at the midpoint of each observation period. We calculated the incidence rate ratio (IRR) by using Poisson regression for specific age groups and observation periods. We calculated vaccine effectiveness as 1 minus the IRR, expressed as a percentage.
We excluded data from 2004–05 from calculations related to meningitis because a transient change in lumbar puncture clinical practice occurred during this period, and we did not analyse meningitis data after 2010 because pneumococcal conjugate vaccine was introduced in 2011, which was expected to reduce the incidence of probable meningitis. We categorised PRP antibody concentrations according to putative threshold protective concentrations, and calculated geometric mean concentrations by year and age category. Pre-2009 serosurveys did not include children aged 13 years or older, so we excluded data from children who were 13 years or older in 2009 from the serological immunity analyses. We present the decline in PRP antibody concentration and reverse cumulative distribution curves according to age category and year.
We did all statistical analyses using Stata, versions 11.2 and 12.0.
Role of the funding source
The funders of the study had no role in study design; in the collection, analysis, and interpretation of data; or the writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
40 482 children younger than 13 years resident in KHDSS were admitted to KCH between 2000 and 2014, 38 206 (94%) of whom had their blood cultured. Although the number of cases of invasive H influenzae type b disease declined after vaccine introduction, the site of culture, sex of patients, and syndrome-specific mortality did not change substantially between the pre-vaccine and routine-use eras (table 1). In children younger than 5 years, the median age of infection with H influenzae type b was 10 months (IQR 5–24) in the prevaccine era and 10·5 months (4–23) in the routine-use era. HIV status was determined for 25 (68%) of the 37 KHDSS children with invasive H influenzae type b disease from 2003 to 2014. Four (16%) of the 25 children with H influenzae type b disease had HIV infection (three in 2003 and one in 2008). For comparison, HIV prevalence in all paediatric admissions to KCH in 2005–14 was 4·3% (807 of 18 767 admissions in whom HIV results were available).
Table 1.
Invasive Haemophilus influenzae disease in children aged 0–12 years in the Kilifi Health and Demographic Surveillance System admitted to the Kilifi County Hospital, 2000–14
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age <5 years | |||||||||||||||||
| Admissions | 3660 | 3591 | 3282 | 2776 | 2716 | 2317 | 2455 | 2130 | 1986 | 2131 | 1905 | 1642 | 1494 | 1001 | 1473 | ||
| With blood culture | 3613 (99%) | 3533 (98%) | 3200 (98%) | 2710 (98%) | 2631 (97%) | 2219 (96%) | 2366 (96%) | 1991 (93%) | 1841 (93%) | 1996 (94%) | 1747 (92%) | 1528 (93%) | 1331 (89%) | 904 (90%) | 1335 (91%) | ||
| With lumbar puncture | 939 (26%) | 866 (24%) | 936 (29%) | 578 (21%) | 351 (13%) | 482 (21%) | 693 (28%) | 611 (29%) | 553 (28%) | 548 (26%) | 532 (28%) | 433 (26%) | 410 (27%) | 260 (26%) | 390 (26%) | ||
| With probable bacterial meningitis | 33 (1%) | 40 (1%) | 52 (2%) | 24 (1%) | 20 (1%) | 24 (1%) | 25 (1%) | 26 (1%) | 18 (1%) | 16 (1%) | 18 (1%) | 18 (1%) | 11 (1%) | 11 (1%) | 5 (<1%) | ||
| Culture-confirmed H influenzae disease | 24 (<1%) | 30 (<1%) | 21 (<1%) | 22 (<1%) | 4 (<1%) | 5 (<1%) | 2 (<1%) | 2 (<1%) | 5 (<1%) | 2 (<1%) | 1 (<1%) | 0 | 3 (<1%) | 2 (<1%) | 3 (<1%) | ||
| Types a, c, d, e, f | 2 (8%) | 1 (3%) | 1 (5%) | 0 | 1 (25%) | 1 (20%) | 0 | 1 (50%) | 2 (40%) | 0 | 0 | 0 | 2 (67%) | 1 (50%) | 1 (33%) | ||
| Non-capsular | 3 (13%) | 1 (3%) | 3 (14%) | 4 (18%) | 0 | 1 (20%) | 0 | 0 | 1 (20%) | 0 | 1 (100%) | 0 | 1 (33%) | 0 | 2 (67%) | ||
