Abstract
Background
Benign prostatic hyperplasia (BPH) is a common, chronic progressive disease. Inflammation is associated with prostatic enlargement and resistance to 5α-reductase inhibitor (5ARI) therapy. Activation of the nuclear factor-kappa B (NF-κB) pathway is linked to both inflammation and ligand-independent prostate cancer progression.
Methods
NF-κB activation and androgen receptor variant (AR-V) expression were quantified in transition zone tissue samples from patients with a wide range of AUASS from incidental BPH in patients treated for low grade, localized peripheral zone prostate cancer to advanced disease requiring surgical intervention. To further investigate these pathways, human prostatic stromal and epithelial cell lines were transduced with constitutively active or kinase dead forms of IKK2 to regulate canonical NF-κB activity. The effects on AR full length (AR-FL) and androgen-independent AR-V expression as well as cellular growth and differentiation were assessed.
Results
Canonical NF-κB signaling was found to be upregulated in late versus early stage BPH, and to be strongly associated with non-insulin dependent diabetes mellitus. Elevated expression of AR-variant 7 (AR-V7), but not other AR variants, was found in advanced BPH samples. Expression of AR-V7 significantly correlated with the patient AUASS and TRUS volume. Forced activation of canonical NF-κB in human prostatic epithelial and stromal cells resulted in elevated expression of both AR-FL and AR-V7, with concomitant ligand-independent activation of AR reporters. Activation of NF-κB and over expression of AR-V7 in human prostatic epithelial cells maintained cell viability in the face of 5ARI treatment.
Conclusion
Activation of NF-κB and AR-V7 in the prostate is associated with increased disease severity. AR-V7 expression is inducible in human prostate cells by forced activation of NF-κB resulting in resistance to 5ARI treatment, suggesting a potential mechanism by which patients may become resistant to 5ARI therapy.
Keywords: NF-κB, Inflammation, BPH, Androgen Receptor, Androgen Receptor Variant 7
INTRODUCTION
Benign Prostatic Hyperplasia (BPH) is the most common urologic disease in men over the age of fifty [1]. Comorbidities of BPH include age, systemic inflammation, autoimmune and inflammatory disease, and individual components of metabolic syndrome [2-5]. There are many potential etiological factors contributing to BPH pathogenesis such as disruption of growth factor and hormone signaling, inflammation, fibrosis, and sympathetic nerve activity [6-9]. Consistent with concepts discussed by other investigators [10, 11] we have recently demonstrated that gene expression profiles of advanced BPH show marked similarities with conditions such as rheumatoid arthritis, psoriasis, and inflammatory bowel disease suggesting the possibility of an autoimmune/inflammatory component to the disease process [12].
There are two major medical approaches for patients presenting with symptoms suggestive of BPH: alpha-adrenergic receptor antagonists (α-blockers) that decrease smooth muscle tone [13] and 5α-reductase inhibitors (5ARI) that reduce the enzymatic conversion of testosterone to dihydrotestosterone, resulting in apoptosis and a decrease of around 25% in total prostate volume [14]. Combination treatment with these therapies was shown to provide a significant reduction in the risk of symptomatic progression. However, nearly 20% of patients display serious adverse complications to these medications and many patients either fail to respond or become resistant over time, with 5-7% progressing to surgical intervention [15]. Given the age and comorbidity profile of this population, many of these patients are not good candidates for surgery [16]. The variability in clinical responses to existing BPH therapies highlights the need to better understand the molecular basis of BPH progression with a view to developing new therapies appropriately targeted to specific patient groups [17].
The eukaryotic nuclear factor-kappa B (NF-κB) transcription factor family regulates the expression of a large variety of genes involved in inflammatory and immune responses as well as cellular growth and development [18]. NF-κB transcription factors are activated as a response to a variety of stress signals, including cytokines and pathogens. Activation of NF-κB proteins is tightly regulated, and inappropriate activation of these signaling pathways has been linked to autoimmunity, chronic inflammation, and various cancers [19, 20]. NF-κB signaling occurs through canonical (p50/RelA) and non-canonical (p52/RelB) pathways resulting in activation of overlapping sets of downstream genes.
The androgen receptor (AR) has been shown to be expressed in the form of C-terminal truncated variants (AR-V) in prostate cancer [21-27]. These AR-Vs lack the ligand-binding domain of full-length AR. AR-V can be constitutively active, driving AR-regulated transcription and promoting tumor progression, even under castrate conditions [23, 28-30]. Expression of AR variant 7 (AR-V7) in circulating prostate tumor cells has been shown to be predictive of resistance to enzalutamide (which interacts with the ligand binding domain of the AR) and abiraterone (which depletes androgen levels) [28]. Gao et al have reported that non-canonical NF-κB signaling induces the expression of AR-V in prostate cancer [31]. We have reported that AR-Vs are also induced by canonical NF-κB signaling resulting in castrate-resistant prostate cancer (CRPC) [32]. Inhibition of NF-κB expression results in AR-V down-regulation and restores sensitivity of CRPC to anti-androgens [32].
Baseline prostate volume is the most reliable predictor of therapeutic failure of BPH and lower urinary tract symptom (LUTS) progression [33] and is most commonly targeted by 5ARI therapy; therefore, our goal is to understand the potential mechanisms of 5ARI resistance. Currently, there is not an established link between AR-V expression and resistance to 5ARI therapy in BPH.
In this study, we investigated the activation of NF-κB and AR-V7 in BPH. We compared human tissue samples from patients who underwent surgery for moderate to severe BPH/LUTS to a cohort of patients with, mildly symptomatic BPH incidental to radical prostatectomy for prostate cancer. We utilized benign human prostate epithelial and stromal cells, to test the presence and consequences of NF-κB activation on AR and AR-V7. To our knowledge, this is the first study to link chronic activation of NF-κB signaling in BPH to increased AR-V7 expression. This provides the basis for a mechanism that could explain why certain patients with BPH fail 5ARI therapy. Our previous study with CRPC demonstrated that inhibition of NF-κB and AR-V7 expression restores responsiveness to medical therapy [34], suggesting that targeting both NF-κB and AR could have an impact in reducing failure of treatment for BPH.
MATERIALS AND METHODS
Cells, reagents, and antibodies
BHPrS1 human prostate stromal cells [35] were cultured in RPMI 1640 (Gibco, Grand Island, NY), 5% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA) and 1% penicillin/streptomycin (Gibco). NHPrE1 prostate epithelial cells [36] were cultured in DMEM/F12 (Gibco) containing either 5% complete fetal bovine serum (FBS) or 5% dextran-coated charcoal-stripped FBS (CS-FBS) (Atlanta Biologicals), 1% penicillin/streptomycin (Gibco), 10ng/ml epidermal growth factor (Sigma Aldrich, St. Louis, MO), 1% insulin-transferrin-selenium (ITS) (Gibco), and 0.4% bovine pituitary extract (Atlanta Biologicals). Primary antibodies against p65, acetyl-p65 (K310) were purchased from Cell Signaling (Beverly, MA), AR (N-20 and C-19) from Santa Cruz Biotechnology (Santa Cruz, CA), AR-V7 from Precision Antibody (Western blotting) (Columbia, MD) and Abcam (IHC)(Cambridge, MA) and, and phospho-p65(S276) from Abcam. Secondary antibodies were purchased from GE Healthcare (Pittsburg, PA).
Matrigel culture
Single cell suspensions of NHPrE1 cells in monolayer culture medium containing 2% growth factor reduced Matrigel (BD Bioscience, Oxford, UK) were seeded at 2000 cells/well in 48 well plate containing 200 μL of medium containing 4% growth factor reduced Matrigel, 5% charcoal- stripped FBS (CS-FBS in DMEM/F12 medium with or without DHT (10−8 M). Cells were incubated at 37°C with replacement of the growth medium containing 4% growth factor reduced Matrigel with/without DHT at day 3. Medium was then changed every 2 days. NHPrE1 cells were extracted from the Matrigel using cell recovery solution (BD Biosciences). Extracted NHPrE1 cells were then dissociated to a single cell suspension using enzymatic disaggregation (0.25% Trypsin EDTA, Sigma).
Viral transduction
The pCFG5-IEGZ retroviral vector, gift from Martin Leverkus (Heidelberg, Germany) containing complementary DNA inserts of IKK2-Empty Vector (EV), IKK2-constitutively active (EE), IKK2-kinase dead (KD) (gifts from Dr. Timothy Blackwell, Vanderbilt), AR-FL and V7 (gift from Dr. Ganesh Raj, University of Texas Southwestern) were used for infection of NHPrE1 and BHPrS1 prostatic epithelial and stromal cells as previously described [37-39]. Briefly, the amphotropic producer cell line φNX was transfected with 10μg of the retroviral vectors by calcium phosphate precipitation. To select transfected producer cells, 0.5μg/ml zeocin (IKK2) (Life Technologies) or blasticidin (AR-FL, V7) (Life Technologies) was added to the culture medium for 3 days to obtain >95% green fluorescent protein-positive producer cells. Cell culture supernatants containing viral particles were generated by incubation of producer cells with DMEM containing 10% FBS overnight. Following filtration at 45μm (Millipore Billerica, MA), culture supernatant was added to NHPrE1 or BHPrS1 cells seeded in six-well plates 24 hours earlier in the presence of 1μg/ml polybrene. After 5 days recovery of bulk-infected cultures, FACS analysis for green fluorescent protein expression and western blot analysis was performed on expanded polyclonal cells to confirm ectopic expression of the respective molecules. Cell lines used were the IKK2-empty vector (NHPrE1-EV and BHPrS1-EV), IKK2-kinase dead (NHPrE1-KD andBHPrS1-KD), or IKK2-constitutively active, (NHPrE1-EE and BHPrS1-EE).
Transient transfection assay
The NGL vector [a NF-κB responsive reporter vector that has luciferase and green fluorescent protein (GFP) reporter genes [40]] was a gift from Dr. Timothy Blackwell (Vanderbilt), ARR2PB-Luc vector [an AR-responsive reporter vector that does not respond directly to NF-κB [41]], and pGL3-PSA-PSAE1-Luc reporters [42] along with p65 and AR siRNA purchased from Cell Signaling (Beverly, MA), Life Technologies (Carlsbad, CA), and Santa Cruz Biotechnology (Santa Cruz, CA) respectively. The transfection efficiency was determined by co-transfecting pRL-CMV containing the Renilla luciferase reporter gene (Promega, Madison, WI). Luciferase activity was determined using the Promega Corp luciferase assay system 24 h after transfection. The values plotted represent the mean of at least three individual samples ± SD.
Cell viability assay
Cell viability assay was performed using CellTiter-Glo (Promega) according to manufacturer's instructions. NHPrE1 cells were seeded at 2000 cells/well in a 96-well plate, grown over 5 and 7 days in the presence and absence of testosterone and Finasteride. CellTiter-Glo measurements were taken at several time points to track cell survival.
