Abstract
Understanding the spatial patterns in species richness gets new implication for biodiversity conservation in the context of climate change and intensified human intervention. Here, we created a database of the geographical distribution of 30,519 vascular plant species and 565 mammal species from 2,376 counties across China and disentangled the determinants that explain species richness patterns both at national and regional scales using spatial linear models. We found that the determinants of species richness patterns varied among regions: elevational range was the most powerful predictor for the species richness of plants and mammals across China. However, species richness patterns in the Qinghai-Tibetan Plateau Region (QTR) are quite unique, where net primary productivity was the most important predictor. We also detected that elevational range was positively related to plant species richness when it is less than 1,900 m, whereas the relationship was not significant when elevational range is larger than 1,900 m. It indicated that elevational range often emerges as the predominant controlling factor within the regions where energy is sufficient. The effects of land use on mammal species richness should attract special attention. Our study suggests that region-specific conservation policies should be developed based on the regional features of species richness.
Biodiversity is distributed heterogeneously over the globe1. Species richness, as one of the major surrogates of biodiversity, reaches the peak around the equator where it is warm and wet, and experiences a decline toward the temperate and polar regions where it is colder and drier2. For more than two centuries, ecologists and biogeographers have been exploring the mechanism that underpins species richness patterns of diverse biological taxa across the earth3,4,5,6,7,8. But there has always been no consensus on this issue. As the climate change and human intervention have already exerted great influences on species richness patterns and even triggered species extinction9,10,11,12,13, this issue gets new implication for biodiversity conservation in this century. Therefore, more intensive and in-depth researches are needed to explore the mechanism of species richness patterns.
Spatial patterns of species richness are the complex product created by the interaction of a series of biotic and abiotic factors7,14. Biotic factors are biological intrinsic characters, e.g. anatomy, physiology, genetics, development and behavior14. Abiotic factors often encompass climate, topography, geographical history15,16,17,18, etc. To date, a plethora of hypotheses have been proposed to identify the environmental factors that explain patterns of species richness. Among them, such hypotheses have received the strongest empirical support, e.g. the energy hypothesis, the environmental stability hypothesis and the habitat heterogeneity hypothesis16,19,20,21,22,23,24. The energy hypothesis posits that water-energy dynamics, ambient energy, and productivity are responsible for species richness gradients16,25,26. The environmental stability hypothesis insists that a stable environment could house more species by accelerating species specialization and ecological niche diversification22,26. The habitat heterogeneity hypothesis states that heterogeneity in habitats is responsible for geographical variation in species richness24 because variability in elevation, landscape or vegetation could make diverse habitats for more species26. Nevertheless, the validity of these hypotheses remains controversial and the environmental factors that predominantly shape species richness patterns require more rigorous verification.
China is one of the countries with the richest biodiversity27,28. It harbors more than 30,000 vascular plant species and 6,300 vertebrate species respectively27,29, accounting for over 10% of the total number of the world30. As a result of obvious disparity in climatological, geographical and topographical features, China is generally categorized into three major regions: the Eastern Monsoon Region (EMR), the Northwestern Arid Region (NAR) and the Qinghai-Tibetan Plateau Region (QTR)31 (Fig. 1). Compared to EMR, species in NAR and QTR show a higher level of endemism and more sensitivity to the climate change32,33,34,35,36, which offers a good opportunity to disentangle the environmental determinants of species richness and get insight into the keystone of biodiversity conservation. Previous studies have made advancement in species richness of one or several taxa in a single region across China32,33,34,35,37,38,39,40. However, a comprehensive study of species richness and its determinants is scarce both at national and regional scales for effective biodiversity conservation in China.
Figure 1. Illustrative map of the Eastern Monsoon Region (EMR), the Northwestern Arid Region (NAR), and the Qinghai-Tibetan Plateau Region (QTR) in the terrestrial and inland water ecosystems of China.
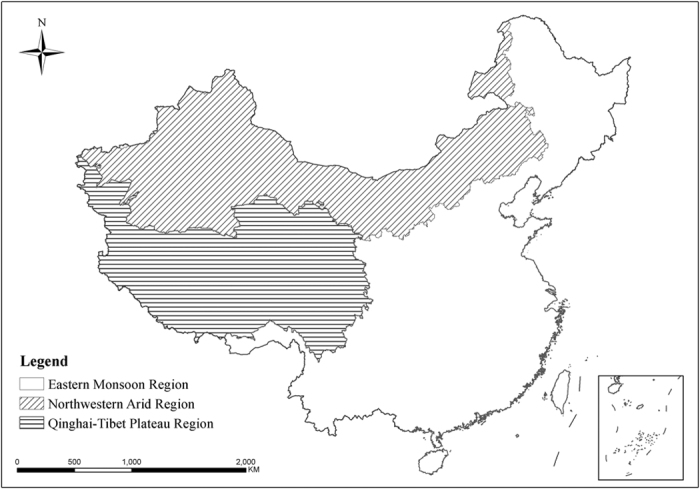
The inset in the right bottom of the figure shows the southern boundary of China, including all islands in the South China Sea. Data on national territory were from the National Administration of Surveying, Mapping and Geoinformation of China (http://www.sbsm.gov.cn/). The map was created using the software Arc GIS 9.3 which was purchased from ESRI-China (http://www.esrichina-bj.cn/) by Nanjing Institute of Environmental Sciences affiliated to the Ministry of Environmental Protection of China, with user number C-801668.
In this study, we created a database of the geographical distribution of 30,519 vascular plant species and 565 mammal species from 2,376 counties in the terrestrial and inland water ecosystems of China, with objectives to disentangle the determinants of species richness of vascular plants and mammals across the whole country and in the three separate regions (EMR, NAR and QTR), and explore its implications for biodiversity conservation both at national and regional scales in China.
Results
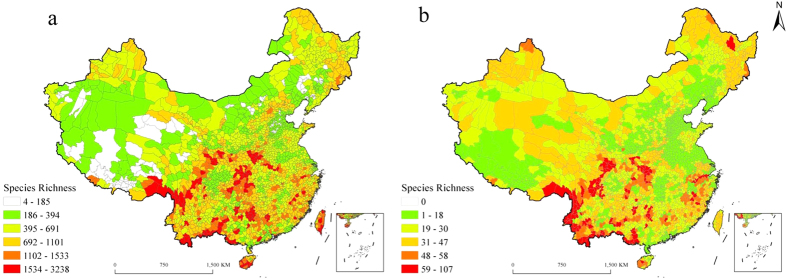
We found that species richness of vascular plants and mammals in China was higher in South China than in North China, and higher in the mountains than in the plains. As for the vascular plants, such regions harbor the highest species richness, i.e., the Min Mountains, the Qionglai Mountains, the Hengduan Mountains, the southeastern Himalaya Mountains, the Qinling Mountains, the Funiu Mountains, the Daba Mountains, the Dabie Mountains, the Wuling Mountains, the Wuyi Mountains, the Nanling Mountains, the Xishuangbanna in Yunnan Province, the mountains of southeastern Yunnan-western Guangxi-southern Guizhou, the mountains of southwestern Guangxi, the mountains of central and southern Hainan, and Taiwanese mountains (Fig. 2). All the hot spots covered 125 assessment units of 17 provinces (autonomous regions), among which, 116 assessment units are from EMR and 9 from QTR. Species richness of vascular plants reached the maximum (i.e., 3238) in Yulong, Yunnan. Species richness of vascular plants ranged from 1102 to 1533 in such regions, i.e., the hills in Zhejiang and Fujian, the hills in southern Anhui, the hills in Guangdong and Guangxi, the Changbai Mountains, and the Taihang Mountains. Species richness in most assessment units of other regions was less than 394, e.g. the Qinghai-Tibet Plateau, the Qaidam Basin, the Tarim Basin, the Inner Mongolian Plateau, the Northeast China Plain, the North China Plain, and the Chengdu Plain. As for the mammals, the distribution pattern of species richness can also be found in Xu et al.41. The hot spots included 49 assessment units of 10 provinces (autonomous regions).
Figure 2. Spatial distribution map of vascular plants and mammals in China.
(a) vascular plants; (b) mammals. Red areas are hotspots defined as the richest 5% of county areas for plant and mammal species. Data on the boundary of assessment units were from the National Administration of Surveying, Mapping and Geoinformation of China (http://www.sbsm.gov.cn/). The map was created using the software Arc GIS 9.3 which was purchased from ESRI-China (http://www.esrichina-bj.cn/) by Nanjing Institute of Environmental Sciences affiliated to the Ministry of Environmental Protection of China, with user number C-801668.