| Type b | 19 (79%) | 28 (93%) | 17 (81%) | 18 (82%) | 3 (75%) | 3 (60%) | 2 (100%) | 1 (50%) | 2 (40%) | 2 (100%) | 0 | 0 | 0 | 1 (50%) | 0 | ||
| H influenzae type b cultured in CSF | 4 (21%) | 13 (46%) | 8 (47%) | 8 (44%) | 1 (33%) | 1 (33%) | 0 | 0 | 1 (50%) | 1 (50%) | 0 | 0 | 0 | 0 | 0 | ||
| Age <24 months | 16 (84%) | 19 (68%) | 10 (59%) | 10 (56%) | 2 (67%) | 1 (33%) | 0 | 1 (100%) | 1 (50%) | 2 (100%) | 0 | 0 | 0 | 1 (100%) | 0 | ||
| Boys | 7 (37%) | 15 (54%) | 6 (35%) | 7 (39%) | 2 (67%) | 1 (33%) | 1 (50%) | 1 (100%) | 0 | 1 (50%) | 0 | 0 | 0 | 1 (100%) | 0 | ||
| Died during the episode | 3 (16%) | 8 (29%) | 5 (29%) | 6 (33%) | 0 | 1 (33%) | 0 | 1 (100%) | 1 (50%) | 0 | 0 | 0 | 0 | 1 (100%) | 0 | ||
| Age 5–12 years | |||||||||||||||||
| Admissions | 459 | 398 | 377 | 416 | 373 | 425 | 419 | 369 | 356 | 395 | 436 | 413 | 324 | 283 | 480 | ||
| With blood culture | 448 (98%) | 378 (95%) | 361 (96%) | 394 (95%) | 328 (88%) | 371 (87%) | 376 (90%) | 326 (88%) | 308 (87%) | 331 (84%) | 380 (87%) | 347 (84%) | 274 (85%) | 226 (80%) | 413 (86%) | ||
| With lumbar puncture | 74 (16%) | 55 (14%) | 68 (18%) | 54 (13%) | 41 (11%) | 37 (9%) | 56 (13%) | 44 (12%) | 50 (14%) | 46 (12%) | 88 (20%) | 65 (16%) | 71 (22%) | 81 (29%) | 96 (20%) | ||
| With probable bacterial meningitis | 7 (2%) | 5 (1%) | 6 (2%) | 10 (2%) | 5 (1%) | 1 (<1%) | 1 (<1%) | 5 (1%) | 4 (1%) | 3 (1%) | 4 (1%) | 3 (1%) | 1 (<1%) | 4 (1%) | 1 (<1%) | ||
| Culture-confirmed H influenzae disease | 1 (<1%) | 2 (<1%) | 2 (<1%) | 2 (<1%) | 1 (<1%) | 1 (<1%) | 1 (<1%) | 0 | 0 | 1 (<1%) | 1 (<1%) | 1 (<1%) | 0 | 0 | 1 (<1%) | ||
| Types a, c, d, e, f | 0 | 0 | 1 (50%) | 0 | 0 | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Non-capsular | 1 (100%) | 0 | 0 | 0 | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 0 | 0 | 1 (100%) | ||
| Type b | 0 | 2 (100%) | 1 (50%) | 2 (100%) | 1 (100%) | 0 | 0 | 0 | 0 | 1 (100%) | 1 (100%) | 0 | 0 | 0 | 0 | ||
| H influenzae type b cultured in CSF | 0 | 1 (50%) | 1 (100%) | 1 (50%) | 1 (100%) | 0 | 0 | 0 | 0 | 1 (100%) | 1 (100%) | 0 | 0 | 0 | 0 | ||
| Boys | 0 | 1 (50%) | 1 (100%) | 1 (50%) | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 1 (100%) | 0 | 0 | 0 | 0 | ||
| Died during the episode | 0 | 1 (50%) | 1 (100%) | 1 (50%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
Data are N and n (%). CSF=cerebrospinal fluid.
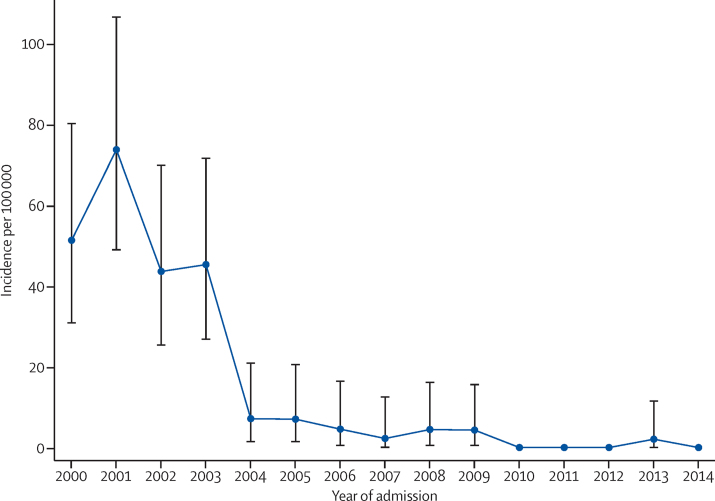
The mean annual incidence of invasive H influenzae type b disease in children younger than 5 years in Kilifi was 62·6 per 100 000 (95% CI 46·0–83·3) in the pre-vaccine era (2000–01) and 4·5 per 100 000 (2·5–7·5) in the routine-use era (2004–14; figure 1), which translates to a vaccine effectiveness of 93% (95% CI 87–96; table 2). Incidences of invasive H influenzae type b disease and meningitis in the early introduction era (2002–03) were significantly higher than in later periods in children younger than 5 years, but rates of invasive H influenzae type b disease were similar in different periods of routine use (table 2). Incidence of invasive H influenzae type b disease in children aged 5–12 years was low in the prevaccine era and remained low in the routine-use era (table 2). In the routine-use era, 13 of the 17 cases of invasive H influenzae type b disease occurred in children younger than 36 months (the age before which serological immunity starts to decline and a comparable metric to that presented in the first Kilifi analysis3). The incidence of non-type-b invasive H influenzae disease (ie, serotype replacement disease) did not increase after introduction of the Hib vaccine (IRR in children aged <5 years, 2000–01 vs 2004–14, was 0·71 (95% CI 0·19–2·61; table 1).
