Abstract
Agarwood, a highly valuable resinous and fragrant heartwood of Aquilaria plants, is widely used in traditional medicines, incense and perfume. Only when Aquilaria trees are wounded by external stimuli do they form agarwood sesquiterpene defensive compounds. Therefore, understanding the signaling pathway of wound-induced agarwood formation is important. Jasmonic acid (JA) is a well-characterized molecule that mediates a plant’s defense response and secondary metabolism. However, little is known about the function of endogenous JA in agarwood sesquiterpene biosynthesis. Here, we report that heat shock can up-regulate the expression of genes in JA signaling pathway, induce JA production and the accumulation of agarwood sesquiterpene in A. sinensis cell suspension cultures. A specific inhibitor of JA, nordihydroguaiaretic acid (NDGA), could block the JA signaling pathway and reduce the accumulation of sesquiterpene compounds. Additionally, compared to SA and H2O2, exogenously supplied methyl jasmonate has the strongest stimulation effect on the production of sesquiterpene compounds. These results clearly demonstrate the central induction role of JA in heat-shock-induced sesquiterpene production in A. sinensis.
The plants of Aquilaria spp. belong to the family Thymelaeaceae, and are typical evergreen trees primarily distributed throughout Southeast Asia. The resinous portion of their branches and trunks, known as agarwood, is widely used in traditional medicine as a digestive, sedative, and anti-emetic and is also popularly used in incense and perfume1,2,3,4,5,6. In the international market, high-quality agarwood is more costly than gold. To date, most agarwood comes from wild Aquilaria resources, which has resulted in severe destruction of the natural Aquilaria forests in almost all the countries where agarwood has been commercially exploited. To protect wild Aquilaria resources and to ensure their sustainability, all species of this genus are listed as endangered species in Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora7.
Because only the wounded Aquilaria tree can produce agarwood1,8,9,10, people have developed various artificial agarwood-inducing methods, such as partly breaking the trunk, making holes with burning chisels and inoculating with fungi11. Of these traditional agarwood-inducing methods, only agarwood obtained from holes made with burning chisels meets the requirements in the Chinese Pharmacopoeia (2010)11,12. However, little is known about the signaling pathway and the regulation mechanisms of wound-induced agarwood formation.
The heat shock response is a conserved cellular defense mechanism in response to elevated temperatures and is observed in cells from bacteria to human. Research has indicated that heat shock induces the accumulation of glucosinolates and several other secondary metabolites derived from the phenylpropanoid pathway, including anthocyanins and sinapine derivatives in Arabidopsis thaliana13. Investigation of Taxus yunnanensis cell suspension cultures has shown that heat shock (40 °C to 50 °C for 30 min) can significantly induce paclitaxel production14. However, the mechanisms by which heat shock affects secondary metabolite production in in vitro cultured plant cells have not been well elucidated.
Jasmonic acid (JA) is a well-characterized long-distance signaling molecule that mediates defensive responses and secondary metabolism in plants15,16,17,18. The biosynthesis of JA in plants is essentially a series of enzyme reactions that is initiated by the release of a substrate, α-linolenic acid (LeA), from the cell membrane. LeA is oxidized to 13-(S)-hydrogen peroxide-linolenic acid (13-HPOT) in plastids by lipoxygenases (LOXs), after which catalysis by allene oxide synthase (AOS) and allene oxide cyclase (AOC) produces 12-oxo-phytodienoic acid (12-O-PDA), which enters the cytoplasm. Subsequently, OPDA is reduced by 12-oxophytodienoate reductase (OPR3), followed by three cycles of β-oxidation, resulting in conversion to JA in the peroxisomes19,20. Thereafter, the volatile methyl jasmonate compound is generated via jasmonic acid carboxyl methyltransferase (JMT). Studies have indicated that expression of these enzymes in the JA biosynthesis pathway greatly affects JA levels in plants. LOX is essential in JA biosynthesis, as different plant species with reduced LOX expression levels have 50–95% reductions in JA levels after wounding21,22, whereas plants that over-express AOS or AOC constitutively do not exhibit elevated JA levels but show increased JA production after wounding or treatment with other stimuli23,24,25,26.
Previous reports have shown that JA plays important roles in sesquiterpene biosynthesis. In rice, MeJA markedly induced the expression of sesquiterpene synthase TPS3 and the release of more than 10 sesquiterpenes, particularly (E)-β-caryophyllene (Oryza sativa)27. Meas et al. showed that exogenous JA treatments promoted expression levels in the artemisinin biosynthetic pathway, ultimately leading to increased artemisinin accumulation in A. annua28. The induction role played by JA in Aquilaria spp. has also been widely demonstrated in recent years. Exogenously applied MeJA in Aquilaria cell suspension cultures or calluses induced biosynthesis and accumulation of sesquiterpenes compounds, especially δ-guaiene2,6,29,30. However, whether these results are directly related, the significance and function of endogenous JA in the agarwood sesquiterpene biosynthetic pathway remains unknown.
In the present study, we treated A. sinensis cell suspension cultures with heat shock, imitating the burn-chisel-drill method used on trees, to investigate how JA affects the accumulation of sesquiterpene compounds. We found that endogenous JA and its methyl ester accumulate rapidly and transiently after heat shock treatment. Correspondingly, the expression of genes in the JA biosynthesis pathway was significantly up-regulated, and sesquiterpene compounds accumulated. A specific inhibitor of JA, nordihydroguaiaretic acid (NDGA), could block these effects. Additionally, when exogenously supplied to A. sinensis cells, methyl jasmonate exhibited the strongest effect on sesquiterpene biosynthesis compared to SA and H2O2. These data demonstrate that JA is a critical signal transducer in the intracellular signal cascade induced by heat shock and that JA ultimately plays a role in the accumulation of sesquiterpene compounds.
