Abstract
Analyzing the proportions of stomatal (SL), mesophyll conductance (MCL) and biochemical limitations (BL) imposed by potassium (K) deficit, and evaluating their relationships to leaf K status will be helpful to understand the mechanism underlying the inhibition of K deficiency on photosynthesis (A). A quantitative limitation analysis of K deficiency on photosynthesis was performed on leaf margins and centers under K deficiency and sufficient K supply treatments of Brassica napus L. Potassium deficiency decreased A, stomatal (gs) and mesophyll conductance (gm) of margins, SL, MCL and BL accounted for 23.9%, 33.0% and 43.1% of the total limitations. While for leaf centers, relatively low limitations occurred. Nonlinear curve fitting analysis indicated that each limiting factor generated at same leaf K status (1.07%). Although MCL was the main component of limitations when A began to fall, BL replaced it at a leaf K concentration below 0.78%. Up-regulated MCL was related to lower surface area of chloroplasts exposed to intercellular airspaces (Sc/S) and larger cytosol diffusion resistance but not the cell wall thickness. Our results highlighted that photosynthetic limitations appear simultaneously under K deficiency and vary with increasing K deficiency intensity.
Potassium (K), one of the macronutrients essential for plant growth and development, is involved in many physiological processes, such as photosynthesis, enzyme activation, water relations, assimilate transport, and protein synthesis1,2. K deficiency profoundly decreased crop yield3,4, thus strategies of survival and improvement would be important for plant growing under adverse conditions. It is a truism that most of the dry matter is formed by leaf photosynthesis (A) which is intimately connected with K status. Multifarious studies have come to a nearly consistent conclusion that leaf A decreases in K-starved plants3,5,6, therefore an even deep comprehending of mechanism underlying the inhibition of K deficiency on A is necessary2.
During photosynthesis, CO2 moves from external atmosphere to the internal leaf air spaces through the stomata, and from there to carboxylation sites inside the chloroplasts7. It is established that stomatal closure is the foremost limitation to CO2 assimilation due to the vital role of K in stomatal aperture8,9. As a major osmotica, K+ accumulation in vacuole is essential for stomatal opening, which had been verified to be initially dropped under K deficiency10. And because of this, Bednarz et al.5 stated that the most limiting resistance to A of Gossypium hirsutum L. came from stomata5. In contrast, K starvation caused a decreased A and stomatal conductance (gs), but an increased intercellular CO2 concentration (Ci) of Carya cathayensis leaves, suggesting that, in addition to gs, mesophyll conductance (gm) and biochemical limitations might be involved in the depression of photosynthesis in K deficient conditions6. Numerous studies have shown that gm is relatively low, leading to great draw-down of chloroplastic CO2 concentration from Ci, and changed along the variation of water status, nitrogen nutrient, irradiance, temperature and CO2 concentration11,12,13,14. Moreover, leaf structures, specific aquaporins, plasma membrane etc. are involved in the determinations of gm15. K starvation might have reduced aquaporin activity16 and increased leaf dry mass per unit area (MA)1,17, therefore, causing a stronger mesophyll diffusion resistance to CO2 delivery18. Besides, K nutrition also known to increase the leaf intercellular air space to enhance gm1.
Additionally, biochemical processes may restrain photosynthesis, particularly under severe and/or long-time K starvation1,5,6. It was reported that Rubisco (ribulose-1,5-bisphosphate carboxylase/oxygenase, EC 4.1.1.39) activity was decreased under K deficiency, becoming a major limiting factor for photosynthesis in Oryza sativa leaves3. Chlorophyll synthesis was observed to be significantly impaired under K deficiency in Eucalyptus grandis leaves1. Moreover, K starvation up-regulated the fraction of electron transport to O2, resulting in an increased reactive oxygen species (ROS)19. Carbohydrate accumulation which may feedback regulation of leaf photosynthesis is more easily observed in K starved leaves20,21. Indeed, the relative contributions of these three limiting processes to photosynthesis under K deficiency and the underlying mechanisms have not been fully explored, due to the complicated physiological processes and variation of dominant limiting factors under differ K deficiencies2,5. No matter what the primary cause of decrease A, the discrepancy between researches was believed to be derived from differ physiological K deficiency severities. For this reason, a comprehensive consideration of whole limiting factors and their relationships with leaf K status seems to be important.
In 2005, Grassi and Magnani proposed a method to accurately quantify photosynthetic limitations by separating the relative controls on A resulting from SL, mesophyll conductance (MCL) and biochemical limitations (BL)22. This method has been successfully applied for evaluating the relative control of leaf A under water stress and during their recovery processes, among inter- and intra-species13,23,24,25. It showed not only great potential for elucidating the magnitude changes of limitations and their dominance in photosynthetic restraints with increasing severity of K deficiency, but also revealing the corresponding critical K concentrations for their transformation.
Winter oilseed rape (Brassica napus L.), a model-plant of winter cover crops who needs substantial amount of potassium to growth was used for a deeply aggregate analysis of K deficiency on photosynthetic limitations26. However, malfunction of physiological processes like photosynthesis is hard to be affected when K concentration above the threshold value (1.5% in dry matter, or less)10. On consideration of the fact that potassium deficiency symptoms, characterized by a chlorosis and even scorch around the periphery can be obviously observed when leaf K concentration below 1.0% in most species27. And the withdrawal K initially occurred at the edge of leaf tip, as tip cells are initially proliferated and oldest28, resulting in different K levels as well as visible distinctions between centers and margins. These natural K gradients are therefore precious for photosynthetic limitation analysis, from which we may seek out the main limiting factors under variable leaf K status and the corresponding threshold values. This phenomenon occurred more frequently under a complex biological and abiological environment system during a long-time and low-temperature wintertide, which may conducive to generate a physiology K deficiency in a K-deficient soil, i.e., it may bring K function into full play29,30. Accordingly, the objectives of the present study were to: (1) estimate the differences of contributions for three limiting factors to photosynthesis between leaf margins and leaf centers, (2) uncover the relationships between photosynthetic limitations and diminishing leaf K status, and therefore the critical K concentration for the predominate restraint transformation, (3) reveal the mechanism underlying the K-induced variation of limiting factors. It is hoped that this research will facilitate a better understanding of the photosynthetic physiological mechanism by which potassium deficiency leads to growth retardation in oilseed rape.
Results
Plant performance, leaf K concentration and net photosynthesis
The total dry matter of the –K treatment decreased significantly by 29.9% on average versus the +K treatment (Table 1). The leaf expansion was also restrained, with a 22.1% and 18.0% decline in the individual leaf dry matter and leaf area, respectively. Leaf K concentration was dramatically influenced by potassium supply and leaf position, which was significantly lower in the –K treatment than in the +K treatment. Meanwhile, within an individual leaf, K concentration was remarkably lower in margins than in centers. The mean net photosynthesis (A) in the leaf margins of the –K treatment was 56.9% that of the +K treatment. However, there was no significant difference between leaf margins and centers under the –K treatment, as well as the two positions under the +K treatment.
Table 1. Effects of K deficiency on plant dry matter, leaf dry matter, leaf area, leaf K concentration, and net CO2 assimilation rate (A) for the two positions in the fifth fully expanded leaves.
| Treatment | Position | Total dry matter (g) | Individual leaf dry matter (g) | Leaf area (cm2) | K concentration (% of dry weight) | A(μmol CO2 m−2 s−1) |
|---|---|---|---|---|---|---|
| –K | margin | 21.1 ± 2.6b1 | 2.31 ± 0.03b | 320 ± 4b | 0.65 ± 0.02b2 | 9.5 ± 0.2b |
| center | 0.98 ± 0.05a | 16.5 ± 0.6a | ||||
| +K | margin | 30.1 ± 1.1a | 2.97 ± 0.16a | 391 ± 8a | 1.32 ± 0.06b*3 | 17.0 ± 0.3a* |
| center | 1.66 ± 0.06a* | 17.3 ± 0.7a |
Values are mean ± SE of six replications for total dry matter, individual leaf dry matter and leaf area, and of four replications for K concentration and A.
1Different letters in the same column of total dry matter, individual leaf dry matter and leaf area indicate significant differences between treatments (P ≤ 0.05).
2Different letters in the same column of K concentration and A indicate significant differences between positions (P ≤ 0.05).
3*shows significant differences between the two K treatment in same position (P ≤ 0.05).