Determination of Androgen Synthesis by Cells
NHPrE1-AR-FL cells where grown in CS media for 5 days and cell pellet and media samples were collected in triplicates and analyzed in 1 analytical run for 5 androgens [testosterone (T), dihydrotestosterone (DHT), dehydroepiandrosterone (DHEA), androstenedione (ASD), and androsterone (AND)] using a modification of a validated high pressure liquid chromatographic (HPLC) assay with tandem mass spectrometric detection (LC/MS/MS) performed Bioanalytics, Metabolomics and Pharmacokinetics (BMPK) Shared Resource as previously described [43].
Prostate Tissue
As part of an Institutional Review Board (IRB)-approved protocol, fresh prostate specimens were collected from 46 consented patients undergoing holmium laser enucleation of the transition zone of the prostate (HoLEP) for symptomatic BPH with LUTS, referred to here as ‘Surgical BPH’. In the majority of cases, these patients had failed medical therapy with an α-blocker, a 5ARI, or both (see Table 1). As a control, a pathologist resected transition zone nodules from 53 patients undergoing radical prostatectomy for small volume, low risk, clinically localized peripheral zone prostate cancer (referred to here as ‘Incidental BPH’) at Vanderbilt University Medical Center (Nashville, TN) as previously described [12]. Low risk prostate cancer was defined as clinical stage T1c, pathologic stage T2a, pre-operative PSA < 10 ng/mL, Gleason Score 3+3=6 or lower, and cancer present in less than 5% of the specimen isolated to the peripheral zone. For all patients, demographic and clinical data was collected including medication history, past medical history (including hypertension, hypercholesterolemia, and diabetes mellitus), demographic and anthropomorphic measurements such as height and weight, and LUTS using the self-reported American Urologic Association Symptom Score (AUASS).
Table 1.
Study population description.
| All pts (n=98) | Incidental (n=53) | Surgical (n=45) | |||||
|---|---|---|---|---|---|---|---|
| Median | 25th, 75th | Median | 25th, 75th | Median | 25th, 75th | p | |
| Age (years) | 63.5 | 58.0, 70.0 | 60.0 | 55.0, 65.0 | 67.0 | 62.0, 72.0 | <0.01 |
| BMI | 28.6 | 25.4, 31.2 | 28.4 | 25.6, 31.9 | 28.7 | 25.3, 30.3 | 0.57 |
| AUASS | 12 | 5, 21 | 7 | 3, 12 | 20 | 15, 25 | <0.01 |
| PSA (ng/ml) | 5.1 | 4.2, 7.3 | 4.8 | 4.3, 6.5 | 5.6 | 3.8, 9.9 | 0.62 |
| Prostate Volume | 60 | 41, 98 | 43 | 30, 57 | 94 | 70, 130 | <0.01 |
| NF-κB-Stromal-(S) | 5.3 | 2.5, 10.7 | 4.6 | 1.7, 10.0 | 6.0 | 4.1, 10.8 | 0.04 |
| NF-κB-Epithelial-(E) | 13.4 | 10.4, 18.7 | 11.9 | 9.3, 14.3 | 16.6 | 13.2, 23.7 | <0.01 |
| AR-FL mRNA | 13.0 | 5.4, 18.4 | 11.6 | 5.4, 17.3 | 14.7 | 5.9, 19.7 | 0.66 |
| AR-V7 mRNA | 86.5 | 15.3, 205.6 | 32.4 | 5.1, 106.5 | 176.5 | 62.0, 425.2 | <0.01 |
| n | % | n | % | n | % | p | |
|---|---|---|---|---|---|---|---|
| BPH TX | |||||||
| α blocker only | 16 | 16.8% | 5 | 9.6% | 11 | 25.6% | <0.01 |
| ARI5 only | 6 | 6.3% | 3 | 5.8% | 3 | 6.7% | |
| 5ARI + α-blocker | 29 | 30.5% | 4 | 7.7% | 25 | 58.1% | |
| None | 44 | 46.3% | 40 | 76.9% | 4 | 9.3% | |
| Comorbidity | |||||||
| NIDDM | 21 | 21.4% | 11 | 20.8% | 10 | 22.2% | 0.86 |
| BMI ≥ 30 | 37 | 37.8% | 22 | 41.5% | 15 | 40.5% | 0.41 |
| AUASS ≥8 | 64 | 68.1% | 25 | 47.2% | 39 | 95.1% | <0.01 |
Table 1: Patient medical records were used to determine Age, BMI, AUASS, PSA, Volume, BPH TX and Comorbidity. Stromal and Epithelial NF-κB activation was determine by quantitated immunofluorescent protein expression. AR-FL and AR-V7 mRNA expression was determine by qPCR. One participant with missing age was dropped from all analyses. Missing data: AUASS (n=4; all in Surgical group), PSA (n=13; all in Surgical group), Volume (n=17; 10 in Incidental and 7 in Surgical groups), alpha-blocker (n=1), ARI5 (n=2). TX: Treatment. p-value : two-sided Wilcoxon Rank Sums test or Chi-squares test comparing differences between Incidental and Surgical groups.
Tissue processing and pathology
After gross pathological examination, all prostate samples used for this study were stored at 4°C and processed within 24 hours. Processing of samples involved flash freezing in liquid nitrogen followed by storage at −80°C until use, as well formalin fixation for paraffin embedding. Samples were reviewed by a pathologist (O.H.) to confirm histologic findings and to exclude those with any foci of cancer.
Quantitative-real-time PCR, Western blot and IHC
For quantitative real-time PCR (qPCR) 50mg flash-frozen tissue was ground using a mortar and pestle in liquid nitrogen and RNA was extracted with Trizol (Ambion) from 46 Surgical BPH and 53 Incidental BPH specimens. Subsequently, 500ng RNA was reverse transcribed into cDNA using RT2 First Strand Kit (Qiagen, Valencia, CA). qPCR was performed using IQ SYBR Green Supermix (BioRad, Hercules, CA) and results were analyzed using BioRad CFX manager software. All results were calculated using ΔΔCt analysis and normalized to GAPDH expression. Primer sequences are listed in Table 2.
Table 2.
Primer Sequences
| Primer set | Oligonucleotidsequence | Annealing temp. (°C) | Amplification cycles |
|---|---|---|---|
| ARFL (V1 and V2)-Fw | ACATCAAGGAACTCGATCGTATCATTGC | 60 | 55 |
| ARFL (V1 and V2)-Rev | TTGGGCACTTGCACAGAGAT | ||
| AR-V1,V2, V3, V4-Fw (AR-P1/P2/P3-Fw) | TGTCACTATGGAGCTCTCACATGTGG | 60 | 55 |
| AR-V1,V2, V3, V4-Rev (AR-P1-Rev) | CACCTCTCAAATATGCTAGACGAATCTGT | ||
| AR-567es-Fw | TGCTGGACACGACAACAA | 60 | 40 |
| AR-567es-Rev | GCAGCTCTCTCGCAATCA | ||
| V7 (AR-P1/P2/P3-Fw) | TGTCACTATGGAGCTCTCACATGTGG | 60 | 60 |
| V7 (AR-P3-Rev) | CTGTGGATCAGCTACTACCTTCAGCTC | ||
| PSA-Fw | GCAGTCTGCGGCGGTGTTCT | 58 | 55 |
| PSA-Rev | GCGGGTGTGGGAAGCTGTGG | ||
| TMPRSS2-Fw | GCACAGCCCACTGTGGTCCC | 58 | 55 |
| TMPRSS2-Rev | CAGAGTAGGCCAGCGGCCAG | ||
| p63-Fw | TTTGTCTGTGTGCTCTGGGA | 55 | 55 |
| p63-Rev | ACTGCCCTGACCCTTACATC | ||
| GAPDH-Fw | TGCACCACCAACTGCTTAGC | 55 | 55 |
| GAPDH-Rev | GGCATGGACTGTGGTCATGAG |
For Western blotting, approximately 50mg of flash frozen human prostate tissue was ground in liquid nitrogen using a mortar and pestle. Protein was extracted with 2% SDS buffer and 30μg protein was run on pre-made 10% polyacrylamide gels (Life Technologies). Primary antibodies were incubated in 5% BSA in TBST overnight at 4°C followed by incubation in secondary antibodies and development using ECL. Membranes were stripped and re-probed with an antibody against β-actin (Sigma).
Immunohistochemistry was performed as previously described [44]. Briefly, 5μm sections were de-waxed, rehydrated and endogenous peroxidases were blocked with hydrogen peroxide. Sections were then boiled in citrate and blocked in 5% serum for 1hr. Primary antibodies were incubated overnight at 4°C at the following concentrations: p-p65-S276 (1:200), AR (N-20 1:200) (C-19 1:50), AR-V7 (1:100)(Abcam). Biotinylated anti-mouse or -rabbit secondary antibodies (DAKO Carpentaria, CA) were incubated for 60min at room temperature after slides were washed for 1hr in PBS. Slides where incubated in ABC-HRP complex (Vector Laboratories Burlingame, CA) for 30min. Bound antibodies where then visualized by incubation with 3,3’ diaminobenzidine tetrahydrochloride (liquid DAB, DAKO). Slides where then rinsed in tap water, counterstained with hematoxylin, and mounted.
Immunofluorescence
Tissues were fixed with 4% paraformaldehyde, permeablized with 0.5% Triton X-100 and incubated with 1% BSA to block non-specific binding. Tissues were then incubated with rabbit anti-phospho-p65 (276) (Abcam) and mouse anti-wide-spectrum cytokeratin (DAKO) and were visualized with anti-rabbit 594-rhodamine-conjugated or anti-mouse FITC-conjugated secondary antibodies (Life Technologies) and nuclei were visualized with DAPI (Vector Laboratories).
Image analysis
Immunostained tissue images were captured using a high throughput Leica SCN400 Slide Scanner automated digital image system from Leica Microsystems. Whole slides were imaged at 20X magnification to a resolution of 0.5 μm/pixel. Tissue cores were mapped using Ariol® Review software. The numbers of positive (brown) and negative (blue) nuclei were determined by analysis of the high-resolution images in the Ariol® software. Fluorescent, immunostained tissue slides were imaged on an Ariol SL-50 automated slide scanner (Leica Biosystems). Tissue cores were imaged at 20X magnification to a resolution of 0.323 μm/pixel. The software used in this work, CellProfiler 2.0, is an open source package for Windows (available from the Broad Institute at www.cellprofiler.org). The software uses a pipeline (available upon request from the authors) of modules designed to automatically identify, quantify, and export the area-shape measurements of cells stained for wide spectrum cytokeratin, phosphorylated p65, and DAPI (nucleus). The pipeline also saves a resultant image with each cell detected (outlined in color). Analysis included total cell count to determine NF-κB positive epithelial cells (wide spectrum cytokeratin, p65-S276 and DAPI) or NF-κB positive stromal cells (p65-S276 and DAPI).