Results
Heat shock treatment induces expression of a set of genes involved in JA biosynthesis, perception and transduction pathways
Making holes in A. sinensis using a burning chisel is a traditional agarwood-inducing method, that agarwood obtained by which could meet the requirements in the Chinese Pharmacopoeia (2010)11,12. We used Solexa technology to sequence A. sinensis materials, in which holes had been made using a burning chisel, so that profiling of gene expression levels could be accomplished (unpublished data). Based on the results obtained, this treatment positively induced the expression of genes involved in JA biosynthesis, including lipoxygenase (LOX), allene oxide synthase (AOS), allene oxide cyclase (AOC) and 12-oxophytodienoate reductase 3 (OPR3). After treatment for 0.5 h, the expression levels of these genes were significantly increased, with fold increases of 23.3, 58.4, 21 and 18.5, respectively. The heat map generated by the hierarchical clustering of genes associated with the JA biosynthesis pathway is shown in Fig. 1. Note that the heat map displays relative expression levels across all the samples analyzed rather than absolute expression levels for each sample. These results led to the hypothesis that JA plays a role in the effects of heat shock treatment.
Figure 1. Heatmap of gene expression levels.
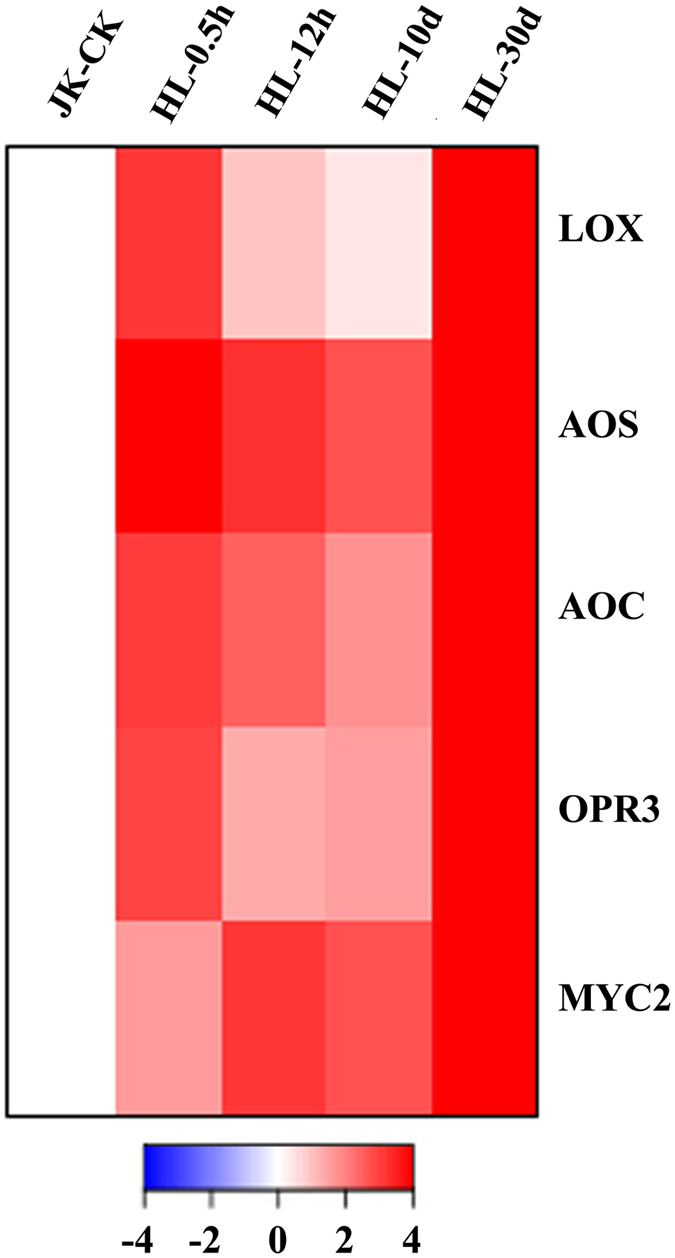
This heatmap is based on hierarchical clustering (P > 0.001) of genes involved in JA biosynthesis and regulation. All gene expression levels were transformed to scores ranging from −4 to 4 and were colored blue, white, or red to represent low, moderate, or high expression levels, respectively. The relative expression levels were scaled based on their mean and do not represent expression levels in comparison with controls.
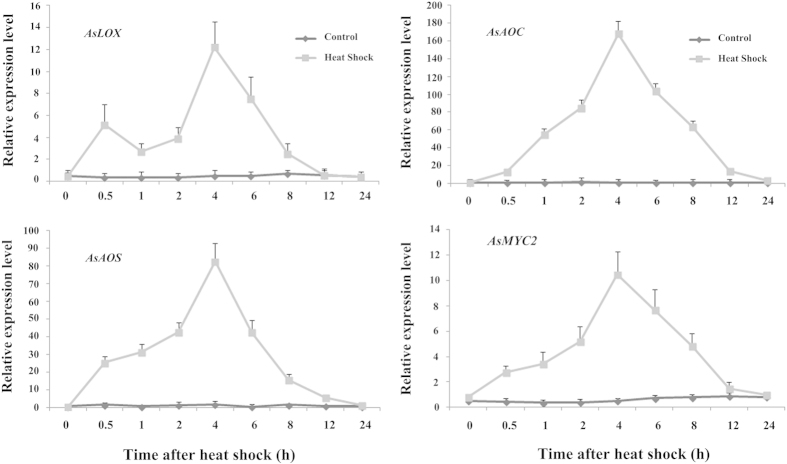
To test this hypothesis, we subjected A. sinensis cell suspension cultures to heat shock treatment, imitating the burn-chisel-drill method utilized on trees, to evaluate the expression levels of genes involved in the JA biosynthesis pathway. As cell suspensions have been reported to exhibit a certain degree of thermo tolerance, cell viability was only affected by temperatures higher than 40 °C; however, cell viability declined significantly at these higher temperatures, and the cells did not exhibit any capacity for growth recovery at temperatures >50 °C14,31,32,33. The heat shock experiment was performed by placing A. sinensis cell suspensions in a 50 °C water bath for 30 min, after which samples were acquired at appointed times for analysis. This treatment induced the expression of LOX, AOS and AOC, which showed similar expression profiles (Fig. 2). Expression of AOS and AOC increased gradually from 0.5 h to 4 h after heat shock treatment, with expression levels increasing 80- and 160-fold, respectively, peaking at 4 h, and subsequently declining gradually within 24 h. By contrast, LOX showed two peaks at 0.5 h and 4 h, peaking at 4 h with a 12-fold increase. This result is consistent with the conclusions of previous studies that the regulation of JA biosynthesis is determined by a positive feedback loop and substrate availability34,35,36,37. When plants are injured, genes involved in JA synthesis are activated, inducing the biosynthesis and accumulation of JA compounds. Correspondingly, wound-induced JA compounds activate the expression of JA biosynthesis genes. This feedback may explain why LOX expression after heat shock treatment exhibits two peaks (Fig. 2). Additionally, the expression profile of MYC2, as a master regulator of most aspects of the JA signaling pathway38,39,40, is similar to those of AOS and AOC (increasing from 0.5 h to 4 h and then declining gradually within 24 h), demonstrating that the JA signaling pathway is activated via the heat shock process.