Figure 1.
Incidence of invasive Haemophilus influenzae type b disease in children younger than 5 years in the Kilifi Health and Demographic Surveillance System, 2000–14
Hib vaccine was introduced in November, 2001. Error bars show 95% CI.
Table 2.
Incidence (per 100 000) of invasive Haemophilus influenzae type b disease and meningitis, and probable bacterial meningitis, in children aged 0–12 years in the Kilifi Health and Demographic Surveillance System, 2000–14
|
Age <2 years |
Age <5 years |
Age 5–12 years |
|||||
|---|---|---|---|---|---|---|---|
| N | Incidence (95% CI) | N | Incidence (95% CI) | N | Incidence (95% CI) | ||
| Invasive H influenzae type b disease | |||||||
| 2000–01 | 35 | 117·1 (81·6–162·9) | 47 | 62·6 (46·0–83·3) | 2 | 4·0 (0·5–14·6) | |
| 2002–03 | 25 | 79·4 (51·4–117·2) | 34 | 44·6 (31·0–61·8) | 3 | 2·9 (0·6–8·6) | |
| 2004–14 | 11 | 8·6 (4·3–15·3) | 14 | 4·5 (2·5–7·5) | 3 | 1·7 (0·3–4·8) | |
| 2004–05 | 5 | 14·5 (4·7–33·8) | 6 | 7·1 (2·6–15·4) | 1 | 1·9 (0·1–10·3) | |
| 2006–09 | 5 | 6·7 (2·2–15·7) | 7 | 3·9 (1·6–8·0) | 1 | 1·6 (0·0–8·8) | |
| 2010–14 | 1 | 5·1 (0·1–28·3) | 1 | 2·1 (0·1–11·5) | 1 | 1·6 (0·0–8·6) | |
| Incidence rate ratio | |||||||
| 2000–01 vs 2002–03 | .. | 0·67 (0·41–1·13) | .. | 0·71 (0·46–1·10) | .. | 0·73 (0·12–4·34) | |
| 2000–01 vs 2004–14 | .. | 0·07 (0·04–0·14) | .. | 0·07 (0·04–0·13) | .. | 0·41 (0·07–2·44) | |
| H influenzae type b meningitis | |||||||
| 2000–01 | 14 | 46·8 (25·6–78·6) | 17 | 22·7 (13·2–36·3) | 1 | 2·0 (0·5–11·2) | |
| 2002–03 | 12 | 38·1 (19·7–66·6) | 15 | 19·1 (10·7–31·4) | 1 | 2·0 (0·1–11·0) | |
| 2006–10 | 2 | 5·3 (0·7–19·2) | 2 | 2·2 (0·3–7·9) | 2 | 1·6 (0·2–5·6) | |
| Incidence rate ratio | |||||||
| 2000–01 vs 2002–03 | .. | 0·81 (0·38–1·76) | .. | 0·84 (0·42–1·68) | .. | 0·97 (0·06–15·57) | |
| 2000–01 vs 2006–10 | .. | 0·11 (0·03–0·50) | .. | 0·09 (0·02–0·42) | .. | 0·77 (0·07–8·54) | |
| Probable bacterial meningitis | |||||||
| 2000–01 | 61 | 204·1 (156·1–262·2) | 73 | 97·3 (76·3–122·3) | 15 | 15·3 (8·6–25·3) | |
| 2002–03 | 67 | 212·9 (165·0–270·3) | 76 | 96·5 (76·0–120·8) | 17 | 16·6 (9·7–26·6) | |
| 2006–10 | 80 | 85·4 (67·7–106·3) | 103 | 45·3 (37·0–55·0) | 17 | 5·5 (3·2–8·8) | |
| Incidence rate ratio | |||||||
| 2000–01 vs 2002–03 | .. | 1·04 (0·74–1·48) | .. | 0·99 (0·72–1·37) | .. | 1·08 (0·54–2·17) | |
| 2000–01 vs 2006–10 | .. | 0·41 (0·30–0·58) | .. | 0·47 (0·35–0·63) | .. | 0·36 (0·18–0·72) | |
N is the number of children with the disease during the corresponding timeframe.
A previous study17 showed that nasopharyngeal carriage of H influenzae type b in children younger than 5 years was 1·7% in 2004, 3 years after Hib vaccine introduction. We obtained nasopharyngeal swabs from 2031 KHDSS residents in the four annual cross-sectional nasopharyngeal swab surveys we did in 2009–12. One (0·2%; identified in the 2012 survey) of 623 children younger than 5 years and two (0·1%; both identified in the 2009 survey) of 1408 individuals aged 5 years or older (mean age 34 years [SD 22·5; range 5–92]), carried H influenzae type b in the nasopharynx.
