Abstract
Individuals with metabolically healthy obesity (MHO) are at relatively low risk for the development of metabolic abnormalities and subclinical atherosclerosis. This study aims to examine whether hepatic fat accumulation determines metabolic phenotype of obesity and associated with subclinical atherosclerosis. A total of 485 obese adults (aged 40–65 years) who received magnetic resonance spectroscopy were divided into metabolically abnormally obesity (MAO) and MHO groups according to metabolic status. MHO individuals had lower levels of intrahepatic triglyceride (IHTG) content and carotid intima-media thickness (CIMT) than MAO individuals. In multivariable linear regression analyses, IHTG content was independently associated with metabolic syndrome components and CIMT. Based on receiver operating characteristic curve analysis, the IHTG content displayed a higher area under the curve (AUC) for detecting the MAO phenotype (AUC = 0.70, 95%CI = 0.65–0.75) and increased CIMT (AUC = 0.60, 95%CI = 0.54–0.66) than BMI, waist circumference, and body fat percent. MHO individuals were 1.9 times (p < 0.001) more likely to have metabolic syndrome per 1 SD change in IHTG content in multivariable-adjusted models. Likewise, the risk for high CIMT increased 29% per 1 SD change in IHTG content [OR (95% CI):1.29(1.01–1.64)]. These findings suggest that hepatic fat is a potential predictor of metabolically unhealthy obesity phenotype and subclinical atherosclerosis.
Obesity is accompanied by a high incidence of type 2 diabetes mellitus and cardiovascular disease (CVD)1,2. The impact of obesity on the development of CVD is mediated through a number of metabolic abnormalities, such as dyslipidemia, hyperglycemia, and hypertension2; however, the incidence of obesity-related metabolic abnormalities varies widely among obese individuals3. Recent interest has focused on a subgroup of obese individuals with normal metabolic phenotypes, referred to as metabolically healthy obesity (MHO)3,4. Increasing evidence suggests that MHO individuals are relatively protected from cardiometabolic disturbances than those with metabolically abnormal obesity (MAO)5,6.
Since the differentiation between the diverse obese phenotypes may have important implications for targeted preventive strategies in practice, an adequate definition and comprehensive characteristics of MHO subtype are of paramount importance for the stratification of obese individuals. Several characteristics have been reported to explain the apparently less deleterious metabolic profile of MHO subjects7. Among them, lower liver enzyme concentrations, lower uric acid, lower inflammatory profile, or higher lipolytic activity have been put forward as determinants of metabolic phenotype6,8,9,10.
In addition, it is well recognized that body fat distribution represents an additional independent determinant of obesity-related cardiometabolic disturbances11,12,13. Evidence suggests that individuals with a selective excess of hepatic fat accumulation are at substantially higher risk of being insulin resistant and having a worse cardiovascular risk profile12,14. Carotid artery intima media thickness (CIMT) is a noninvasive surrogate marker of subclinical atherosclerosis15,16, and is linked to various traditional risk factors and adverse cardiovascular outcomes17,18. However, information regarding whether hepatic fat accumulation determines the metabolic phenotype of obesity and is associated with increased CIMT is not available. In the present study, we set out to identify whether hepatic fat content is a determinant of the metabolic phenotype of obesity and its related subclinical atherosclerosis.
Results
Table 1 summarizes the mean levels of study variables by subtypes of obese subjects. Within the sample, 41.2%(200/485) of participants were metabolically healthy obese. Compared with MAO subjects, MHO subjects had a favorable metabolic profile, including lower levels of BMI, fasting plasma glucose, postprandial glucose, systolic blood pressure, diastolic blood pressure, triglyceride, total cholesterol, LDL-c, and HOMA-IR, and higher levels of HDL-c. Also, MHO subjects had lower CIMT compared with MAO subjects (0.70 ± 0.14 mm vs. 0.76 ± 0.16 mm, p < 0.001). There was no difference in body fat percent between the two groups. Of interest, MHO subjects had lower intrahepatic triglyceride (IHTG) content than MAO subjects (10.5 ± 9.3% vs.16.3 ± 9.9%, p < 0.001). Additionally, MHO subjects had lower levels of uric acid and liver enzymes, including ALT, AST, and GGT, than MAO subject (All p < 0.01).