Figure 2. Expression levels of genes after heat shock treatment.
Cultures of A. sinensis cells in suspension were subjected to heating to 50 °C for 30 min. After this heat shock treatment, the culture flasks were returned to the gyratory shaker at 25 °C for continued shaking and were sampled at appointed times (0 h, 0.5 h, 2 h, 4 h, 6 h, 12 h, 24 h, and 3d). Healthy cells in suspension culture that were not subjected to heat shock treatment were used as controls. Expression levels of AsLOX, AsAOS, AsAOC and AsMYC2 were assayed using real-time PCR analysis and AsGADPH as the internal control. All genes were up-regulated in response to heat shock treatment. Each value is the mean ± SE of 3 independent biological replicates.
Endogenous jasmonic acid and its methyl ester increase rapidly after heat shock, while NDGA blocks it
Studies have shown that jasmonate is rapidly synthesized only in response to external stimuli, such as treatments with fungi, pathogens, insects or herbivory41,42,43,44,45. Furthermore, the wound-induced rise in JA is transient and appears before the expression of LOX, AOS and AOC46,47. To investigate whether heat shock promotes the production of endogenous JA, the JA contents of three samples (healthy control, heat shock, and heat shock plus NDGA) were determined using GC-MS. As shown in Fig. 3, after heat shocking the cell suspension cultures in a 50 °C water bath, a transient increase in the concentration of JA was observed, which peaked 30 min after heat shock treatment with a value of 112.09 ng/g [fresh weight], and approximately 6-fold increase compared with the healthy control (no heat shock). Subsequently, the JA concentration gradually declined to a normal level at 12 h, indicating that the induction of JA results in rapid transient accumulation after heat shock and is, therefore, an early defense response. By contrast, almost no difference was observed between healthy (no heat shock treatment) and JA-inhibitor-containing cell cultures.
Figure 3. Time course of endogenous jasmonates induced by heat shock treatment.
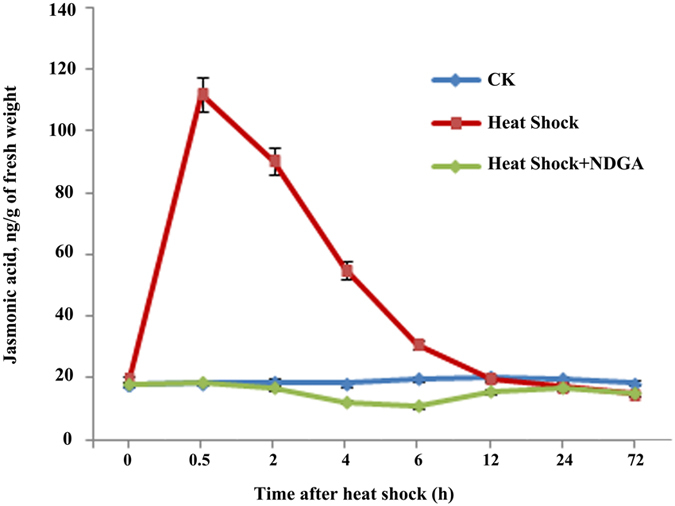
A. sinensis cells cultured in suspension containing or not containing 1.0 μM nordihydroguaiaretic acid (NDGA), an inhibitor of JA biosynthesis, were subjected to heating at 50 °C for 30 min. After the heat shock treatment, the culture flasks were returned to the gyratory shaker at 25 °C for continued shaking and were sampled at appointed times (0 h, 0.5 h, 2 h, 4 h, 6 h, 12 h, 24 h, and 3d). Healthy cells cultured in suspension that did not receive heat shock treatment were used as controls. Endogenous jasmonates were extracted and analyzed by GC-MS. Heat shock treatment rapidly induced JA production in A. sinensis cell suspensions, and NDGA, a specific inhibitor of JA, could block this induction, as demonstrated by the lack of JA accumulation. CK indicates the healthy control; Heat Shock indicates NDGA-free treatment in a 50 °C water bath; Heat Shock + NDGA indicates NDGA treatment in a 50 °C water bath.