We developed spatial linear models (SLM) for the species richness of vascular plants and mammals across China (Table 1). We identified elevational range was the most important predictor for vascular plants (z = 15.68, p < 0.001) and mammals (z = 9.59, p < 0.001). Water-energy variables (mean annual precipitation, temperature and net primary productivity), environmental stability (temperature annual range and precipitation seasonality) and main land cover type also played important roles in explaining the variance of the species richness of vascular plants and mammals across China (Table 1). These core predictors together explain 61% and 53% of the variance of the two taxa, respectively. When considering all 19 environmental variables, change in model fit was very small (Δr2 = 0.01) compared to SLM models with six variables (Table 1). Therefore, the best SLM models were robust.
Table 1. SLM multivariate models for the residuals of species richness of vascular plants and mammals in the terrestrial and inland water ecosystems of China.
| Model | Predictors | Whole China | EMR | NAR | QTR | |
|---|---|---|---|---|---|---|
| Vascular plants | ||||||
| Model with 6 predictors | Elevational range | z | 15.68*** | 9.19*** | 6.34*** | 4.15*** |
| Net primary productivity | z | 4.83*** | — | — | 5.59*** | |
| Normalized difference vegetation index | z | — | — | 5.47*** | — | |
| Maximum temperature of the warmest month | z | 3.64*** | −2.72** | — | — | |
| Minimum temperature of the coldest month | z | — | 6.22*** | — | −1.72 | |
| Annual potential evapotranspiration | z | — | −1.95 | — | — | |
| Mean diurnal range | z | — | — | 0.85 | — | |
| Temperature annual range | z | — | — | — | 0.03 | |
| Mean annual precipitation | z | 6.80*** | — | — | — | |
| Precipitation of the driest quarter | z | — | — | −0.33 | 1.37 | |
| Precipitation seasonality | z | −3.83*** | −2.35* | −1.79 | −2.18* | |
| Main land cover type | z | −4.11*** | −2.23* | −1.48 | — | |
| AIC | −1147.1 | −1606.7 | −147.5 | 153.6 | ||
| Fitted values | r2 | 0.61 | 0.58 | 0.53 | 0.51 | |
| Moran’s I | −0.06 | −0.02 | −0.08 | 0.00 | ||
| 19-predictor model | AIC | −1202.6 | −1626.8 | −148.7 | 167.4 | |
| Fitted values | r2 | 0.62 | 0.60 | 0.58 | 0.55 | |
| Mammals | ||||||
| Model with 6 predictors | Elevational range | z | 9.59*** | 6.71*** | 3.54*** | 3.79*** |
| Mean annual precipitation | z | — | 6.22*** | — | — | |
| Precipitation of the driest quarter | z | — | — | 0.62 | — | |
| Precipitation of the wettest quarter | z | — | — | −1.17 | — | |
| Net primary productivity | z | 8.65*** | 0.10 | — | 4.46*** | |
| Mean annual dryness | z | — | — | — | 2.39* | |
| Normalized difference vegetation index | z | — | — | 3.27** | — | |
| Maximum temperature of the warmest month | z | 2.67*** | −4.83*** | — | — | |
| Minimum temperature of the coldest month | z | — | — | — | −0.59 | |
| Temperature annual range | z | −2.64** | — | — | — | |
| Precipitation seasonality | z | −2.09* | 1.12 | −0.44 | −4.05*** | |
| Main land cover type | z | −7.11*** | −4.02*** | — | — | |
| Number of land cover types | z | — | — | −0.21 | — | |
| Mean elevation | z | — | — | — | −2.09* | |
| AIC | −1505.0 | −1443.3 | −170.5 | −83.4 | ||
| Fitted values | r2 | 0.53 | 0.54 | 0.36 | 0.68 | |
| Moran’s I | −0.02 | −0.01 | −0.00 | 0.01 | ||
| 19-predictor model | AIC | −1524.2 | −1446.4 | −173.7 | −70.0 | |
| Fitted values | r2 | 0.54 | 0.55 | 0.43 | 0.68 | |
We divided the area of China into three regions: the Eastern Monsoon Region (EMR, n = 1995), the Northwestern Arid Region (NAR, n = 210), and the Qinghai-Tibet Plateau Region (QTR, n = 171). Species richness and all continuous variables were log10-transformed. Data on residuals of species richness were used to remove the effects of area (*Pr(>|z|) <0.05, ** Pr(>|z|) <0.01, ***Pr(>|z|) <0.001).
We also established SLM models for species richness of vascular plants and mammals in EMR, NAR, and QTR, respectively. Changes in fitted values between SLM models with six predictors and those with 19 environmental variables were small (Δr2 ranges from 0.00 to 0.07) (Table 1). Therefore, these best SLM models were robust. Elevational range, the most important predictor of plant and mammal species richness across China, becomes the second or third important predictor of species richness of vascular plants and mammals in QTR. The value of z decreased from 15.68 across China to 4.15 in QTR for plant species, and from 9.59 across China to 3.79 in QTR for mammal species (Table 1). Net primary productivity became the most important factor affecting the distribution of plant and mammal species (z = 5.59 and 4.46, p < 0.001, respectively) in QTR.
We analyzed sampling bias in the dataset of this study. We identified 2010 counties that are ‘under-sampled’ based on the inventory incompleteness higher than 0.0542. Among the 2010 counties, the number of vascular plant species in the dataset of this study was higher in 1749 counties (87%), identical in two counties (approximately 0%) and less in 259 counties (13%) than that of Yang et al.42 (Fig. 3). The mean and standard deviation in the difference of vascular plant species richness between the two datasets were 305.2 and 314.3, respectively. Besides, we compared the number of vascular plant species per county between 217 counties in the dataset of this study and 217 nature reserves. As a result, we found that the number of vascular plant species in these counties and nature reserves were basically approximate (r = 0.92, P < 0.01, Fig. 4). From the above two aspects, it suggests that the accuracy of the data on species geographical distribution at county level was greatly improved and sampling bias can be reduced to a larger extent.
Figure 3. Difference in the number of vascular plant species between the dataset of this study and that of Yang et al. 42.
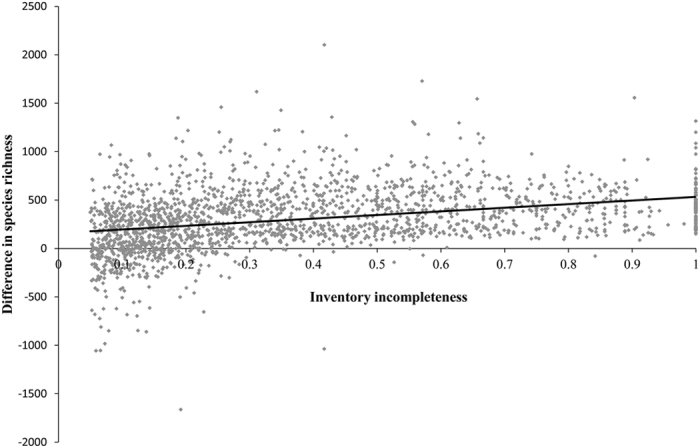
X-axis is the inventory incompleteness of vascular plants based on Yang et al.42 and Y-axis is the number of vascular plant species in the dataset of this study minus that of Yang et al.42. The mean and standard deviation in the difference in the number of vascular plant species between the two datasets were 305.2 and 314.3, respectively. Among the 2010 ‘under-sampled’ counties based on the definition of Yang et al.42, the inventory completeness in 87% of counties was greatly improved.
Figure 4. Comparison between the number of vascular plant species in 217 counties in the dataset of this study and the number of vascular plant species in 217 relevant nature reserves.
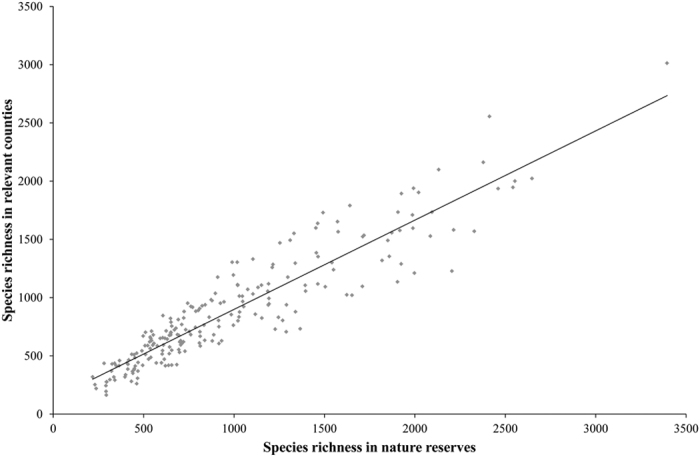
The species numbers of vascular plant species in nature reserves were compiled from the full-surveyed information. These 217 nature reserves are nested within the relevant counties. The number of vascular plant species in relevant counties and nature reserves was basically approximate (r = 0.92, P < 0.01).