Statistical Analysis
Chi-square and the Wilcoxon-Rank Sum tests were used for univariate comparisons of study characteristics between Surgical and Incidental patients. Primary analyses of differences in NF-κB, AR, or AR-V7 expression across Incidental and Surgical groups were performed within a linear regression model that allowed us to adjust for differences in age, body mass index (BMI), and Non-insulin dependent diabetes mellitus (NIDDM) between groups. Additional regression analyses investigated the age- and group-adjusted associations between NF-κB, AR, and AR-V7 levels with BMI, NIDDM, and 5ARI and α-blocker use. Tissue markers were natural log transformed prior to analysis to normalize these distributions as necessary to meet model assumptions, and adjusted mean biomarker values were then back transformed such that geometric means are reported. A two-sided p-value of 0.05 or less was considered statistically significant. A One-Way ANOVA test was used for comparisons of characteristics between IKK2-empty vector (EV), IKK2-kinase dead (KD), and IKK2-constitutively active (EE) gene expression. A Wilcoxon-Rank Sum test was used when comparing inhibition of NF-κB in EE to EV. Correlation between AR-FL, AR-V7, and AUASS and TRUS volume was evaluated by the determination of the Spearman correlation coefficient (GraphPad Prism).
RESULTS
Activation of NF-κB signaling is associated with clinical progression of BPH
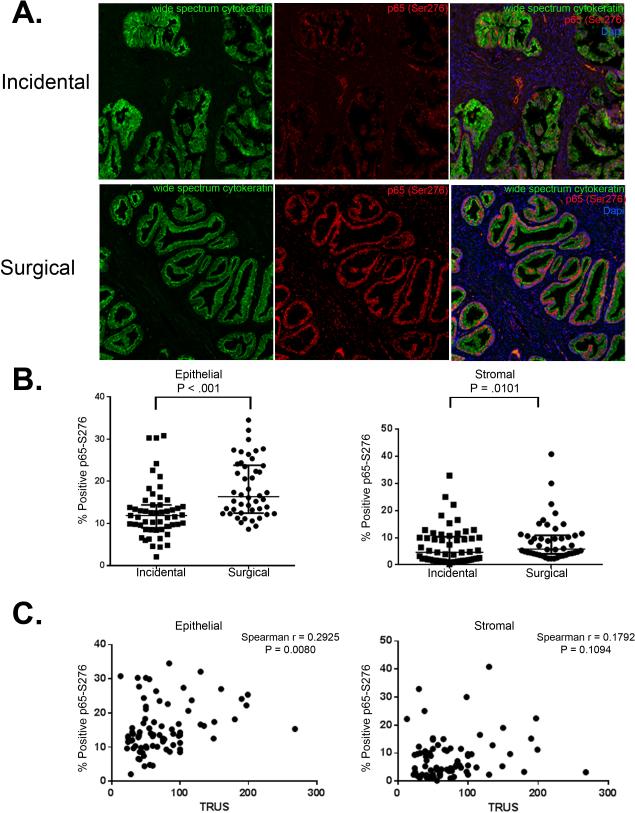
Progressive BPH is commonly associated with inflammation [45], and indeed has molecular similarities to other autoimmune inflammatory conditions [12]. The NF-κB pathway is a molecular program that plays an important role in the regulation of inflammation. An unbiased high resolution scanning analysis of immunofluorescent nuclear phospho-p65 localization (indicating canonical NF-κB signaling) was performed to compare NF-κB activation in 46 clinically advanced Surgical BPH specimens versus 53 Incidental BPH specimens. As illustrated in a representative example in Figure 1A, and quantified in Figure 1B there is significantly higher nuclear phospho-p65 in the more advanced Surgical samples compared to Incidental BPH. Nuclear p65 levels were also higher within the epithelial vs. stromal compartment in both clinical groups, and the epithelial compartment in Surgical BPH patients displayed the highest p65 levels (P < 0.01). We next investigated whether activated NF-κB expression was associated with increased LUTS. We examined all Incidental and Surgical BPH patients (n=99), and analyzed patients whose TRUS (TransRectal UltraSound) volume measurements was included in our data set (n=81). We looked at NF-κB activation by compartment and saw that epithelial NF-κB activation significantly correlated with TRUS volume while there was no significant correlation with stromal NF-κB activation and TRUS volume. (Figure 1C).
Figure 1.
NF-κB expression is significantly higher in symptomatic BPH. Representative images of immunofluorescence staining for the epithelium using wide spectrum cytokeratin (green) and activated NF-κB (p65-S276 red) in 99 BPH patients. A. Examples of immunofluorescence for wide spectrum cytokeratin (green), p65-S276 (red), and DAPI (blue) in Incidental and Surgical BPH. B. Quantitative analysis of immunofluorescence staining of 53 Incidental and 46 Surgical samples for p65-S276 positivity in wide-spectrum cytokeratin-positive epithelium and wide spectrum cytokeratin-negative stroma showing a significant increase in NF-κB activation in both the epithelium and stroma of Surgical versus Incidental patients. C. Spearman's correlation coefficient analysis of NF-κB activation (p65-S276) in wide-spectrum cytokeratin-postive epithelium showing a significant correlation between NF-κB expression and TRUS volume and wide spectrum cytokeratin-negative in the stroma and TRUS volume. Bars are presented as medians, p-value: two-sided Wilcoxon Rank Sums test.
BPH progression is associated with increased expression of AR-V7
Recent studies have linked NF-κB activation to androgen receptor expression, and in particular to the expression of AR variants, in castrate-resistant prostatic cancer [31, 34, 46]. Given that most advanced BPH patients are refractory to 5ARI therapy, we examined the expression of both full length and variant forms of the AR in Surgical and Incidental BPH patient samples. When compared to Incidental BPH patients, Surgical BPH patients were significantly older, had higher AUASS scores, larger prostate volumes, and were more likely to have taken a 5ARI or an α-blocker (Table 1).
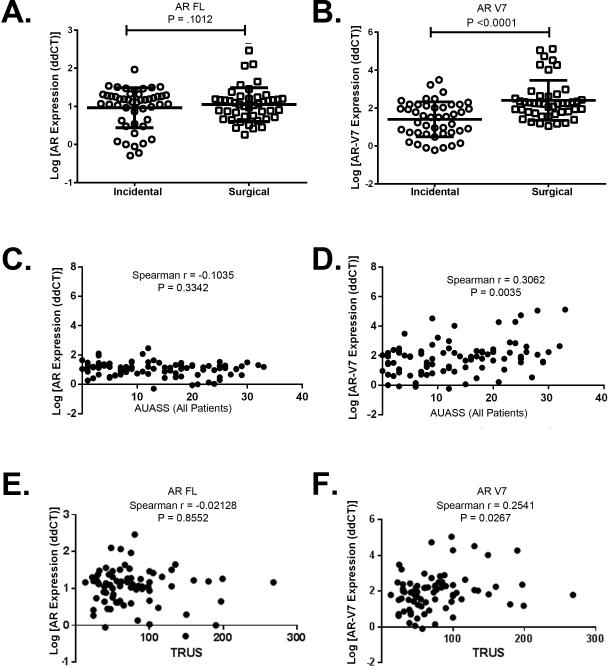
Immunohistochemical analysis with an N-terminal AR antibody did not show differences by clinical group (Supp. Figure 1, Table 1). This was confirmed using qPCR with primers for full length AR (Figure 2A). Following previous studies [17, 47], we examined the expression of a series of AR variants (V1, V2, V3, V4, V567es, and V7 - see Table 2 for primer data). Only AR-V7 was expressed at detectible levels. qPCR quantitation of AR-V7 showed a significant increase (Figure 2B) (P < 0.001) in the Surgical vs. Incidental BPH samples. Immunohistochemistry was used to localize both AR-FL and to confirm the presence of nuclear AR-V7 protein using a specific antibody for this variant and was confirmed using Western blotting (Supp. Figure 1).
Figure 2.
AR-V7 expression is significantly higher in advanced BPH. A. qPCR analysis of AR-FL showed no significant difference between cohorts. B. qPCR analysis of AR-V7 showed there is a significant increase in expression in the Surgical cohort. Bars are presented as medians, p-value: non-parametric Mann-Whitney test. C. Spearman's correlation coefficient analysis of AR mRNA expression and AUASS showing no correlation. D. Spearman's correlation coefficient analysis of AR-V7 mRNA expression and AUASS showing a significant correlation between increased AR-V7 mRNA expression and AUASS. E. Spearman's correlation coefficient analysis of AR mRNA expression and TRUS volume showing no correlation. F. Spearman's correlation coefficient analysis of AR-V7 mRNA expression and TRUS volume showing a significant correlation between increased AR-V7 expression and TRUS volume.
AR-V7 correlates with AUASS scores and TRUS volume
We next investigated whether AR-FL or AR-V7 mRNA expression was associated with increased LUTS. We examined all Incidental and Surgical BPH patients (n=99), and analyzed patients whose AUASS score was included in our data set (n=89). While there was no significant correlation between AR-FL expression and AUASS (Figure 2C), there was a significant correlation between increased AUASS and AR-V7 expression (P < 0.0035) (Figure 2D). To use a non-subjective measurement to determine the association between AR-FL and AR-V7 mRNA expression with increased LUTS, we used TRUS volumes that were included in our data set (n=81). While there was no significant correlation between AR-FL expression and TRUS volume (Figure 2E), there was a significant correlation between increased TRUS volume and AR-V7 expression (P = 0.0267).
Inflammation is associated with increased AR expression
To determine whether adjacent inflammation is associated with increased AR and NF-κB expression we performed immunohistochemistry for inflammatory cells, AR, and activated NF-κB (p-p65). Inflammatory markers such as CD3ε and CD4 were used to determine naïve T-lymphocytes and T-helper lymphocytes respectively. As shown in representative images in Supplemental Figure 2, epithelial cells adjacent to areas with low inflammation (Supp. Figure 2A) had lower expression levels of AR and activated NF-κB. In areas with high areas of inflammation (Supp. Figure 2B) correlated with increased expression of AR and NF-κB. Incidental disease samples displayed relatively low levels of AR expression and NF-κB activation while Surgical BPH patients displayed increased expression of AR and NF-κB in inflamed areas.
NF-κB activation and decreased AR expression are associated with NIDDM
Given that obesity and non-insulin dependent diabetes mellitus (NIDDM) is associated with BPH risk [48, 49], we examined whether NF-κB and AR-V7 expression were associated with BMI or NIDDM (Table 3). NF-κB expression was significantly elevated in both the stroma and epithelium of diabetic versus non-diabetic patients (Table 3). Obesity, defined as a BMI > 30, was not associated with NF-κB activation after controlling for NIDDM. Patterns were similar within Incidental and Surgical BPH groups, with no significant difference in epithelial or stromal cell NF-κB activity with either high or low BMI. In contrast, AR-FL expression was significantly lower in Surgical BPH patients with NIDDM, while differences in AR-V7 expression levels were not significantly associated with BMI or NIDDM.
Table 3.