Table 1. Clinical and blood biochemical characteristics by subtypes of obese subjects.
| Variables | Overall | Metabolically healthy obesity | Metabolically abnormal obesity | P-value |
|---|---|---|---|---|
| Sample size | 485 | 200 | 285 | |
| Age (years) | 54.1 ± 7.1 | 53.4 ± 7.2 | 54.5 ± 7.1 | 0.082 |
| Gender (female, n, %) | 352 (73) | 152 (76) | 200 (70) | 0.157 |
| BMI (kg/m2) | 27.1 ± 2.7 | 26.4 ± 2.6 | 27.5 ± 2.7 | <0.001 |
| Waist circumference (cm) | 94.1 ± 6.7 | 92.9 ± 6.3 | 94.9 ± 6.8 | 0.001 |
| Current smokers (n, %) | 74(15) | 30(15) | 44(15) | 0.895 |
| Current alcohol drinking (n, %) | 133(27.4) | 58(29.0) | 75(26.3) | 0.514 |
| Physical activity (Met/h. week) | 8.0(23.1–46.2) | 23.6(8.8–52.3) | 23.1(7.7–46.2) | 0.768 |
| Systolic BP (mmHg) | 129.5 ± 16.1 | 121.4 ± 13.5 | 135.2 ± 15.4 | <0.001 |
| Diastolic BP (mmHg) | 77.7 ± 10.1 | 73.2 ± 8.5 | 80.9 ± 9.9 | <0.001 |
| Triglyceride (mmol/L) | 1.60(1.17–2.18) | 1.19(0.93–1.47) | 1.97(1.60–2.65) | <0.001 |
| Total cholesterol (mmol/L) | 5.76 ± 1.00 | 5.56 ± 0.89 | 5.89 ± 1.04 | <0.001 |
| LDL- cholesterol (mmol/L) | 3.76 ± 0.98 | 3.65 ± 0.89 | 3.84 ± 1.04 | 0.039 |
| HDL-cholesterol (mmol/L) | 1.31 ± 0.26 | 1.41 ± 0.25 | 1.23 ± 0.23 | <0.001 |
| Fasting glucose (mmol/L) | 5.52 ± 0.52 | 5.27 ± 0.40 | 5.70 ± 0.51 | <0.001 |
| 2-h glucose (mmol/L) | 7.85 ± 1.93 | 7.13 ± 1.57 | 8.36 ± 2.00 | <0.001 |
| HOMA-IR | 2.84(1.94–4.01) | 2.09(1.53–2.95) | 3.32(2.46–4.65) | <0.001 |
| ALT (U/L) | 25.6 ± 13.6 | 22.4 ± 12.4 | 27.9 ± 14.0 | <0.001 |
| AST (U/L) | 23.6 ± 6.8 | 22.8 ± 6.1 | 24.2 ± 7.2 | 0.027 |
| GGT (U/L) | 35.5 ± 23.1 | 30.8 ± 20.6 | 38.8 ± 24.2 | <0.001 |
| Uric acid (mmol/L) | 346.6 ± 92.0 | 318.1 ± 83.4 | 366.6 ± 92.6 | <0.001 |
| Body fat percent (%) | 33.8 ± 5.6 | 34.0 ± 5.4 | 33.7 ± 5.8 | 0.594 |
| CIMT(mm) | 0.73 ± 0.16 | 0.70 ± 0.14 | 0.76 ± 0.16 | <0.001 |
| IHTG content (%) | 13.9 ± 10.1 | 10.5 ± 9.3 | 16.3 ± 9.9 | <0.001 |
CIMT = carotid intima-media thickness; BMI = body mass index; HOMA-IR = homeostasis model assessment of insulin resistance; IHTG content = intrahepatic triglyceride content;
Data are presented as the mean ± SD or median (interquartile range).
