Abstract
Study Objectives:
To examine whether patients with restless legs syndrome demonstrate specific alterations in cardiovascular autonomic control.
Methods:
Patients with moderate-severe restless legs syndrome (n = 20, 80% female) and controls (n = 20) matched for age, sex, body mass index, and free of hypertension and cardiovascular disease were enrolled. We assessed cardiovagal baroreflex gain via the modified Oxford technique, sympathetically mediated vascular responses to isometric exercise to fatigue, bradycardiac response to Valsalva maneuver, and respiratory sinus arrhythmia during paced breathing. Standard electrocardiography, beat-by-beat arterial pressure, respiration, and popliteal blood flow velocity were recorded continuously.
Results:
Resting blood pressure and heart rate were similar between groups. However, baroreflex gain averaged 14.3 ± 1.4 msec/mm Hg in restless legs syndrome and was lower than in controls (22.6 ± 3.5 msec/mm Hg, P = 0.04). Hemodynamic responses to isometric exercise were similar between groups, though participants with restless legs syndrome had lower leg blood flow (P < 0.001), with greater leg vascular resistance (P < 0.0001), before and during isometric exercise. Respiratory sinus arrhythmia and Valsalva ratios were similar between groups. Neither baroreflex gain nor vascular resistance was correlated with sleep duration, sleep quality, or symptom duration.
Conclusion:
Patients with restless legs syndrome demonstrate compromised cardiovagal control, specific to the arterial baroreflex, with greater peripheral vascular resistance, potentially due to heightened sympathetic outflow. These autonomic alterations may directly relate to the higher prevalence of cardiovascular disease in restless legs syndrome.
Citation:
Bertisch SM, Muresan C, Schoerning L, Winkelman JW, Taylor JA. Impact of restless legs syndrome on cardiovascular autonomic control. SLEEP 2016;39(3):565–571.
Keywords: baroreflex, sleep, heart rate variability, restless legs syndrome, periodic leg movements of sleep
Significance.
Moderate-severe restless legs syndrome (RLS) is prevalent in 2–4% of adults and is associated with increased cardiovascular risk. However, the physiological mechanisms by which RLS may impact development of cardiovascular disease have been largely unexplored. We investigated whether patients with moderate-severe RLS (n = 20) exhibited impaired autonomic cardiovascular control compared to matched controls (n = 20). The RLS group demonstrated reduced cardiovagal baroreflex gain and greater peripheral vascular resistance, suggesting RLS may impair vagal control and heighten sympathetic outflow- autonomic impairment, which independently contributes to cardiovascular risk. Thus, alterations in autonomic cardiovascular control may be one mechanism by which RLS contributes to increased cardiovascular risk. Future studies are needed to determine whether treating RLS may improve cardiovascular control, and thereby reduce cardiovascular risk.
INTRODUCTION
Restless legs syndrome (RLS) is a common sensorimotor neurologic disorder characterized by an uncontrollable urge to move the legs, often associated with discomfort and/or dysesthesias. Recently, several large epidemiologic studies,1–4 though not all,5,6 have demonstrated RLS is associated with cardiovascular disease, with greatest risk among those who have more frequent symptoms3,7 and an RLS diagnosis for ≥ 3 years.1
Despite these findings, few have investigated the physiologic pathways by which RLS may elevate cardiovascular risk. Previous studies have focused on the cardiovascular alterations that coincide with periodic leg movements of sleep (PLMS),8–11 which are present in > 80% of patients with RLS. Other work has suggested lesser cardiac vagal modulation12,13 and lesser heart rate response to orthostatic stress in RLS.12 Though these emergent data indicate that cardiovascular autonomic responses may be altered in RLS, studies directly probing cardiovascular autonomic control in RLS are sparse.
We sought to determine if persons with moderate-severe RLS, without cardiovascular comorbidities, exhibit compromised cardiovascular autonomic control in comparison to age-, sex-, and BMI-matched controls. Specifically, we assessed resting hemodynamics, arterial baroreflex gain via the modified Oxford technique, sympathetically-mediated vasoconstrictor responses to isometric exercise, respiratory sinus arrhythmia magnitude, and bradycardiac response to the Valsalva maneuver. From this array of assessments, we sought to investigate whether RLS imparts broad alterations in cardiovascular control, or acts more specifically, by altering a particular autonomic limb (parasympathetic vs. sympathetic) or impacting a distinct response.