Sesquiterpene compounds accumulate after heat shock, whereas NDGA significantly inhibits sesquiterpene production
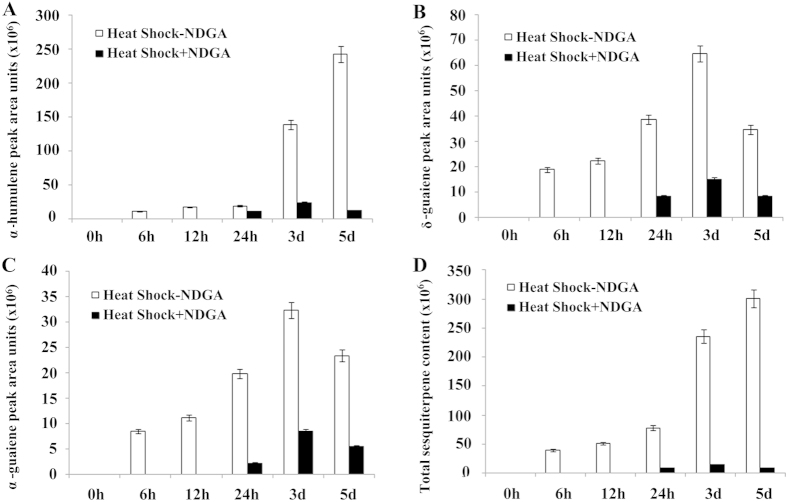
To confirm the role of endogenous JA in heat-shock-induced agarwood formation, volatile oils were extracted from the three samples (healthy control, heat shock, heat shock plus NDGA) via a solid-phase micro-extraction method and were analyzed by GC-MS. No sesquiterpene compounds were detected in the healthy control sample (Supplementary Fig. S1), whereas three sesquiterpene compounds were detected in the heat shock samples, (Supplementary Fig. S2A,B). Of these, the α-humulene content was the highest (Fig. 4A), followed by δ-guaiene and α-guaiene (Fig. 4B,C). Interestingly, these were all induced beyond 6 h after heat shock treatment, and their relative contents increased smoothly until 72 h. Maximum δ-guaiene and α-guaiene contents were obtained 3 d after heat shock treatment, whereas the maximum α-humulene content was reached 5 d after heat shock treatment, with a 19-fold increase compared with the control sample. By contrast, very little volatile oil content was detected beyond 24 h after heat shock treatment in the samples containing NDGA. The total sesquiterpene content in the heat shock samples increased 9-fold at 24 h, 15-fold at 3 d, and 35-fold at 5 d compared with that of the NDGA-containing samples (Fig. 4D), clearly demonstrating that production of endogenous JA plays a critical role in heat-shock-induced sesquiterpene production. It is noteworthy that, although we observed no increase in JA levels in the NDGA-containing samples until 72 h after heat shock treatment (Fig. 3), volatile oils were still detected 24 h after heat shock treatment. A possible explanation for this phenomenon is that the biosynthesis of sesquiterpene is not solely dependent on the JA signaling pathway. Although JA generation is completely inhibited or does not accumulate to detectable levels within 24 h after heat shock treatment, other signaling molecules may function alongside sesquiterpene production in a defensive response.
Figure 4. Changes in sesquiterpene compounds in heat-shock-treated cells in suspension.
The fused silica fiber of the Solid Phase Micro Extraction (SPME) was introduced into the headspace above the ground samples (4-g each), where this fiber adsorbed volatile components for 30 min. The adsorbed components were analyzed by GC-MS. The relative contents of sesquiterpene compounds were reflected by the peak areas generated. (A) Relative amount of α-humulene at different time points, as determined by peak areas. (B) Relative amount of δ-guaiene at different time points, as determined by peak areas. (C) Relative amount of α-guaiene at different time points, as determined by peak areas. (D) Total amount of sesquiterpene compounds obtained after heat shock treatment or heat shock treatment with NDGA. The hollow column indicates heat shock samples, while the solid column indicates heat shock samples containing NDGA.
Exogenous MeJA affects the production of agarwood sesquiterpene more strongly compared to SA and H2O2
To further demonstrate that the production of JA and methyl jasmonate triggers the biosynthesis of sesquiterpene defense compounds in plant cell suspension cultures, we treated A. sinensis calluses with 100 μM MeJA, SA or H2O2 to examine the effects of exogenous signal molecules on the production of violate oils. GC-MS analysis (Supplementary Fig. S3) revealed that the species and amounts of violate ingredients change greatly in both healthy and treated samples, with alkanes comprising an abundant proportion of the volatile oils in the healthy samples, whereas treated samples were rich in sesquiterpenes and aromatic constituents (Table 1). The peak areas indicated relative contents, with the healthy sample weighing in at 14.512, whereas the values for the MeJA-, H2O2- and SA-treated samples were 905.9, 383.95 and 85.24, respectively. Production of sesquiterpene compounds (β-elemene, β-humulene, α-guaiene, α-humulene, δ-guaiene) significantly increased in response to MeJA, H2O2 and SA treatments, with the greatest increasing observed for MeJA, followed by H2O2 (SA exhibited the weakest induction). It is noteworthy that of these sesquiterpene compounds, β-elemene, α-guaiene and δ-guaiene are also products of sesquiterpene synthase ASS1 catalysis6; meanwhile, α-humulene, δ-guaiene and α-guaiene are induced by heat shock treatment (Fig. 4), demonstrating the crucial role of JA in inducing the biosynthesis of agarwood sesquiterpene.
Table 1. Violates components in different treated samples.
| TR Sesquiterpene and aromatics | Compounds | RI | Peaks areas (x108) |
|||
|---|---|---|---|---|---|---|
| Healthy | H2O2 treated | MeJA treated | SA treated | |||
| 12.209 | β-elemene | 1198.425 | – | 7.89 | 39.89 | – |
| 12.808 | β-humulene | 1212.378 | – | 2.73 | 7.94 | – |
| 13.008 | α-guaiene | 1217.037 | 5.52 | 109.3 | 248.6 | 17.5 |
| 13.483 | α-humulene | 1228.101 | – | 13.29 | 86.96 | 12.73 |
| 13.583 | Naphthalene | 1230.431 | 3.01 | 5.07 | 6.75 | 1.39 |
| 14.161 | Bicyclo[5.3.0]decane | 1243.894 | – | 2.56 | 8.66 | – |
| 14.461 | δ-guaiene | 1250.883 | 5.95 | 242.7 | 507.1 | 53.62 |
| Total peak areas | 14.512 | 383.95 | 905.9 | 85.24 | ||
| Alkanes | ||||||
| 10.774 | Tridecane | 970.216 | 2.41 | 0.939 | 1.75 | 1.02 |
| 12.196 | Tetradecane | 1198.122 | 7.36 | – | – | 0.672 |
| 14.056 | Pentadecane | 1241.449 | 11.13 | 4.63 | 14.02 | 3.99 |
| 16.776 | Hexadecane | 1626.551 | 4.58 | 2.46 | 9.39 | 1.81 |
| 20.994 | Heptadecane | 1668.993 | 3.73 | – | 7.05 | – |
| Total peak areas | 29.21 | 8.029 | 32.21 | 7.492 | ||
‘TR’ indicates retention time; ‘RI’ indicates retention indices which were calculated against C8–C40 n-alkanes on the non-polar VF-5MS column; ‘–’ indicates not detected.