Stomatal conductance
Potassium deficiency led to a significant decline of the mean stomatal conductance (gs) in leaf margins, which was 63.6% that of the +K treatment. However, the mean gs value of the leaf centers was not influenced by K nutrient (Table 2). There was a significantly lower gs in leaf margins than in leaf centers under the –K treatment, whilst the gs values of these two positions were the same under the +K treatment. Despite a decrease of the gs value in leaf margins of the –K treatment, the intercellular CO2 concentrations (Ci) value was raised, and the mean Ci were similar to those of other groups.
Table 2. Effects of K deficiency on stomatal conductance (gs), intercellular CO2 concentrations (Ci), stomatal frequency, length, and width, single stomatal pore area and total stomatal pore area of the two positions in the lower epidermis of the fifth fully expanded leaves.
| Treatment | Position | gs(mol H2O m−2 s−1) | Ci(μmol CO2mol−1) | Stomatal frequency (no. mm−2) | Stomatal length (μm) | Stomatal width (μm) | Single stomatal pore area (μm2) | Total stomatal pore area (10−3 mm2 mm−2) |
|---|---|---|---|---|---|---|---|---|
| −K | margin | 0.142 ± 0.006b1 | 257 ± 6a | 334.1 ± 7.0a | 9.11 ± 0.07a | 2.91 ± 0.02b | 20.87 ± 0.15b | 6.97 ± 0.05b |
| center | 0.231 ± 0.012a | 240 ± 5a | 334.7 ± 8.5a | 9.94 ± 0.04a | 3.49 ± 0.03a | 27.30 ± 0.12a | 9.14 ± 0.04a | |
| +K | margin | 0.223 ± 0.017a*2 | 238 ± 7a | 335.7 ± 14.4a | 10.01 ± 0.05a | 3.68 ± 0.02a* | 28.90 ± 0.14a* | 9.70 ± 0.05a* |
| center | 0.238 ± 0.011a | 231 ± 3a | 338.5 ± 7.9a | 10.66 ± 0.04a | 3.54 ± 0.02a | 29.64 ± 0.15a | 10.03 ± 0.05a* |
Images were taken at a magnification of ×500 with a scanning electron microscope. Values are mean ± SE of four replications for gs, Ci, of 20 replications for stomatal frequencies, and of 300 replications for stomatal lengths, stomatal widths, stomatal pore areas and total stomatal pore areas.
1Different letters in the same column at a given treatment indicate significant differences between positions (P ≤ 0.05).
2*shows significant differences between the two K treatment in same position (P ≤ 0.05).
Potassium supply and leaf position had no effects on stomatal frequency and stomatal length (Table 2). However, stomatal width was significantly decreased in the –K treatment, especially in the leaf margins where the width decreased by 20.9% as compared with the +K treatment. Stomatal pore area was therefore considerably decreased due to K deficiency, particularly in the leaf margins with a 28.0% decline in the single stomatal pore area. Nevertheless, the stomatal length and width as well as the stomatal pore area showed no significant difference in these two positions under the +K treatment.
Mesophyll conductance
Despite a dramatic decrease in the mean mesophyll conductance (gm) in the leaf margins of the –K treatment, the mean chloroplastic CO2 concentrations (Cc) was 9.4% higher than that of the +K treatment (Table 3). The mean gm and Cc values were similar in the leaf centers of different K treatments, as well as between the two positions in the +K treatment. Potassium niutrient and leaf position did not affect mean intercellular CO2 compensation point (Ci*) and mitochondrial respiration rate in the light (Rd), however, the mean chloroplastic CO2 compensation point (Γ*) was significantly increased in leaf margins of the –K treatment, but showed no statistical differences among the other three groups.
Table 3. Effects of K deficiency on mesophyll conductance (gm), chloroplastic CO2 concentrations (C c), intercellular CO2 compensation point (C i *), mitochondrial respiration rate in the light (R d), and chloroplastic CO2 compensation point (Γ*) in the two positions of the fifth fully expanded leaves.
| Treatment | Position | gm(mol CO2m−2s−1) | Cc(μmol CO2mol−1) | Ci* (μmol CO2mol−1) | Rd(μmol CO2 m−2 s−1) | Γ* (μmol CO2mol−1) |
|---|---|---|---|---|---|---|
| −K | margin | 0.084 ± 0.002b1 | 139 ± 3a | 36.2 ± 0.8a | 0.95 ± 0.03a | 46.5 ± 1.6a |
| center | 0.151 ± 0.008a | 132 ± 5b | 35.4 ± 0.9a | 0.92 ± 0.03a | 41.7 ± 2.1a | |
| +K | margin | 0.163 ± 0.003a*2 | 127 ± 2a* | 33.5 ± 1.4a | 0.89 ± 0.05a | 40.1 ± 1.5a* |
| center | 0.174 ± 0.018a | 130 ± 7a | 32.4 ± 1.7a | 0.87 ± 0.04a | 39.4 ± 2.0a |
Ci* and Rd were measured by Laisk method, Γ* was calculated according to the equation Γ* = Ci* + Rd/gm. Values are mean ± SE of four replications for gm, Cc, and Ci-Cc, of three replications for Ci*, Rd and Γ*.
1Different letters in the same column at a given treatment indicate significant differences between positions (P ≤ 0.05).
2*shows significant differences between the two K treatment in same position (P ≤ 0.05).
Biochemical characteristics
The mean maximum rate of electron transport (Jmax) and maximum rate of carboxylation (Vc,max) in the leaf margins of the –K treatment were the lowest, and minor changes were observed among the other three treatments (Table 4). However, the mean Jmax/Vc,max in leaf margins of the –K treatment was dramatically increased compared with the mean values of the other three groups in the range from 1.42 to 1.46. The variation of photosynthetic parameters was verified by chemical analyses (Table 4). A significant decline of leaf chlorophyll concentration was found in the –K treated leaves, especially in the leaf margins, with a 31.1% decrease. Furthermore, Rubisco activity was dramatically decreased in leaf margins of the –K treatment, but it was the same in the leaf centers of the –K treatment and the two positions of the +K treatment. Potassium deficiency caused severe ROS production in leaf margins where O2.− generation rate increased by 22.8%, and meanwhile, POD activity increased by 25.5%.
Table 4. Effects of K deficiency on the maximum rate of electron transport (J max), maximum rate of carboxylation (V c,max), ratio between J max and V c,max (J max/V c,max) estimated from A-C c curves, chlorophyll concentration, Rubisco activity, O2 .− generation rate, and POD activity in the two positions of the fifth fully expanded leaves.
| Treatment | Position | Jmax(μmol m−2s−1) | Vc,max(μmol m−2s−1) | Jmax/Vc,max | Chlorophyll (g m−2) | Rubisco activity (U g−1 FW) | O2.− generation rate (nmol g−1 FW min−1) | POD activity (U g−1 FW min−1) |
|---|---|---|---|---|---|---|---|---|
| −K | margin | 114.5 ± 9b1 | 70.9 ± 4.8b | 1.61 ± 0.12a | 0.42 ± 0.03b | 0.29 ± 0.02b | 8.77 ± 0.35a | 2868 ± 176a |
| center | 166.2 ± 10a | 113.9 ± 4.5a | 1.46 ± 0.03b | 0.54 ± 0.02a | 0.36 ± 0.01a | 6.97 ± 0.15b | 2511 ± 74b | |
| + K | margin | 170.6 ± 12a* | 120.5 ± 5.9a* | 1.42 ± 0.06a* | 0.61 ± 0.05a* | 0.33 ± 0.01a* | 7.18 ± 0.22a* | 2275 ± 111a* |
| center | 167.5 ± 9a | 118.0 ± 5.6a | 1.42 ± 0.13a | 0.64 ± 0.04a* | 0.35 ± 0.01a | 6.61 ± 0.33a | 2085 ± 148a* |
Values are mean ± SE of four replications.
1Different letters in the same column at a given treatment indicate significant differences between positions (P ≤ 0.05).
2*shows significant differences between the two K treatment in same position (P ≤ 0.05).
The relationship between relative A, g s and g m with leaf K concentration
A significant curvilinear relationship between relative A, gs or gm and leaf K concentrations is shown in Fig. 1. The relative values increased with increasing leaf K concentration, and remained stable when the leaf K concentration was beyond a certain concentration. Here a photosynthesis-based concentration threshold with the relative values reaching 95.0% of the maxima was defined. The relative A values increased rapidly with increasing leaf K concentration when it was less than 1.07% (Fig. 1a), and varied little when the leaf K concentration was above 1.07%. Therefore, the K concentration (1.07%) was used to evaluate the relative gs and gm (Fig. 1b,c), and the calculated result (93.7% and 94.3%) was close to 95.0%, indicating that this threshold value was acceptable for gs and gm.