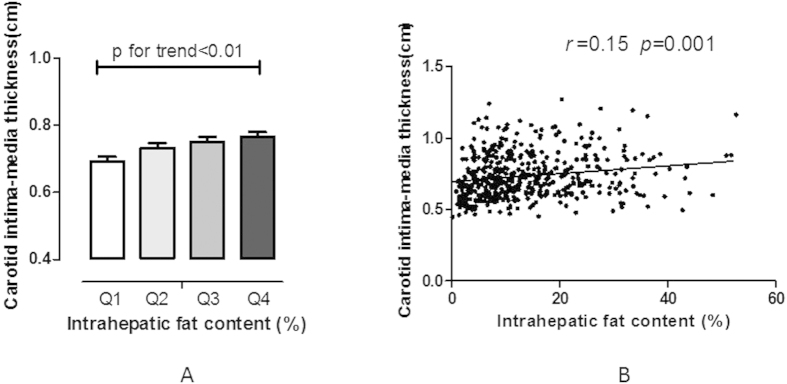
As shown in Fig. 1, Pearson correlation analysis was performed to investigate the association of IHTG content and CIMT. Results showed that CIMT was significantly positively correlated with IHTG content. By dividing the distribution of IHTG content into quartiles, CIMT gradually increased with the increase in IHTG content (p < 0.001 for trend).
Figure 1. Relationship between intrahepatic triglyceride content (IHTG) and carotid intima-media thickness (CIMT).
(A) Levels of CIMT by quartile of IHTG; (B) correlations of CIMT and IHTG, adjusted for age, gender, and smoking. CIMT = carotid intima-media thickness; IHTG content = intrahepatic triglyceride content.
Table 2 presents results of linear regression analyses of metabolic risk factors and IHTG content on MetS and CIMT. In simple linear regression models, IHTG content and HOMA-IR were significantly associated with MetS components. IHTG content was also significantly associated with CIMT, while BMI and HOMA-IR showed no significant association with CIMT. In multivariable linear regression models, BMI, HOMA-IR and IHTG content were all significantly associated with MetS components, after adjustment for age, gender, current smoking, alcohol consumption, physical activity, BMI, hypertension, fasting plasma glucose, triglyceride, HDL-c, and HOMA-IR. Meanwhile, only IHTG content was significantly associated with CIMT.
Table 2. Standardized regression coefficients of metabolic risk factors and intrahepatic triglyceride content on metabolic syndrome and CIMT.
| Independent variables | Unadjusted |
Adjusteda |
||||
|---|---|---|---|---|---|---|
| β | SE | P | β | SE | P | |
| MetS components | ||||||
| BMI | 0.067 | 0.051 | 0.189 | 0.147 | 0.051 | 0.004 |
| HOMA-IR | 0.401 | 0.052 | <0.001 | 0.153 | 0.050 | 0.003 |
| IHTG content | 0.182 | 0.047 | <0.001 | 0.102 | 0.045 | 0.023 |
| Model R2(%) | 0.209 | 0.447 | ||||
| CIMT | ||||||
| BMI | 0.008 | 0.003 | 0.333 | 0.007 | 0.008 | 0.412 |
| HOMA-IR | 0.011 | 0.008 | 0.169 | 0.007 | 0.008 | 0.405 |
| IHTG content | 0.021 | 0.008 | 0.009 | 0.016 | 0.007 | 0.030 |
| Model R2(%) | 0.038 | 0.248 | ||||
CIMT = carotid intima-media thickness; IHTG content = intrahepatic triglyceride content; BMI = body mass index; HOMA-IR = homeostasis model assessment of insulin resistance; β = standardized regression coefficient; SE = standard error.
aadjusted for age, sex, smoking, alcohol consumption, physical activity, BMI, hypertension, glucose, triglyceride, HDL-c and HOMA-IR.
Table 3 presents results of area under the curve (AUC) calculations for detecting metabolic abnormities and increased CIMT. Based on ROC curve analysis, IHTG content displayed a significantly higher AUC for detecting the MAO phenotype (AUC = 0.70, 95%CI = 0.65–0.75) than BMI, waist circumference, and total body fat. Additionally, IHTG content also displayed a significantly higher AUC for detecting increased CIMT (AUC = 0.60, 95%CI = 0.54–0.66) than total body fat.