METHODS
All procedures were approved by the Institutional Review Board at Spaulding Rehabilitation Hospital, with other institutional review boards ceding review (i.e., Beth Israel Deaconess Medical Center; Brigham and Women's Hospital). All participants provided written informed consent.
Study Participants
Men and women with moderate to severe RLS (n = 20) and healthy controls (n = 20) were recruited from the greater Boston area and local sleep clinics. Telephone screening was conducted using the International RLS Study Group diagnostic criteria. Diagnosis of RLS, which included ruling out of RLS “mimics,” were confirmed during 1 to 2 independent physician interviews. All participants were aged 20–65 years, with a body mass index 18.5 to < 35 kg/m2, and a normal resting electrocardiogram (EKG). Moderate to severe RLS was defined as symptoms ≥ 15 nights/month, within the past month or prior to initiation of RLS medication. Exclusion criteria included: (1) previously diagnosed hypertension, current antihypertensive medication use, or high blood pressure (systolic > 140 or diastolic > 90 mm Hg) at either study visit, (2) evidence of another sleep disorder (e.g., obstructive sleep apnea screening via Berlin Questionnaire), (3) self-reported prevalent cardiovascular or peripheral vascular disease, (4) history of diabetes, (5) weight change > 10 lbs within past 6 months, (6) self-reported tobacco use or illicit substance use determined by urine toxicology screen, (7) consumption of > 10 alcoholic drinks/ week for women or > 15 alcoholic drinks/week for men, (8) use of antipsychotics, stimulants, or tricyclic antidepressants, (9) pregnancy, (10) premenopausal women with irregular menses or amenorrhea, and (11) family history of RLS (controls only). Additionally, participants with RLS were excluded if they were unwilling or unable to discontinue all RLS medications for 32 hours prior to testing.
Measurements
A standard EKG (Dash 2000, General Electric), beat-by-beat photoplethysmographic arterial pressure (Portapres, Finapres Medical Systems), and popliteal artery blood flow velocity, recorded from a 4-MHz Doppler probe (Multidop T2, DWL), were recorded continuously. Oscillometric brachial pressures (DASH 2000, General Electric) were taken every 3 minutes in the contralateral arm to ensure photoplethysmographic finger pressure calibration throughout the protocol. Respiratory depth and frequency were monitored and recorded using a respiratory transducer band around the midchest. Tibialis anterior muscle electromyography (EMG) was recorded bilaterally (Powerlab ML-880, ADInstruments, Colorado Springs, CO). All signals were digitized and stored at 1,000 Hz (PowerLab, ADInstruments).
Protocol
All studies were conducted in the morning after participants fasted overnight and refrained from strenuous exercise for > 48 h, as well as from alcohol and caffeine for 24 h. All RLS medications were discontinued at midnight on the penultimate night before testing, at least 32 h prior to testing. Premenopausal women were studied during the follicular phase of their menstrual cycle.
Baseline recordings were obtained for 10 minutes. All participants were supine throughout the protocol. Baroreflex function was tested via 2 trials of the modified Oxford method: sequential intravenous bolus injections of 100 μg sodium nitroprusside followed 60 seconds later by 150 μg phenylephrine hydrochloride.14 This technique initially lowers blood pressure below the baroreflex threshold and subsequently increases pressure through the threshold, linear, and possibly, saturation ranges.15 Ten minutes of recovery was allotted after each trial. Participants then performed sustained isometric hand-grip exercise at 30% maximum voluntary contraction (MVC) to fatigue. Participants were provided continuous visual and auditory feedback to ensure maintenance of target force until exhaustion, which was defined as a decrease in handgrip force of > 10% below the target for > 2 sec, despite verbal encouragement and attainment of a maximal perceived exertion. Sustained isometric handgrip exercise provides a physiologic stimulus that generates a highly reproducible pressor response with sympathetically-mediated increases in calf vascular resistance, which are progressive throughout exercise to fatigue.16 After a 10 to 15 min recovery period, participants then performed 3 Valsalva maneuvers, with 2 min of rest between each maneuver. Participants inhaled deeply and then exhaled through a tube, with a pressure gauge attached, and maintained a target exhalation pressure of 40 mm Hg for 15 seconds. Lastly, participants performed paced breathing via audio instruction at 0.25 Hz (15 breaths/min) for 5 minutes. Though it has limitations,17 phasic respiratory modulation of vagal outflow results in respiratory sinus arrhythmia, which is proportional to the mean level of cardiac vagal outflow. Controlled paced breathing at 15 breaths/min allows for accurate quantification of respiratory sinus arrhythmia amplitude by limiting variations in respiration that strongly influence respiratory sinus arrhythmia.18
All participants completed the International Physical Activity Questionnaire (IPAQ),19 the Pittsburgh Sleep Quality Index (PSQI),20 and had blood drawn. To quantify RLS severity, participants completed the International Restless Legs Syndrome Rating Scale (IRLS) at the screening visit.21 All participants wore bilateral leg actigraphs (PAM-RL, Philips Respironics, Bend, OR)22 to detect periodic leg movements for 5 nights prior to testing. We averaged the sum of movements/h of each leg for the first 4 nights recorded (baseline), as well as the night before testing. Sleep diaries were used to aid with editing of overnight PAM-RL data and to provide information on self-reported sleep duration on the night prior to testing.