We found that the consistent results were obtained in GLM models based on different proportions of samples (60%, 70%, 80% and 90%) compared to that of multivariate models in target regions (100%) (Table S4). The top six environmental variables that reached statistical significance for the most times remained unchanged in GLM models based on the above sets of data (from 60% to 100%). For instance, as for vascular plants in whole China, the top six environmental variables were elevational range, net primary productivity, precipitation seasonality, mean annual precipitation, maximum temperature of the warmest month, and main land cover type. As for mammals in whole China, the top six environmental variables were elevational range, net primary productivity, main land cover type, maximum temperature of the warmest month, precipitation seasonality, and temperature annual range (Table S4).
We detected that the correlation coefficients between elevational range and some other environmental variables (i.e. precipitation, temperature and productivity) across China were less than 0.2 (Table S1). Similarly, the correlation coefficients between elevational range and other environmental variables in QTR were less than 0.3, except the minimum temperature of the coldest month and the number of main land types (Table S1). In addition, the effect of elevational range on plant species richness was not linear but marginal beyond certain threshold. As is shown in Fig. 5, elevational range was positively related to vascular plant species richness when elevational range is less than 1,900 m, whereas the relationship was marginal when elevational range is larger than 1,900 m. Areas with elevational range larger than 1,900 m are mostly low in annual potential evapotranspiration (less than 1,000 mm) and are mainly distributed in southwestern and northwestern part of China (Fig. 6).
Figure 5. Relationship between elevational range and vascular plant species richness.
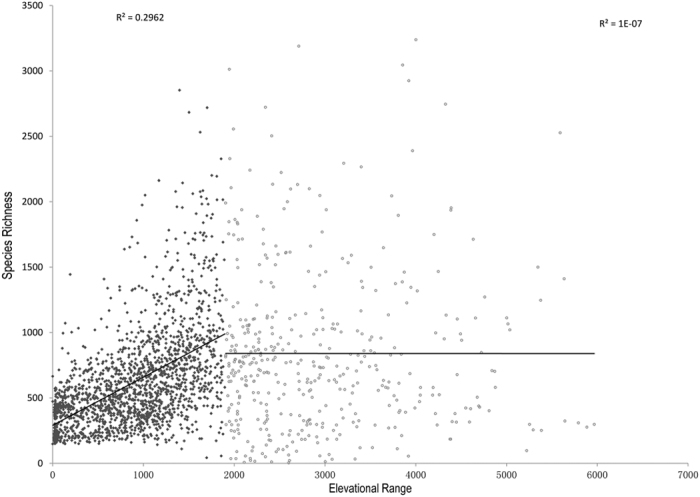
Counties with elevational range larger than 6,000 m were excluded in the analysis. The relationship is significant when elevational range is less than 1,900 m (R2 = 0.2962, F = 815.2, P < 0.001), beyond which the relationship is marginal (R2 = 0.0000, F = 0.02, P = 0.89).
Figure 6. Areas (grey) with elevational range larger than 1,900 m mainly distributed in southwestern and northwestern part of China.
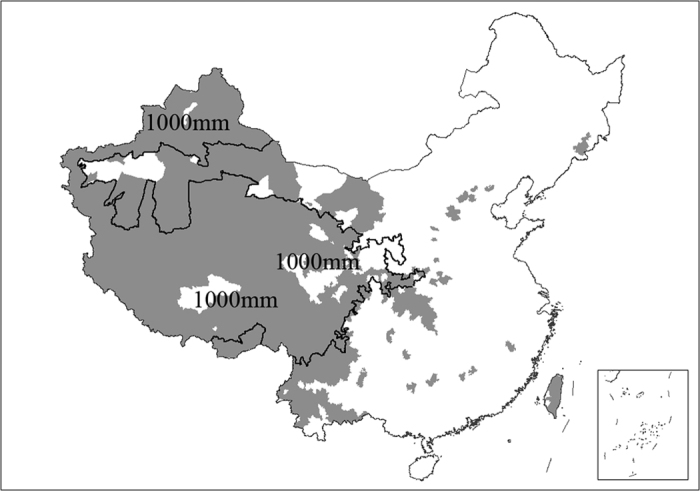
Line indicates low-energy areas where annual potential evapotranspiration is around 1,000 mm. The inset in the right bottom of the figure shows the southern boundary of China, including all islands in the South China Sea. Data on national territory were from the National Administration of Surveying, Mapping and Geoinformation of China (http://www.sbsm.gov.cn/). The map was created using the software Arc GIS 9.3 which was purchased from ESRI-China (http://www.esrichina-bj.cn/) by Nanjing Institute of Environmental Sciences affiliated to the Ministry of Environmental Protection of China, with user number C-801668.
Discussion
According to the methods in the study of Jetz and Rahbek43 and Kreft & Jetz24, we assigned importance of some variables to species richness based on the z-score in SLM models. The higher z-score of a variable shows its more remarkable effect on species richness and the more important role of its relevant hypothesis. We found that elevational range was the most dominant factor of vascular plant species richness across China. Similar findings were reported in previous studies of vascular plants in the nature reserves of China44, the Jura Mountains of Switzerland45 and the Iberian Peninsula46. These similar findings, coupled with our results may be illustrated by the fact that all the above study sites are mostly composed of mountainous regions with relatively higher elevational range. Mountains provide a wide variety of habitats for species formation and specialization and buffering against climate change47,48. More microscopically, elevational transects in the mountains are nested within a biogeographic region and form a test system where the flora and fauna have gone through a similar geological and evolutionary history for the development of diverse species49. Kreft and Jetz24 identified potential evapotranspiration and the number of wet days per year as the two most important predictors of species richness of vascular plants across 1,032 geographic regions worldwide. The disparity between our conclusions may result from the different study regions and assessment units: the study of Kreft and Jetz24 was at global scale based on the assessment unit of about 12,100 km2 while our study was at national scale based on the assessment unit of about 3908.7 km2. Wang et al.38 stated that the mean temperature of the coldest quarter was the strongest predictor of species richness of woody plants in China. The different conclusions from our studies are probably due to the fact that spatial autocorrelation was not accounted for by GLM models in Wang et al.’s study, while our results were based on SLM models in which spatial autocorrelation was accounted for. Qian40 found that temperature seasonality was the best predictor of woody species richness in China based on the assessment unit of province, which is obviously larger than county, since the effect of habitat heterogeneity on species richness is stronger at fine scales than that at broad scales50,51.
In the three major regions of China, we detected that elevational range was not the most prominent predictor for the species richness of vascular plants in EMR. Our results demonstrated that elevational range was positively correlated with species richness when elevational range was less than 1,900 m, beyond which the relationship was not significant (Fig. 5). QTR fall within grey patches (elevational range is larger than 1,900 m; Fig. 6). The grey patches contain mostly low-energy regions where annual potential evapotranspiration is less than 1,000 mm. It revealed that in the regions with low availability of energy, species richness was often correlated with energy-related variables instead of elevational range. Conversely, elevational range often took the predominant role within the regions with sufficient energy for the survival and development of species. It is basically consistent with the previous studies in North America where mammals’ species richness is determined by habitat heterogeneity in the high-energy regions6. However, elevational range also greatly contributes to species richness of vascular plants and mammals (Table 2) and is slightly associated with most of other environmental variables both at national and regional scales (Table S1). It suggests that elevational range is a robust and relatively independent determinant at different scales. Zhao and Fang44 got the similar conclusion based on the study of vascular plants in nature reserves of the subtropical forest region, temperate forest region, temperate steppe and desert region, and the Qinghai-Tibet Plateau region. Since elevational range is vital to species richness, it suggests that habitat heterogeneity should be taken seriously in the work of biodiversity conservation.