Figure 1. Relationship between relative photosynthetic parameters and leaf K concentration.
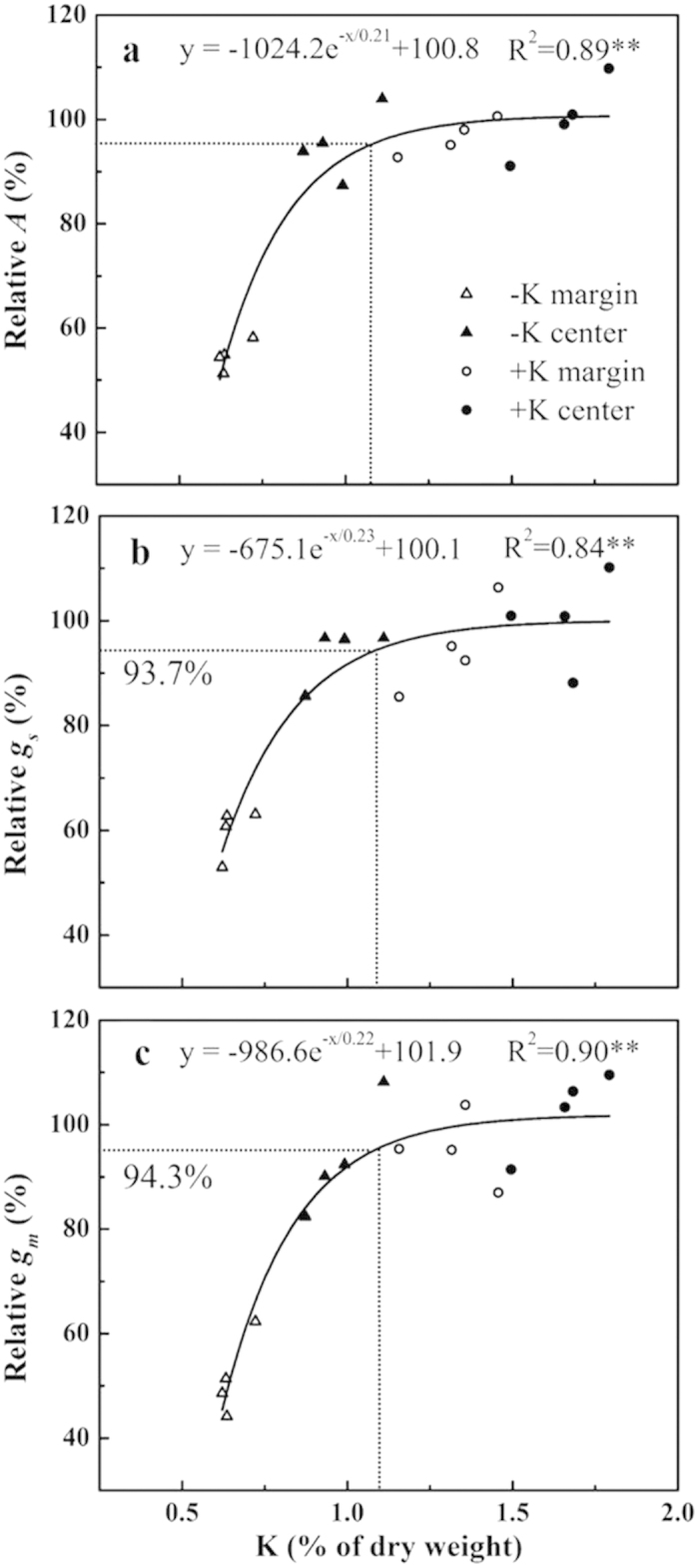
Relative (a) photosynthesis rate (A), (b) stomatal conductance (gs), (c) mesophyll conductance (gm). The values were the relative proportion of measured values over the mean values of K-sufficient leaf centers. Each point represents one leaf measurement. Open triangles and closed triangles represent the values of leaf margin and leaf center under the –K treatment, while open circles and closed circles represent those of the +K treatment. Equations, regression coefficients, and significance are shown when P ≤ 0.05 (*P ≤ 0.05; **P ≤ 0.01).
Quantitative limitation analysis
The restrictions of Amax in the –K leaves posed by stomatal (SL), mesophyll conductance (MCL) and biochemical limitations (BL) are presented in Fig. 2a. In symptomatic margins, total limitations reached a value of 46.9%, and the contribution of SL, MCL and BL represented 23.9%, 33.0% and 43.1% of total limitations, respectively. By contrast, despite the relatively low limitation (4.8%) in the leaf center, MCL contributed a primary limitation to Amax. Accordingly, the dominant limitations changed from symptomatic leaf margins to centers. The relationship between relative limitations and leaf K concentrations were further analyzed (Fig. 2b). All the limitations declined precipitously with the leaf K concentration increased from 0.6 to 1.07% (according to the K-based concentration threshold), particularly the BL with the maximum slope of the fitted curve, but they gradually decrease as leaf K concentration continues to increase. Their relative contribution also varied with the change of the leaf K status. While MCL largely predominated at the leaf K concentration of less than 1.07%, BL replaced it when the K concentration was below 0.78% (leaf K concentration of the intersection point between BL and MCL fitted curves).
Figure 2. Photosynthetic limitations and their response to leaf K concentration.
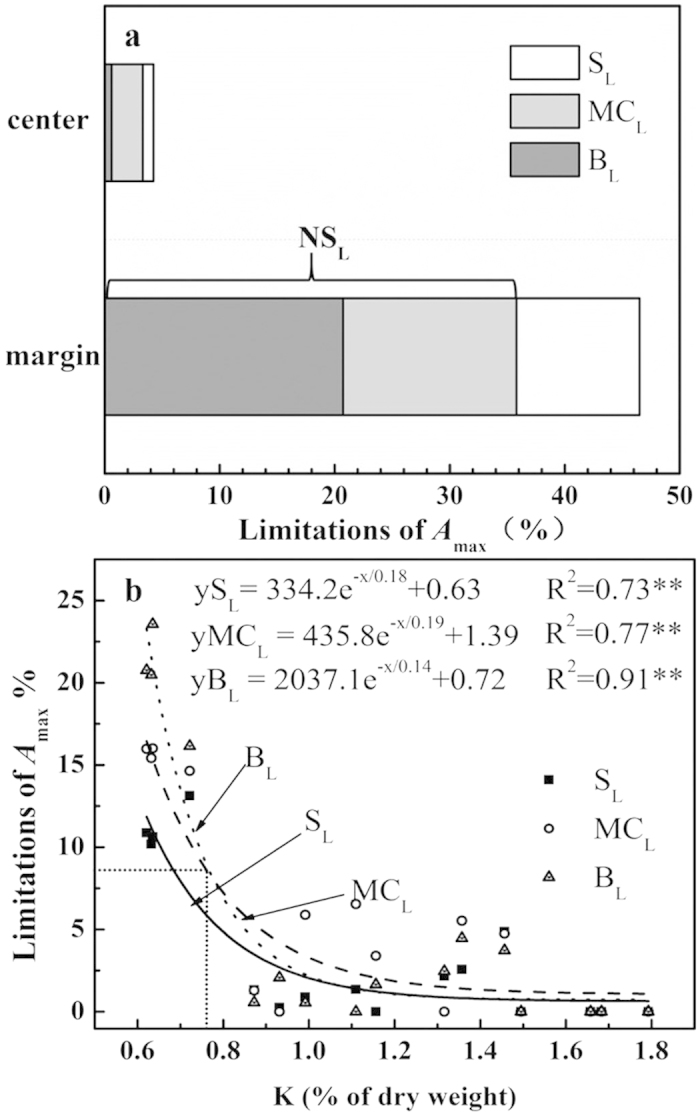
(a) Quantitative limitation analysis of photosynthetic CO2 assimilation in leaf margins and centers under the –K treatment. Values are mean ± SE of four replicates per position. The open, gray, and dark gray bars represent the percentages of stomatal (SL), mesophyll conductance (MCL), and biochemical (BL) limitations, respectively. (b) Relationships between limitations and leaf K concentration. Each point with the same shape represents a single leaf (n = 16). The symbols are as follows: SL, closed squares; MCL, open circles; BL, closed triangles. Solid, dash, and dot lines are regression curves of SL, MCL, and BL, respectively. Equations, regression coefficients, and significance are shown when P ≤ 0.05 (*P ≤ 0.05; **P ≤ 0.01).