Discussion
Numerous studies have shown that mechanical wounds, chemical wounds, and fungal infection can induce the formation of agarwood in A. sinensis8,9,10,48,49,50,51, and agarwood is widely used in traditional medicine, incense, and perfumes across Asia, the Middle East, and Europe1,2,3,4,5,6. Despite the economic and pharmacological value of agarwood, studies regarding the mechanism by which such injuries induce agarwood formation are rare.
A previous study in our laboratory showed that holes made by a burning chisel in an A. sinensis tree can produce high-quality agarwood that meets the requirements of the Chinese Pharmacopoeia (2010)11,12. From the Solexa analysis, we found that expression levels of genes involved in JA biosynthesis and regulation pathways were almost up-regulated by heat shock wounding (Fig. 1), indicating that JA may act as a signal molecule. It was proposed the effect of heat shock is to initiate the JA synthesis pathway, which generates a long distance transport of signaling molecules, maybe JA itself or its derivatives.
Previous studies have shown that JA is involved in fungus-induced secondary metabolism41,52,53,54. The present study provides evidence that JA plays a critical role in regulating heat-shock-induced agarwood sesquiterpene formation, in agreement with previous studies. The results herein demonstrate that endogenous JA and its methyl ester accumulate rapidly and transiently after A. sinensis cell suspensions are treated with a 50 °C water bath for 30 min (Fig. 4). Correspondingly, we monitored three mainly sesquiterpene compounds 6 h after heat shock treatment and found that they accumulated within 72 h (Fig. 4). A specific inhibitor of JA, NDGA, could block the JA signaling pathway and markedly reduce the accumulation of sesquiterpene compounds (Figs 3 and 4), clearly demonstrating that sesquiterpene formation is JA-dependent. Correspondingly, the expression levels of the principle genes involved in JA biosynthesis (LOX, AOS and AOC), which are located in a companion cell-sieve element complex55,56, were up-regulated after heat shock treatment (Figs 1 and 2). Based on these findings, it is possible that heat shock triggers the synthesis and accumulation of JA, which is mobile within the phloem and becomes a secondary signal that induces the expression of defense genes. However, this induction of defense genes depends on JA perception and transduction but not the capacity of JA synthesis. In support of our hypothesis, MYC2, a master of JA signaling, was also up-regulated (Figs 1 and 2). Studies have shown that JA-induced biosynthesis of nicotine in tobacco57, sesquiterpene in Arabidopsis58,59, and alkaloid in Catharanthus roseus60,61 are all mediated by transcription factor MYC2. However, whether the regulation mechanism in A. sinensis is consistent with these other studies is unclear and requires further study. We now know that this elicitation process occurs, with gene activation ultimately leading to the synthesis of JA and the activation of the JA signaling pathway. JA and its derivatives have an integral role in the cascade of events that occur during the elicitation process, either directly or indirectly activating the genes involved in sesquiterpene biosynthesis. This explains how agarwood can be induced in an entire tree rather than being formed only in proximity to an injured site.
It is noteworthy that even though gene expression and JA production in the samples containing the JA inhibitor were hardly changed compared with the healthy control sample, violate oils still accumulated to a small degree in the former (Fig. 4). These results imply that, although heat-shock-induced sesquiterpene biosynthesis is JA-dependent, other signal molecules may also be involved in this process. A burst of reactive oxygen species (ROS) is a common event in both biotic and abiotic induced synthesis and the accumulation of secondary metabolites, the wound signaling of which H2O2 has been proposed to play a role62,63,64,65. The phytohormones SA and JA are also recognized play crucial roles in regulating the defensive signaling network62,66,67. More importantly, studies have shown that agarwood sesquiterpene production is induced by these signaling molecules. For instance, H2O2 induced the formation of vessel occlusions and sesquiterpene as a result of pruning68, and Liu et al. found that H2O2 promoted programmed cell death and SA accumulation during the induced production of sesquiterpenes in cell suspension cultures of A. sinensis69. SA induced the production of three sesquiterpene compounds (α-guaiene, α-humulene and δ-guaiene) in A. sinensis cell suspensions2, and MeJA induced the expression of sesquiterpene synthase and produced three sesquiterpenes in A. sinensis cell suspension cultures or calluses2,3,6,29. However, the specific roles of these compounds are not fully understood.
In the present study, we compared the effect of exogenously applied JA with those of SA and H2O2 on the induction of agarwood sesquiterpene biosynthesis, and our results agree with previous reports2,6,29,30,68,69. All of them induced the accumulation of sesquiterpene compounds, but MeJA exhibited the strongest induction role, with an effect that was 3-times greater than that of H2O2 and more than 10-times greater than that of SA. These results clearly demonstrate the crucial role played by JA in sesquiterpene production.
The results of the present study also demonstrate that sesquiterpene production is dependent on the endogenous production of JA and is strongly elicited by exogenously applied JA. Thus, the experiments herein have identified JA as a crucial signal transducer in heat-shock-induced agarwood formation. This information facilitates our understanding of the potential mechanism by which wounds induce the formation of high-quality agarwood and provide new clues for improving agarwood-inducing techniques.
Materials and Methods
Plant materials and treatments
Cell suspensions of A. sinensis were grown in Murashige-Skoog media on a gyratory shaker (110 rpm) at 25 °C in the dark for 15 days prior to heat shock treatments. All heat shock experiments were carried out in a water bath with a removable shaking carriage (DSHZ-300A, China). Cell suspensions with and without 1.0 μM nordihydroguaiaretic acid (NDGA), an inhibitor of JA biosynthesis, were subjected to a water bath at 50 °C for 30 min. After heat shock treatment, the culture flasks were returned to the gyratory shaker at 25 °C and shaking was continued. The cultures were then sampled at pointed times (0 h, 0.5 h, 2 h, 4 h, 6 h, 12 h, 24 h, and 3 d). After filtering with a 100-mesh filter and gassing with a pump, the samples were immediately frozen in liquid nitrogen and then stored at −80 °C. For every treatment, three independent repetitions of the biological experiments were performed.