Data Analysis
Baseline mean heart rate, blood pressure (oscillometric brachial blood pressure), and leg blood flow/resistance (ultrasound popliteal flow velocity) were derived. Baroreflex gain was estimated from the relation of systolic pressure to RR interval. Analysis began at the lowest pressure value after phenylephrine administration and ended at peak pressure. This selection of data points often encompasses both threshold and saturation regions of the sigmoid relationship.15 Our aim, however, was to determine linear gain with exclusion of these regions. Therefore, we used piecewise linear regression23 applied to raw data points (Figure 1). The model requires ≥ 5 data points to define the presence of threshold and/or saturation (if any) and excludes them from assessment to derive a robust linear gain. Mean baroreflex gain was derived from the average of the 2 trials, weighted by the r-square of each relation.23 For the isometric handgrip exercise data, since time to fatigue varies across subjects and cardiovascular responses are progressive throughout the duration of exercise, hemodynamic variables were examined as a percentage of time to fatigue rather than absolute time.24,25 Data were averaged for 5 time intervals representing 0–20%, 20–40%, 40–60%, 60–80%, and 80–100% of time to exhaustion. The Valsalva ratio was derived from the ratio of RR interval during phase IV to RR interval during phase III. Respiratory sinus arrhythmia values were derived from power spectral analyses based on the Welch algorithm of averaging periodograms (DaDisp, DSP Development Corporation, Newton, MA) from the 300-sec RR interval time series during paced breathing.26 To explore potential interaction by RLS medication status, we performed secondary analyses by medication status, when statistically appropriate given the small number of participants using medications.
Figure 1.
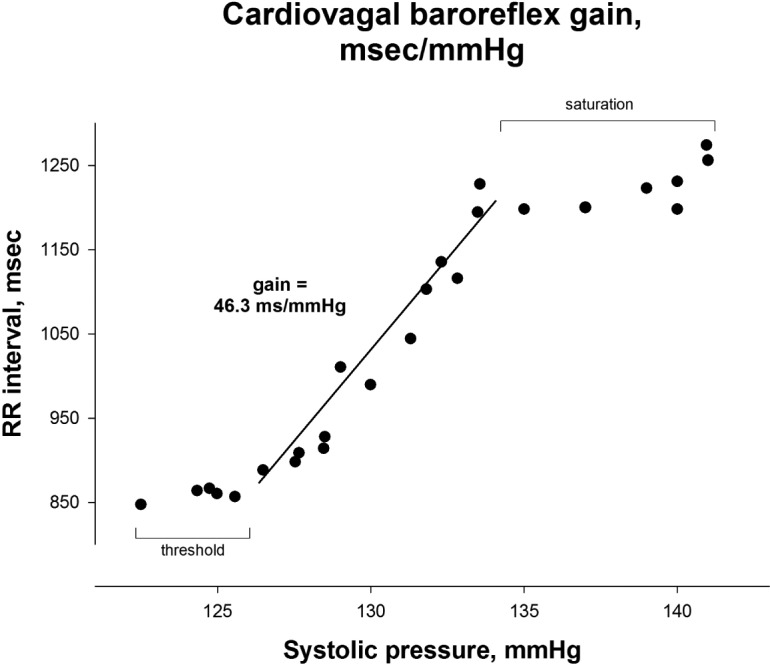
An example of baroreflex gain data derived from the phenylephrine induced pressure rise during the modified Oxford technique.