Adjusted mean biomarker values by BMI and NIDDM, within clinical groups.
| All Pts | Incidental | Surgical | |||||
|---|---|---|---|---|---|---|---|
| Mean# | P | Mean## | P | Mean## | P | ||
| NF-κB-S | BMI < 30 | 6.7 | 0.489 | 6.4 | 0.78 | 7.2 | 0.19 |
| BMI ≥ 30 | 7.6 | 5.9 | 9.7 | ||||
| NIDDM | 10.5 | <0.01 | 10.7 | <0.01 | 10.5 | 0.07 | |
| No NIDDM | 4.8 | 3.5 | 6.6 | ||||
| NF-κB-E | BMI < 30 | 16.1 | 0.18 | 12.8 | 0.13 | 20.2 | 0.78 |
| BMI ≥ 30 | 17.9 | 15.5 | 19.7 | ||||
| NIDDM | 22.9 | <0.01 | 19.5 | <0.01 | 25.7 | <0.01 | |
| No NIDDM | 12.6 | 10.2 | 15.4 | ||||
| AR-FL | BMI < 30 | 9.0 | 0.54 | 11.8 | 0.99 | 7.4 | 0.64 |
| BMI ≥ 30 | 10.5 | 11.8 | 8.7 | ||||
| NIDDM | 7.8 | 0.11 | 11.8 | 0.99 | 5.1 | 0.03 | |
| No NIDDM | 12.3 | 11.8 | 12.7 | ||||
| AR-V7 | BMI < 30 | 53.1 | 0.09 | 17.1 | 0.17 | 167.2 | 0.46 |
| BMI ≥ 30 | 127.7 | 44.7 | 308.6 | ||||
| NIDDM | 67.3 | 0.50 | 28.4 | 0.95 | 140.8 | 0.31 | |
| No NIDDM | 100.8 | 26.9 | 366.4 | ||||
Table 3: Patient medical records were used to determine BMI and NIDDM. Stromal (S) and Epithelial (E) NF-κB activation was determine by quantitated immunofluorescent protein expression. AR-FL and AR-V7 mRNA expression was determine by qPCR.
adjusted geometric mean:- NF-κB and AR variables were natural log transformed prior to analysis, and p values derive from analysis with each marker as the dependent variable in a linear model that included group, BMI, NIDDM, and age. -Biomarkers were then back transformed to produce adjusted mean values.— Thus, p-values represent the likelihood of a difference in biomarker value between each category, adjusted for the other parameters in the model.
Similar approach, but models run separately within each diagnostic group.
Since androgen ablation can lead to the expression of AR-Vs in prostate cancer [30], we also examined whether medication used to treat BPH was associated with NF-κB activation, AR-FL or AR-V7 expression (Table 4). There was a significant increase in AR-V7 in patients with a history of α-blocker use (Table 4), whether in Incidental or Surgical BPH groups. While this result certainly warrants future investigation, we note that α-blockers are the front line therapy for BPH symptoms and this may simply reflect an underlying biology of the disease where patients with high levels of AR-V7 may be more likely to show symptoms that are treatable with α-blockers.
Table 4.
Adjusted Mean biomarker values by BPH medication history
| Mean | ||||
|---|---|---|---|---|
| Marker | Drug(s) | All | Inci | Surg |
| NF-κB-S | 5ARI only | 4.9 | 5.3 | 3.9 |
| α blocker only | 5.3 | 2.0 | 9.7 | |
| 5ARI + α-blocker | 5.2 | 3.1 | 7.1 | |
| None | 6.2 | 5.1 | 3.4 | |
| P | 0.90 | 0.26 | 0.04 | |
| NF-κB-E | 5ARI only | 12.4 | 12.7 | 12.2 |
| α blocker only | 15.3 | 11.8 | 19.4 | |
| 5ARI + α-blocker | 13.2 | 7.1 | 17.5 | |
| None | 14.5 | 11.9 | 14.9 | |
| P | 0.64 | 0.30 | 0.24 | |
| AR-FL | 5ARI only | 14.1 | 4.4 | 31.2 |
| α blocker only | 13.7 | 21.3 | 10.2 | |
| 5ARI + α-blocker | 11.5 | 43.7 | 8.1 | |
| None | 9.1 | 9.9 | 16.2 | |
| P | 0.69 | 0.01 | 0.24 | |
| AR-V7 | 5ARI only | 22.9 | 1.8 | 150.9 |
| α blocker only | 450.8 | 142.5 | 1290.5 | |
| 5ARI + α-blocker | 92.4 | 60.4 | 212.2 | |
| None | 45.9 | 20.0 | 51.9 | |
| P | 0.01 | 0.06 | 0.11 | |
Table 4: Patient medical records were used to determine medication history. Stromal and Epithelial NF-κB activation was determine by quantitated immunofluorescent protein expression. AR-FL and AR-V7 mRNA expression was determine by qPCR. Control for group and age; Group specific analyses control for age; p-values – omnibus test of significant variability within the group being tested, simply indicating whether or not there is a significant association between drug use and biomarker. Specific mean values may be based on only a handful of subjects. -Check n values from table 1. Early associations between 5ARI and NF-κB were due to confounding by differences in 5ARI use between groups. Once group differences are controlled for, 5ARI is no longer associated.
Activation of NF-κB upregulates AR-FL and AR-V7 expression in benign prostate epithelial and stromal cells
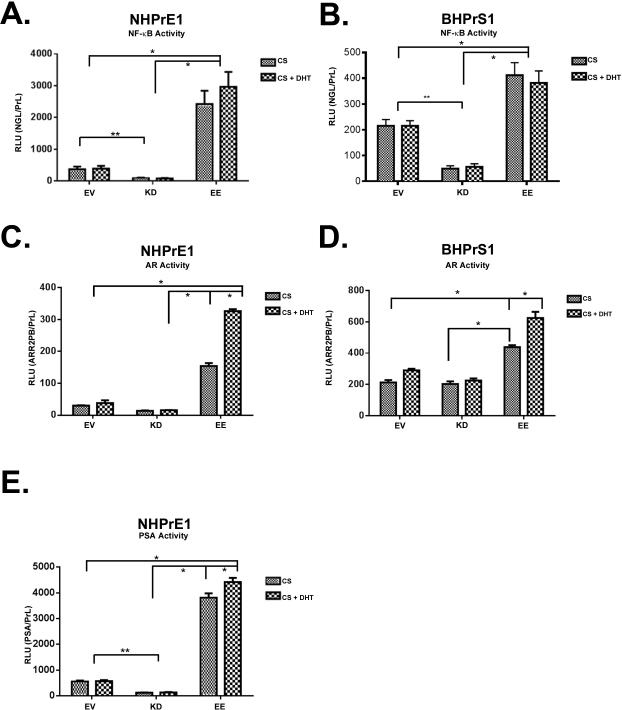
Activation of AR-Vs by NF-κB has previously been described in prostate cancer but not in BPH. To determine whether chronic activation of NF-κB results in increased expression of AR and influenced cell growth and function, we retrovirally transduced benign human prostatic NHPrE1 (epithelial) and BHPrS1 (stromal) cells [35, 36], using empty vector (EV), kinase dead IKK2 (KD), or constitutively active IKK2 (EE) retroviral constructs. KD will block NF-κB activity while EE will increase NF-κB activity. Cells were grown in charcoal-stripped FBS (CS) in the presence/absence of 10−8 M DHT. Activation of NF-κB signaling was assessed using the NGL reporter, a plasmid with an NF-κB responsive element coupled to GFP/Luciferase. We confirmed the successful transduction and increased expression of NF-κB signaling in NHPrE1 cells (Figure 3A) and BHPrS1 cells (Figure 3B). As expected, the background level of NF-κB activity was suppressed by the KD construct and enhanced by the constitutively active EE form of IKK2. The presence or absence of DHT had no significant effect on the activation of NF-κB.
Figure 3.
Chronic activation of NF-κB increases AR activity. NF-κB reporter activity (NGL) vector was transduced into benign epithelial (NHPrE1) and stromal (BHPrS1) cell lines to determine NF-κB activity plus/minus 10−8M DHT after cell lines were transduced with IKK2- empty vector (EV), kinase dead (KD), or constitutively active (EE) constructs. A. NF-κB activity in NHPrE1-EE cell line was significantly increased when compared to -EV and the NHPrE1-KD cell line showing a significant decrease in activity when compared to -EV. B. NF-κB activity in BHPrS1-EE cell line was significantly increase when compared to -EV and BHPrS1-KD cell line showing a significant decrease in activity when compared to -EV. A-B cell lines were reported as relative luciferase units (RLU) normalized to the transfection control Renilla luciferase reporter (pRL) plus/minus 10−8 M dihydrotestosterone (DHT) in charcoal stripped serum (CS). C-D. AR activity was significantly increased in -EE cell lines when compared to -EV and -KD in both the presence and absence of 10−8M DHT. C-D. AR reporter activity (ARR2PB) in (C) NHPrE1 and (D) BHPrS1 cell lines were normalized to Renilla luciferase (pRL) plus/minus 10−8 M DHT. E. Prostate specific antigen (PSA) promoter activity was significantly increased in NHPrE1-EE when compared to -EV and NHPrE1-KD PSA activity was significantly decreased when compared to -EV. PSA activity in NHPrE1 cells using the PSA luciferase promoter was normalized to Renilla luciferase reporter (pRL) plus/minus 10−8 M dihydrotestosterone (DHT) in CS. Error bars are presented as mean +/− SD. Significant differences are compared to EV and are indicated in the graph. p-value: One-Way ANOVA. (* = P < 0.01, ** = P < 0.05).
Having established that the transductions were effective, we used the ARR2PB-Luc reporter that responds to activation of the AR but has no binding sites for NF-κB. Both NHPrE1 and BHPrS1 showed significant activation of the reporter construct when NF-κB signaling was constitutively activated even in the absence of androgens (Figures 3C and D). Stromal cells have a stronger basal reporter activity and a smaller induction of the reporter with DHT without the expression of NF-κB, likely reflecting basal expression of low levels of AR in culture.
NHPrE1 epithelial cells, which do not normally express AR in vitro, showed a clearer induction of signal. Addition of DHT increased the activity of the androgen-driven reporter in both epithelial and stromal cells (Figure 3C and D). We repeated this experiment using a PSA promoter driven luciferase reporter. This construct contains AR responsive elements and also an NF-κB responsive element, previously identified in the PSA gene [50]. Activation of NF-κB drove increased PSA promoter activity that was only slightly increased by the addition of androgens (Figure 3E). These data demonstrate that cells in which NF-κB is active are able to activate AR reporters in the absence of ligand, but retain an additional and greater ligand-driven response.