Table 3. ROC curve analysis for detecting metabolic abnormities and increased CIMT.
| Test variables | MAO phenotype |
Increased CIMT |
||||
|---|---|---|---|---|---|---|
| AUC | 95%CI | P | AUC | 95%CI | P | |
| BMI | 0.62 | 0.57–0.67 | <0.001 | 0.56 | 0.50–0.62 | <0.001 |
| Waist circumference | 0.59 | 0.54–0.64 | <0.001 | 0.56 | 0.50–0.62 | <0.001 |
| Body fat | 0.49d,e | 0.44–0.54 | <0.001 | 0.44d,e | 0.38–0.50 | <0.001 |
| IHTG content | 0.70a,b,c | 0.65–0.75 | <0.001 | 0.60c | 0.54–0.66 | <0.001 |
ROC = receiver operating characteristic; MAO = metabolically abnormal obesity; CIMT = carotid intima-media thickness; IHTG content = intrahepatic triglyceride content; BMI = body mass index; AUC = area under the curve; CI = confidence interval;
ap value < 0.05 for the comparison AUC of IHTG content versus AUC of BMI;
bp value < 0.05 for the comparison AUC of IHTG content versus AUC of waist circumference;
cp value < 0.05 for the comparison AUC of IHTG content versus AUC of body fat;
dp value < 0.05 for the comparison AUC of BMI versus AUC of body fat;
ep value < 0.05 for the comparison AUC of Waist circumference versus AUC of body fat.
The multivariable-adjusted odds ratios (ORs) for the association between IHTG content and MetS or increased CIMT are shown in Table 4. MHO subjects were 1.86 times(p < 0.001) more likely to have MetS per 1 SD increase in IHTG content, after adjustment for age, gender, current smoking, alcohol consumption, physical activity, and BMI, and this relationship remained significant after further adjusting for HOMA-IR and total body fat [OR(95% CI):1.46(1.13–1.88)]. Likewise, IHTG content was significantly associated with increased CIMT, after adjusting for age, gender, current smoking, alcohol consumption, physical activity, BMI, HOMA-IR, and total body fat. After further adjusting for metabolic components, the risk for high CIMT significantly increased by 29% per 1 SD change in IHTG content [OR (95% CI):1.29(1.01–1.64)].
Table 4. Odds ratios for increased CIMT and metabolic syndrome according to hepatic triglyceride content.
| Metabolic syndrome |
Increased CIMT |
|||
|---|---|---|---|---|
| OR(95% CI) | P-value | OR(95% CI) | P-value | |
| Model 1 | 1.86(1.46–2.37) | <0.001 | 1.31(1.05–1.63) | 0.017 |
| Model 2 | 1.46(1.13–1.88) | 0.004 | 1.27(1.01–1.60) | 0.043 |
| Model 3 | — | — | 1.29(1.01–1.64) | 0.045 |
OR = odds ratio; CI = confidence interval; BMI = body mass index; HOMA-IR = homeostasis model assessment of insulin resistance; CIMT = carotid intima-media thickness;
Model 1: adjusted for age, gender, smoking, alcohol consumption, physical activity, and BMI.
Model 2: Model 1+adjusted for HOMA-IR and body fat.
Model 3: Model 2+adjusted for metabolic components, including glucose, triglyceride, HDL-c and blood pressure.
Discussion
Numerous studies have reported that MHO subjects were at low risk of obesity-related cardiometabolic disturbances5,6. However, comprehensive characteristics of the MHO subtype are not well determined. In particular, information regarding whether hepatic fat accumulation determines the metabolic profiles of distinct subtypes of obesity, in relation to subclinical CVD profile, is not available. In the present study, the MHO subtype had significantly lower levels of CIMT and IHTG content than the MAO subtype. Furthermore, IHTG content was independently associated with metabolic abnormalities and increased CIMT in obese adults. This study provides strong evidence that hepatic fat accumulation plays a role as a considerable discriminator between MAO and MHO subtypes in relation to increased CIMT.
Evidence has suggested that MHO individuals were relatively protected from CVD as compared to MAO individuals5,6. In a 15-year follow-up study, subjects with the MHO phenotype did not have increased CVD mortality and all-cause mortality19. Ki-Chul Sung et al. reported that, among 14,384 South Koreans, MHO individuals had no increased risk of pre-clinical atherosclerosis determined by CAC score20. Marini and colleagues suggested that MHO subjects had an intermediate cardiovascular risk profile that fell between MAO subjects and healthy non-obese subjects5. In contrast, several studies have questioned the apparently healthy metabolic profile of MHO, showing that it may not translate into lower morbidity and mortality4,21,22. Our data support a lower risk of atherosclerosis among MHO individuals than MAO individuals. Accordingly, strategies and treatments for the primary prevention of CVD in the MHO subtype could be relaxed due to lower risk of CVD.