Statistical Analysis
Group comparisons of all variables were using t-tests, except isometric handgrip data, which were assessed by two-way (group and time) repeated measures ANOVA. Spearman rank correlation coefficients for all pairs of variables were performed. Significance for all tests was set a priori at P < 0.05. Data are presented as mean ± standard error of the mean.
RESULTS
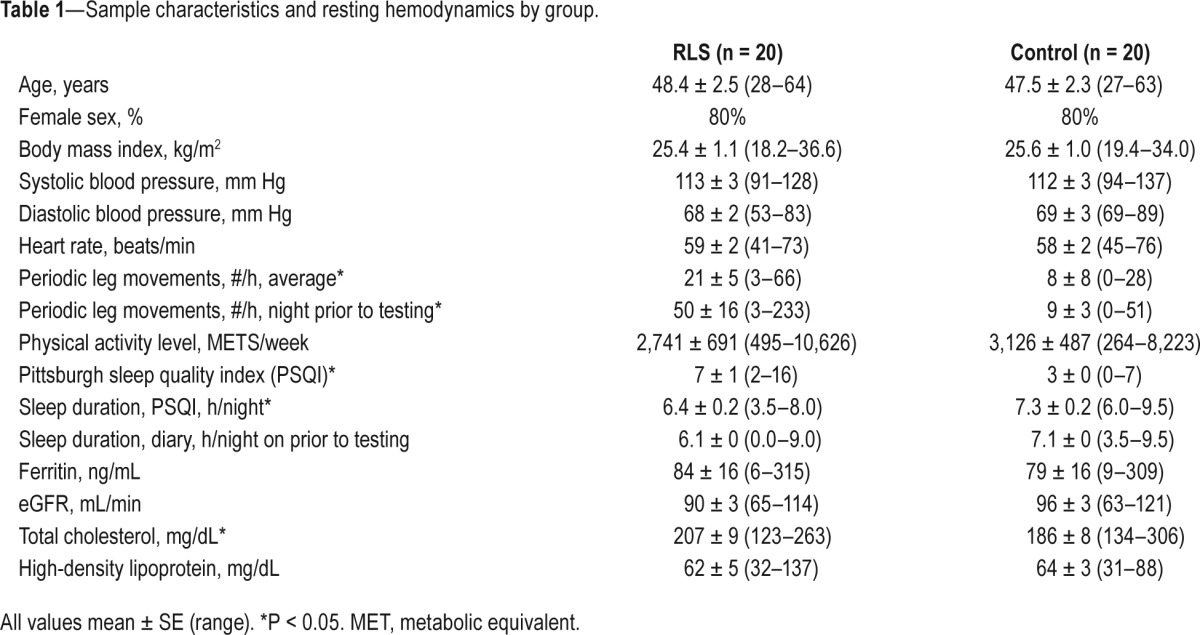
Patients with RLS had moderate symptoms at baseline (mean IRLS score 16.2 ± 1.6). Eight (40%) participants used at least 1 RLS medication (gabapentin, pramipexole, pregabalin, ropinirole, and oxycodone), with 2 participants taking > 1 medication. Duration of symptoms ranged from 1 to 47 years (mean 16 ± 2 years). Participant characteristics are shown in Table 1. By design, there were no differences in age, sex, or body mass index. Baseline resting blood pressure and heart rate, physical activity levels, and total sleep time on the night prior to testing (diary) did not differ between groups. Those with RLS reported poorer sleep quality, shorter average sleep duration (PSQI), and objectively had more periodic leg movements. Non-fasting total cholesterol was higher in the RLS group; high-density lipoprotein, glomerular filtration rate, and ferritin did not differ.
Table 1.
Sample characteristics and resting hemodynamics by group.