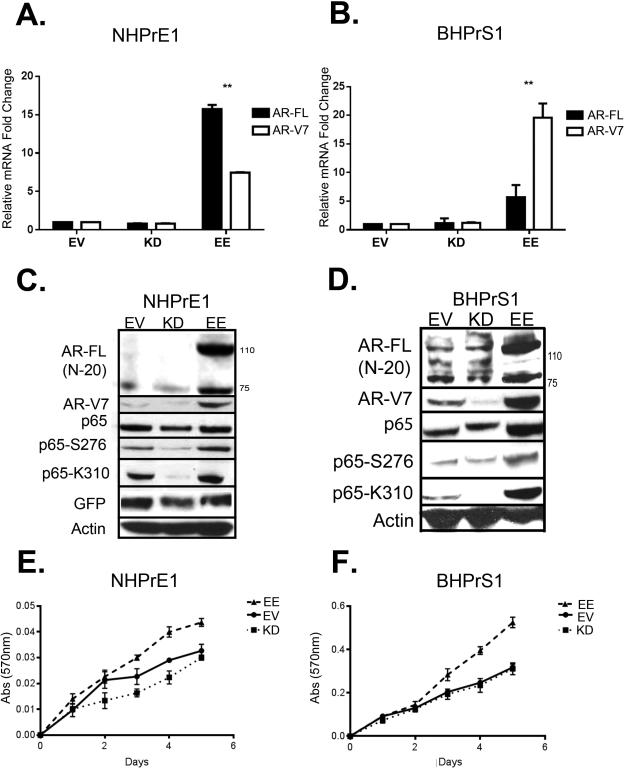
We examined the expression of AR variants V1, V2, V3, V4, V567es, and V7 in the retrovirally transduced cells lines. Consistent with our observations in human tissue samples, the only significant (P < 0.01) increase in expression of variants was in AR-V7 (Supp. Figure 3A, Figure 4A and B). We performed Western blot analysis to examine the relationship between NF-κB activity and the expression of AR-FL and AR-V7. This Western blot analysis (Figure 4C and D) confirmed the luciferase reporter data shown in Figure 3, showing increased phosphorylation of p65 in cells with constitutive activation of NF-κB (EE) and suppression of total and phosphorylated p65 by the kinase dead (KD) construct. Both AR-FL and AR-V7 proteins were upregulated in cells in which NF-κB signaling was constitutively activated. To evaluate the biological effects of NF-κB on proliferation, we performed growth assays. Constitutive activation of NF-κB in prostate epithelial and stromal cells was associated with an increase in proliferation starting by three days in culture in the absence of androgens, compared to the empty vector and kinase dead NF-κB (Figure 4E and F).
Figure 4.
Chronic activation of NF-κB induces AR and AR-V7 expression in benign epithelial and stromal cells. To determine whether transduction of the IKK2 construct affected AR-FL and/or AR-V7 expression at the message and protein level we performed quantitative real time PCR (qPCR), western blot analysis and assessed proliferation in transduced cell lines. qPCR analysis of (A) NHPrE1 and (B) BHPrS1 cell lines of AR-FL and AR-V7 mRNA expression normalized to -EV, showing a significant increase in AR-FL and AR-V7 expression in -EE cell lines when compared to -EV. Western blot analysis of showing increased expression of AR/ARV (N-20), AR-V7, p65, p65-S276, p65-S536, and p65K310 in the -EE cell line when compared to -EV in (C) NHPrE1 and (D) BHPrS1 cell lines. Quantitative analysis of growth is made by a crystal violet assay using absorbance at 570 nm in (E) NHPrE1, showing a significant increase in growth in the -EE cell line and a significant decrease in growth in the -KD cell line when compared to EV, and (F) BHPrS1-EE cell line showing a significant increase in growth compared to -EV. Error bars are presented as means +/− SD. Significant differences are compared to EV and are indicated in the graph p-value: One-Way ANOVA. (*= P < 0.05), **=P < 0.01)
To determine whether the increase proliferation was due to NF-κB or AR-FL we used a cell viability assay based on quantitation of ATP, using cellular metabolism as a surrogate for cell number. AR expression was knocked down in NHPrE1-EV and NHPrE1-EE cell lines. Cells were grown in charcoal-stripped (CS) serum in vehicle or in the presence of 10−7 M Testosterone (T). There was a significant increase in proliferation in EE (P < 0.001) and EE-AR knockdown cells (P < 0.003) when compared to EV and EV-AR knockdown respectively (Supp. Figure 3B). This highlights that the increased proliferation is due to both the expression of NF-κB and expression of AR and increased proliferation when NF-κB and AR are coexpressed.
Chronic Activation of NF-κB induces resistance to a 5ARI
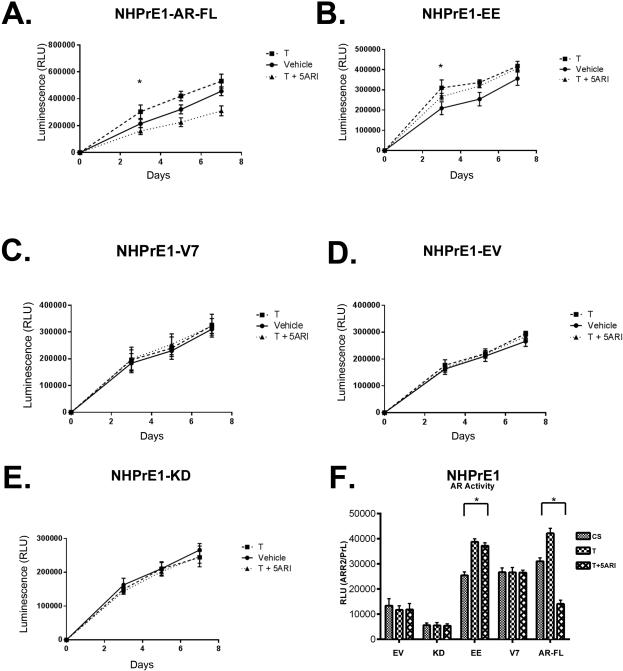
NHPrE1-V7 and NHPrE1-AR-FL were established by drug selection following retroviral transduction with the AR-V7 (V7) and AR-FL overexpression constructs, respectively. To examine whether chronic activation of NF-κB affected cellular response to a 5ARI, we assessed total metabolic activity. NHPrE1-EV, NHPrE1-KD, NHPrE1-EE, NHPrE1-V7, and NHPrE1-AR-FL cells were grown in charcoal-stripped (CS) serum in the presence/absence of 10−9 M Testosterone (T), or 10−9 M T combined with 10−7 M Finasteride (5ARI) or ethanol vehicle as control over a seven day period (Fig 5A-E). T is used rather than DHT since we are accessing the ability of 5ARI to block the conversion of T to DHT. Overall, the activation of NF-κB and overexpression of AR-V7 were resistant to a 5ARI, due to the displaying no decrease in viability in response to a 5ARI.
Figure 5.
Chronic activation of NF-κB induces increased cell viability in response to a 5ARI. (A-E) NHPrE1 cell lines where treated with plus/minus 10−9 Testosterone (T) and 10−7 M Finasteride (5ARI). A. NHPrE1-AR-FL when compared to vehicle had a significant increase in viability in the presence of T and a significant decrease in the addition of T and a 5ARI. B. NHPrE1-EE cells had a significant increase in viability in the presence of plus/minus T and 5ARI when compared to vehicle. C-E. NHPrE1-V7,-EV, and -KD cell lines had no significant difference in viability in the presence of plus/minus T and 5ARI. F. AR reporter activity (ARR2PB) NHPrE1 cell lines were normalized to Renilla Luciferase (pRL) plus/minus 10−9 T and 10−7 M 5ARI. Indicating that AR activity was significantly increased in NHPrE1-EE cells plus/minus T and 5ARI and AR activity was significantly decreased in NHPrE1-AR-FL cell in in the presence of T and 5ARI. NHPrE1-AR-V7, -EV, and -KD had no significant change in AR activity plus/minus T and 5ARI. Error bars are presented as mean +/− SD. Significant differences are compared to EV and are indicated in the graph. p-value: One-Way ANOVA. (* = P < 0.01).
NHPrE1-AR-FL cells had a significant increase in viability in the presence of testosterone over vehicle and had a significant decrease in the presence of a 5ARI (P < 0.05) (Figure 5A). This highlights that these cells are responsive to a 5ARI. NHPrE1-EE cells had an increase in cell viability at three days with the addition of testosterone; however, the addition of a 5ARI did not significantly change overall metabolic activity (Figure 5B). Testosterone alone or in combination with the 5ARI did not affect viability of NHPrE1-V7 cells (Figure 5C). As expected, NHPrE1-EV (Figure 5D) and -KD (Figure 5E) cells did not respond to a 5ARI as these cells express little to no AR.
To examine the effects of chronic activation of NF-κB on epithelial cell function, NHPrE1 cell lines where transfected with the ARR2PB-Luc reporter. This reporter responds to activation of the AR but, unlike the PSA promoter, has no binding sites for NF-κB. NHPrE1-EE and NHPrE1-V7 showed similar increased activation of the reporter construct in the absence of T. As expected, testosterone increased this signal in the NHPrE1-EE cells (which express elevated AR-FL in addition to AR-V7), but not in the NHPrE1-V7 cells. Cells in which only the AR-FL was overexpressed showed a response to testosterone that, as expected, could be down-regulated by the addition of 5ARI (Figure 5F). The NHPrE1-EV and NHPrE1-KD cell lines express little to no AR in 2D culture and therefore would not be expected to respond to the presence of androgens or a 5ARI. These data suggest that chronic activation of NF-κB can induce expression of AR-V7, and that the expression of AR-V7 can confer resistance to the effects of a 5ARI.
To determine if NHPrE1-EV cells express the enzyme 5α-reductase isoforms -1, -2, and -3 (SRD5A1, -2, -3) we perform qPCR of NHPrE1-EV cell grown in charcoal-stripped (CS) serum to determine the relative quantities of mRNA expression. As shown in Supplemental Figure 4A, NHPrE1-EV cell express all three isoforms; however, SRD5A2 is expressed significantly higher than SRD5A1 and SRD5A3 (P < 0.0001). To determine if NHPrE1-AR-FL cells produce de novo synthesis of androgens we used two different approaches. One method used a pharmacological inhibitor to androgen synthesis: abiraterone acetate (Abi), in relation to vehicle, and the second method used was high pressure liquid chromatographic (HPLC) to detect the levels of 5 endogenous androgens. As shown in Supplemental figure 4B, NHPrE1-AR-FL cells grown in CS or CS with Abi treatment resulting in significantly reduce cell viability across a range on concentrations, although concentrations higher than 6μM became toxic to the cells. Also, 5 endogenous androgens [testosterone (T), dihydrotestosterone (DHT), dehydroepiandrosterone (DHEA), androstenedione (ASD), and androsterone (AND)] were quantitated in NHPrE1-AR-FL cells grown in CS serum over a 5 day period. Individual androgen results for the calibrators and QCs are reported in Supplemental Table I. Analysis of NHPrE1-AR-FL showed that these cells can produce T and DHT (Supp. Figure 4C). ASD, AND, and DHEA were below the lower limit of quantitation. This demonstrates that NHPrE1-AR-FL cells can manufacture limited amounts of androgens.