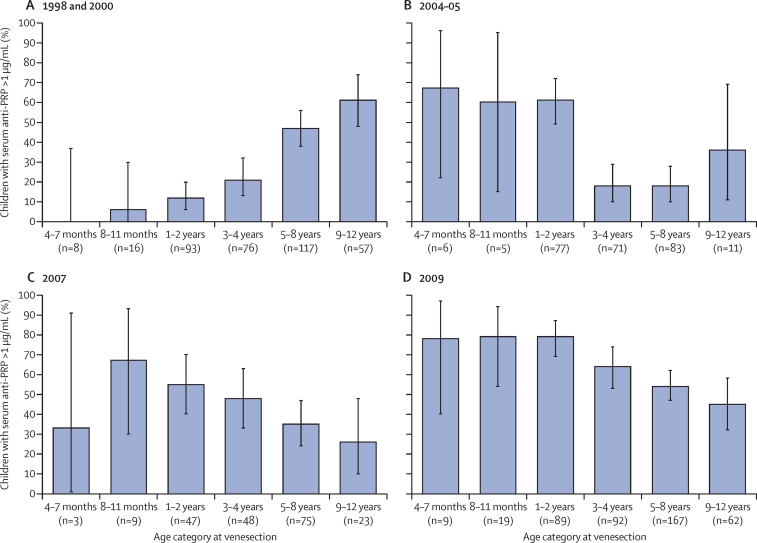
To assess serological immunity, we tested available stored serum samples from children younger than 12 years from four convenience samples (367 samples from 1998 and 2000, 253 samples from 2004–05, and 205 samples from 2007), and a further 438 samples from our age-stratified, random sample of KHDSS children in 2009. The pattern of immunity in children in the community is shown in Figure 2, Figure 3 and the appendix. The proportion of children with an anti-PRP concentration of greater than 1 mg/mL (the putative threshold for long-term protection from invasive H influenzae type b disease) increased with age in the prevaccine surveys in 1998 and 2000, from very low proportions of infants (one [4%] of 24]) aged 4–7 months and 8–11 months (those at highest risk of invasive H influenzae type b disease) up to 35 (61%; 95% CI 48–74) of 57 children aged 9–12 years (figure 2).18 After Hib vaccine introduction, a large proportion of children had protective concentrations of antibody (92 [79%; 95% CI 70–86] of 117 children aged 4–35 months had long-term protective anti-PRP concentrations in 2009) and the proportion with long-term protective anti-PRP concentrations did not start to decline until after 36 months of age (figure 2). We noted a similar pattern when assessing the geometric mean concentrations (appendix).
Figure 2.
Children with anti-PRP concentrations of >1 μg/mL, by age group and survey year
Data for 1998 and 2000 combined (A), 2004–05 (B), 2007 (C), and 2009 (D). The proportion of children aged 4–7 months in (A) is 0%. Error bars show 95% CI. PRP=polyribosylribitol phosphate.
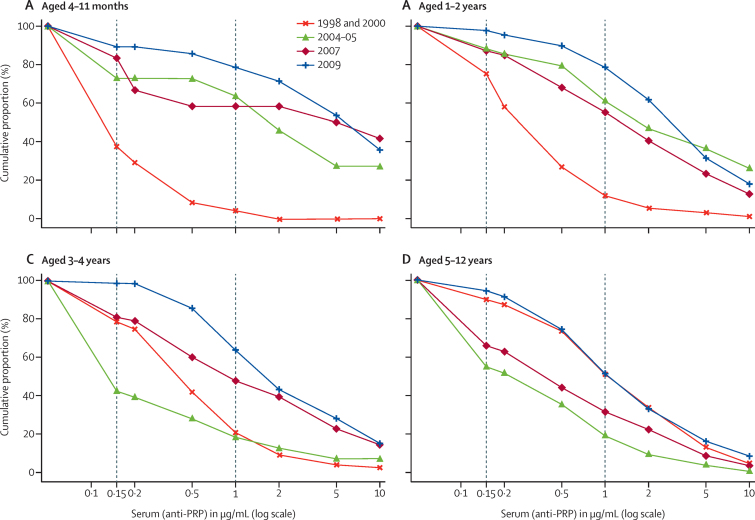
Figure 3.
Reverse cumulative proportions of children with anti-PRP concentrations that exceed thresholds, by age group and survey year
Data for children aged 4–11 months (A) 1–2 years (B), 3–4 years (C), and 5–12 years (D). Vertical lines indicate thresholds for short-term (0·15 μg/mL) and long-term (1 μg/mL) protection against invasive H influenzae type b disease.
The proportions of children of various ages exceeding anti-PRP concentrations in the years before and after vaccine introduction are shown in the reverse cumulative distribution (RCD) curves (figure 3). The proportions of children aged 4 months to 2 years exceeding anti-PRP concentrations of 0·15 μg/mL and 1 mg/mL were almost uniformly greater in the years after vaccine introduction than in the prevaccine surveys (ie, the RCD curves shift up and to the right after vaccine introduction). In children aged 3–4 years, serological protection declined in 2004–05, but by 2007, population immunity was greater than in prevaccine years. Children aged 5–12 years had a transient decline in serological protection during 2004–05 and 2007, but the RCD curves suggest the same extent of population protection in 2009 as in prevaccine years.
Discussion
We report sustained control of paediatric invasive H influenzae type b disease, to the point of near elimination, in Kilifi, Kenya, in the 13 years after the introduction of Hib vaccine into the routine infant vaccination schedule without a booster dose. Kenya was one of three countries in Africa that were first to include the Hib vaccine in their routine childhood immunisation programme, and this study provides evidence of a robust and durable effect of the vaccine programme. Worldwide, 46 of 54 high-income countries give a booster dose of Hib vaccine, whereas booster doses are used by only six of 78 low-income and lower-middle-income countries and 22 of 57 upper-middle-income countries.4 This disparity is a reflection of the fact that support from Gavi, the Vaccine Alliance, for introduction of the Hib vaccine in the poorest countries of the world, which began in 2000, is aligned with the WHO recommendation for routine infant vaccination with Hib as a three-dose primary series.