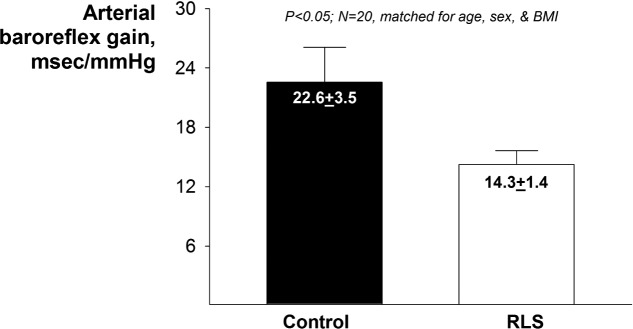
Baroreflex gain was, on average, 20% lower in those with RLS (P < 0.05; Figure 2). When we stratified our analyses by use of RLS medications (i.e., washout/no washout), baroreflex sensitivity was similarly lower in both RLS groups compared to their respective matched controls: RLS not using medications (13.9 ± 1.4 msec/mm Hg) vs. control (22.3 ± 4.3 msec/mm Hg); RLS using medications/washout (14.9 ± 2.6 msec/mm Hg) vs. control (23.0 ± 2.7 msec/mm Hg).
Figure 2.
Group averages for baroreflex gain. Values are mean ± SEM.
Across the entire sample, there was no correlation between baroreflex gain and frequency of periodic leg movements (4-night average or 1-night before study), sleep quality, average sleep duration (from PSQI), or sleep duration the night before the study (from diary). Among participants with RLS, we found no association between baroreflex gain and RLS severity with the IRLS scale, RLS duration, or periodic leg movements. Interestingly, we did find an association between baroreflex gain and blood pressure in the RLS group only (r = −0.50, P < 0.05).
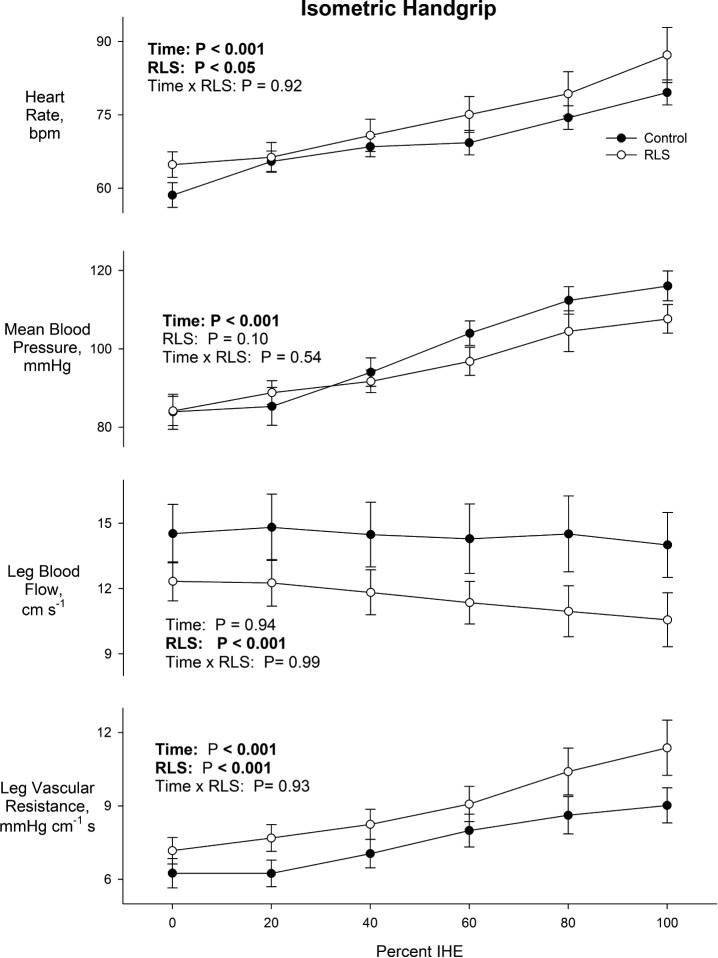
At rest, leg blood flow was lower (12.3 ± 0.73 vs 15.2 ± 0.89 cm/sec, P < 0.05) and leg vascular resistance higher in RLS participants (7.24 ± 0.45 vs 5.81 ± 0.36 mm Hg/cm/sec, P < 0.05). These results were largely unchanged in analyses stratified by use of RLS medications and their respective controls. As not all participants were able to sustain isometric exercise to fatigue without ancillary contraction in the leg, thereby confounding measurement of leg blood flow, adequate data were obtained for 11/20 matched pairs during isometric exercise. Lower leg blood flow and greater leg vascular resistance in the RLS group was maintained throughout isometric exercise to fatigue (Figure 3), though heart rate and blood pressure response to exercise were similar between groups. Given the limited number of matched pairs, further subgroup analyses by medication status were not performed. The Valsalva ratio was similar in RLS participants and controls (1.58 ± 0.11 vs. 1.49 ± 0.09, P = 0.30). Likewise, respiratory sinus arrhythmia did not differ between groups (5,199 ± 1,615 vs. 7,676 ± 2,059 msec2, P = 0.38).