Table 2. Summary of main environmental variables in China and its three regions.
| Main environmental variables | Whole China | EMR | NAR | QTR | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Mean annual precipitation (mm) | 921.3 | 488.0 | 1029.1 | 448.9 | 253.6 | 136.6 | 483.4 | 249.3 |
| Precipitation of the wettest quarter (mm) | 474.0 | 206.5 | 522.7 | 180.8 | 158.8 | 97.0 | 293.0 | 130.8 |
| Precipitation of the driest quarter (mm) | 60.7 | 58.6 | 70.4 | 59.1 | 9.1 | 7.7 | 11.0 | 9.2 |
| Mean annual dryness | 85.3 | 40.1 | 93.2 | 37.1 | 28.8 | 16.7 | 63.2 | 28.0 |
| Mean annual temperature (0.1 °C) | 123.5 | 61.9 | 140.5 | 49.6 | 54.5 | 32.3 | 10.0 | 35.7 |
| Max temperature of the warmest month (0.1 °C) | 287.6 | 45.4 | 299.5 | 28.1 | 274.9 | 29.8 | 162.8 | 30.4 |
| Min temperature of the coldest month (0.1 °C) | −58.9 | 102.7 | −34.9 | 92.7 | −189.8 | 39.6 | −178.5 | 48.6 |
| Annual potential evapotranspiration (mm) | 1055.8 | 157.4 | 1091.4 | 130.7 | 952.1 | 134.3 | 767.1 | 109.3 |
| Net primary productivity (gC·m−2·a−1) | 437.9 | 231.8 | 488.6 | 208.8 | 129.1 | 79.4 | 225.3 | 195.7 |
| Annual actual evapotranspiration (mm) | 713.1 | 274.8 | 780.5 | 232.3 | 258.8 | 137.8 | 485.9 | 194.9 |
| Normalized difference vegetation index | 494.7 | 135.0 | 527.6 | 98.5 | 303.8 | 163.2 | 346.0 | 167.3 |
| Temperature annual range (0.1 °C) | 346.5 | 86.4 | 334.4 | 83.9 | 464.7 | 31.3 | 341.3 | 38.2 |
| Mean diurnal range (0.1 °C) | 101.5 | 23.7 | 95.4 | 20.0 | 130.5 | 10.6 | 137.6 | 14.8 |
| Temperature seasonality | 8725.3 | 2635.6 | 8522.7 | 2573.9 | 11971.4 | 1243.9 | 7103.1 | 1154.4 |
| Precipitation seasonality | 81.3 | 23.5 | 78.7 | 23.0 | 90.6 | 22.6 | 100.5 | 17.9 |
| Elevational range (m) | 1247.3 | 1086.0 | 1024.8 | 812.1 | 1884.0 | 1533.8 | 3060.8 | 1211.3 |
| Mean elevation (m) | 860.5 | 1100.9 | 541.7 | 609.4 | 1308.9 | 515.4 | 4029.8 | 842.7 |
| Number of land cover types | 6.5 | 2.1 | 6.4 | 2.1 | 6.8 | 2.4 | 7.0 | 2.2 |
EMR: Eastern Monsoon Region, n = 1995; NAR: Northwestern Arid Region, n = 210; QTR: Qinghai-Tibet Plateau Region, n = 171. There are significant differences (one-way ANOVA analysis, p < 0.05) in means of these variables among the three regions.
Net primary productivity was considered as the most significant determinant for vascular plant species richness in QTR. The result was supported by the study of alpine meadow by Wang et al.33 in the same region. As the QTR and the Himalayas underwent accelerating uplift through the Quaternary, the interior became progressively desiccated as the influx of Indian Ocean moisture was constrained28. Thus, annual precipitation is low in most parts of QTR and unevenly distributed, e.g. 100–300 mm at the center of QTR and 3000 mm in the eastern part of QTR52. Besides, it is also characterized by low temperatures: the annual mean air temperature is −1.7 °C53. Accordingly, the richest plant species occur in the parts where the conditions of water and heat are sufficient for species formation and specialization. Hence, low temperature, little precipitation, and the strong variability make the productivity-related factors responsible for species richness patterns in the region and the biodiversity monitoring using the productivity-related indicators is urgently needed. Similarly, we found that normalized difference vegetation index (NDVI) was the second most prominent determinant of vascular plant species richness in NAR. It is basically consistent with the results of such previous studies of vascular plants in Kenya, Israel and USA54,55,56 since all the study regions harbor drier and colder environment. Li et al.34 identified that water availability was mostly correlated with plant species richness and supported the water-energy dynamics hypothesis. This result is similar to our study because NDVI and mean annual precipitation are highly correlated in NAR (r = 0.77, p < 0.01). Interestingly, some plants (e.g., Lomatogoniopsis) have been progressively evolving drought-tolerance or cold-adapted features, resulting in a drastic shift and uniqueness in the distribution of plant communities in the above regions. Thus, the endemic species’ ecological and evolutionary history should be highlighted in the making and implementation of conservation strategy51,57.
As for mammals, elevational range was also discovered as the most important predictor of species richness across China. Similar to vascular plants, determinants that dominantly shape the species richness pattern were also found to vary at different scales. At the regional scale, elevational range remains the most important predictor for mammals in EMR and NAR, however became the third important predictor in QTR. Mean annual precipitation was identified as the second most important determinants for mammals in EMR. It can be explained by precipitation gradients along latitude, with rich precipitation in the South where mammal species richness is high and low precipitation in the North where mammal species richness is low. Net primary productivity was the most important predictor for mammals in QTR. The reason is similar for plants in QTR. Net primary productivity is low in interior parts of QTR because the uplift of the Himalayas makes the interior become progressively desiccated28, resulting in low species richness there. Meanwhile, higher species richness of mammals occurs in the eastern and southeastern parts of QTR where net primary productivity is higher due to relatively sufficient precipitation and heat there (Fig. 2). As a result of the low ability of mammals to utilize the low water content forage in dry regions58, the efficiency of food chain is obviously lower than that with permanent water sources. The shortage of water and food makes the survival and specialization of mammals difficult in the extremely stressing status. Thus, mammal species richness is higher in the wetter regions than in the drier regions. The global climate change greatly influences the annual precipitation in QTR59,60 and tends to alter the species richness pattern accordingly, which should be particularly emphasized in the conservation of mammal species. In addition, precipitation seasonality was responsible for species richness in QTR. It is consistent with the previous study of a savannah large mammal community in the Amboseli ecosystem, Africa58. Both the study regions harbor the arid or semi-arid climate and show strong precipitation seasonality52. It is also discovered that main land cover type has emerged as the key factors to impact the mammal species richness in the whole country (z = −7.11) and EMR (z = −4.02) (Table 1). The result can be explicitly explained by the recent conspicuous changes in land use pattern across China, such as in the tropical mountains of Xishuangbanna, Yunnan and the farming-pasturing interlock region of northern China61,62. Hence, the effects of land use on mammal species richness should attract special attention.
Sampling bias may introduce errors into the results of our study. Two types of errors are possible, i.e., omission errors and commission errors. Here, we illustrated the sources of errors as follows: 1) Omission errors. The current dataset is much more prone to omission errors. Like most of the published databases, our database also has the ubiquitous shortcoming that sampling efforts are not uniform in space63,64,65,66. Most records of species distribution are derived from opportunistic collections without a unified sampling strategy to cover the full variation of environmental conditions in the entire target region. Sampling biases are common as records can be spatially biased towards more popular species or easily accessible regions63,67. Some species receive special attention, or are easy to be detected. Some regions are close to researchers, or have more research funding68,69. Some poorly known species and regions are most likely to be affected by the above limitations. As detailed surveys across the entire possible range are barely conducted due to lack of resources70,71, a lot of species that are actually present have not yet been recorded. As only a few counties in China have been surveyed with the aim of generating complete species lists, omission errors occur in the data on species distribution in some counties of China72. By contrast, well-surveyed regions are less likely to have omission errors. 2) Commission errors. Species may be misidentified or the locations may be wrongly recorded, which result in commission errors63,69. Species occurrence is not fixed especially for mammals as they move dynamically in time and space as a consequence of changing biotic and abiotic conditions. Due to change of habitats, such as transformation of forests into croplands, and wetlands into rice paddies, the distribution of species is likely to change. Thus, data on species distribution based on literatures or specimens may overestimate species distribution and then lead to commission errors in this analysis.