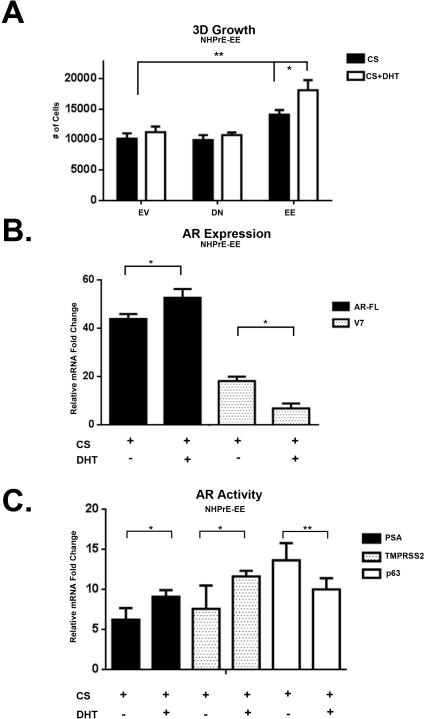
Chronic Activation of NF-κB increases 3D growth
To determine whether chronic activation of NF-κB affected growth in a 3D organoid culture model that we previously described [51], NHPrE1-EV, NHPrE1-KD, and NHPrE1-EE cells were seeded in triplicate in 48 well plates in the absence or presence of DHT (Supp. Figure 5). Figure 6A shows a significant (P <0.01) increase in the number of NHPrE1-EE cells in the presence of DHT. AR-FL and AR-V7 expression in the NHPrE1-EE cells normalized to NHPrE1-EV (Figure 6B) showed a significant increase (P = 0.002) in AR-FL expression and a significant reduction (P = 0.001) of AR-V7 expression 24 hours after treatment with DHT. PSA and TMPRSS2, two genes transcriptionally regulated by AR, have a significant increase in expression (P = 0.01 and 0.012 respectively - Figure 6C) in the presence of DHT. These data suggest that chronic activation of NF-κB is able to significantly induce AR activity in a ligand-independent manner. A concomitant reduction in the relative expression of p63 (a marker of basal phenotype prominent in 2D culture) in the presence of androgens reflects increased differentiation and organization of the organoids in 3D culture (Supp. Figure 5).
Figure 6.
3D Growth of NHPrE1-EE induces AR expression. A. Quantitative analysis of NHPrE1 cells grown in matrigel over a ten day period in absence or presence of DHT (10−8 M) showing a significant increase in growth in NHPrE1-EE in the presence/absence of DHT when compared to -EV. B. qPCR of AR-FL and AR-V7 expression in NHPrE1-EE cells in the absence or presence of DHT (10−8 M) normalized to NHPrE1-EV showing a significant increase in AR-FL mRNA expression in the presence of DHT and a significant decrease in AR-V7 mRNA expression in the presence of DHT. C. AR activity in NHPrE1-EE was significantly increased in the presence of DHT. AR activity was analyzed by qPCR for the induction of PSA and TMPRSS2. p63, was used as basal cell differentiation marker and was significantly decreased in the presence of DHT when normalized to NHPrE1-EV GAPDH. Error bars are presented as means +/− SD. Significant differences are compared to NHPrE1-EV and are indicated in the graph (A: p-value: One-Way ANOVA, B and C: p-value: Two way ANOVA (*= P < 0.01), **=P < 0.05)
Inhibition of NF-κBabrogates AR signaling
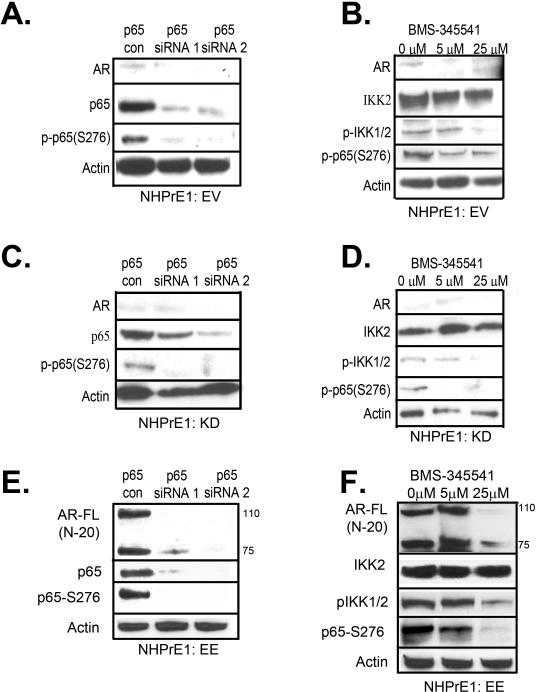
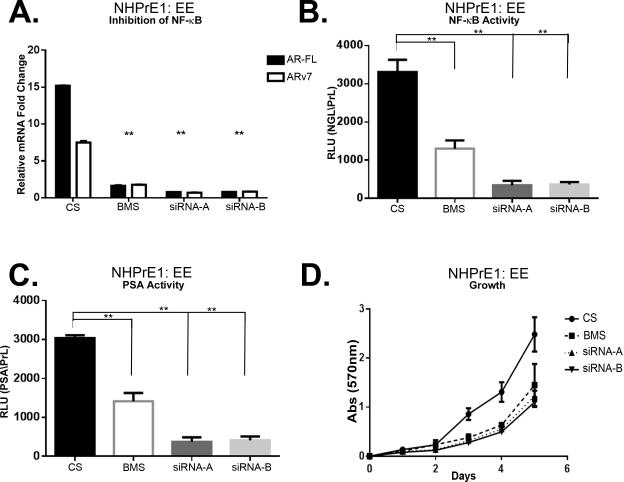
To better understand the specific role of canonical NF-κB signaling in regulating AR expression in benign prostate cells, we performed a series of experiments aimed at abrogating critical downstream effectors. Since the constitutive activation of NF-κB in the EE-transduced cells is regulated by a modified form of IKK2, we used two alternative approaches to suppress NF-κB signaling. We used silencing of NF-κB, through siRNA targeting of p65, and the allosteric inhibition of IKK2 with the chemical compound BMS-345541. We determined whether these approaches had an effect on AR-FL expression or the proliferation of NHPrE1-EE cells and controls (Figure 7 and 8). As shown in Figure 7, inhibition of NF-κB by p65 siRNA or BMS-345541 (25 μM) was sufficient to reduce p65 phosphorylation (and total p65 in the case of the siRNA approach). Significantly, inhibition of p65 by siRNA (Figure 7E) or BMS-345541 (Figure 7F) was sufficient to reduce AR-FL and AR-V7 protein levels. siRNA knockdown or chemical inhibition of NF-κB also decreased AR-FL and AR-V7 mRNA expression as determined by qPCR (Figure 8A). While use of the BMS inhibitor resulted in an approximately 50% reduction in NGL luciferase expression, use of p65 siRNA resulted in almost complete abrogation of NGL activity (Figure 8B). We also found that suppression of NF-κB by either method reduced AR action in the absence of DHT (reflecting a reduction in AR-V levels and associated constitutive activity) using the PSA promoter luciferase reporter (Figure 8C). Next we assessed whether inhibition of NF-κB in NHPrE1-EE cells had an effect on proliferation. As shown in Figure 8D, significant (P <0.05) inhibition of the proliferation of NHPrE1-EE cells was seen by 3 days in culture and was maintained until the end of the experiment at 5 days. These data confirm that chronic activation of NF-κB in prostate epithelial cells can regulate proliferation through expression of AR-FL and AR-V7.
Figure 7.
Inhibition of NF-κB decreases AR-FL and AR-V7 protein expression. (A,C,E) Western blot confirming knockdown of NF-κB in NHPrE1-EV,-KD and -EE cell lines by transfection of p65-siRNA (20 μM). Showing decreased p65 protein expression and activity (p-p65 S276) (B,D,F) Western blot confirming the inhibition of NF-κB in NHPrE1-EV, -KD, and -EE by a highly selective IKK2 inhibitor BMS-345541 (5 and 25 μM). IKK2 expression is not changed with the addition of the BMS-345541, however the phosphorylation of IKK2 and p65 activity (p-p65 S276) were decreased.
Figure 8.
Inhibition of NF-κB decreases AR-FL and AR-V7 mRNA expression and AR activity. A. qPCR of NHPrE1-EE for AR-FL and AR-V7 transfected with p65-siRNA or incubated with BMS-345541 showing a significant decrease in both AR-FL and AR-V7 mRNA expression. AR-FL and AR-V7 are normalized to NHPrE1-EV in charcoal stripped media (CS). (B) NF-κB activity in NHPrE1-EE was significantly decreased when transfected with p65-siRNA or treated with BMS-345541 when compared to NHPrE1-EE cell grown in CS. Reported as RLU normalized to pRL. C. AR activity was significantly decreased when transfected with p65-siRNA or incubated with BMS-345541. Reported as prostate specific antigen (PSA) normalized to pRL. D. Quantitative analysis of growth was significantly decrease when transfected with p65-siRNA or incubated with BMS-345541. Analysis by crystal violet at absorbance 570 nM of NHPrE1-EE cell lines transfected with p65-siRNA or incubated with BMS-345541. Error bars are presented as means +/- SD. Significant differences are compared to CS and are indicated in the graph. p-value: One-Way ANOVA (** = P < 0.01).
DISCUSSION
Benign prostatic hyperplasia and associated LUTS represent a common health problem resulting in significant morbidity and associated patient care costs [1, 52]. The most common medical approaches for patients with BPH/LUTS are α-adrenergic blockers and 5α-reductase inhibitors. While often initially effective in relieving LUTS, many patients see a slow and continued progression of their disease [15]. As a result, even though the routine use of 5ARIs and α-adrenergic inhibitors has reduced the number of patients that undergo surgical intervention, surgery remains common with approximately 260,000 such interventions performed annually [53]. These patients are usually older, often with comorbidities such as obesity and diabetes, and thus are often not prime candidates for surgery. An understanding of the underlying biological factors that result in disease progression should lead to new approaches to help prevent resistance to therapy with subsequent disease progression and the need for surgical intervention.
BPH has long been considered to be a product of androgen action upon an aging prostate. However, longitudinal epidemiological studies from numerous patient cohorts that controlled for related hormone levels have shown that androgen levels are unlikely to be solely responsible [54]. Separate mechanisms are almost certainly in play in many patients [48, 55]. Furthermore, comorbidities associated with inflammation appear to play an important role in disease progression [56] potentially contributing to cell proliferation and resistance to current therapies. There is broad recognition in the field that BPH represents a variety of pathologies, which may lead to prostatic inflammation, increased prostate volume and therapeutic resistance [45, 57]. Baseline prostate volume and PSA levels are the most reliable clinical predictors of acute urinary retention and surgical intervention for BPH/LUTS [33] and 5ARIs are the most widely used therapy for treating prostatic enlargement. Accordingly, our goal is to understand the mechanisms that cause resistance to 5ARI therapy.
We have developed a repository of human prostate transition zone tissues representing a range of BPH/LUTS severity. A previous analysis of this resource demonstrated that increased expression of AP-1 transcription factors was associated with advanced disease [12]. Consistent with observations from other groups, our data suggested a possible autoimmune inflammatory component to BPH [10, 11].
In this study we examined the activation of the p65/RelA, the canonical NF-κB pathway, as well as the expression of constitutively active AR variants in BPH and BPH-derived cell lines. Increased activation of canonical NF-κB was seen in both the epithelial and stromal cells of human BPH and was increased in more advanced disease. Since NF-κB activation has also been linked to the expression of AR-V in prostate cancer [31, 34], we examined the expression of a panel of AR-Vs in BPH. Significant expression of AR-V7 mRNA and protein was found while the other AR variants were either not expressed or were detected at very low levels. We also saw increased expression of AR-FL associated with canonical NF-κB activation adjacent to areas of inflammation compared to areas lacking inflammation. To the best of our knowledge, this is the first report on the expression of AR variants in human BPH and suggests a mechanism for escape from growth regulation by 5ARI therapy.