After introduction of the Hib vaccine, a marked reduction in H influenzae type b disease has been documented in many developed and developing regions; however, opportunities to examine the sustainability of the vaccine impact in the absence of a booster dose have been scarce.1 Our results are consistent with findings in western regions of The Gambia of near elimination of paediatric H influenzae type b meningitis 14 years after introduction of Hib vaccine administered at 2, 3, and 4 months of age and in the absence of a booster dose.8 As in The Gambia, our study occurred in a setting with high vaccine coverage: in the KHDSS, coverage with three doses of the Hib vaccine was 91% in children aged 12 months in 2002, 88% in children aged 9–23 months in 2004, 95% in children aged 12 months in 2007, and 93% in 2013 in those aged 12–23 months resident in the KHDSS since birth who had vaccine cards available (Scott JAG, unpublished).19, 20 Additional evidence of sustained disease control in the absence of a booster dose is provided by data from South America, where the incidence of H influenzae type b meningitis 6–10 years after vaccination introduction was similar in four countries, two of which used a booster dose and two of which did not.21
By contrast, evidence of waning immunity has prompted three countries—the UK, Mexico, and South Africa—to add a booster dose of Hib vaccine, after they initially recommended only a primary series. In response to low levels of anti-PRP concentrations, persistence of H influenzae type b nasopharyngeal carriage, and rising rates of invasive disease, the UK introduced a booster dose of Hib vaccine for children aged 12–15 months in 2006, 10 years after introducing infant Hib vaccine with a catch-up campaign but without a booster dose.5 In Mexico, in 2006, results of a cross-sectional study showed that only 40–50% of 110 children aged 12–23 months had anti-PRP concentrations greater than 1 mg/mL.7 92% of these children had received the full Hib vaccine primary course given as the combination pentavalent vaccine, the same combination vaccine as is used in Kenya. On the basis of these results, Mexico, an upper-middle-income country, introduced Hib booster vaccine in 2007. In 2010, 11 years after introducing Hib vaccine as a three-dose primary series, South Africa also introduced a booster dose of the combination vaccine containing Hib. Although the booster dose was primarily for polio prevention, it was hoped that the booster would reduce the number of Hib vaccine failures in South African children (135 [51%] of 263 cases of invasive H influenzae type b disease in 2003–09 were classified as being the result of vaccine failures, of which 55% occurred in children aged 18 months or older).22 In 2015, a resurgence of invasive H influenzae type b disease was reported in eastern regions of The Gambia where coverage with three doses of Hib vaccine was 91% in children aged 12 months, suggesting that a three-dose primary series in the absence of a booster dose might not be providing sustained disease control in this setting.23 The reason for this resurgence was unclear, but the authors of the report speculated that it could have been related to waning immunity, continued transmission, or a change in malaria prevalence.
The reason a booster dose is needed to achieve sustained control of disease in some settings but not others remains unclear. Tetanus-toxoid-conjugated Hib vaccine is used widely in developing countries—both in settings with sustained control of H influenzae type b disease and in those with evidence of waning immunity—so the vaccine formulation is unlikely to explain the different patterns of disease. Vaccinated individuals are less likely to be carriers of H influenzae type b and are therefore less likely to transmit the infection. However, reductions in carriage and transmission also result in fewer opportunities for natural acquisition of anti-H influenzae type b antibodies or for boosting of such antibodies. This might have been the reason anti-H influenzae type b antibodies declined in adults after routine use of Hib vaccine in children in the UK.24 In The Gambia from 1997 to 2002, introduction and widespread use of Hib vaccine was associated with a decline in nasopharyngeal carriage of H influenzae type b from 12% to 0·25% in children younger than 5 years.25 In 2010, oropharyngeal H influenzae type b carriage, as detected by culture, was estimated to be 0·9% in children aged 12–23 months in eastern regions of The Gambia.8 This is slightly higher than the carriage prevalence detected by culture of nasopharyngeal swabs reported herein. Culture of oropharyngeal swabs and PCR-based methods might be more sensitive for the detection of H influenzae type b than are nasopharyngeal swabs;26, 27 however, carriage in KHDSS residents was also low when PCR for H influenzae type b was done on both oropharyngeal and nasopharyngeal swabs collected from children aged 2–59 months enrolled as controls in a multisite study of pneumonia aetiology in 2011–13 (three [<1%] of 856 children; Hammitt LL and Scott JAG, unpublished). Although older children and adults can serve as a reservoir for transmission, the prevalence of H influenzae type b carriage was very low in these age groups in Kilifi. On the basis of these data, the opportunities for natural boosting in Kilifi are rare, as is the risk of exposure.