To reduce the sampling bias of species distribution data, we first organized more than 20 expert meetings and invited over 50 senior experts that are specialized in taxonomy and ecology of nearly all the specific taxa included in this study and rich experienced in field survey of vascular plants and mammals to review the data of spatial distribution of each species at the county level across China. Our invited experts carefully checked the list of species names. More importantly, they also comprehensively rectified the distribution information of each species. Through this process, the errors especially commission errors derived from sampling bias have been reduced as far as possible. In a second step, we made comparison of the inventory completeness of vascular plants between this study and the published study of Yang et al.42. Among the 2010 ‘under-sampled’ counties based on the definition of Yang et al.42, the inventory completeness in 87% of counties was greatly improved. Moreover, we compared the number of vascular plant species per county among 217 counties in the dataset of this study with species numbers that were compiled from the full-surveyed information from 217 nature reserves nested within the relevant counties. The result showed that the number of vascular plant species in these counties and nature reserves were basically approximate. Thus, we can comprehensively reduce sampling bias to a larger extent and improve the accuracy of the data on species geographical distribution at the county level.
To further test the impact of sampling bias on the robustness of the final (GLM) models, we performed a bootstrap procedure with stratified random sampling73,74,75,76. This method has been reasonably applied to explore the effects of sampling bias on the research result in the study of Muir et al.77. We selected subsets of samples (60%, 70%, 80% and 90%) from the target regions (i.e., whole China, EMR, NAR and QTR respectively) using stratified random sampling with a bootstrap procedure, and compared the multivariate models based on the subsets of samples with that of target regions (100%). When p < 0.05, the regression coefficient is considered statistically significant. This process was replicated 1000 times with randomly generated samples. We counted the number of times the regression coefficient of each variable reached statistical significance. The top six variables were selected according to the number of times each variable reached statistical significance among 1000 times. We found that the consistent results were obtained in GLM models based on different proportions of samples (from 60% to 90%), as compared with that of multivariate models in target regions (100%). The top six environmental variables that reached statistical significance for the most times remained unchanged in GLM models based on the above sets of data (from 60% to 100%) (Table S4). Therefore, we may basically conclude that the impact of sampling bias on multivariate models can be effectively controlled and the multivariate models are robust in this study. Though this method is still in the infant stage, we believe that it can offer a new way to test the effects of sampling bias.
In summary, our study provides insights into spatial patterns in species richness of vascular plants and mammals at national and regional scales. The relative contribution of variables that explain species richness patterns varied among regions. Elevational range was the most important predictor for plants and mammals across China. However, the uplift of the Himalayas makes spatial patterns of species richness in QTR quite unique, where net primary productivity was the most controlling factor for plants and mammals. Elevational range is often independent at different scales and emerges as the predominant controlling factor in the regions with sufficient energy for the survival and development of species. Therefore, we suggest that region-specific conservation policies should be developed based on the regional features of species richness.
Methods
Study area
We studied the species richness in the terrestrial and inland water ecosystems across China. To get insight into this issue at the regional scale, we further made research in the major three regions (EMR, NAR and QTR) based on Zhao’s geographical regionalization system31 (Fig. 1), because the obvious disparity in climatological, geographical and topographical features makes the unique distribution pattern of biodiversity and the different responses of biodiversity to climate change and human intervention occur in the three regions31,34,78,79. EMR, accounting for about 46.6% of the total terrestrial area of China, dominated by monsoon climate, is characterized by humid climate, significant change in temperature along latitude and great human intervention, with elevation mostly lower than 1,000 m. NAR, accounting for 29.1% of the total terrestrial area of China, mostly grasslands and deserts, is mainly of arid and semi-arid climate, with high precipitation variability, plateaus of approximately 1,000 m, and some mountains higher than 3,000 m. QTR, accounting for 24.3% of the total terrestrial area of China, harbors the world’s largest and highest plateau, with an average elevation of more than 4,000 m, thin air, low temperature and strong wind52.
Species richness data
We created a database of the geographical distribution of 30,519 vascular plant species and 565 mammal species from 2,376 counties in the terrestrial and inland water ecosystems of China. Species in marine ecosystems, exotic species, and cultivated or bred species in botanical gardens, zoos or farms were excluded. The checklist of vascular plants was derived from Species 2000, Catalogue of Life China edited by the Biodiversity Committee, CAS (Checklist 2011)80 (Appendix S1). The checklist of mammal species was based on the complete information compiled by Wang81 and Jiang et al.82 (Appendix S2). The species distribution information in the database was mainly compiled from literatures from 1970 to 2012 (including national floras and faunas, e.g., Flora Reipulicae Popularis Sinicae83 [Supplementary information], Flora of China84, Higher Plants of China85 [Supplementary text], Fauna Sinica.Mammalia86,87, regional and provincial monographs on floras and faunas, e.g., Flora Yunnanica88 [Supplementary text], Mammals of Beijing89, and numerous studies in biodiversity, e.g., the study of Cheng and Xiao90) and collection information of specimens in herbaria of more than 20 institutes and universities. Some recent ground observation information of plants and mammals was also integrated into the database based on records of field surveys by experts from more than 11 institutes of the Chinese Academy of Sciences and over 14 universities. We then invited more than 50 experts specialized in different specific taxa to review the information of species distribution across the whole country. In order to get a finer view of species richness, we used ‘county’ as the basic assessment unit. Such units were respectively treated as one assessment unit, i.e., the urban area of a municipality, the urban area of a capital city in a province and autonomous region, the urban area of a city at prefectural level and a special administrative region (e.g., Hong Kong and Macau) because the presence of species in such regions was mostly recorded in the above units instead of county. In total, 2,376 assessment units (thereafter named as counties) were included for the analysis.
Environmental data
We selected 19 environmental variables that were considered to mostly explain species richness patterns of vascular plants and mammals from previous studies according to the core hypotheses24,25,26,39,91, including: (1) mean annual precipitation; (2) precipitation of the wettest quarter; (3) precipitation of the driest quarter; (4) mean annual dryness; (5) mean annual temperature; (6) maximum temperature of the warmest month; (7) minimum temperature of the coldest month; (8) annual potential evapotranspiration; (9) annual actual evapotranspiration; (10) net primary productivity; (11) normalized difference vegetation index; (12) mean diurnal range; (13) temperature seasonality; (14) temperature annual range; (15) precipitation seasonality; (16) elevational range; (17) mean elevation; (18) main land cover type, and (19) number of land cover types. These above environmental variables were classified into five categories (hypotheses) (Table S2), i.e. water-energy dynamics hypothesis (environmental variables 1 to 4), ambient energy (temperature) hypothesis (environmental variables 5 to 8), productivity hypothesis (environmental variables 9 to 11), environmental stability hypothesis (environmental variables 12 to 15), and habitat heterogeneity hypothesis (environmental variables 16 to 19). Although species richness gradients are also attributed to differences in evolutionary history according to the historical hypothesis92,93, we did not test the historical hypothesis in this study as it was difficult to evaluate the historical effects and incorporate historical factors into regression models94.
Data on these environmental variables were from public sources that are often used by peer-reviewed literatures. Data on climate variables were from WorldClim-Global Climate Data (http://www.worldclim.org) with a resolution of 30” × 30”. Data on potential evapotranspiration (PET) were from the website of the Consortium for Spatial Information (CGIAR-CSI) of the Consultative Group on International Agricultural Research (http://www.cgiar-csi.org) (30” × 30” resolution). Data on annual actual evapotranspiration (AET) were from the UNEP website (http://www.grid.unep.ch/data/download/gnv183.zip) (0.5° × 0.5° resolution). Data on the normalized difference vegetation index (NDVI) (250 m × 250 m resolution) and topography (90 m × 90 m resolution) were from SRTM90 of Global Land Cover Facility (http://glcf.umd.edu/data/). Data on net primary productivity (NPP) were from the NASA/EOS Project of the University of Montana (http://www.ntsg.umt.edu/project/mod17#data-product) (30” × 30” resolution). Data on land- cover type were from the website of the European Space Agency (http://bioval.jrc.ec.europa.eu/products /glc2000/glc2000.php) (30” × 30” resolution). Data on administrative boundary of counties were from the National Archives for Surveying and Mapping of China (http://ngcc.sbsm.gov.cn/article/en/or/an/). We obtained values of environmental variables in a county by calculating the average value of the variables among all pixels in the county. Elevational range was calculated as the maximum minus the minimum elevation recorded in a county. Main type of land cover was estimated by the majority of land cover type in a county. Mean annual dryness was calculated as the mean annual precipitation divided by the annual potential evapotranspiration95. The mean values of environmental variables between 1950 to 2000 were used to conduct multivariate analysis of species richness data24.
Data on major environmental variables in the whole country and three separate regions (EMR, NAR and QTR) were listed in Table 2.