Because the differentiation of the diverse obese phenotypes may have important therapeutic implications, an adequate definition for the stratification of obese phenotypes is of importance. A challenge in evaluating the health implications of the MHO phenotype is the lack of an adequate determination of what factors characterize this phenotype. Several studies have suggested that body fat distribution might be the most prominent factor explaining the variance of metabolic profile of obesity23,24. A cross-sectional study of 150 postmenopausal women reported that body composition represented a determinant of metabolic subtypes of obesity24. In the current study, we found that the MHO subtype had lower hepatic fat content than the MAO subtype. Consistently, Stefan and colleague reported that MHO individuals had lower level of ectopic fat in the liver compared with MHO individuals in 314 subjects3. Importantly, our data demonstrated that IHTG content was independently associated with MetS components in obese subjects. Furthermore, our results indicated that IHTG content, but not total body fat, was an independent determinant of metabolic phenotype of obesity.
In addition, elevated fat accumulation in the liver has been shown to be accompanied by atherosclerosis14. Consistently, we found that IHTG content was independently associated with CIMT, irrespective of total body fat. Of note, individuals were 1.29 times more likely to have increased CIMT per 1 SD increase in IHTG content. Indeed, excess hepatic fat accumulation results from the inability of adipose tissue to appropriately store excess energy11; thereby it is no surprise that hepatic fat content could predict metabolic profile of obesity. On the other hand, excess hepatic fat accumulation leads to increased expression of multiple inflammatory factors and adipocytokines, which are associated with increased risk of CVD11. Therefore, our data suggested that increased hepatic fat accumulation may determine metabolic phenotype with subclinical atherosclerosis in obese adults.
Increased liver enzymes and uric acid concentrations have also been proposed as metabolic factors explaining the differences between MHO and MAO subtype10,24,25. Consistently, we found that MHO subjects had lower levels of serum ALT, AST, GGT, and uric acid than MAO subjects. On the other hand, previous studies indicated that increased hepatic fat accumulation was associated with elevated liver enzymes (i.e. ALT, AST or GGT) and serum uric acid24,26,27. Thus, our data supported the notion that hepatic fat accumulation represented a paramount determinant of metabolic phenotypes of obesity.
This community-based, cross-sectional study provided an opportunity to determine the role of hepatic triglyceride content in predicting the MAO phenotype and subclinical atherosclerosis. There are several limitations to the current study. First, given its cross-sectional design, it is not possible to determine a causal relationship among hepatic fat accumulation and the development of the MAO phenotype and increased CIMT. Second, hepatic triglyceride content was determined by 1H-MRS measurement, instead of biopsy-proven steatosis, steatohepatitis, or fibrosis. However, hepatic triglyceride content is a more sensitive measure of fat accumulation in the liver28.
In conclusion, out data suggested that hepatic fat content represented an independent determinant of the MHO phenotype. In particular, hepatic fat content was independently associated with MetS and increased CIMT. These results support the notion that hepatic fat accumulation predicts the MAO phenotype and subclinical atherosclerosis. These findings underscore the role of hepatic fat accumulation in the identification of high risk obese phenotypes and the importance of controlling fatty liver for the prevention of CVD.
Methods
Study participants
We recruited participants from the Lianqian community, Xiamen, China from April 2011 to December 2013. The details of the study design and methods have been previously reported29. In brief, a total of 485 adult obese subjects (waist circumference ≥ 90 cm for men or 80 cm for women) who were randomly chosen to receive magnetic resonance spectroscopy (1H-MRS) for the measurement of hepatic fat content were included in the analysis. All participants completed a uniform questionnaire including histories of diabetes, malignancy, cardiovascular disease, smoking status, alcohol consumption, and physical activity. The following participants were excluded: 1) those with any clinical evidence of cirrhosis, biliary obstructive diseases, or other secondary chronic liver diseases (e.g. alcohol intake ≥140 g/week for men or 70 g/week for women currently or in the past 6 months, acute or chronic viral hepatitis, autoimmune hepatitis and/or the use of hepatotoxic medications, such as corticosteroids) and 2) those with established or newly diagnosed type 2 diabetes. The subjects were stratified into metabolically healthy obesity (MHO) and metabolically abnormal obesity (MAO) groups. MHO subjects were defined as those with obesity and one or no metabolic abnormality; MAO subjects were defined as those who were obese and had at least 2 of the following metabolic abnormalities: glucose concentrations ≥5.6 mmol/L or antidiabetes medication use; systolic blood pressure ≥130 mmHg, diastolic blood pressure ≥5 mmHg, or antihypertensive medication use; triglyceride concentrations ≥1.7 mmol/L; and HDL-cholesterol levels <1.03 mmol/L for men and <1.29 mmol/L for women or lipid-lowering medication use as reported previously30,31.