Figure 3.
Hemodynamic response to isometric handgrip exercise (IHE) as a function of time to fatigue (20% increments) in the RLS and CON groups. Values are mean ± SEM
DISCUSSION
We found that moderate to severe RLS is associated with lower baroreflex gain and higher leg vascular resistance. These findings were not attributable to differences in sleep quality, sleep duration, or periodic leg movements, suggesting RLS may directly contribute to alterations in cardiovascular autonomic control. Other indices of cardiovagal control, respiratory sinus arrhythmia, and Valsalva ratio, did not vary by RLS status. Hence, our findings indicate that the relation between RLS and cardiovascular autonomic control is specific to the arterial baroreflex and to vascular resistance. Therefore, these specific alterations may serve as a mechanism by which RLS elevates cardiovascular risk.
Recent epidemiologic data suggest RLS is associated with coronary artery disease and stroke, as well as incident coronary heart disease.1–4 Among the largest prospective studies evaluating the association between RLS and cardiovascular risk to date, the Nurses' Health Study found that physician-confirmed RLS was associated with an increased risk of incident coronary heart disease, independent of other risk factors, among women with RLS for ≥ 3 years.1
Our findings are consistent with 1 previous study demonstrating ∼40% reduction in heart rate response to head-up tilt, in untreated patients with RLS;13 a response partially mediated by the baroreflex. Baroreflex gain is also reduced in other populations with elevated cardiovascular risk, such as those with diabetes and hypertension.27,28 In fact, the ∼20% difference in gain between our groups is similar to differences in gain between normotensive and hypertensive individuals.28 Furthermore, baroreflex gain is inversely associated with subclinical coronary artery disease, life-threatening arrhythmias, and cardiovascular mortality following myocardial infarction.29–32 Interestingly, we found a correlation between baroreflex gain and resting blood pressure in RLS, such that lower gain was associated with higher pressure. This suggests that the lower baroreflex gain in patients with RLS may result in a blood pressure that is relatively higher, but below the threshold of overt disease.
Although it has been surmised that the sympathetic system is perturbed in RLS, no studies have explored this possibility to date. We used sustained isometric handgrip to fatigue to assess sympathetically-mediated vascular resistance. Sustained handgrip at 25% to 35% MVC to fatigue markedly augments sympathetic nervous outflow to inactive skeletal muscle and is reflected in concurrent progressive increases in calf vascular resistance.16,33 Our data demonstrate that participants with RLS have increased calf vascular resistance during isometric handgrip exercise, as well as higher vascular resistance at rest. While these findings may or may not reflect systemic increases in sympathetic outflow in RLS, they do suggest enhanced sympathetic outflow to skeletal muscle. However, alterations in other factors influencing vascular resistance, such as nitric oxide (NO), could also explain our findings. NO concentration may be lower in RLS34 and variants in neuronal nitric synthase in RLS have been described.35 Though the physiologic underpinnings of our findings have yet to be elucidated, increased sympathetic outflow to skeletal muscle and/or altered bioavailability of NO may indicate elevated cardiovascular risk in RLS. Of note, while recent studies have observed differences in skin sympathetic response and partial pressure of oxygen in the calves of persons with RLS, autonomic control of the skin correlates poorly with sympathetic vascular control.36–38 Additionally, anecdotal reports have suggested that reduced leg blood flow contributes to PLMS.39,40
We also explored alterations in other, less robust measures of cardiovascular autonomic regulation, heart rate variability, and the Valsalva ratio. Our results are consistent with 2 previous studies,12,13 which demonstrated that high frequency heart rate variability, the index most closely reflective of respiratory sinus arrhythmia, does not differ between participants with RLS and controls. One of these studies also failed to find differences in other standard indices of heart rate variability (i.e., standard deviation of all RR intervals [SDNN], root mean square of successive differences [SDNN]), although they did find the triangular index, a less robust, geometric representation of heart rate variability, was reduced in RLS.13 Conversely, our results contrast that study's finding of reduced heart rate response to Valsalva maneuver in RLS compared to controls.13 Although they did find group differences, it is possible their findings could be due to other factors influencing heart rate response, such as age and hypertension. Since we used phase III of the maneuver, which represents vagal activation, and they used phase II, we are not able to meaningfully compare our results with these prior data.41 Therefore, it does not appear that RLS impacts all aspects of cardiovascular regulation, but rather it specifically perturbs the arterial baroreflex and resting peripheral vascular resistance.