Assessing inventory completeness
We made an assessment of the inventory completeness of vascular plants in this study before the model test in order to control the sampling bias as much as possible. First, we compared the inventory completeness of our database at the county level with that of the study of Yang et al.42. Due to lack of enough specimen information, we were not able to directly compute the smoothed species accumulation curves42. Instead, we calculated the values of ‘mean’ and ‘standard deviation’ in the difference in the number of vascular plant species between the above two datasets. If the data quality of species was improved in the ‘under-sampled’ counties defined by Yang et al.42 to a larger extent, we considered that the inventory completeness in such counties was improved as much as possible. In addition, we also compared the number of vascular plant species per county among 217 counties in the dataset of this study with species numbers that were compiled from the well-surveyed information from 217 nature reserves. These 217 nature reserves are distributed within relevant 217 counties, respectively. If the species richness of vascular plants among counties in the dataset of our study was basically approximate to that from the well-surveyed nature reserves, we considered that a good level of inventory completeness was achieved in such counties.
Statistical analyses
In the first step, we performed Spearman (two-sided) correlation analysis between any two environmental variables in each hypothesis in order to reduce multicollinearity among the variables. Besides, we analyzed potential single predictors of species richness using univariate regression models. Then, we identified strongly intercorrelated variables (Spearman’s coefficient >0.7) and retained the variables that explained more deviance in univariate regression models24,96,97. Thus, we selected predictors for each hypothesis and reduced the correlation among predictors in each category (Table S2).
In the second step, we established generalized linear models (GLM) with the selected predictors from the first step43, and calculated the statistical significance at the p < 0.05 for the regression coefficient of each variable. This process was replicated 1000 times. We counted the number of times the regression coefficient of each variable reached statistical significance. The top six variables were selected according to the percentage of a variable reaching statistical significance among 1000 times (Table S3).
In the third step, to avoid inflation of type I errors and invalid parameter estimate owning to spatial autocorrelation, we established spatial linear models (SLM) using the six variables selected in the second step. The simultaneous autoregressive (SAR) models were used to account for spatial autocorrelation. Among the three different SAR model types (spatial error = SARerr, lagged = SARlag and mixed = SARmix), we employed SARerr when dealing with spatially autocorrelated species distribution data98. We tested a set of possible lag distances (50, 100, 200, 400, 600, 800 and 1,000 km) for each model and determined the degree of spatial autocorrelation in the residuals of models using Moran’ s I coefficient. SARerr with a lag distance of 100 km accounted best for the spatial structure in the data set according to the minimum value of AIC. As r2 values are not directly provided for SAR models, we assessed the maximum model fit based on a pseudo-r2 value, that is calculated as the squared Pearson correlation coefficient between predicted and observed (species richness) values98. By testing z value for its significance, we examined the contribution of each predictor to the residuals of species richness in the best-fit SLM43. Finally, we compared multivariate regressions of six predictors with that of 19 variables, and detected if changes in model fit (Δr2) occur to assess the robustness of best-fit SLM43.
Species richness, areas and environmental variables were log10-transformed in all analyses unless otherwise stated. Statistical analyses were carried out using the free software packages R, version 2.1599,100 unless otherwise stated. As the large changes in county area (mean: 3908.7 km2; standard deviation: 9287.6 km2) may influence patterns of species richness93, we regressed species richness on county area and obtained residuals of species richness101, and data on the residuals of species richness were used in these three steps to avoid effects of area. In addition, we analyzed the correlation between elevational range and species richness (counties where elevational range larger than 6,000 m were excluded from the correlation analysis) using split-line regression techniques6.
Area-effect test
Data on the residuals of species richness were used to establish multivariate models, although this may lead to biased parameter estimates102. We also established SLM multivariate models using raw data and treated area as a variable in the model. In this process, influence of area on environmental variables (especially elevational range and the number of landcover types) was considered. We found that no obvious difference existed in multivariate models between the two methods (Table S5). Thus, the results from the residuals of species richness were presented in this study.
Test the impact of sampling bias on the robustness of multivariate models
The performance of multivariate models may be influenced by geographical sampling bias42,64 though the limitations in the database were reduced to the lowest extent. To test the impact of sampling bias on the robustness of GLM models, we performed a bootstrap procedure with stratified random sampling74,76,77. To ensure the thorough coverage of environmental conditions in the study region, we adopted the stratification system based on the phytogeographic regions in China103 for vascular plants and zoogeographical regions in China52 for mammals. Two principles were observed in this procedure: the first is that the target regions (i.e., whole China, EMR, NAR and QTR respectively) should remain unchanged, and the second is that sampling units (i.e., the basic assessment unit) should be randomly selected41. We illustrated the procedure as follows: (1) Stratified random sampling was used to generate a sample of 60% of the total dataset from each strata of target regions (whole China, EMR, NAR and QTR respectively)77; (2) We fitted a GLM model based on the subset of data (60%); (3) When p < 0.05, the regression coefficient is considered statistically significant; (4) The above steps from (1) to (3) were repeated 1000 times with randomly generated samples. We summed the number of times each variable reached the statistical significance based on the regression coefficient and selected the top six variables based on the percentage of a variable reaching statistical significance in the procedure with 1000 replicates; (5) We then randomly resampled 70%, 80% and 90% of total dataset respectively, and repeated the above steps from (1) to (4). If consistent environmental variables were finally obtained from GLM models based on different proportions of samples (60%, 70%, 80% and 90%), as compared with that of multivariate models in the target regions (100%), we can effectively control the impact of sampling bias on the models using stratified random sampling and verify the robustness of multivariate models.
Additional Information
How to cite this article: Xu, H. et al. Disentangling the determinants of species richness of vascular plants and mammals from national to regional scales. Sci. Rep. 6, 21988; doi: 10.1038/srep21988 (2016).
Supplementary Material
Acknowledgments
We thank the more than 500 field biologists and conservation staff from the Chinese Academy of Sciences, universities, research institutes and government bodies who have made the species richness data sets possible. We are grateful to Walter Jetz from Yale University for his discussion and comments on the manuscript. We are grateful to Dr. Wenjing Yang for her provision of data on vascular plant species in China that were used in her published work and Prof. Zhi Wang from Nanjing Institute of Environmental Sciences under the Ministry of Environmental Protection of China for his provision of data on vascular plant species in nature reserves. We are also grateful to Prof. H. N. Qin from the Institute of Botany for their provision of species checklist. This work was funded by the National Key Technologies Research and Development Programme (environmental project 201409061, grants 2008BAC 39B06 and 2008BAC39B01) and the Biodiversity Conservation Programme of China.
Footnotes
Author Contributions H.X., M.C. and L.C. designed the study; M.C. and H.X. developed the methods; J.W., P.C., L.C., M.C., J.L., Y.W., Z.L. and H.D. collected the data; H.X., M.C., Y.W., Y.C. and J.L. conducted the analyses; H.X., Y.C., M.C. and L.C. wrote the paper.