Written informed consent was obtained from each participant. The study protocol was approved by the Institutional Review Board of the First Affiliated Hospital of Xiamen University. The methods were carried out in accordance with the approved guidelines.
Clinical and biochemical measurements
Anthropometric measurements included height, weight, waist circumference, and blood pressure. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters. Waist circumference was measured at the level of the umbilicus. Blood pressure (BP) was assessed in triplicate using an electronic sphygmomanometer (OMRON Company).
All blood samples were obtained after 12 h of fasting. Plasma glucose, triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), and uric acid levels were measured by enzymatic colorimetric methods with a Hitachi 7450 analyzer (Hitachi, Tokyo, Japan).Fasting plasma glucose concentrations and 2-h glucose concentrations were measured using the glucose oxidase method. Low-density lipoprotein cholesterol (LDL-C) was calculated by Friedewald’s formula. Serum ALT and AST were measured by standard enzymatic methods. Serum GGT was measured by the Szasz-Persijn method. Serum insulin concentrations were measured using electrochemiluminiscence immunoassay (Roche Elecsys Insulin Test, Roche Diagnostics, Mannheim, Germany). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated by the following formula: fasting serum insulin (mIU/L) × fasting plasma glucose (mmol/L)/22.5.
Body fat mass was determined using the HOLOGIC whole body DXA system (Hologic Inc., Bedford, MA). Carotid intima-media thickness (CIMT) was measured with high-resolution ultrasonography as previously described32.
Measurement of hepatic triglyceride content
Intrahepatic triglyceride (IHTG) content was determined by magnetic resonance spectroscopy (1H-MRS; Avanto 3.0-T, Siemens AG, Erlangen, Germany). This non-invasive measurement of hepatic triglyceride content is reproducible. Images of a sagittal, coronal, and axial cube of 2 cm3 volume in the right lobe of the liver were acquired. Quantification of the spectra (water and methylene resonances) was performed as described previously33,34. Areas of resonance from water protons and methylene groups in fatty acid chains were obtained with a time-domain nonlinear fitting routine using commercial software (Syngo spectroscopy VB15, Siemens AG). The percent of IHTG content was calculated as the ratio of the area under the resonance peak for methylene groups in fatty acid chains of IHTG and the combined area under the resonance peaks for methylene groups and water.
Statistical analysis
All statistical analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC). Metabolic syndrome (MetS) was defined according to International Diabetes Federation (IDF) diagnostic criteria35. These criteria included: 1)central obesity defined as waist circumference ≥90 cm in men or ≥80 cm in women; 2)plus two or more of the following: (a) low serum HDL-c (HDL-c < 1.03 mmol/L in men or <1.29 mmol/L in women), (b) hypertriglyceridemia (TG ≥ 1.7 mmol/L), (c) hypertension (BP ≥ 130/85 mmHg or treatment of previously diagnosed hypertension), or (d) dysglycemia (fasting plasma glucose ≥5.6 mmol/L or previously diagnosed type 2 diabetes).Increased CIMT was defined as average CIMT ≥ 0.8 mm36,37.The subjects were classified into four quartiles according to IHTG content(Quartile 1, <6.5%, Quartile 2, 6.5–11.0%, Quartile 3, 11.0–19.5%, and Quartile 4, ≥19.5%).