In Kilifi, in the years after vaccine introduction, naturally acquired antibody has been replaced by vaccine-induced antibody. For infants and young children, this has meant greater serological protection, with 79% (95% CI 59–92) of children aged 4–11 months (historically at greatest risk of invasive disease) now having concentrations greater than 1 mg/mL, the threshold associated with long-term H influenzae type b-specific protection.18 Older children, who in the prevaccine era had naturally acquired immunity, went through a transitional period (2004–05 and 2007) during which both geometric mean concentrations and proportions exceeding protective thresholds were lower than in the prevaccine era. As surveys of older children started to include those who had been vaccinated, and vaccine-induced antibody was persisting into late childhood, measures for children aged 5–12 years resumed their prevaccine levels by 2009. In essence, the vaccine has led to improvements in population immunity in the youngest, highest-risk age groups without compromising immunity in older children. The low number of serosurvey participants in the youngest age groups is a limitation of these data. Although the 2009 survey participants were selected at random from KHDSS records, earlier surveys were convenience samples, which might have resulted in imbalances in representation of children in KHDSS as a whole. Additionally, the longer duration of freezing could have degraded antibody in older samples. These limitations notwithstanding, these results are promising for the prospect of continued effectiveness of Hib vaccine against invasive disease in older children and for maintenance of herd immunity in this setting. However, continued observation is needed, because the proportion of older children (aged 9–12 years) with anti-PRP concentrations greater than 1 mg/mL in Kilifi in 2009 (28 [45%] of 62 children) was similar to eastern regions of The Gambia (55% of 9–14 year-olds), where a resurgence in disease has been noted.
Our immunogenicity data are similar to other studies' data from low-income or lower-middle-income countries. In vaccine trials in Niger and Nepal, 83–88% and 100% of infants, respectively, had post-primary vaccination concentrations of anti-PRP above 1 mg/mL, declining to 67–75% and 64%, respectively, by late infancy.28, 29 In Mali, 2 years after vaccine introduction and with coverage at 81%, 82% of infants aged 6–7 months had anti-PRP concentrations greater than 1 mg/mL.30 In the same setting the following year, antibody decline did not begin until after 2 years of age.31 Our results also lend support to the previously observed findings that children in developing countries generate higher anti-PRP concentrations in response to vaccination than those in developed countries such as the UK.32 Proposed reasons for this include higher background environmental H influenzae type b exposure in developing countries and exposure to bacterial polysaccharides that cross-react with the PRP capsular polysaccharide of H influenzae type b.33, 34, 35, 36 Exposure to potentially cross-reactive organisms such as Escherichia coli or serogroup 6 pneumococci is likely to be higher in developing countries without access to improved water and sanitation or with higher overall pneumococcal carriage prevalence and density than in high-income or middle-income settings.13, 37, 38, 39 The level and effects of this exposure could change with improvements in water and sanitation and expanding use of pneumococcal conjugate vaccines. Continued surveillance will monitor whether effective control of disease persists or whether shifts in epidemiology (eg, disease occurring in older children, fewer opportunities for natural boosting) will necessitate a booster dose.
The findings reported herein do not address the possible benefit of a booster dose of Hib vaccine in settings with different epidemiological characteristics from Kilifi (eg, higher HIV prevalence, lower vaccine coverage, exposure to highly unvaccinated populations that might have high rates of carriage). Because the reasons why some settings require a booster dose of Hib vaccine to maintain control of disease and others do not are not well understood, local epidemiological data is vital to guide vaccine policy. In the absence of long-standing surveillance for invasive H influenzae type b disease, low H influenzae type b carriage prevalence in large studies that include both children and adults and use sensitive methods of detection, or high prevalence of protective antibody concentrations throughout the ages of highest risk for H influenzae type b disease, would provide evidence of ongoing protection.
Over the past 25 years, 189 countries, including 73 countries eligible for support from Gavi, the Vaccine Alliance, have introduced a Hib-containing vaccine into their national immunisation programme for children. A booster dose of Hib vaccine is recommended in most high-income countries, whereas most low-income countries have followed the WHO EPI schedule, which does not include a booster dose. In this study, we found that use of Hib vaccine according to the EPI schedule led to near elimination of paediatric invasive H influenzae type b disease, with no evidence of resurgent disease in older children in whom immunity might be expected to wane without a booster dose. Indeed, immunogenicity data show that immunity persists through the age of greatest risk of disease for most children and that antibody concentrations in older children, although lower than in young children, are similar to concentrations reported in the prevaccine era. In sum, we found no evidence to support introduction of a booster dose of Hib vaccine into the Kenyan EPI at this time.
Acknowledgments
Acknowledgments
We thank the residents of the Kilifi Health and Demographic Surveillance System and the dedicated team of fieldworkers, administrative staff, clinicians, and laboratory staff who worked on this study. This report is published with the permission of the Director of the Kenya Medical Research Institute. LLH, JAGS, and SCM have received grants from the Gavi, the Vaccine Alliance. JAGS is funded by the Wellcome Trust (fellowship number 98504). RJC has received a European Society for Paediatric Infectious Diseases award, and the National Institute for Health Research funded her Academic Clinical Fellowship.
Contributors
LLH, TK, SS, NM, and JAGS were responsible for the design and conduct of the study. RJC, AK, SCM, PB, and DG did the laboratory analyses. LLH, RJC, AK, AM, and JAGS analysed the data, and LLH, RJC, AM, SCM, PB, DG, and JAGS interpreted it. LLH, RJC, SCM, DG, and JAGS were responsible for the writing of the report.