Analysis of the clinical samples revealed a number of interesting correlations in detection of NF-κB and AR-V7. We determined that patients with more advanced disease showed higher levels of both epithelial and stromal nuclear p-p65 (NF-κB activation) and also increased AR-V7 transcript levels. We determined that increased AR-V7 levels positively correlate with AUASS and TRUS volume. We also determined that increased activation of epithelial cell NF-κB correlates with increased TRUS volume. This suggests a linkage between disease progression, activation of NF-κB and expression of AR-V7. We hypothesized that obesity would also be linked to these same outcomes, but based on a binary analysis (BMI < 30 vs. BMI ≥ 30) there was no significant relationship between obesity and either NF-κB or AR-V7 expression after adjusting for differences in NIDDM prevalence between groups. In contrast, NIDDM was positively associated with NF-κB expression. Past studies have found direct associations between diabetes and BPH progression [58]. This might suggest that specific forms of systemic stress exert distinct influences in the pathogenesis of BPH, and reinforces the idea that, although prostatic hyperplasia is associated with both obesity and diabetes, these conditions may act through separate or overlapping mechanisms [2]. We also saw an association between α-blocker treated patients as having increased NF-κB activation and AR-V7 expression. Conflicting reports in literature suggest that α-adrenergic receptors are both pro-inflammatory [59-61] and anti-inflammatory [62-64]. Our data would be consistent with the latter scenario, that α-adrenergic receptors are anti-inflammatory and that patients on an α-blocker would have NF-κB activation increased. This could also support our observations of increased AR-V7 levels in this patient population. The role of α-blocker to alter inflammation needs to be elucidated further.
We performed a series of 2D and 3D in vitro experiments to investigate whether activation of NF-κB can result in the expression of both AR full length (AR-FL) and AR-V7 in benign epithelial and stromal cell lines. The findings were consistent in epithelial and stromal cell lines in that the constitutive activation of NF-κB resulted in the coordinate expression of both AR-FL and AR-V7. This is consistent with observations in prostate cancer where both forms of the androgen receptor are regulated together by the NF-κB pathway [34].We next performed a series of in vitro experiments to determine if NF-κB and specifically AR-V7 can induce resistance to a 5ARI. These findings demonstrated that forced activation of NF-κB and over expression of AR-V7 are able to induce resistance to a 5ARI. We also showed by HLPC LC/MS/MS analysis that NHPrE1-AR-FL cells can undertake androgen synthesis. This could suggest another mechanism by which BPH patients could fail 5ARI therapy. Stromal cells are an important component of BPH, which is often considered to be a disease of the stromal tissue with epithelial growth as a secondary consequence. This follows the early observations of McNeal who suggested the reawakening of fetal mesenchymal potential contributed to the development of BPH, a concept subsequently validated by Cunha and colleagues in rodent and tissue recombination models [65, 66]. The activation of NF-κB presented here is consistent with the idea that inflammation plays a role in BPH progression as well as providing a potential molecular mechanism of resistance to 5ARIs via activation of AR-V7 expression. AR-V7 has been shown to modulate expression of a number of tumor-promoting autocrine/paracrine growth factors in prostate cancer [47]. However, it is likely that this is a multifactorial process in which underlying comorbidities play a complex role. There may well be a number of alternative and possibly complementary pathways involved in this process. For example, our previous observations that AP-1 transcription factors (molecules known to be related to several immune/inflammatory conditions) are highly enriched in symptomatic BPH [12]. AP-1 transcription factors are post-transcriptionally regulated by upstream factors such as NF-κB, JNK, ERK, and p38 [67] and therefore activation of NF-κB can upregulate not only AR-FL and AR-V7 but also AP-1 factors which can serve as AR co-factors to regulate transcription, which could lead to the development and progression of therapy-resistant BPH. These pathways might be interrelated, with no individual change (for example, androgen synthesis, activation of stress factors, expression of AR-V7) being sufficient to allow escape from therapy but that these individual changes may be additive and allow for eventual regrowth of the prostate in the face of therapy.
In summary, the observations presented here provide a potential mechanism to explain the previously observed links between prostatic inflammation and 5ARI resistance [45], whereby aberrant activation of NF-κB drives AR-V7 expression resulting in ligand independent activation of AR resulting in 5ARI resistance. BPH is a complex condition and there are likely multiple pathways in play in individual patients relating to common comorbidities including diabetes, obesity and metabolic syndrome. An understanding of the stress pathways active in individual patients may provide a route to appropriately tailor therapy and avoid the profile of resistance and subsequent progression to surgery that is seen in many patients.
Supplementary Material
Acknowledgements
The authors thank Dr. Timothy Blackwell (Vanderbilt) and Dr. Ganesh Raj (UT Southwestern) their kind gifts of reagents. Prostate tissue was provided by the Cooperative Human Tissue Network (CHTN) - Western Division. We thank the NorthShore Histology and Imaging Core for help with the immunohistochemical localization of AR and AR-V. The LC-MS/MS analysis was conducted by the Bioanalytics, Metabolomics and Pharmacokinetics (BMPK) Shared Resource of Roswell Park Cancer Institute, and partially supported by National Cancer Institute (NCI) grant P30CA016056.
Grant Acknowledgement: This work is supported through grants from the National Institutes of Health to DWS (DK098277), RJM (R01-CA076142 and R01-DK055748) and SWH (DK103483, DK067049, DK097782 and DK104280). The work was supported in part by the Vanderbilt-Ingram Cancer Center CCSG award No. P30 CA068485 from the National Cancer Institute through the use of the Translational Pathology Shared Resource. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Platz EA, Joshu CE, Mondul AM, Peskoe SB, Willett WC, Giovannucci E. Incidence and progression of lower urinary tract symptoms in a large prospective cohort of United States men. J Urol. 2012;2:496–501. doi: 10.1016/j.juro.2012.03.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammarsten J, Peeker R. Urological aspects of the metabolic syndrome. Nat Rev Urol. 2011;9:483–494. doi: 10.1038/nrurol.2011.112. [DOI] [PubMed] [Google Scholar]
- 3.McNeal J. Pathology of benign prostatic hyperplasia. Insight into etiology. Urol Clin North Am. 1990;3:477–486. [PubMed] [Google Scholar]
- 4.Rohrmann S, De Marzo AM, Smit E, Giovannucci E, Platz EA. Serum C-reactive protein concentration and lower urinary tract symptoms in older men in the Third National Health and Nutrition Examination Survey (NHANES III). Prostate. 2005;1:27–33. doi: 10.1002/pros.20110. [DOI] [PubMed] [Google Scholar]
- 5.Schenk JM, Calip GS, Tangen CM, Goodman P, Parsons JK, Thompson IM, Kristal AR. Indications for and use of nonsteroidal antiinflammatory drugs and the risk of incident, symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol. 2012;2:156–163. doi: 10.1093/aje/kwr524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fibbi B, Penna G, Morelli A, Adorini L, Maggi M. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int J Androl. 2010;3:475–488. doi: 10.1111/j.1365-2605.2009.00972.x. [DOI] [PubMed] [Google Scholar]
- 7.McVary KT, Rademaker A, Lloyd GL, Gann P. Autonomic nervous system overactivity in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2005;4(Pt 1):1327–1433. doi: 10.1097/01.ju.0000173072.73702.64. [DOI] [PubMed] [Google Scholar]
- 8.Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011;1:29–41. doi: 10.1038/nrurol.2010.207. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Nieves JA, Macoska JA. Prostatic fibrosis, lower urinary tract symptoms, and BPH. Nat Rev Urol. 2013;9:546–550. doi: 10.1038/nrurol.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;5:1202–1216. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Madigan AA, Sobek KM, Cummings JL, Green WR, Bacich DJ, O'Keefe DS. Activation of innate anti-viral immune response genes in symptomatic benign prostatic hyperplasia. Genes Immun. 2012;7:566–572. doi: 10.1038/gene.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin-Tsai O, Clark PE, Miller NL, Fowke JH, Hameed O, Hayward SW, Strand DW. Surgical intervention for symptomatic benign prostatic hyperplasia is correlated with expression of the AP-1 transcription factor network. Prostate. 2014;6:669–679. doi: 10.1002/pros.22785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lepor H, Kazzazi A, Djavan B. alpha-Blockers for benign prostatic hyperplasia: the new era. Curr Opin Urol. 2012;1:7–15. doi: 10.1097/MOU.0b013e32834d9bfd. [DOI] [PubMed] [Google Scholar]
- 14.Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G, Aria A, Investigators AS. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002;3:434–441. doi: 10.1016/s0090-4295(02)01905-2. [DOI] [PubMed] [Google Scholar]
- 15.McConnell JD, Roehrborn CG, Bautista OM, Andriole GL, Jr., Dixon CM, Kusek JW, Lepor H, McVary KT, Nyberg LM, Jr., Clarke HS, Crawford ED, Diokno A, Foley JP, Foster HE, Jacobs SC, Kaplan SA, Kreder KJ, Lieber MM, Lucia MS, Miller GJ, Menon M, Milam DF, Ramsdell JW, Schenkman NS, Slawin KM, Smith JA, G. the Medical Therapy of Prostatic Symptoms Research The Long-Term Effect of Doxazosin, Finasteride, and Combination Therapy on the Clinical Progression of Benign Prostatic Hyperplasia. The New England Journal of Medicine. 2003;25:2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 16.Lu-Yao GL, Barry MJ, Chang CH, Wasson JH, Wennberg JE. Transurethral resection of the prostate among Medicare beneficiaries in the United States: time trends and outcomes. Prostate Patient Outcomes Research Team (PORT). Urology. 1994;5:692–698. doi: 10.1016/s0090-4295(94)80208-4. discussion 698-699. [DOI] [PubMed] [Google Scholar]
- 17.Hu DG, Hickey TE, Irvine C, Wijayakumara DD, Lu L, Tilley WD, Selth LA, Mackenzie PI. Identification of androgen receptor splice variant transcripts in breast cancer cell lines and human tissues. Hormones & cancer. 2014;2:61–71. doi: 10.1007/s12672-014-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;11:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 19.Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;51:6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 20.Toubi E, Shoenfeld Y. Toll-like receptors and their role in the development of autoimmune diseases. Autoimmunity. 2004;3:183–188. doi: 10.1080/08916930410001704944. [DOI] [PubMed] [Google Scholar]