Discussion
Limitations imposed by K deficiency occur at the same time
In the present study, A in leaf margins were weakened by K deficiency. Generally, the declining A is considered to be limited by stomatal and mesophyll resistances to CO2 diffusion, and biochemical obstacles13,22. Here we demonstrated that gs, gm and biochemical activities were profoundly restricted as A down-regulated. Stomatal conductance which determine the vital step of CO2 diffuse from the atmosphere to the interior of leaf was markedly decreased in –K leaf margins, as reported for Eucalyptus grandis1, Gossypium hirsutum17, and Oryza sativa3. This is mainly because the lack of vacuole K to keep stomatal aperture by providing driving force to promote water inpour into the guard cell vacuole31. The declined A, to a certain extent, revealed that the K in cytoplasm identified as biochemical functional component was below the critical value10. Therefore, malfunction of physiological process could come with limited A.
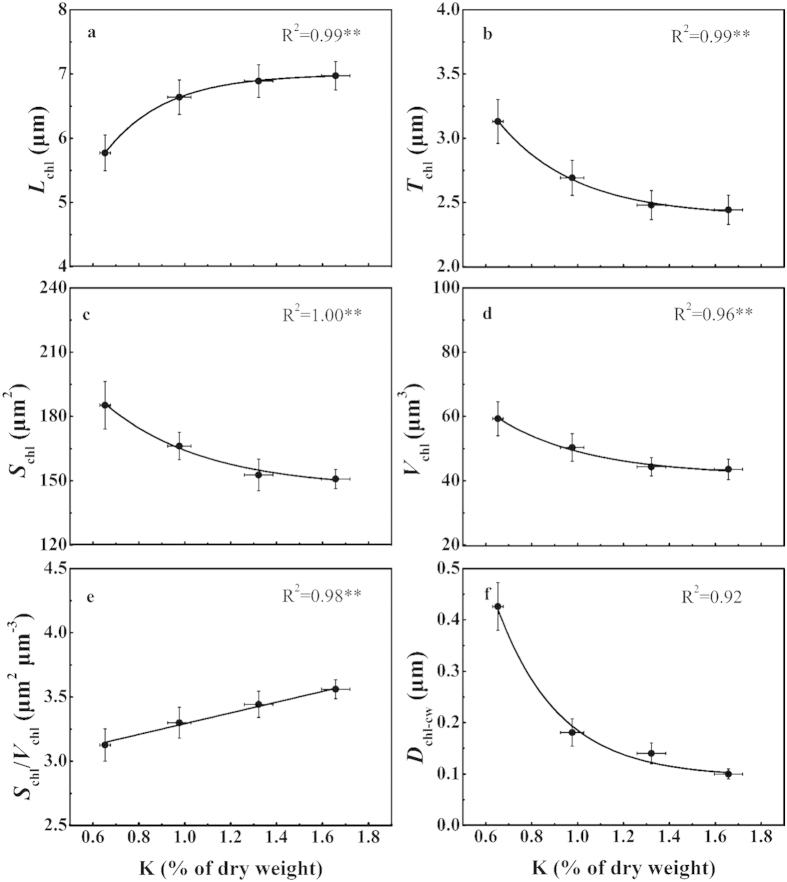
Likewise, gm was decreased in parallel with A. Indeed, gm might be down-regulated by increasing leaf dry mass per area (MA)7,23, however, in the present study, there was no remarkable difference in MA between the –K and +K leaves (Table 5; Supplementary Fig. S1). Cell wall thickness (Tcell-wall) and surface area of chloroplasts exposed to intercellular airspaces (Sc/S) are reported to be the most substantial anatomical traits in determining gm23,32. However, significant differences in mesophyll cell wall surface area exposed to intercellular airspace per leaf area (Sm/S) and Sc/S, but not Tcell-wall between leaf margins of two K treatments were observed (Table 5). Besides, chloroplast size33 has also been proved to influence gm. In the present study, though the chloroplast length (Lchl) decreased under lowest K status, the thickness (Tchl), surface area (Schl) and volume (Vchl) of chloroplast were largely increased, however, the Schl/Vchl was smaller (Fig. 3). The chloroplast enlarging under lowest K concentration was not completely same to that discovered under low nitrogen conditions33,34. The increase of Tchl was more likely to be based on the sacrifice of length owing to roughly circular envelope (Fig. 3a,b; Supplementary Fig. S2). Mathematically, ellipsoidal chloroplasts, combining with an increscent chloroplast number (see Supplementary Fig. S3) were more probably to have longer length of chloroplasts facing the cell wall than swollen even sphere envelopes. Furthermore, the resistance along diffusion pathway length in cytoplasm (distance of chloroplast from cell wall, Dchl-cw) and stroma (taken as half of the chloroplast thickness) account for 10–50% of gm limitation23, which however, reported only up to 22% of liquid phase resistance (rliq) by Evans et al. in 199435. Low K status strongly increased Tchl and Dchl-cw (Fig. 3b,f), accordingly, the corresponding resistance would be increased. It is therefore proved that the decreased gm is primary due to the reduced Sc/S and larger cytosol diffusion resistance but not Tcell-wall. More evidences may seek from the influence of K on plasma membrane and chloroplast envelope conductance32, carbonic anhydrase and aquaporins that participated in determination of gm14,15,23.
Table 5. Effects of K deficiency on leaf thickness (T leaf), mesophyll cell wall thickness (T cell-wall), mesophyll cell wall surface area exposed to intercellular airspace per leaf area (S m/S), and surface area of chloroplasts exposed to intercellular airspaces (S c/S) in the two positions of the fifth fully expanded leaves.
| Treatment | Position | Tleaf(μm) | Tcell-wall(μm) | Sm/S(m2 m−2) | Sc/S(m2 m−2) |
|---|---|---|---|---|---|
| −K | margin | 305 ± 27.7a | 0.167 ± 0.017a | 12.9 ± 1.8b | 8.6 ± 1.1b |
| center | 310 ± 19.2a | 0.170 ± 0.033a | 17.1 ± 2.4a | 12.8 ± 0.9a | |
| + K | margin | 309 ± 31.3a | 0.172 ± 0.049a | 17.4 ± 3.3a*2 | 13.1 ± 1.6a* |
| center | 312 ± 22.0a | 0.176 ± 0.045a | 18.2 ± 2.6a | 13.5 ± 2.1a |
Data are mean ± SE of eight replications for Sm/S and Sc/S, at least thirty replications for Tleaf and Tcell-wall.
1Different letters in the same column at a given treatment indicate significant differences between positions (p ≤ 0.05).
2* shows significant differences between the two K treatment in same position (p ≤ 0.05).
Figure 3. The relationship between chloroplast characteristics and leaf K concentration.
(a) Chloroplast length (Lchl), (b) thickness (Tchl), (c) surface area (Schl), (d) volume (Vchl), (e) Schl/Vchl, (f) distance of chloroplast from the cell wall (Dchl-cw). Values are mean ± SE of four replicates for K concentration and at least thirty replicates for microstructure parameters. Regression coefficients and significance are shown when P ≤ 0.05 (*P ≤ 0.05; **P ≤ 0.01).
It should be noted that A, gs or gm started to decline almost at the same time with an extremely similar leaf K status. By another way, the quantitative analysis of limitations indicated that three limiting factors coexist when K concentration below 1.07%. This was similar to the results reported by Grassi and Magnani22 and Tezara et al.36 in plants suffering from water stress. However, the investigation carried out by Galmés et al.13 revealed that BL of Hypericum balearicum and Phlomis italica still remained zero under mild water stress even if the total limitation reached 20–30%. The present finding highlights that all photosynthetic limitations simultaneously occur when leaf is in a physiological K-deficiency state.
Limitations vary with increasing K deficiency intensity
The leaf K concentration threshold value observed in this study was 1.07%, in consistent with the range of 0.5 to 2.0% reported by Leigh and Wyn Jones37. Quantitative limitation analysis gives insight into the contributions of different photosynthetic limitations, revealing that the BL and MCL accounted for the majority of total limitations in K-starved leaf margins and centers, respectively. This is mainly ascribed to the discrepancy of relative severity of K deficiency5,6. As has been stated in the previous studies that some irreversible damages, such as impaired ATP synthesis, depressed Rubisco activity, and cell damage occurred when the limiting A, for the most part, is attributed to BL3,38. Some of which were verified in the present study, such as degraded chloroplast, limited photoassimilate transportation (see Supplementary Fig. S2), and increased O2.− generation rate under severe K deficiency. The obstacle of these physiological processes alleviated as K deficient stress mitigating, however, the role of MCL on A began to stand out.