Determinations of endogenous jasmonate
Endogenous JA was determined according to the method of Zhang et al.70. Four grams of cells sampled from A. sinensis cell suspension cultures were ground in liquid nitrogen and then transferred to a 15-ml Eppendorf tube containing 7 ml of 80% methanol, to which was immediately added 100 ng of 9,10-dihydrojasmonic acid (DHJA), which was synthesized from JA or its methyl ester by catalytic hydrogenation with Pd/charcoal, as internal standards and 1.0 mg diethyldithiocarbamate (DDTC) as antioxidant. The samples were then extracted at −20 °C overnight. After vacuum filtration, the samples were frozen and thawed three times and then centrifuged for 20 min at 12,000 rpm. The supernatant was stirred with non-soluble polyvinyl pyrrolidone. Subsequently, samples were extracted three times with ethyl acetate, after which they were evaporated at 35 °C to dryness. The residues were dissolved in 100 μL of methanol, and an excess of diazomethane in ether (1 mL) was added. After 30 min at room temperature, the sample was transferred to a capillary. Sample aliquots of 3 μL were analyzed by gas chromatography/mass spectrometry (GC/MS) (Agilent 7890BGC-5977AMS) under the following conditions: linear He flow at 23 cm/s; column temperature gradient: 50 °C for 1 min, 50–160 °C at 30 °C/min, 160–200 °C at 5 °C/min, 200–290 °C at 30 °C/min, 290 °C for 5 min; electron potential, 70 eV. Methyl jasmonate content was determined based on the relationship by which the ratio of the peak area equals the concentration ratio: NJA = (0.1542 × A224/A226 + 0.0146) × NDHJA. NJA and NDHJA represent the concentrations of JA and DHJA, respectively; A224 and A226 represent the peak areas of JA (224 m/z) and DHJA (226 m/z), respectively.
GC-MS analysis of sesquiterpene components in A. sinensis cell suspensions and calluses
GC-MS analysis was performed using a Varian 450 GC (USA) equipped with a VF-5MS capillary column (internal diameter, 30 m × 0.25 mm; film thickness, 0.25 μm), and a Varian 300 mass spectrometer with an ion-trap detector in full-scan mode under election impact ionization (70 eV). The carrier gas was helium, and the flow rate was 1 mL/min. The injections were performed in splitless mode at 250 °C. Samples were powdered, requiring the same weight for placement into the 12-mL sample bottle, and balanced for 30 min in 60 °C water. The fused silica fiber used for Solid Phase Micro Extraction (SPME) was introduced into the headspace above the sample, and volatile components were adsorbed for 30 min, followed by a thermal desorption process in which the SPME fiber was introduced into the injection port of the gas chromatographic system. The program was immediately started, and the fiber was removed after 10 min. The GC-MS conditions and identification of sesquiterpenes compounds pertained to previously described procedures4. The two fibers were injected separately and run in the same program, and relative components were obtained via normalization of peak areas without applying correction factors. We did not use an internal standard because of the difficulty in choosing one that was suited for both samples. Peaks areas pertained to the amounts of compounds in the two different samples to some degree, as the weight of the two samples and the analytical methods were identical.
Quantitative real-time PCR (RT-PCR) analysis
For To analyze gene expression levels, total RNA was isolated from treated cell suspensions or calluses. A 2-μg aliquot was subjected to first-strand synthesis using M-MLV reverse transcriptase (Promega, USA) and an oligo (dT18) primer. The suitabilities of the oligonucleotide sequences, in terms of annealing efficiencies, were evaluated in advance using the Primer 5.0 program. A fragment of the glyceraldehyde-3-phosphate dehydrogenase (GADPH) gene71 was also amplified as an internal control. The qRT-PCR analysis was performed using a BioRad Real-Time System CFX96TM C1000 Thermal Cycler (Singapore). Amplification of the target genes was monitored during every cycle by detecting SYBR-green fluorescence. The Ct (threshold cycle), defined as the PCR cycle at which a statistically significant increase in reporter fluorescence was first detected, was used to measure the starting copy numbers of each target gene. Relative quantitation of each target gene expression level was performed using the comparative Ct method72 (comparing the CTs of the target genes with that of the housekeeping gene using 2−ΔΔCT). All experiments were repeated at least 3 times, such that there were 3 independent repetitions of each biological experiment. The primers used in this study are listed in Supplementary Table S1.
Additional Information
How to cite this article: Xu, Y.-H. et al. Jasmonic acid is a crucial signal transducer in heat shock induced sesquiterpene formation in Aquilaria sinensis. Sci. Rep. 6, 21843; doi: 10.1038/srep21843 (2016).
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81173539, 31100220, 81573525).
Footnotes
Author Contributions Y.X.: Participated in the design of the study, analyzed the data, and drafted the manuscript. Y.L.: Performed the experiments, participated in the design of the study and analysis of the data. Z.Z.: Helped to analyze the data. J.L. and P.S.: Prepared the A sinensis cell suspension. Z.G. and C.S.: Participated in the discussion of the result and helped to revise the manuscript. J.W.: Initiated the project, helped conceive the study, and revised the manuscript.
References
- Persoon G. A. Growing ‘the wood of the gods’: agarwood production in southeast Asia. Smallholder Tree Growing for Rural Development and Environmental Service. Advance in Agroforesty 5, 245–262 (2008). [Google Scholar]
- Okudera Y. & Ito M. Production of agarwood fragrant constituents in Aquilaria calli and cell suspension cultures. Plant Biotechnology 26, 307–315(2009). [Google Scholar]
- Kakino M. et al. Laxative effects of agarwood on low-fiber diet-induced constipation in rats. BMC Complementary and Alternative Medcine 10, 68–75 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. Q. et al. Comparison of compositions and antimicrobial activities of essential oils from chemically stimulated agarwood, wild agarwood and healthy Aquilaria sinensis (Lour.) Gilg trees. Molecules 16, 4884–4896 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. Q. et al. Chemical constituents of agarwood originating from the endemic genus Aquilaria plants. Chem Biodivers 9(2), 236–250 (2012). [DOI] [PubMed] [Google Scholar]
- Xu Y. H. et al. Identification of genes related to agarwood formation: transcriptome analysis of healthy and wounded tissues of Aquilaria sinensis. BMC Genomics 14, 227 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- CITES. Amendments to Appendix I and II of CITES. Bangkok, Thailand: Proceedings of Thirteenth Meeting of the Conference of the Parties, 2–14 (2004).