- 21.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;13:5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;6:2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, Bova GS, Luo J. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;1:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu R, Isaacs WB, Luo J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. Prostate. 2011;15:1656–1667. doi: 10.1002/pros.21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, Page ST, Coleman IM, Nguyen HM, Sun H, Nelson PS, Plymate SR. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;8:2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, Kim K, Sawyers CL. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proceedings of the National Academy of Sciences. 2010;39:16759–16765. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Morrissey C, Sun S, Ketchandji M, Nelson PS, True LD, Vakar-Lopez F, Vessella RL, Plymate SR. Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PLoS ONE. 2011;11:e27970. doi: 10.1371/journal.pone.0027970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, Lotan TL, Zheng Q, De Marzo AM, Isaacs JT, Isaacs WB, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;11:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;2:483–489. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Z, Chen S, Sowalsky AG, Voznesensky OS, Mostaghel EA, Nelson PS, Cai C, Balk SP. Rapid Induction of Androgen Receptor Splice Variants by Androgen Deprivation in Prostate Cancer. Clin Cancer Res. 2014:1590–1600. doi: 10.1158/1078-0432.CCR-13-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nadiminty N, Tummala R, Liu C, Yang J, Lou W, Evans CP, Gao AC. NF-kappaB2/p52 induces resistance to enzalutamide in prostate cancer: role of androgen receptor and its variants. Mol Cancer Ther. 2013;8:1629–1637. doi: 10.1158/1535-7163.MCT-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin R, Yamashita H, Yu X, Wang J, Franco OE, Wang Y, Hayward SW, Matusik RJ. Inhibition of NF-kappa B signaling restores responsiveness of castrate-resistant prostate cancer cells to anti-androgen treatment by decreasing androgen receptor-variant expression. Oncogene. 2015;28:3700–3710. doi: 10.1038/onc.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roehrborn CG. Definition of at-risk patients: baseline variables. BJU Int. 2006:7–11. doi: 10.1111/j.1464-410X.2006.06098.x. discussion 21-12. [DOI] [PubMed] [Google Scholar]
- 34.Jin R, Yamashita H, Yu X, Wang J, Franco OE, Wang Y, Hayward SW, Matusik RJ. Inhibition of NF-kappa B signaling restores responsiveness of castrate-resistant prostate cancer cells to anti-androgen treatment by decreasing androgen receptor-variant expression. Oncogene. 2014 doi: 10.1038/onc.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franco OE, Jiang M, Strand DW, Peacock J, Fernandez S, Jackson RS, 2nd, Revelo MP, Bhowmick NA, Hayward SW. Altered TGF-beta signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 2011;4:1272–1281. doi: 10.1158/0008-5472.CAN-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang M, Strand DW, Fernandez S, He Y, Yi Y, Birbach A, Qiu Q, Schmid J, Tang DG, Hayward SW. Functional Remodeling of Benign Human Prostatic Tissues In Vivo by Spontaneously Immortalized Progenitor and Intermediate Cells. Stem Cells. 2010:344–356. doi: 10.1002/stem.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diessenbacher P, Hupe M, Sprick MR, Kerstan A, Geserick P, Haas TL, Wachter T, Neumann M, Walczak H, Silke J, Leverkus M. NF-kappaB inhibition reveals differential mechanisms of TNF versus TRAIL-induced apoptosis upstream or at the level of caspase-8 activation independent of cIAP2. J Invest Dermatol. 2008;5:1134–1147. doi: 10.1038/sj.jid.5701141. [DOI] [PubMed] [Google Scholar]
- 38.Leverkus M, Sprick MR, Wachter T, Denk A, Brocker EB, Walczak H, Neumann M. TRAIL-induced apoptosis and gene induction in HaCaT keratinocytes: differential contribution of TRAIL receptors 1 and 2. J Invest Dermatol. 2003;1:149–155. doi: 10.1046/j.1523-1747.2003.12332.x. [DOI] [PubMed] [Google Scholar]
- 39.Marienfeld R, Berberich-Siebelt F, Berberich I, Denk A, Serfling E, Neumann M. Signal-specific and phosphorylation-dependent RelB degradation: a potential mechanism of NF-kappaB control. Oncogene. 2001;56:8142–8147. doi: 10.1038/sj.onc.1204884. [DOI] [PubMed] [Google Scholar]
- 40.Everhart MB, Han W, Sherrill TP, Arutiunov M, Polosukhin VV, Burke JR, Sadikot RT, Christman JW, Yull FE, Blackwell TS. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J Immunol. 2006;8:4995–5005. doi: 10.4049/jimmunol.176.8.4995. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Thomas TZ, Kasper S, Matusik RJ. A small composite probasin promoter confers high levels of prostate- specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo. Endocrinology. 2000;12:4698–4710. doi: 10.1210/endo.141.12.7837. [DOI] [PubMed] [Google Scholar]
- 42.Gao N, Zhang J, Rao MA, Case TC, Mirosevich J, Wang Y, Jin R, Gupta A, Rennie PS, Matusik RJ. The role of hepatocyte nuclear factor-3 alpha (Forkhead Box A1) and androgen receptor in transcriptional regulation of prostatic genes. Mol Endocrinol. 2003;8:1484–1507. doi: 10.1210/me.2003-0020. [DOI] [PubMed] [Google Scholar]
- 43.Wilton JH, Titus MA, Efstathiou E, Fetterly GJ, Mohler JL. Androgenic biomarker profiling in human matrices and cell culture samples using high throughput, electrospray tandem mass spectrometry. Prostate. 2014;7:722–731. doi: 10.1002/pros.22792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strand DW, Jiang M, Murphy TA, Yi Y, Konvinse KC, Franco OE, Wang Y, Young JD, Hayward SW. PPARgamma isoforms differentially regulate metabolic networks to mediate mouse prostatic epithelial differentiation. Cell Death Dis. 2012:e361. doi: 10.1038/cddis.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nickel JC, Roehrborn CG, O'Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol. 2008;6:1379–1384. doi: 10.1016/j.eururo.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang L, Altuwaijri S, Deng F, Chen L, Lal P, Bhanot UK, Korets R, Wenske S, Lilja HG, Chang C, Scher HI, Gerald WL. NF-kappaB regulates androgen receptor expression and prostate cancer growth. Am J Pathol. 2009;2:489–499. doi: 10.2353/ajpath.2009.080727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun F, Chen HG, Li W, Yang X, Wang X, Jiang R, Guo Z, Chen H, Huang J, Borowsky AD, Qiu Y. Androgen receptor splice variant AR3 promotes prostate cancer via modulating expression of autocrine/paracrine factors. J Biol Chem. 2014;3:1529–1539. doi: 10.1074/jbc.M113.492140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parsons JK. Modifiable risk factors for benign prostatic hyperplasia and lower urinary tract symptoms: new approaches to old problems. J Urol. 2007;2:395–401. doi: 10.1016/j.juro.2007.03.103. [DOI] [PubMed] [Google Scholar]
- 49.Parsons JK. Benign Prostatic Hyperplasia and Male Lower Urinary Tract Symptoms: Epidemiology and Risk Factors. Current bladder dysfunction reports. 2010;4:212–218. doi: 10.1007/s11884-010-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen CD, Sawyers CL. NF-kappa B activates prostate-specific antigen expression and is upregulated in androgen-independent prostate cancer. Mol Cell Biol. 2002;8:2862–2870. doi: 10.1128/MCB.22.8.2862-2870.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strand DW, Degraff DJ, Jiang M, Sameni M, Franco OE, Love HD, Hayward WJ, Lin-Tsai O, Wang AY, Cates JM, Sloane BF, Matusik RJ, Hayward SW. Deficiency in Metabolic Regulators PPARgamma and PTEN Cooperates to Drive Keratinizing Squamous Metaplasia in Novel Models of Human Tissue Regeneration. Am J Pathol. 2013;2:449–459. doi: 10.1016/j.ajpath.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saigal CS, Joyce G. Economic costs of benign prostatic hyperplasia in the private sector. J Urol. 2005;4:1309–1313. doi: 10.1097/01.ju.0000152318.79184.6f. [DOI] [PubMed] [Google Scholar]
- 53.Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2005;4:1256–1261. doi: 10.1097/01.ju.0000155709.37840.fe. [DOI] [PubMed] [Google Scholar]
- 54.Arrighi HM, Metter EJ, Guess HA, Fozzard JL. Natural history of benign prostatic hyperplasia and risk of prostatectomy. The Baltimore Longitudinal Study of Aging. Urology. 1991;1(Suppl):4–8. doi: 10.1016/0090-4295(91)80191-9. [DOI] [PubMed] [Google Scholar]
- 55.Parsons JK, Carter HB, Partin AW, Windham BG, Metter EJ, Ferrucci L, Landis P, Platz EA. Metabolic Factors Associated with Benign Prostatic Hyperplasia. J Clin Endocrinol Metab. 2006;7:2562–2568. doi: 10.1210/jc.2005-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schenk JM, Kristal AR, Arnold KB, Tangen CM, Neuhouser ML, Lin DW, White E, Thompson IM. Association of symptomatic benign prostatic hyperplasia and prostate cancer: results from the prostate cancer prevention trial. Am J Epidemiol. 2011;12:1419–1428. doi: 10.1093/aje/kwq493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roehrborn CG. Combination medical therapy for lower urinary tract symptoms and benign prostatic hyperplasia. Rev Urol. 2005:S43–51. [PMC free article] [PubMed] [Google Scholar]
- 58.Breyer BN, Sarma AV. Hyperglycemia and insulin resistance and the risk of BPH/LUTS: an update of recent literature. Curr Urol Rep. 2014;12:462. doi: 10.1007/s11934-014-0462-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maestroni GJ. Dendritic cell migration controlled by alpha 1b-adrenergic receptors. J Immunol. 2000;12:6743–6747. doi: 10.4049/jimmunol.165.12.6743. [DOI] [PubMed] [Google Scholar]
- 60.Heijnen CJ, van der Voort C. Rouppe, van de Pol M, Kavelaars A. Cytokines regulate alpha(1)-adrenergic receptor mRNA expression in human monocytic cells and endothelial cells. J Neuroimmunol. 2002;1-2:66–72. doi: 10.1016/s0165-5728(02)00034-6. [DOI] [PubMed] [Google Scholar]
- 61.Perez DM, Papay RS, Shi T. alpha1-Adrenergic receptor stimulates interleukin-6 expression and secretion through both mRNA stability and transcriptional regulation: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB. Molecular pharmacology. 2009;1:144–152. doi: 10.1124/mol.108.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farmer P, Pugin J. beta-adrenergic agonists exert their “anti-inflammatory” effects in monocytic cells through the IkappaB/NF-kappaB pathway. American journal of physiology. Lung cellular and molecular physiology. 2000;4:L675–682. doi: 10.1152/ajplung.2000.279.4.L675. [DOI] [PubMed] [Google Scholar]
- 63.Romero-Sandoval EA, McCall C, Eisenach JC. Alpha2-adrenoceptor stimulation transforms immune responses in neuritis and blocks neuritis-induced pain. J Neurosci. 2005;39:8988–8994. doi: 10.1523/JNEUROSCI.2995-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;4:595–638. [PubMed] [Google Scholar]
- 65.McNeal JE. Prostate Anatomy and BPH Morphogenisis. Prog. Clin. Biol. Res. 1984:27–54. [PubMed] [Google Scholar]
- 66.Hayward SW, Haughney PC, Rosen MA, Greulich KM, Weier HU, Dahiya R, Cunha GR. Interactions between adult human prostatic epithelium and rat urogenital sinus mesenchyme in a tissue recombination model. Differentiation. 1998;3:131–140. doi: 10.1046/j.1432-0436.1998.6330131.x. [DOI] [PubMed] [Google Scholar]
- 67.Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;11:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.