Declaration of interests
LLH has received research funding from GlaxoSmithKline Biologicals and Pfizer, and has participated in a Scientific Input Engagement for Merck. DG has received research funding from GlaxoSmithKline Biologicals, Sanofi, and Merck. All other authors declare no competing interests.
Supplementary Material
References
- 1.Watt JP, Chen S, Santosham M. Haemophilus influenzae type b conjugate vaccine: review of observational data on long term vaccine impact to inform recommendations for vaccine schedules. World Health Organization; Geneva: 2012. [Google Scholar]
- 2.Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev. 2000;13:302–317. doi: 10.1128/cmr.13.2.302-317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cowgill KD, Ndiritu M, Nyiro J. Effectiveness of Haemophilus influenzae type b conjugate vaccine introduction into routine childhood immunization in Kenya. JAMA. 2006;296:671–678. doi: 10.1001/jama.296.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO WHO vaccine-preventable diseases: monitoring system. 2014 global summary. http://apps.who.int/immunization_monitoring/globalsummary/schedules (accessed Feb 8, 2015).
- 5.Johnson NG, Ruggeberg JU, Balfour GF. Haemophilus influenzae type b reemergence after combination immunization. Emerg Infect Dis. 2006;12:937–941. doi: 10.3201/eid1206.051451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trotter CL, McVernon J, Andrews NJ, Burrage M, Ramsay ME. Antibody to Haemophilus influenzae type b after routine and catch-up vaccination. Lancet. 2003;361:1523–1524. doi: 10.1016/s0140-6736(03)13172-8. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez RS, Mascarenas C, Conde-Glez CJ. Serological protection induced by Haemophilus influenzae type B conjugate vaccine in Mexican children: is a booster dose of the vaccine needed? Clin Vaccine Immunol. 2010;17:1639–1641. doi: 10.1128/CVI.00249-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howie SR, Oluwalana C, Secka O. The effectiveness of conjugate Haemophilus influenzae type B vaccine in The Gambia 14 years after introduction. Clin Infect Dis. 2013;57:1527–1534. doi: 10.1093/cid/cit598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO Haemophilus influenzae type b (Hib) vaccination position paper—July 2013. Wkly Epidemiol Rec. 2013;88:413–426. [PubMed] [Google Scholar]
- 10.Scott JA, Bauni E, Moisi JC. Profile: The Kilifi Health and Demographic Surveillance System (KHDSS) Int J Epidemiol. 2012;41:650–657. doi: 10.1093/ije/dys062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National AIDS and STI Control Program (NASCOP) Kenya . Kenya AIDS indicator survey 2012: final report. National AIDS and STI Control Program; Nairobi, Kenya: 2014. [Google Scholar]
- 12.Maaroufi Y, De Bruyne JM, Heymans C, Crokaert F. Real-time PCR for determining capsular serotypes of Haemophilus influenzae. J Clin Microbiol. 2007;45:2305–2308. doi: 10.1128/JCM.00102-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammitt LL, Akech DO, Morpeth SC. Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: findings from cross-sectional carriage studies. Lancet Glob Health. 2014;2:e397–e405. doi: 10.1016/S2214-109X(14)70224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bejon P, Mwacharo J, Kai O. A phase 2b randomised trial of the candidate malaria vaccines FP9 ME-TRAP and MVA ME-TRAP among children in Kenya. PLoS Clin Trials. 2006;1:e29. doi: 10.1371/journal.pctr.0010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mwangi TW, Ross A, Snow RW, Marsh K. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J Infect Dis. 2005;191:1932–1939. doi: 10.1086/430006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phipps DC, West J, Eby R, Koster M, Madore DV, Quataert SA. An ELISA employing a Haemophilus influenzae type b oligosaccharide-human serum albumin conjugate correlates with the radioantigen binding assay. J Immunol Methods. 1990;135:121–128. doi: 10.1016/0022-1759(90)90264-v. [DOI] [PubMed] [Google Scholar]
- 17.Abdullahi O, Nyiro J, Lewa P, Slack M, Scott JA. The descriptive epidemiology of Streptococcus pneumoniae and Haemophilus influenzae nasopharyngeal carriage in children and adults in Kilifi district, Kenya. Pediatr Infect Dis J. 2008;27:59–64. doi: 10.1097/INF.0b013e31814da70c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kayhty H, Peltola H, Karanko V, Makela PH. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1983;147:1100. doi: 10.1093/infdis/147.6.1100. [DOI] [PubMed] [Google Scholar]
- 19.Moisi JC, Kabuka J, Mitingi D, Levine OS, Scott JA. Spatial and socio-demographic predictors of time-to-immunization in a rural area in Kenya: Is equity attainable? Vaccine. 2010;28:5725–5730. doi: 10.1016/j.vaccine.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ndiritu M, Cowgill KD, Ismail A. Immunization coverage and risk factors for failure to immunize within the Expanded Programme on Immunization in Kenya after introduction of new Haemophilus influenzae type b and hepatitis b virus antigens. BMC Public Health. 2006;6:132. doi: 10.1186/1471-2458-6-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia S, Lagos R, Munoz A. Impact of vaccination against Haemophilus influenzae type b with and without a booster dose on meningitis in four South American countries. Vaccine. 2012;30:486–492. doi: 10.1016/j.vaccine.2011.10.101. [DOI] [PubMed] [Google Scholar]