The relationship between relative limitations and leaf K concentration verified that, at a leaf K concentration of less than 1.07%, MCL represented the main component of limitations, but BL replaced it when leaf K concentration below 0.78%. This pattern, to a lesser extent, could be found in plants suffering from water stress which suggested that the variation of limitations depends on the stress intensity and duration22. Regrettably, the present study failed to reveal whether or not there is a critical concentration in the shifting process from SL predominance to MCL predominance. Further studies focusing on the photosynthetic limitations of rapeseed leaves subjected to a serial K gradient may help to elucidate this issue.
Methods
Study site and growth conditions
A field experiment was conducted in Wuxue county, Hubei province, central China (30° 06′46″N, 115° 36′9″E) during the 2013–2014 oilseed rape growing season. The mean temperature of the season was 13.8 °C, and the average temperature during winter (from December 2013 to February 2014) was 5.9 °C. The total precipitation during oilseed rape cropping season was 660.7 mm, with wintertide accounting for 26.1% of the total. The soil was a sandy loam with pH 5.3, organic matter 30.5 g kg−1, total N 1.7 g kg−1, NH4OAc-K 42.5 mg kg−1, Olsen-P 15.7 mg kg−1 and hot-water soluble B 0.78 mg kg−1 in the topsoil layer (0–20 cm). As stated by Zou, the soil belongs to a K-deficient type, which would cause yield reduction without K fertilizer addition4.
Experimental design
A complete randomized block design was set up with two treatments and four replicates. The treatments were: (1) Sufficient K supply treatment (+K), with a K fertilizer recommendation rate of 120 kg K2O ha–1 which was tested and well-proved to ensure the optimal growth and yield formation of oilseed rape based on field experiments in this region39. (2) K deficiency treatment (–K), with no K fertilizer applied throughout the growing season.
To ensure that nutrients other than K did not limit plant K uptake, 180 kg N ha−1, 90 kg P2O5 ha−1, and 1.6 kg B ha−1 were applied for these two treatments. The N, P, K, B fertilizers used in the experiment consisted of urea (46% N), superphosphate (12% P2O5), potassium chloride (60% K2O), and borax (10.8% B). The N fertilizer was applied in three splits: 60% prior to transplanting, i.e., BBCH (Biologische Bundesantalt, Bundessortenamt and Chemische Industrie) 15–1640, 20% at the over-wintering stage (i.e., BBCH 29), and 20% at the initiation of stem elongation (i.e., BBCH 30). Besides, all the P, K, B fertilizers were applied as basal fertilizers. The experimental field was plowed and leveled with a rotary tiller, and basal fertilizers were incorporated during the process. The plot measured 20 m2 with a length of 10 m and a width of 2 m.
The oilseed rape cultivar was Zhongshuang 11, supplied by Oil Crops Research Institute of the Chinese Academy of Agricultural Sciences. Rapeseeds were sown in prepared seedbeds on 16 September 2013, and then, on 22 October, about 36 d after sowing, oilseed-rape seedlings with five to six leaves (i.e., BBCH 15–16, 3–4 g dry weight plant−1) were uniformly selected and transplanted by hand in double rows spaced approximately 0.3 m apart with 0.2–0.3 m between plants, corresponding to 112 500 plants ha–1. The oilseed rape was grown under rain-fed conditions. Meanwhile, weeds, pests and disease stresses were controlled by spray herbicides, insecticide and fungicide so that no obvious weeds, insect pests, and diseases infestation occurred during cropping season.
Plant and leaf tagging
There was an obvious phenotypic difference in plants between the –K and +K treatments 60 d after transplanting. The discrepancy was highlighted in the fifth to ninth fully expanded leaves (with a total average of 9 fully expanded leaves (i.e., BBCH 19) in both treatments) from apex downwards, specifically, obvious etiolation symptoms around the periphery in K-deficient leaves and asymptomatic leaves in the +K treatment. For each treatment, 24 fifth fully expanded leaves and six uniform plants were tagged in each of the four replicate plots for destructive and non-destructive analysis described later in the methods.
Leaf gas exchange and fluorescence measurements
Leaf gas exchange and chlorophyll fluorescence were measured simultaneously at either leaf margins (Fig. 4, about 2 cm of leaf surface from the margin) or leaf centers (the rest part between half-elliptic and vertical dashed lines), using a portable, open circuit, infrared gas analysis system (Li-6400, Li-Cor Inc., Lincoln, NE, USA) equipped with an integrated leaf chamber fluorometer (Li-6400–40). Measurements were performed on at least four randomly selected leaves of both treatments in the late morning (11:00–12:30) under a light-saturating photosynthetic photon flux density (PPFD) of 1200 μmol m−2s−1 (with 90% red light and 10% blue light). CO2 concentration in the leaf chamber (Ca) was set at 400 μmol mol−1 air, leaf temperature was controlled at 25 ± 0.2 °C, relative humidity was between 50 and 60%, and the flow rate was 500 μmol s−1. In addition to net photosynthesis (A), stomatal conductance to water vapour (gs) and intercellular CO2 concentration (Ci), the incorporated fluorometer allowed determination the steady-state fluorescence yield (Fs) under actinic light and maximum fluorescence (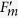 ) during light-saturating pulse (0.8 s) of approx. 8000 μmol m–2 s–1. The relative A, gs and gm values were the relative proportion of measured values over the mean values of K-sufficient leaf centers.
) during light-saturating pulse (0.8 s) of approx. 8000 μmol m–2 s–1. The relative A, gs and gm values were the relative proportion of measured values over the mean values of K-sufficient leaf centers.
Figure 4. Leaves under the –K and +K treatments.
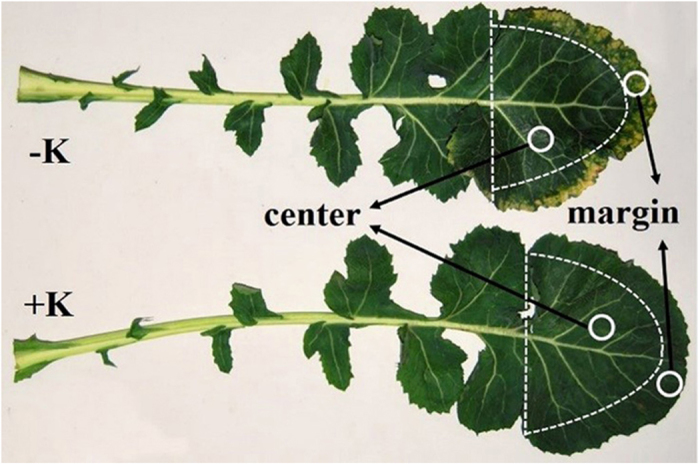
The vertical dash lines divide leaf into two parts, the leaf apexes are separated into leaf margins and leaf centers by half-elliptic lines. White circles indicate the gas exchange measuring positions.
A/Ci curves were measured on the two positions that had been previously acclimated to saturating light conditions for 20 min. The CO2 concentration (Ca) in the gas exchange chamber was reduced stepwise from 400 to 300, 250, 200, 150, 100, 50 μmolCO2 mol−1, and then increased from 50 to 400, 600, 800, 1000, 1200, 1500, 1800 μmol CO2 mol−1 at a constant PPFD of 1200 μmol m−2s−1 at 25 ± 0.2 °C, and 50–60% relative humidity. In all cases, the parameters were recorded after the gas exchange rate stabilized at the given Ca. At least four leaves were performed in each treatment.
The actual photochemical efficiency of photosystem II (ΦPSII) was then determined as follows41:
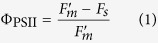 |
The electron transport rate (J) can be calculated as:
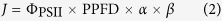 |
Where α is the leaf absorptance, and β is the fraction of light distributed to PSII. As routinely assumed, α was taken as 0.8542,43 and β was taken as 0.544,45. A sensitivity analysis of J biases resulting from rough assumption of α and β on gm variations was also conducted (See Supplementary Table S5).
Mesophyll conductance was estimated according to Harley et al. from combined gas exchange and chlorophyll fluorescence measurements46.