The χ2-test was used for comparison of categorical variables between groups. Analyses of covariance were performed using general linear models (GLM) to test differences in study variables between MHO and MAO groups or different quartiles of IHTG contents. The correlation of IHTG content with CIMT was analyzed by Pearson correlation. Receiver operating characteristic (ROC) curve analysis was applied to evaluate the utility of several anthropometric measures, body fat, and IHTG content to determine metabolic phenotypes and subclinical atherosclerosis. Multivariable linear regression models were used to examine the association between IHTG content and CIMT or individual MetS components as continuous variables, adjusted for age, sex, smoking, alcohol consumption, physical activity, BMI, hypertension, glucose, triglyceride, HDL-c, and HOMA-IR. Multivariable logistic regression models were used to examine the association between IHTG content and MetS or increased CIMT, adjusted for age, gender, smoking, alcohol consumption, physical activity, BMI, HOMA-IR, body fat and metabolic components.
Additional Information
How to cite this article: Zhang, H. et al. Hepatic fat content is a determinant of metabolic phenotypes and increased carotid intima-media thickness in obese adults. Sci. Rep. 6, 21894; doi: 10.1038/srep21894 (2016).
Acknowledgments
This study was supported by grants from the China Natural Science Foundation (81570785), Young Talent Foundation of Fujian Provincial Health Department (2013-ZQN-ZD-31), and the Xiamen Systems Biology Research Program for Metabolic Disease (3502Z20100001). Dr. Huijie Zhang was partially supported by a research training grant (D43TW009107) from the John E Fogarty International Center of the National Institutes of Health, Bethesda, MD. We also acknowledge the editorial assistance of Miss Katherine Obst.
Footnotes
Author Contributions H.Z., Z.M., S.Y. and X.L. generated the hypothesis, directed implementation, and wrote the manuscript. L.P., Y.X. and J.S. contributed to analytic strategy and statistical analyses. Z.H., Z.C., Q.S., C.L. and M.L. supervised the field activities and data collection and edited the manuscript.
References
- Hu F. B. et al. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med 351, 2694–2703 (2004). [DOI] [PubMed] [Google Scholar]
- Juonala M. et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med 365, 1876–1885 (2011). [DOI] [PubMed] [Google Scholar]
- Stefan N. et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 168, 1609–1616 (2008). [DOI] [PubMed] [Google Scholar]
- Kramer C. K., Zinman B. & Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med 159, 758–769 (2013). [DOI] [PubMed] [Google Scholar]
- Marini M. A. et al. Metabolically healthy but obese women have an intermediate cardiovascular risk profile between healthy nonobese women and obese insulin-resistant women. Diabetes Care 30, 2145–2147 (2007). [DOI] [PubMed] [Google Scholar]
- Gomez-Ambrosi J. et al. Increased cardiometabolic risk factors and inflammation in adipose tissue in obese subjects classified as metabolically healthy. Diabetes Care 37, 2813–2821 (2014). [DOI] [PubMed] [Google Scholar]
- Phillips C. M. Metabolically healthy obesity: definitions, determinants and clinical implications. Rev Endocr Metab Disord 14, 219–227 (2013). [DOI] [PubMed] [Google Scholar]
- Perreault M. et al. A distinct fatty acid profile underlies the reduced inflammatory state of metabolically healthy obese individuals. PLoS One 9, e88539,(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naukkarinen J. et al. Characterising metabolically healthy obesity in weight-discordant monozygotic twins. Diabetologia 57, 167–176 (2014). [DOI] [PubMed] [Google Scholar]
- Mangge H. et al. Uric acid best predicts metabolically unhealthy obesity with increased cardiovascular risk in youth and adults. Obesity (Silver Spring) 21, E71–77 (2013). [DOI] [PubMed] [Google Scholar]
- Shulman G. I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 371, 1131–1141 (2014). [DOI] [PubMed] [Google Scholar]
- Arsenault B. J. et al. Visceral adipose tissue accumulation, cardiorespiratory fitness, and features of the metabolic syndrome. Arch Intern Med 167, 1518–1525 (2007). [DOI] [PubMed] [Google Scholar]
- Despres J. P. & Lemieux I. Abdominal obesity and metabolic syndrome. Nature 444, 881–887 (2006). [DOI] [PubMed] [Google Scholar]