References
- Gaston K. J. Global patterns in biodiversity. Nature 405, 220–227 (2000). [DOI] [PubMed] [Google Scholar]
- Kerkhoff A. J., Moriarty P. E. & Weiser M. D. The latitudinal species richness gradient in New World woody angiosperms is consistent with the tropical conservatism hypothesis. Proceedings of the National Academy of Sciences 111, 8125–8130 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianka E. R. Latitudinal gradients in species diversity: A review of concepts. The American Naturalist 100, 33–46 (1966). [Google Scholar]
- Heck K. L. Jr. & Wetstone G. S. Habitat complexity and invertebrate species richness and abundance in tropical seagrass meadows. Journal of Biogeography 4, 135–142 (1977). [Google Scholar]
- Currie D. J. & Paquin V. Large-scale biogeographical patterns of species richness of trees. Nature 329, 326–327 (1987). [Google Scholar]
- Kerr J. T. & Packer L. Habitat heterogeneity as a determinant of mammal species richness in high-energy regions. Nature 385, 252–254 (1997). [Google Scholar]
- Rahbek C. & Graves G. R. Multiscale assessment of patterns of avian species richness. Proceedings of the National Academy of Sciences 98, 4534–4539 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallina S. M. et al. Global relationship between phytoplankton diversity and productivity in the ocean. Nat. Commun. 5, 10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson L. R. & Prasad A. M. Potential changes in tree species richness and forest community types following climate change. Ecosystems 4, 186–199 (2001). [Google Scholar]
- Malcolm J. R., Liu C., Neilson R. P. & Hansen L. LEE H. Global warming and extinctions of endemic species from biodiversity hotspots. Conservation Biology 20, 538–548 (2006). [DOI] [PubMed] [Google Scholar]
- Moreno-Rueda G. & Pizarro M. The relative influence of climate, environmental heterogeneity, and human population on the distribution of vertebrate species richness in southeastern Spain. Acta Oecologica 32, 50–58 (2007). [Google Scholar]
- Butchart S. H. M. et al. Global biodiversity: Indicators of recent declines. Science 328, 1164–1168 (2010). [DOI] [PubMed] [Google Scholar]
- Dawson T. P., Jackson S. T., House J. I., Prentice I. C. & Mace G. M. Beyond predictions: biodiversity conservation in a changing climate. Science 332, 53–58 (2011). [DOI] [PubMed] [Google Scholar]
- Brown J. H. Macroecology: Progress and prospect. Oikos 87, 3–14 (1999). [Google Scholar]
- Brown J. H. Mammals on mountainsides: Elevational patterns of diversity. Global Ecology and Biogeography 10, 101–109 (2001). [Google Scholar]
- Francis A. P. & Currie D. J. A globally consistent richness-climate relationship for angiosperms. The American Naturalist 161, 523–536 (2003). [DOI] [PubMed] [Google Scholar]
- Rahbek C. The role of spatial scale and the perception of large-scale species-richness patterns. Ecology Letters 8, 224–239 (2005). [Google Scholar]
- Buckley L. B. & Jetz W. Environmental and historical constraints on global patterns of amphibian richness. Proceedings of the Royal Society B: Biological Sciences 274, 1167–1173 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie D. J. Energy and large-scale patterns of animal- and plant-species richness. The American Naturalist 137, 27–49 (1991). [Google Scholar]
- Rohde K. Latitudinal gradients in species diversity: the search for the primary cause. Oikos 65, 514–527 (1992). [Google Scholar]
- Allen A. P., Brown J. P. & Gillooly J. F. Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science 297, 1545–48 (2002). [DOI] [PubMed] [Google Scholar]
- Qian H. & Ricklefs R. E. Taxon richness and climate in angiosperms: Is there a globally consistent relationship that precludes region effects? The American Naturalist 163, 773–779 (2004). [DOI] [PubMed] [Google Scholar]
- Clarke A. & Gaston K. J. Climate, energy and diversity. Proceedings of the Royal Society B: Biological Sciences 273, 2257–2266 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft H. & Jetz W. Global patterns and determinants of vascular plant diversity. Proceedings of the National Academy of Sciences 104, 5925–5930 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins B. A. et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117 (2003). [Google Scholar]
- Luo Z. et al. Environmental effects on vertebrate species richness: Testing the energy, environmental stability and habitat heterogeneity hypotheses. PLoS ONE 7, e35514 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Wang S. & Xue D. Biodiversity conservation in China: Legislation, Plans and Measures. Biodiversity & Conservation 8, 819–837 (1999). [Google Scholar]
- Xu H. et al. Biodiversity congruence and conservation strategies: A national test. BioScience 58, 632–639 (2008). [Google Scholar]
- Tang Z., Wang Z., Zheng C. & Fang J. Biodiversity in China’s mountains. Frontiers in Ecology and the Environment 4, 347–352 (2006). [Google Scholar]
- Liu J. et al. Protecting China’s biodiversity. Science 300, 1240–1241 (2003). [DOI] [PubMed] [Google Scholar]
- Zhao S. Q. A new scheme for comprehensive geographical regionalization in China. Acta Geogr Sin 38, 1–10 (1983). [Google Scholar]
- Wang G., Wang Z., Zhou Q. & Zhong W. Relationship between species richness of small mammals and primary productivity of arid and semi-arid grasslands in north China. Journal of Arid Environments 43, 467–475 (1999). [Google Scholar]
- Wang Z., Tang Z. & Fang J. Altitudinal patterns of seed plant richness in the Gaoligong Mountains, southeast Tibet, China. Diversity and Distributions 13, 845–854 (2007). [Google Scholar]
- Li L. et al. Species richness patterns and water-energy dynamics in the drylands of Northwest China. PLoS ONE 8, e66450 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Yang X. & Tang Z. Patterns of species diversity and phylogenetic structure of vascular plants on the Qinghai-Tibetan Plateau. Ecology and Evolution 3, 4584–4595 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J., Zhang J. Q., Nie Z. L., Zhong Y. & Sun H. Evolutionary diversifications of plants on the Qinghai-Tibetan Plateau. Frontiers in Genetics 5, 4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X. et al. Geographic patterns and environmental correlates of terrestrial mammal species richness in China. Biodiversity Science 17, 652–663 (2009). [Google Scholar]
- Wang Z., Fang J., Tang Z. & Lin X. Patterns, determinants and models of woody plant diversity in China. Proceedings of the Royal Society B: Biological Sciences 278, 2122–2132 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H. & Kissling W. D. Spatial scale and cross-taxon congruence of terrestrial vertebrate and vascular plant species richness in China. Ecology 91, 1172–1183 (2010). [DOI] [PubMed] [Google Scholar]
- Qian H. Environmental determinants of woody plant diversity at a regional scale in China. PLoS ONE 8, e75832 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, H. et al. Determinants of mammal and bird species richness in China based on habitat groups. PLoS ONE 10(12): e0143996. doi: 10.1371/journal.pone.0143996 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. J., Ma K. P. & Kreft H. Geographical sampling bias in a large distributional database and its effects on species richness–environment models. Journal of Biogeography 40, 1415–1426 (2013). [Google Scholar]
- Jetz W. & Rahbek C. Geographic range size and determinants of avian species richness. Science 297, 1548–1551 (2002). [DOI] [PubMed] [Google Scholar]
- Zhao S. & Fang J. Patterns of species richness for vascular plants in China’s nature reserves. Diversity and Distributions 12, 364–372 (2006). [Google Scholar]
- Dufour A., Gadallah F., Wagner H. H., Guisan A. & Buttler A. Plant species richness and environmental heterogeneity in a mountain landscape: effects of variability and spatial configuration. Ecography 29, 573–584 (2006). [Google Scholar]
- Lobo J. M., Castro I. & Moreno J. C. Spatial and environmental determinants of vascular plant species richness distribution in the Iberian Peninsula and Balearic Islands. Biological Journal of the Linnean Society 73, 233–253 (2001). [Google Scholar]
- Moeslund J. E., Arge L., Bøcher P. K., Dalgaard T. & Svenning J. C. Topography as a driver of local terrestrial vascular plant diversity patterns. Nordic Journal of Botany 31, 129–144 (2013). [Google Scholar]
- Poulsen B. Avian richness and abundance in temperate Danish forests: tree variables important to birds and their conservation. Biodiversity & Conservation 11, 1551–1566 (2002). [Google Scholar]
- Körner C. Why are there global gradients in species richness? Mountains might hold the answer. Trends. Ecol. Evol. 15, 513–514 (2000). [Google Scholar]
- Field R. et al. Spatial species-richness gradients across scales: a meta-analysis. Journal of Biogeography 36, 132–147 (2009). [Google Scholar]
- Wang Z., Rahbek C. & Fang J. Effects of geographical extent on the determinants of woody plant diversity. Ecography 35, 1160–1167 (2012). [Google Scholar]