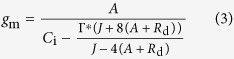 |
where A, Ci and J were determined as previously described for each treatment, mitochondrial respiration rate in the light (Rd) and the intercellular CO2 compensation point (Ci*) were measured by Laisk method, as described by Brooks and Farquhar47. Briefly, the A/Ci curves generated with PPFD values of 75, 150, 500 μmol m−2s−1, respectively, with each having five different Ca in chamber (i.e. 50, 80, 100, 120 and 150 μmol CO2 mol−1). A linear regression was then fitted to each A/Ci curve. The x-axis and y-axis of intersection point of three A/Ci curves were defined as Ci* and Rd48. The Γ* is the chloroplastic CO2 photocompensation point calculated from Ci* and Rd as:
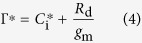 |
For each data point generated, we checked whether it met the range of 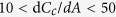 46. The CO2 concentration in the chloroplast stroma (Cc) was calculated as:
46. The CO2 concentration in the chloroplast stroma (Cc) was calculated as:
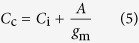 |
Therefore, A-Ci curves were converted into A-Cc curves. On the basis of Cc, the maximum rate of Rubisco-catalysed carboxylation (Vc,max), and the maximum rate of electron transport (Jmax) as defined by Farquhar et al.49, were calculated11,50.
Since variable J method is sensitive to many sources of errors, e.g. (1) Γ* and Rd biases; (2) a wrong assumption of p1 and p2; (3) biases in the measurements of Ci, A, and J, a sensitivity analysis would be great values to improve the confidence in gm estimates and following limitation calculations51. Following the method of Harley et al.46, we used actual Γ*, Rd and J values calculated in this study and a deviation from the measured values to analyze the effects of Γ*, Rd and J on gm estimates (see Supplementary Table S1, S3, S5). RuBP regeneration can be limited by either insufficient NADPH or ATP, according to Farquhar model, A and J can be linked as follows:
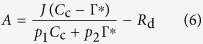 |
For insufficient NADPH, p1 = 4 and p2 = 8; for insufficient ATP, p1 = 4.5 and p2 = 10.5 or p1 = 4 and p2 = 9.33. Finally, the sensitivity analysis for photosynthetic limitations was conducted basing on these calculated gm values (see Supplementary Table S2, S4, S6). The analysis showed that the gm was significantly affected by varying Γ* and Rd (see Supplementary Table S1), p1 and p2 inputs (see Supplementary Table S3) and J biases (see Supplementary Table S5). However, the gm variation derived from Γ*, Rd, J, p1 and p2 biases, did not cause profound effects on photosynthetic limitations (see Supplementary Table S2, S4, S6). In addition, gm appears to be strikingly affected by Ci12,14,51, nevertheless, the similar Ci in different treatments and positions here seems to have no impact on gm (Table 3). Therefore, the results obtained was unlikely to be altered by these methodological artifacts.
Plant dry matter, leaf area and dry matter
Six tagged leaves and six tagged plants in each plot were used to determine the individual leaf area, dry matter, and total dry matter. Each leaf was digitally scanned using an Epson ES-1200C scanner (Epson, Long Beach, CA, USA), and the area determined using ImageJ software (National Institutes of Health, Bethesda, Maryland, USA)1. Individual leaf dry matter and total dry matter were weighed after oven drying at 65 °C for 48 h.
Biochemical analysis
Twelve tagged leaves per plot were picked immediately after the determination of photosynthesis. They were divided into two parts along vertical dashed lines (Fig. 4), followed by dissecting the leaf apexes into leaf margins and leaf centers, and removing all the veins. A portion of segments were immersed in liquid N and then stored at −78 °C, and the rest were used for leaf K concentration determination. There were four replications for biochemical determinations.
Leaf segments (2 g) were oven dried at 65 °C for 48 h. After that, about 0.15 g dried leaves were milled and digested with H2SO4-H2O2 as described by Thomas et al.52, and K concentration in digestion solution was measured by a flame photometer (M-410, Cole-Parmer, Chicago, IL, USA).
The Rubisco extracts were prepared according to Weng et al. with minor modifications3. Briefly, leave segments (0.2 g) were ground to a powder using a chilled mortar and pestle with liquid N2 and a small amount of quarzsand, followed by homogenization with 4 mL pre-cooled extraction buffer containing 50 mM Tris-HCl (pH 7.5), 1 mM EDTA,10 mM MgCl2, 12.5% (v/v) glycerol, 10 mM (v/v) β-mercaptoethanol and 1% (w/v) PVP-40 (soluble PVP) at 0–4 °C. The homogenate was centrifuged for 15 min at 15 000 g at 4 °C, and then the supernatant was immediately used to determine the activity of ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco, EC 4.1.1.39) by an enzyme-linked immunosorbent assay method with a RuBPcase ELISA kit (CK-E91697P, Shanghai jijin Chemistry and Technology Co., Ltd, China) according to the manufacturer’s instructions. The chlorophyll concentration was determined according to the method of Huang et al.53.
Superoxide radical O2.− production rate was measured by monitoring the nitrite formation from hydroxylamine in the presence of O2.− according to Elstner and Heupel54. A 0.5 g aliquot of leaf margins and centers was ground and homogenized in 5 mL of 65 mM pre-cooled phosphate buffer (pH 7.8), followed by centrifuging the homogenate at 10,000 g for 15 min at 4 °C and mixing 0.5 mL of the supernatant with phosphate buffer (0.5 mL) and 0.1 mL of 10 mM hydroxylamine hydrochloride. This mixture was incubated at 25 °C for 20 min, followed by the addition of 1 mL of 58 mM sulfanilic acid and 1 mL of α-naphthylamine, and then another 20 min incubation at 25 °C. The as-prepared solution was shaken with equal volume of ether, followed by centrifuging the mixture at 10,000 g for 3 min and measuring the absorbance of the pink water phase at 530 nm. The activity of POD (EC 1.11.1.7) was determined using the guaiacol oxidation method55.
Anatomical analysis
Another six tagged leaves per treatment were collected, and removed all the veins for anatomical analysis. The stomatal size and frequency were measured in six sub-samples either for leaf margin or center. The materials were prepared as described by Meng et al.56. Briefly, leaf samples (about 1cm in length and 1 cm in width) were fixed in 2.5% glutaraldehyde (v/v) at 4 °C for 2 h, and washed twice in 0.1 M phosphate buffer (pH 6.8). Next, they were sequentially dehydrated in ethanol (30%, 50%, 70%, 80%, 90%, 95%, and 100%) for 10 min at each gradient concentration, with 100% ethanol repeated twice. After further drying and spraying with gold, the as-treated leaf samples were observed and photographed with a scanning electron microscope (JSM-5310LV, Jeol Co, Tokyo, Japan). Images were taken of the lower leaf surface for five microscope fields per sub-sample at a magnification of ×500. The number of stomata was counted in each field (a total of 20 measurements of stomatal frequency for each position) as described by Battie-Laclau et al.1, and the stomatal frequency was calculated by dividing the stomatal count by the area of the field of view57. Moreover, the length and width of ten stomata selected at random were measured in each field. Assuming the stomatal pore as an ellipse, the total stomatal pore area was calculated (stomatal frequency × π × 0.25 × stomatal length × stomatal width).
Leaf segments (1–2 mm2) were cut from each part and fixed with 2.5% glutaraldehyde (v/v) in 0.1 M phosphate buffer (pH 7.4) for 4 h, followed by washing twice in the same buffer for 30 min and postfixing with 2% osmium tetroxide for 4 h at 4 °C. Next, the samples were dehydrated with an ethanol series (10–100%) and in propylene oxide, followed by embedding them in Epon 812 resin.
For the light microscope observation, they were cut into 1 μm transverse sections by LKB-5 ultramicrotome 359 (LKB Co., Ltd., Uppsala, Sweden), and stained with 0.5% toluidine blue. Micrographs were captured at a magnification of ×400 with a Nikon Eclipse E600 microscope equipped with a Nikon 5 MP digital microscope camera DS-Fi1 (Nikon Corporation, Kyoto, Japan). There were four samples per treatment. For each samples, three cross-sections were chosen to measure their thickness (Tleaf), mesophyll cell wall surface area exposed to intercellular airspace per leaf area (Sm/S), and surface area of chloroplasts exposed to intercellular airspaces (Sc/S) according to Tosens et al. (2012)32.
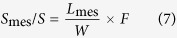 |
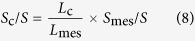 |
Where Lmes and Lc are the length of mesophyll cell wall exposing to intercellular air space and chloroplast surface area touching the intercellular air space. W is the width of measured cross-section. F is the curvature correction factor which was obtained as the weight average of palisade and spongy mesophyll.