- Targher G., Day C. P. & Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 363, 1341–1350 (2010). [DOI] [PubMed] [Google Scholar]
- Cohen G. I. et al. Relationship between carotid disease on ultrasound and coronary disease on CT angiography. JACC Cardiovasc Imaging 6, 1160–1167 (2013). [DOI] [PubMed] [Google Scholar]
- Nambi V. et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol 55, 1600–1607 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeboah J. et al. Mediation of cardiovascular risk factor effects through subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 34, 1778–1783 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi T. Z. & Lee M. S. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging 7, 1025–1038 (2014). [DOI] [PubMed] [Google Scholar]
- Calori G. et al. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care 34, 210–215 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung K. C., Cha S. C., Sung J. W., So M. S. & Byrne C. D. Metabolically healthy obese subjects are at risk of fatty liver but not of pre-clinical atherosclerosis. Nutr Metab Cardiovasc Dis 24, 256–262 (2014). [DOI] [PubMed] [Google Scholar]
- Kuk J. L. & Ardern C. I. Are metabolically normal but obese individuals at lower risk for all-cause mortality? Diabetes Care 32, 2297–2299,(2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Song Y., Chen Y., Hui R. & Zhang W. Combined effect of obesity and cardio-metabolic abnormality on the risk of cardiovascular disease: a meta-analysis of prospective cohort studies. Int J Cardiol 168, 4761–4768 (2013). [DOI] [PubMed] [Google Scholar]
- Karelis A. D., St-Pierre D. H., Conus F., Rabasa-Lhoret R. & Poehlman E. T. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab 89, 2569–2575 (2004). [DOI] [PubMed] [Google Scholar]
- Peppa M. et al. Body composition determinants of metabolic phenotypes of obesity in nonobese and obese postmenopausal women. Obesity (Silver Spring) 21, 1807–1814 (2013). [DOI] [PubMed] [Google Scholar]
- Messier V. et al. Metabolically healthy but obese individuals: relationship with hepatic enzymes. Metabolism 59, 20–24 (2010). [DOI] [PubMed] [Google Scholar]
- Lin H. et al. Liver Fat Content Is Associated with Elevated Serum Uric Acid in the Chinese Middle-Aged and Elderly Populations: Shanghai Changfeng Study. PLoS One 10, e0140379 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. et al. Serum alanine aminotransferase independently correlates with intrahepatic triglyceride contents in obese subjects. Dig Dis Sci 59, 2470–2476 (2014). [DOI] [PubMed] [Google Scholar]
- Wong V. W. et al. Incidence of non-alcoholic fatty liver disease in Hong Kong: a population study with paired proton-magnetic resonance spectroscopy. J Hepatol 62, 182–189 (2015). [DOI] [PubMed] [Google Scholar]
- Pan L. L. et al. Intrahepatic triglyceride content is independently associated with chronic kidney disease in obese adults: A cross-sectional study. Metabolism 64, 1077–1085 (2015). [DOI] [PubMed] [Google Scholar]
- Velho S., Paccaud F., Waeber G., Vollenweider P. & Marques-Vidal P. Metabolically healthy obesity: different prevalences using different criteria. Eur J Clin Nutr 64, 1043–1051 (2010). [DOI] [PubMed] [Google Scholar]
- Stefan N., Haring H. U., Hu F. B. & Schulze M. B. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol 1, 152–162 (2013). [DOI] [PubMed] [Google Scholar]
- Johnson H. M. & Stein J. H. Measurement of carotid intima-media thickness and carotid plaque detection for cardiovascular risk assessment. J Nucl Cardiol 18, 153–162 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang H. J. et al. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J Hepatol 59, 557–562 (2013). [DOI] [PubMed] [Google Scholar]
- Frimel T. N., Deivanayagam S., Bashir A., O’Connor R. & Klein S. Assessment of intrahepatic triglyceride content using magnetic resonance spectroscopy. J Cardiometab Syndr 2, 136–138 (2007). [DOI] [PubMed] [Google Scholar]
- Alberti K. G., Zimmet P. & Shaw J. The metabolic syndrome--a new worldwide definition. Lancet 366, 1059–1062 (2005). [DOI] [PubMed] [Google Scholar]
- Cuspidi C. et al. Role of echocardiography and carotid ultrasonography in stratifying risk in patients with essential hypertension: the Assessment of Prognostic Risk Observational Survey. J Hypertens 20, 1307–1314 (2002). [DOI] [PubMed] [Google Scholar]
- de Groot E. et al. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation 109, III33–38 (2004). [DOI] [PubMed] [Google Scholar]