- Zhang R. Z. Zoogeography of China. Science Press, Beijing, China, pp. 8, 487 (2011). [Google Scholar]
- Gu S. et al. Energy exchange between the atmosphere and a meadow ecosystem on the Qinghai–Tibetan Plateau. Agricultural and Forest Meteorology 129, 175–185 (2005). [Google Scholar]
- Oindo B. O. & Skidmore A. K. Interannual variability of NDVI and species richness in Kenya. International Journal of Remote Sensing 23, 285–298 (2002). [Google Scholar]
- Gillespie T. W. Predicting woody-plant species richness in tropical dry forests: a case study from South Florida, USA Ecological Applications 15, 27–37 (2005). [Google Scholar]
- Levin N., Shmida A., Levanoni O., Tamari H. & Kark S. Predicting mountain plant richness and rarity from space using satellite-derived vegetation indices. Diversity and Distributions 13, 692–703 (2007). [Google Scholar]
- Forest F. et al. Preserving the evolutionary potential of floras in biodiversity hotspots. Nature 445, 757–760 (2007). [DOI] [PubMed] [Google Scholar]
- Western D. Water availability and its influence on the structure and dynamics of a savannah large mammal community. African Journal of Ecology 13, 265–286 (1975). [Google Scholar]
- Zhao L. et al. Changes of climate and seasonally frozen ground over the past 30 years in Qinghai–Xizang (Tibetan) Plateau, China. Global and Planetary Change 43, 19–31 (2004). [Google Scholar]
- Wu S. H. et al. Climate changes in the Tibetan Plateau during the last three decades. Acta Geographica Sinica 60, 3–11 (2005). [Google Scholar]
- Guo H., Padoch C., Coffey K., Chen A. & Fu Y. Economic development, land use and biodiversity change in the tropical mountains of Xishuangbanna, Yunnan, Southwest China. Environmental Science & Policy 5, 471–479 (2002). [Google Scholar]
- Liu J., Liu M., Zhuang D., Zhang Z. & Deng X. Study on spatial pattern of land-use change in China during 1995-2000. Science in China Series D 46, 373–384 (2003). [Google Scholar]
- Boitani L. et al. What spatial data do we need to develop global mammal conservation strategies? Phil. Trans. R. Soc. B 366, 2623–2632 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortal J., Lobo J. M. & Jim_enez-Valverde A. Limitations of biodiversity databases: case study on seed-plant diversity in Tenerife, Canary Islands. Conservation Biology 21, 853–863 (2007). [DOI] [PubMed] [Google Scholar]
- Soberón J., Jim_enez R., Golubov J. & Koleff P. Assessing completeness of biodiversity databases at different spatial scales. Ecography 30, 152–160 (2007). [Google Scholar]
- Beck J. & Schwanghart W. Comparing measures of species diversity from incomplete inventories: an update. Methods in Ecology and Evolution 1, 38–44 (2010). [Google Scholar]
- Boakes E. H. et al. Distorted views of biodiversity: spatial and temporal bias in species occurrence data. PLoS Biol. 8, e1000385 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C., Kreft H., Guralnick R. & Jetz W. Global priorities for an effective information basis of biodiversity distributions. Nature Communications 6, 8221 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondinini C., Wilson K. A., Boitani L., Grantham H. & Possingham H. P. Tradeoffs of different types of species occurrence data for use in systematic conservation planning. Ecology Letters 9, 1136–1145 (2006). [DOI] [PubMed] [Google Scholar]
- Haila Y. & Margules C. R. Survey research in conservation biology. Ecography 19, 323–331 (1996). [Google Scholar]
- Pressey R. L. Conservation planning and biodiversity: assembling the best data for the job. Conserv. Biol. 18, 1677–1681 (2004). [Google Scholar]
- Qian H. & Ricklefs R. E. Latitude, tree species diversity and the metabolic theory of ecology. Global Ecology and Biogeography 20, 362–365 (2011). [Google Scholar]
- Austin P. C. & Tu J. V. Bootstrap methods for developing predictive models. Am. Stat. 58, 131–137 (2004). [Google Scholar]
- Rizopoulos D. bootStepAIC: Model selection by bootstrapping the stepAIC() procedure R package version 1.2-0. http://cran.r-project.org, (2009) (Date of access: 19/02/2015).
- Martins I. S., Proença V. & Pereira H. M. The unusual suspect: Land use is a key predictor of biodiversity patterns in the Iberian Peninsula. Acta Oecologica 61, 41–50 (2014). [Google Scholar]
- Tille Y. Sampling: Functions for drawing and calibrating samples. R package version 2.7. 2015. http://cran.r-project.org, (2015) (Date of access: 10/09/2015).
- Muir P. R., Wallace C. C., Done, T. & Aguirre J. D. Limited scope for latitudinal extension of reef corals. Science 348, 1135–1138 (2015). [DOI] [PubMed] [Google Scholar]
- Xu J. et al. The melting Himalayas: Cascading effects of climate change on water, biodiversity, and livelihoods. Conservation Biology 23, 520–530 (2009). [DOI] [PubMed] [Google Scholar]
- Ding J. J., Liu D. Z., Li C. W. & Jiang Z. G. Spatial variation in species richness of birds and mammals in mainland China. Acta Ecologica Sinica 32, 343–350 (2012). [Google Scholar]
- The Biodiversity Committee of Chinese Academy of Sciences. The Catalogues of Life China 2011 Annual Checklist. Beijing: Science Press (2011). [Google Scholar]
- Wang Y. X. A Complete Checklist of Mammal Species and Subspecies in China: a Taxonomic and Geographic Reference. China Forestry Publishing House, Beijing, China (2003). [Google Scholar]
- Jiang Z. et al. China’s mammalian diversity. Biodiversity Science 23(3), 351–364 (2015). [Google Scholar]
- Editorial Committee of Flora Reipublicae Popularis Sinicae. Flora Reipulicae Popularis Sinicae (Vol. 1-80). Science Press, Beijing (1959–2004). [Google Scholar]
- Wu Z. Y., Raven P. H. & Hong D. Y. Flora of China. Missouri Botanical Garden Press, St. Louis. http://flora.huh.harvard.edu/china/mss/treatments.htm (1994–2006) (Date of access: from 21/05/2009 to 3/11/2012).
- Fu L. G. et al. Higher Plants of China (Vol. 2-13). Qingdao Publishing House, Qingdao (1999–2004). [Google Scholar]
- Gao Y. T. Fauna Sinica Mammalia (Vol VIII), Carnivora. Science Press, Beijing, pp. 1–377 (1987). [Google Scholar]
- Luo Z. X. Fauna Sinica Mammalia (Vol VI), Rodentir, Cricetidae. Science Press, Beijing, pp. 1–522 (2000). [Google Scholar]
- Editorial Committee of Flora Yunnanica. Flora Yunnanica (Vol. 1–21). Science Press, Beijing (1977–2006). [Google Scholar]
- Chen W., Gao W. & Fu B. Q. Mammals of Beijing. Beijing Press, Beijing, 1–304 (2002). [Google Scholar]
- Cheng R. M. & Xiao W. F. Biodiversity of main coniferous forests at low elevation of Three Gorges Reservoir Area. Chinese Journal of Applied Ecology 16, 1791–1794 (2005). [PubMed] [Google Scholar]
- Hortal J., Rodríguez J., Nieto-Díaz M. & Lobo J. M. Regional and environmental effects on the species richness of mammal assemblages. Journal of Biogeography 35, 1202–1214 (2008). [Google Scholar]
- Qian H. & Ricklefs R. E. Global concordance in diversity patterns of vascular plants and terrestrial vertebrates. Ecology Letters 11, 547–553 (2008). [DOI] [PubMed] [Google Scholar]
- Mittelbach G. G. et al. Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecology Letters 10, 315–331 (2007). [DOI] [PubMed] [Google Scholar]
- Fielding A. H. & Haworth P. F. Testing the generality of bird–habitat models. Conservation Biology 9, 1446–1481 (1995). [Google Scholar]
- Trabucco A. & Zomer R. J. Global Aridity Index (Global-Aridity) and Global Potential Evapo-Transpiration (Global-PET) Geospatial Database. CGIAR Consortium for Spatial Information. http://www.cgiar-csi.org/, (2009) (Date of access: 19/02/2014).
- Graf R. F., Bollmann K., Suter W. & Bugmann H. The importance of spatial scale in habitat models: Capercaillie in the Swiss Alps. Landscape Ecology 20, 703–717 (2005). [Google Scholar]
- Benitez-Lopez A., Vinuela J., Hervas I., Suarez F. & Garcia J. T. Modelling sandgrouse (Pterocles spp.) distributions and large-scale habitat requirements in Spain: implications for conservation. Environmental Conservation 41, 132–143 (2014). [Google Scholar]
- Kissling W. D. & Carl G. Spatial autocorrelation and the selection of simultaneous autoregressive models. Global Ecology and Biogeography 17, 59–71 (2008). [Google Scholar]
- Mac Nally R. Multiple regression and inference in ecology and conservation biology: further comments on identifying important predictor variables. Biodiversity and Conservation 11, 1397–1402 (2002). [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. (R Foundation for Statistical Computing, Vienna, Austria). http://www.r-project.org, (2012) (Date of access: 19/02/2014).
- Lamoreux J. F. et al. Global tests of biodiversity concordance and the importance of endemism. Nature 440, 212–214 (2006). [DOI] [PubMed] [Google Scholar]
- Freckleton R. P. On the misuse of residuals in ecology: regression of residuals vs. multiple regression. Journal of Animal Ecology 71, 542–545 (2002). [Google Scholar]
- Wu Z. Y., Sun H., Zhou Z. K., Li D. Z. & Peng H. Floristics of Seed Plants from China. Science Press, Beijing, China, pp. 52–108 (2010). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.