For the ultrastructural observations, ultrathin sections (90 nm) were examined with a transmission electron 360 microscope (H-7650, Hitachi, Japan) after staining with 2.0% uranyl acetate (w/v) and lead citrate. Cell wall thickness (Tcell-wall), chloroplast length (Lchl) and thickness (Tchl) were measured from at least 30 chloroplasts. Chloroplasts were assumed as ellipsoids, and chloroplast surface area (Schl) and volume (Vchl) were calculated according Cesaro formula58:
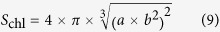 |
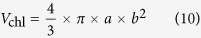 |
where 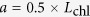 ;
; 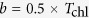 . Distance of chloroplast from cell wall (Dchl-cw) was determined according to Tomás et al.23.
. Distance of chloroplast from cell wall (Dchl-cw) was determined according to Tomás et al.23.
Quantitative limitation analysis
The limitations (stomatal limitations, SL; mesophyll conductance limitations, MCL; biochemical limitations, BL) imposed by K deficiency on photosynthesis were investigated by analyzing the leaf margins and centers under two treatments using the quantitative limitation analysis method proposed by Grassi and Magnai22. Relative changes in light-saturated assimilation is expressed in terms of relative changes in stomatal, mesophyll conductance, and biochemical capacity as Equation (11).
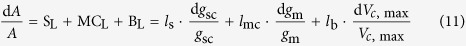 |
where ls, lmc, and lb are the corresponding relative limitations calculated as Eqns from (12) to (14), gsc is stomatal conductance to CO2 (gs/1.6), and Vc,max is maximum rate of carboxylation estimated from A-Ci curve.
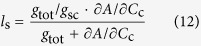 |
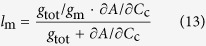 |
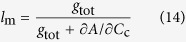 |
where gtot is the total conductance to CO2 from leaf surface to carboxylation sites determined as Equation (15). By following Tomás et al.23, 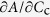 was calculated as slope of A-Cc response curves over a Cc range of 50–100 μmol mol–1.
was calculated as slope of A-Cc response curves over a Cc range of 50–100 μmol mol–1.
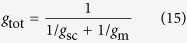 |
Then the relative change of A, gsc, gm and Vc,max in Equation (11) can be approximated by Chen et al.59.
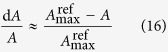 |
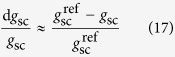 |
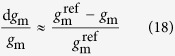 |
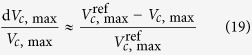 |
where 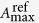 ,
, 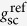 ,
, 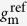 and
and 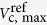 are the reference values of net CO2 assimilation rate, stomatal conductance and mesophyll conductance, and the rate of carboxylation, defined as maximun value measured under light saturation. In the original reference, the authors used the maximum value of seasonal Amax under light-saturated conditions as a reference to assess the photosynthetic limitations of A for each determination. In the current study, the maximum A was generally reached, concomitantly with gs, gm and Vc,max in the leaf centers with the +K treatment, and the mean values of the +K treatments was thus used as a reference, i.e., there was no limitation present in the leaf centers under the +K treatment. Whenever one of the three parameters was higher in either one of the rest treatment than that of the reference, its corresponding limitation was set to zero. In this way, the limitations in the leaf margins and centers under the –K treatment could be quantified. Finally, non-stomatal limitations were defined as the sum of mesophyll conductance limitations and biochemical limitations (NSL = MCL + BL).
are the reference values of net CO2 assimilation rate, stomatal conductance and mesophyll conductance, and the rate of carboxylation, defined as maximun value measured under light saturation. In the original reference, the authors used the maximum value of seasonal Amax under light-saturated conditions as a reference to assess the photosynthetic limitations of A for each determination. In the current study, the maximum A was generally reached, concomitantly with gs, gm and Vc,max in the leaf centers with the +K treatment, and the mean values of the +K treatments was thus used as a reference, i.e., there was no limitation present in the leaf centers under the +K treatment. Whenever one of the three parameters was higher in either one of the rest treatment than that of the reference, its corresponding limitation was set to zero. In this way, the limitations in the leaf margins and centers under the –K treatment could be quantified. Finally, non-stomatal limitations were defined as the sum of mesophyll conductance limitations and biochemical limitations (NSL = MCL + BL).
Statistical analysis
One-way analysis of variance (ANOVA) was caiculated usig SPSS 18.0 software (SPSS, Chicago, IL, USA). The mean values were compared using the least significant difference (LSD) test (P < 0.05). Graphics and regression analysis were performed using the OriginPro 8.5 software (OriginLab Corporation, Northampton, MA, USA).
Additional Information
How to cite this article: Lu, Z. et al. Differences on photosynthetic limitations between leaf margins and leaf centers under potassium deficiency for Brassica napus L. Sci. Rep. 6, 21725; doi: 10.1038/srep21725 (2016).
Supplementary Material
Acknowledgments
This work was supported by the earmarked fund for China Agriculture Research System (CARS-13); the Special Fund for Agro-scientific Research in the Public Interest (201203013); and the PhD Candidate Research Innovation Project of Huazhong Agricultural University (2014bs17).
Footnotes
Author Contributions Z.L., T.R. and J.L. conceived and designed the experiment. Z.L. and Y.P. performed the experiments, Z.L. analyzed the data, wrote the main manuscript text and prepared all of the figures, and Z.L., T.R., Y.P., X.L., R.C. and J.L. reviewed and approved the manuscript.
References
- Battie-Laclau P. et al. Photosynthetic and anatomical responses of Eucalyptus grandis leaves to potassium and sodium supply in a field experiment. Plant cell environ. 37, 70–81 (2014). [DOI] [PubMed] [Google Scholar]
- Erel R. et al. Modification of non-stomatal limitation and photoprotection due to K and Na nutrition of olive trees. J. Plant physiol. 177, 1–10 (2015). [DOI] [PubMed] [Google Scholar]
- Weng X. Y., Zheng C. J., Xu H. X. & Sun J. Y. Characteristics of photosynthesis and functions of the water-water cycle in rice (Oryza sativa) leaves in response to potassium deficiency. Physiol. plantarum 131, 614–621 (2007). [DOI] [PubMed] [Google Scholar]
- Zou J., Lu J. W., Li Y. S. & Li X. K. Regional evaluation of winter rapeseed response to K fertilization, K use efficiency, and critical level of soil K in the Yangtze River Valley. Sci. Agric. Sin. 10, 911–920 (2011). [Google Scholar]
- Bednarz C., Oosterhuis D. & Evans R. Leaf photosynthesis and carbon isotope discrimination of cotton in response to potassium deficiency. Environ. Exp. Bot. 39, 131–139 (1998). [Google Scholar]
- Jin S. H. et al. Effects of potassium supply on limitations of photosynthesis by mesophyll diffusion conductance in Carya cathayensis. Tree physiol. 31, 1142–1151 (2011). [DOI] [PubMed] [Google Scholar]
- Flexas J., Ribas-Carbó M., Diaz-Espejo A., Galmés J. & Medrano H. Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ. 31, 602–621 (2008). [DOI] [PubMed] [Google Scholar]
- Lebaudy A. et al. Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels. P. Natl. Acad. Sci. USA 105, 5271–5276 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés Z. et al. Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. P. Natl. Acad. Sci. USA 111, 1806–1814 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan-Meille L. & Pellerin S. Shoot and root growth of hydroponic maize (Zea mays L.) as influenced by K deficiency. Plant Soil 304, 157–168 (2008). [Google Scholar]
- Bernacchi C. J., Portis A. R., Nakano H., von Caemmerer S. & Long S. P. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol. 130, 1992–1998 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexas J. et al. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ. 30, 1284–1298 (2007). [DOI] [PubMed] [Google Scholar]
- Galmés J., Medrano H. & Flexas J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 175, 81–93 (2007). [DOI] [PubMed] [Google Scholar]
- Xiong D. L. et al. Rapid responses of mesophyll conductance to changes of CO2 concentration, temperature and irradiance are affected by N supplements in rice. Plant Cell Environ. doi: 10.1111/pce.12558 (2015). [DOI] [PubMed] [Google Scholar]