- Ng L. T., Chang Y. S. & Kadir A. A. A review on agar (gaharu) producing Aquilaria species. Journal of Tropical Forest Product. 2(2), 272e285 (1997). [Google Scholar]
- Itoh T. et al. Structure and artificial induction of aloe wood. The Fifth Pacific Regional Wood Anatomy Conference. Abstracts of Papers and Posters. IAWA Journal 23(4), 466–467 (2002). [Google Scholar]
- Pojanagaroon S. & Kaewrak C. Mechanical methods to stimulate aloes wood formation in Aquilaria crassna Pierre Ex H. LEC. (Kritsana) trees. ISHS Acta Horticuturae 676, 161–166 (2005). [Google Scholar]
- Liu Y. Y. et al. Whole-tree agarwood-inducing technique: an efficient novel technique for producing high-quality agarwood in cultivated Aquilaria sinensis trees. Molecules 18, 3086–3106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- China pharmacopoeia Committee. The Pharmacopoeia of People’s Republic of China (I). Chemical Industry Press, Beijing, p. 172 (2010). [Google Scholar]
- Bennett R. N. et al. The tu8 mutation of Arabidopsis thaliana encoding a heterochromatin protein 1 homolog causes defects in the induction of secondary metabolite biosynthesis. Plant Biology 7, 348–357 (2005). [DOI] [PubMed] [Google Scholar]
- Zhang C. & Fevereiro P. S. The effect of heat shock on Paclitaxel production in Taxus yunnanensis cell suspension cultures: role of abscisic acid pretreatment. Biotechnology and Bioengineering 96(3), 506–514 (2007). [DOI] [PubMed] [Google Scholar]
- Reymond P. & Farmer E. E. Jasmonate and salicylate as global signals for defense gene expression. Current Opinion in Plant Biology 1, 404–411 (1998). [DOI] [PubMed] [Google Scholar]
- Leon J., Rojo E. & Sánchez-Serrano J. J. Wound signalling in plants. Journal of Experimental Botany 52, 1–9 (2001). [DOI] [PubMed] [Google Scholar]
- Howe G. A. & Jander G. Plant immunity to insect herbivores. Annual Review of Plant Biololgy 59, 41e66 (2008). [DOI] [PubMed] [Google Scholar]
- Mithofer A. & Boland W. Plant defense against herbivores: chemical aspects. Annual of Review Plant Biology 63, 431e450 (2012). [DOI] [PubMed] [Google Scholar]
- Wasternack C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Annals of Botany 100, 681–697 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller A. & Stintzi A. Enzymes in jasmonate biosynthesis–structure, function, regulation. Phytochemistry 70, 1532–1538 (2009). [DOI] [PubMed] [Google Scholar]
- McConn M. et al. Jasmonate is essential for insect defense in Arabidopsis. Proceedings of National Academy of Sciences, USA 94, 5473–5477 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halitschke R. & Baldwin I. T. Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. Plant Journal 36, 794–807 (2003). [DOI] [PubMed] [Google Scholar]
- Laudert D., Schaller F. & Weiler E. W. Transgenic Nicotiana tabacum and Arabidopsis thaliana plants overexpressing allene oxide synthase. Planta 211, 163–165 (2000). [DOI] [PubMed] [Google Scholar]
- Sivasandar S., Sheldricd B. & Rothstein S. J. Expression of allene oxide synthaxe determines defense gene activation in tomato. Plant Physiol 122(4), 1335–1342 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. H. et al. A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant Journal 31, 1–12 (2002). [DOI] [PubMed] [Google Scholar]
- Stenzel I. et al. Allene oxide cyclase dependence of the wound response and vascular bundle specific generation of jasmonates in tomato – amplification in wound-signalling. The Plant Journal 33, 577–589 (2003a). [DOI] [PubMed] [Google Scholar]
- Cheng A. X. et al. The rice (E)-beta-caryophyllene synthase (OsTPS3) accounts for the major inducible volatile sesquiterpenes. Phytochemistry 68, 1632–1641 (2007b). [DOI] [PubMed] [Google Scholar]
- Maes L. et al. Dissection of the phytohormonal regulation of trichome formation and biosynthesis of the antimalarial compound artemisinin in Artemisia annua plants. New Phytologist 189, 176–189 (2011). [DOI] [PubMed] [Google Scholar]
- Ito M., Okimoto K., Yagura T. & Honda G. Induction of sesquiterpenoid production by methyl jasmonate in Aquilaria sinensis cell suspension culture. Journal of Essential Oil Research 17, 175–180 (2005). [Google Scholar]
- Kumeta Y. & Ito M. Characterization of δ-guaiene synthases from cultured cells of Aquilaria, responsible for the formation of the sesquiterpenes in agarwood. Plant Physiology 154, 1998–2007 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. T., Wallner S. J. & Waddell J. W. Heat stress responses in cultured plant cells. Plant Physiology 74, 944–946 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin D. D. et al. Heat stress-responsive transcriptome analysis in heat susceptible and tolerant wheat (Triticum aestivum L.) by using wheat genome array. BMC Genomics 9, 432 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Correia J., Azevedo H., Lino-Neto T. & Tavares R. M. Understanding heat stress tolerance of suspended cells in the model plant populus euphratica. International Scholarly Research Network ISRN Forestry, doi: 10.5402/2012/243694 (2012). [DOI] [Google Scholar]
- Kazan K. & Manners J. M. Jasmonate Signaling: Toward an Integrated View. Plant Physiology 146, 1459–1468 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes H. D., Koetje D. S. & Fanceschi V. R. Expression, activity and cellular accumulation of methyl-jasmonate-responsive lipoxygenase in soybean seedling. Plant Physiology 100, 433–443 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J. Jasmonate passes muster: a receptor and targets for the defense hormone. Annual Review in Plant Biology 60, 183–205 (2009a). [DOI] [PubMed] [Google Scholar]
- Browse J. The power of mutants for investigating jasmonate biosynthesis and signaling. Phytochemistry 70, 1539–1546 (2009c). [DOI] [PubMed] [Google Scholar]