- 22.von Gottberg A, Cohen C, Whitelaw A. Invasive disease due to Haemophilus influenzae serotype b ten years after routine vaccination, South Africa, 2003–2009. Vaccine. 2012;30:565–571. doi: 10.1016/j.vaccine.2011.11.066. [DOI] [PubMed] [Google Scholar]
- 23.Mackenzie GA, Ikumapayi UN, Scott S. Increased disease due to Haemophilus influenzae type b: population-based surveillance in eastern Gambia, 2008–2013. Pediatr Infect Dis J. 2015;34:e107–e112. doi: 10.1097/INF.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 24.McVernon J, Trotter CL, Slack MP, Ramsay ME. Trends in Haemophilus influenzae type b infections in adults in England and Wales: surveillance study. BMJ. 2004;329:655–658. doi: 10.1136/bmj.329.7467.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adegbola RA, Secka O, Lahai G. Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunisation with a Hib conjugate vaccine: a prospective study. Lancet. 2005;366:144–150. doi: 10.1016/S0140-6736(05)66788-8. [DOI] [PubMed] [Google Scholar]
- 26.Michaels RH, Poziviak CS, Stonebraker FE, Norden CW. Factors affecting pharyngeal Haemophilus influenzae type b colonization rates in children. J Clin Micro. 1976;4:413–417. doi: 10.1128/jcm.4.5.413-417.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odutola A, Antonio M, Owolabi O. Comparison of the prevalence of common bacterial pathogens in the oropharynx and nasopharynx of Gambian infants. PLoS One. 2013;8:e75558. doi: 10.1371/journal.pone.0075558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campagne G, Garba A, Schuchat A. Response to conjugate Haemophilus influenzae B vaccine among infants in Niamey, Niger. Am J Trop Med Hyg. 1998;59:837–842. doi: 10.4269/ajtmh.1998.59.837. [DOI] [PubMed] [Google Scholar]
- 29.Metz JA, Hanieh S, Pradhan R. Evaluation of Haemophilus influenzae type b vaccine for routine immunization in Nepali infants. Pediatr Infect Dis J. 2012;31:e66–e72. doi: 10.1097/INF.0b013e31824a9c37. [DOI] [PubMed] [Google Scholar]
- 30.Sow SO, Tapia MD, Diallo S. Haemophilus influenzae type B conjugate vaccine introduction in Mali: impact on disease burden and serologic correlate of protection. Am J Trop Med Hyg. 2009;80:1033–1038. [PubMed] [Google Scholar]
- 31.Hutter J, Pasetti MF, Sanogo D, Tapia MD, Sow SO, Levine MM. Naturally acquired and conjugate vaccine-induced antibody to Haemophilus influenzae type b (Hib) polysaccharide in Malian children: serological assessment of the Hib immunization program in Mali. Am J Trop Med Hyg. 2012;86:1026–1031. doi: 10.4269/ajtmh.2012.11-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly DF, Moxon ER, Yu LM, Pollard AJ. Anti-polyribosylribitol phosphate antibody concentrations and avidities in children since the start of Haemophilus influenzae type b immunization of infants in the United Kingdom. Clin Vaccine Immunol. 2009;16:246–252. doi: 10.1128/CVI.00023-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradshaw MW, Schneerson R, Parke JC, Jr, Robbins JB. Bacterial antigens cross-reactive with the capsular polysaccharide of Haemophilus influenzae type b. Lancet. 1971;1:1095–1096. doi: 10.1016/s0140-6736(71)91837-x. [DOI] [PubMed] [Google Scholar]
- 34.Neter E. Antigenic relationship between H. influenzae type b and pneumococcus type VI. Proc Soc Exp Biol Med. 1943;52:289–292. [Google Scholar]
- 35.Schneerson R, Bradshaw M, Whisnant JK, Myerowitz RL, Parke JC, Jr, Robbins JB. An Escherichia coli antigen cross-reactive with the capsular polysaccharide of Haemophilus influenzae type b: occurrence among known serotypes, and immunochemical and biologic properties of E coli antisera toward H influenzae type b. J Immunol. 1972;108:1551–1562. [PubMed] [Google Scholar]
- 36.Zepp HD, Hodes HL. Antigenic relation of type b H. influenzae to type 29 and type 6 pneumococci. Proc Soc Exp Biol Med. 1943;52:315–317. [Google Scholar]
- 37.Adegbola RA, DeAntonio R, Hill PC. Carriage of Streptococcus pneumoniae and other respiratory bacterial pathogens in low and lower-middle income countries: a systematic review and meta-analysis. PLoS One. 2014;9:e103293. doi: 10.1371/journal.pone.0103293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morpeth SC. Understanding the dynamics and spread of pneumococcal infection from healthy carriage to pneumonia and invasive disease, in Kilifi, Kenya. PhD thesis, Open University, 2014.
- 39.van den Bergh MR, Spijkerman J, Swinnen KM. Effects of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D-conjugate vaccine on nasopharyngeal bacterial colonization in young children: a randomized controlled trial. Clin Infect Dis. 2013;56:e30–e39. doi: 10.1093/cid/cis922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.