- Kaldenhoff R. Mechanisms underlying CO2 diffusion in leaves. Curr. Opin. Plant Biol. 15, 276–281 (2012). [DOI] [PubMed] [Google Scholar]
- Kanai S. et al. Potassium deficiency affects water status and photosynthetic rate of the vegetative sink in green house tomato prior to its effects on source activity. Plant sci. 180, 368–374 (2011). [DOI] [PubMed] [Google Scholar]
- Wang N. et al. Genotypic variations in photosynthetic and physiological adjustment to potassium deficiency in cotton (Gossypium hirsutum). J. Photochem. Photobiol. B: Biol. 110, 1–8 (2012). [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. et al. Do we underestimate the importance of leaf size in plant economics? Disproportional scaling of support costs within the spectrum of leaf physiognomy. Ann. Bot. 100, 283–303 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 168, 521–530 (2005). [Google Scholar]
- Paul M. J. & Pellny T. K. Carbon metabolite feedback regulation of leaf photosynthesis and development. J. Exp. Bot. 54, 539–547 (2003). [DOI] [PubMed] [Google Scholar]
- Araya T., Noguchi K. & Terashima I. Effects of carbohydrate accumulation on photosynthesis differ between sink and source leaves of Phaseolus vulgaris L. Plant cell physiol. 47, 644–652 (2006). [DOI] [PubMed] [Google Scholar]
- Grassi G. & Magnani F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 28, 834–849 (2005). [Google Scholar]
- Tomás M. et al. Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. J. Exp. Bot. 64, 2269–2281 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gago J. et al. Photosynthesis limitations in three fern species. Physiol. plantarum 149, 599–611 (2013). [DOI] [PubMed] [Google Scholar]
- Muir C. D., Hangarter R. P., Moyle L. C. & Davis P. A. Morphological and anatomical determinants of mesophyll conductance in wild relatives of tomato (Solanum sect. Lycopersicon, sect. Lycopersicoides; Solanaceae). Plant cell environ. 37, 1415–1426 (2014). [DOI] [PubMed] [Google Scholar]
- Ren T. et al. Potassium-fertilizer management in winter oilseed-rape production in China. J Plant Nutr. Soil Sci. 176, 429–440 (2013). [Google Scholar]
- Gierth M. & Mäser P. Potassium transporters in plants–involvement in K+ acquisition, redistribution and homeostasis. FEBS lett. 581, 2348–2356 (2007). [DOI] [PubMed] [Google Scholar]
- Gonzalez N., Vanhaeren H. & Inzé D. Leaf size control: complex coordination of cell division and expansion. Trends Plant Sci. 17, 332–340 (2012). [DOI] [PubMed] [Google Scholar]
- Zörb C., Senbayram M. & Peiter E. Potassium in agriculture-status and perspectives. J. Plant Physiol. 171, 656–669 (2014). [DOI] [PubMed] [Google Scholar]
- Fanaei H., Galavi M., Kafi M. & Bonjar A. G. Amelioration of water stress by potassium fertilizer in two oilseed species. Int. J. Plant Prod. 3, 41–54 (2009). [Google Scholar]
- Peiter E. The plant vacuole: emitter and receiver of calcium signals. Cell Calcium 50, 120–128 (2011). [DOI] [PubMed] [Google Scholar]
- Tosens T., Niinemets Ü., Westoby M. & Wright I. J. Anatomical basis of variation in mesophyll resistance in eastern Australian sclerophylls: news of a long and winding path. J. Exp. Bot. 63, 5105–5119 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. et al. Does chloroplast size influence photosynthetic nitrogen use efficiency? PloS ONE 8, e62036 doi: 10.1371/journal.pone.0062036 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong D. L. et al. SPAD-based leaf nitrogen estimation is impacted by environmental factors and crop leaf characteristics. Scientific Reports 5, 13389 doi: 10.1038/srep13389 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. R., von Caemmerer S., Setchell B. A. & Hudson G. S. The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Aust. J. Plant Physiol. 21, 475–495 (1994). [Google Scholar]
- Tezara W., Mitchell V., Driscoll S. & Lawlor D. Effects of water deficit and its interaction with CO2 supply on the biochemistry and physiology of photosynthesis in sunflower. J. Exp. Bot. 53, 1781–1791 (2002). [DOI] [PubMed] [Google Scholar]
- Leigh R. A. & Wyn Jones R. G. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 97, 1–13 (1984). [Google Scholar]
- Flexas J., Bota J., Loreto F., Cornic G. & Sharkey T. D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 Plants. Plant Biology 6, 269–279 (2004). [DOI] [PubMed] [Google Scholar]
- Li Y. S. et al. Study on response to potassium (K) application and recommendation of optimal K rates for rapeseed in Hubei. Chin. J. Oil Crop Sci. 30, 469–475 (2008). [Google Scholar]
- Lancashire P. D. et al. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 119, 561–601 (1991). [Google Scholar]
- Genty B., Briantais J. M. & Baker N. R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. BBA-Gen. Subjects 990, 87–92 (1989). [Google Scholar]
- Li Y., Gao Y. X., Xu X. M., Shen Q. R. & Guo S. W. Light-saturated photosynthetic rate in high-nitrogen rice (Oryza sativa L.) leaves is related to chloroplastic CO2 concentration. J. Exp. Bot. 60, 2351–2360 (2009). [DOI] [PubMed] [Google Scholar]
- Manter D. K. & Kerrigan J. A/Ci curve analysis across a range of woody plant species: influence of regression analysis parameters and mesophyll conductance. J. Exp. Bot. 55, 2581–2588 (2004). [DOI] [PubMed] [Google Scholar]
- Albertsson P.-A. A quantitative model of the domain structure of the photosynthetic membrane. Trends Plant Sci. 6, 349–354 (2001). [DOI] [PubMed] [Google Scholar]
- Warren C. R. The photosynthetic limitation posed by internal conductance to CO2 movement is increased by nutrient supply. J. Exp. Bot. 55, 2313–2321 (2004). [DOI] [PubMed] [Google Scholar]
- Harley P. C., Loreto F., Di Marco G. & Sharkey T. D. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol. 98, 1429–1436 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A. & Farquhar G. D. Effect of temperature on the CO2/O2 specificity of ribulose-1, 5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta 165, 397–406 (1985). [DOI] [PubMed] [Google Scholar]
- von Caemmerer S., Evans J., Hudson G. & Andrews T. J. The kinetics of ribulose-1,5-bisphosphate carboxylase/oxygenase in vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta 195, 88–97 (1994). [Google Scholar]
- Farquhar G. D., von Caemmerer S. V. & Berry J. A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90 (1980). [DOI] [PubMed] [Google Scholar]
- Sharkey T. D., Bernacchi C. J., Farquhar G. D. & Singsaas E. L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 30, 1035–1040 (2007). [DOI] [PubMed] [Google Scholar]
- Gu L. H. & Sun Y. Artefactual responses of mesophyll conductance to CO2 and irradiance estimated with the variable J and online isotope discrimination methods. Plant Cell Environ. 37, 1231–1249 (2014). [DOI] [PubMed] [Google Scholar]
- Thomas R. L., Sheard R. W. & Moyer J. R. Comparison of conventional and automated procedures for nitrogen, phosphorus, and potassium analysis of plant material using a single digestion. Agron. J. 59, 240–243 (1967). [Google Scholar]
- Huang Z. A., Jiang D. A., Yang Y., Sun J. W. & Jin S. H. Effects of nitrogen deficiency on gas exchange, chlorophyll fluorescence, and antioxidant enzymes in leaves of rice plants. Photosynthetica 42, 357–364 (2004). [Google Scholar]
- Elstner E. F. & Heupel A. Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal. Biochem. 70, 616–620 (1976). [DOI] [PubMed] [Google Scholar]
- Chance B. & Maehly A. Assay of catalases and peroxidases. Methods enzymol. 2, 764–775 (1955). [DOI] [PubMed] [Google Scholar]
- Meng F. J., Peng M., Pang H. y. & Huang F. L. Comparison of photosynthesis and leaf ultrastructure on two black locust (Robinia pseudoacacia L.). Biochem.l Syst. Ecol. 55, 170–175 (2014). [Google Scholar]
- Craven D., Gulamhussein S. & Berlyn G. Physiological and anatomical responses of Acacia koa (Gray) seedlings to varying light and drought conditions. Environ. Exp. Bot. 69, 205–213 (2010). [Google Scholar]
- Makino A., Mae T. & Chira K. Colorimetric measurement of protein stained with coomassie brilliant blue ron sodium dodecyl sulfate–polyacrylamide gel electrophoresis by eluting with formamide. Agric. Biol. Chem. 50, 1911–1912 (1986). [Google Scholar]
- Chen T. W. Kahlen K. & Stützel H. Disentangling the contributions of osmotic and ionic effects of salinity on stomatal, mesophyll, biochemical and light limitations to photosynthesis. Plant cell environ. doi: 10.1111/pce.12504 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.