- Boter M., Ruíz-Rivero O., Abdeen A. & Prat S. Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes Development 18, 1577–1591 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O., Chico J. M., Sánchez-Serrano J. J. & Solano R. JASMONATE- INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16, 1938–1950 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrecht B. et al. MYC2 differentially modulates diverse jasmonate dependent functions in Arabidopsis. Plant Cell 19, 2225–2245 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlach H., Muller M. J., Kutchan T. M. & Zenk M. H. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proceedings of National Academy of Sciences, USA 89, 2389–2393 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Alme´ras E. & Krishnamurthy V. Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Current Opinion in Plant Biology 6, 372–378 (2003). [DOI] [PubMed] [Google Scholar]
- Howe G. A. Jasmonates as signals in the wound response. Journal of Plant Growth Regulation 23, 223–237 (2004). [Google Scholar]
- Schilmiller A. L. & Howe G. A. Systemic signaling in the wound response. Current Opinion in Plant Biology 8, 369–377 (2005). [DOI] [PubMed] [Google Scholar]
- Wasternack C. Oxilipins: biosynthesis, signal transduction and action. In: Hedden P., Thomas S. eds Plant hormone signaling. Annual Plant Reviews, 185–228 (2006). [Google Scholar]
- Howe G. A. et al. Cytochrome P450-dependent metabolism of oxylipins in tomato. Cloning and expression of allene oxide synthase and fatty acid hydroxide lyase. Plant Physiology 123(2), 711–724 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel I. et al. Jasmonate biosynthesis and the allene oxide cyclase family of Arabidopsis thaliana. Plant Molecular Biology 51, 895–911 (2003b). [DOI] [PubMed] [Google Scholar]
- Rahman M. A. & Basak A. C. Agar production in agar trees by artificial inoculation and wounding. Bano Bigan Patrika 9(1/2), 97–93 (1980). [Google Scholar]
- Nobuchi T. & Somkid S. Preliminary observation of Aquliaria crassna wood associated with the formation of aloeswood bult. Kyoto University Forests, 63, 226–235 (1991). [Google Scholar]
- Patent (US6848211). Cultivated Agarwood (2001).
- Blanchette R. & Heuveling V. B. H. Cultivated agarwood. US 7638145B2, 2009.
- Nojiri H. et al. Involvement of jasmonic acid in elicitor-induced phytoalexin production in suspension-cultured rice cells. Plant Physiology 110(2), 387–392 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel B. N. & Brooks D. M. Cross talk between signaling pathways in pathogen defense. Current Opinion of Plant Biology 5(4), 325–331 (2002). [DOI] [PubMed] [Google Scholar]
- Ren C. G. & Dai C. C. Jasmonic acid is involved in the signaling pathway for fungal endophyte-induced volatile oil accumulation of Atractylodes lancea plantlets. BMC Plant Biology 12, 128 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hause B. et al. Tissue specific oxylipin signature of tomato flowers: allene oxide cyclase is highly expressed in distinct flower organs and vascular bundles. Plant Journal 24, 113–126 (2000). [DOI] [PubMed] [Google Scholar]
- Hause B. et al. Enzymes of jasmonate biosynthesis occur in tomato sieve elements. Plant Cell and Physiology 44, 643–648 (2003a). [DOI] [PubMed] [Google Scholar]
- Shoji T. & Hashimoto T. Tobacco MYC2 Regulates Jasmonate-Inducible Nicotine Biosynthesis Genes Directly and By Way of the NIC2-Locus ERF Genes. Plant Cell Physiology 52(6), 1117–1130 (2011). [DOI] [PubMed] [Google Scholar]
- Hong G. J. et al. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 24(6), 2635–2648 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran Li. et al. Virulence Factors of Geminivirus Interact with MYC2 to Subvert Plant Resistance and Promote Vector Performance. Plant Cell 26, 4991–5008 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttipanta N. et al. The transcription factor CrWRKY1 positively regulates the terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Plant Physiology 157, 2081–2093 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. et al. The basic helix–loop–helix transcription factor CrMYC2 controls the jasmonate-responsive expression of the ORCA genes that regulate alkaloid biosynthesis in Catharanthus roseus. The Plant Journal 67, 61–71 (2011). [DOI] [PubMed] [Google Scholar]
- Zhao J., Davis L. C. & Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnology Advance 23, 283–333 (2005). [DOI] [PubMed] [Google Scholar]
- Ross C., Kupper F. C. & Jacobs R. S. Involvement of reactive oxygen species and reactive nitrogen species in the wound response of Dasycladus vermicularis. Chemistry Biology 13, 353–364 (2006). [DOI] [PubMed] [Google Scholar]
- Angelini R. et al. Involvement of polyamine oxidase in wound healing. Plant Physiology 146, 162–177 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. et al. ROS signaling: the new wave? Trends in Plant Science 16, 300–309 (2011). [DOI] [PubMed] [Google Scholar]
- Koornneef A. & Pieterse C. M. J. Cross talk in defense signaling. Plant Physiology 146, 839–844 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse C. M. J. et al. Hormonal modulation of plant immunity. Annual Review of Cell Devolepmental Biology 28, 489–521 (2012). [DOI] [PubMed]
- Zhang Z. et al. Hydrogen peroxide induces vessel occlusions and stimulates sesquiterpenes accumulation in stems of Aquilaria sinensis. Plant Growth of Regulation 72, 81–87 (2014).
- Liu J., Xu Y. H., Zhang Z. & Wei J. H. Hydrogen peroxide promotes programmed cell death and salicylic acid accumulation during the induced production of sesquiterpenes in cultured cell suspensions of Aquilaria sinensis. Functional Plant Biology 42(4), 337–346 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang F. J. et al. Study on the extraction purification and quantification of jasmonic acid, abscisic acid and indole-3-acetic acid in plants. Phytochemical Analysis 19(6), 560–567 (2008). [DOI] [PubMed] [Google Scholar]
- Gao Z. H. et al. Selection and validation of reference genes for studying stress-related agarwood formation of Aquilaria sinensis. Plant Cell Report 31, 1759e1768 (2012). [DOI] [PubMed] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using realtime quantitative PCR and the 2-deltadelta CT method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.