Mechanisms that might induce lesser cardiovagal baroreflex gain and greater sympathetically- mediated vascular resistance in RLS are not well understood. Several putative mechanistic pathways have been implicated, including repetitive exposure to surges in blood pressure following PLMS, RLS-related sleep disruption and its consequent cardiovascular alterations, or a central controller in the brainstem that induces both RLS symptoms and alters integrated cardiovascular regulation.42,43 While previous data demonstrate that blood pressure and heart rate elevations following PLMS are augmented in RLS,44,45 we found no association between periodic leg movements and either baroreflex function or calf vascular resistance. Furthermore, while our findings lend credence to RLS independently contributing to altered cardiovascular autonomic control, our results, somewhat surprisingly, do not suggest these effects are related to sleep duration, sleep quality, or RLS duration. However, our measures of sleep were crude, using only self-reported data over the past month (PSQI) and a single night of diary data. Similarly, RLS symptom duration is a crude measure, due to symptom waxing/waning severity. This may partially explain the discrepancy between our finding of lack of association between RLS symptom duration and cardiovascular autonomic control, and prior research suggesting an association between duration of RLS diagnosis (as opposed to RLS symptom duration) ≥ 3 years and incident coronary artery disease in women.1 Hence, further research is needed to improve our understanding of the mechanistic pathways by which RLS impairs cardiovascular autonomic control. Investigating the relationship between RLS and cardiovascular control may additionally further our understanding of the mechanisms underlying this sensorimotor disorder.
Strengths and Limitations
Our study had a number of strengths, including physician-diagnosis of RLS, matched controls, and direct assessment of baroreflex gain. We also objectively measured periodic leg movements during the sleep period. This study also had certain limitations. Although we matched on age, sex, and BMI, we were not able to concurrently adjust for other potential confounders, which could impact our findings. Though we had participants washout of all RLS medications prior to testing, the washout period may have been too short given known cardiovascular effects of dopamine agonists; however, only 3 participants used dopaminergics. Our sample size precluded us from further exploring the potential differential influences of RLS duration and PLMS on our outcomes by medication status. Despite this limitation, our subgroup analyses did not support that RLS differentially impacts baroreflex sensitivity, nor vascular resistance, by previous medication use and washout. Additionally, we were only able to assess self-reported measures of sleep duration and quality, and therefore did not account for potential differences in objectively measured sleep. Although we measured periodic leg movements, we were not able to assess their influence on arousals from sleep, which may have a greater influence on blood pressure fluctuations.8,9 By design, we studied participants with moderate to severe RLS, and hence the distribution of severity may have been too narrow to effectively evaluate the influence of RLS severity on our findings. Finally, we studied participants who were healthy, non-smokers, free of cardiovascular and peripheral vascular disease and symptoms, and thus our results may not be generalizable to other populations with RLS.
CONCLUSION
Our results demonstrate that RLS is associated with both reduced cardiovagal baroreflex gain and increased calf vascular resistance, and that these effects were independent of sleep quality, sleep duration, or periodic leg movements. These results suggest that RLS specifically impacts the baroreflex and peripheral vascular resistance, and not cardiovascular autonomic control more generally. Future work should seek to elucidate the physiologic mechanisms underlying reduced baroreflex control and the factors mediating greater vascular peripheral resistance in RLS.
DISCLOSURE STATEMENT
Supported for this study was provided by Schwarz Pharma Ltd. and by NIH (K23AT005104). This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102), and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health. Dr. Winkelman has consulting/advisory arrangements with UCB/Schwarz Pharma, Impax Pharmaceuticals, Xenoport, and Oakstone Publishing, and has received research support from UCB/Schwarz Pharma, GlaxoSmithKline, and Impax Pharmaceuticals.
The other authors have indicated no financial conflicts of interest. The work for the study was performed at Spaulding Hospital Cambridge and Massachusetts General Hospital.
REFERENCES
- 1.Li Y, Walters AS, Chiuve SE, Rimm EB, Winkelman JW, Gao X. Prospective study of restless legs syndrome and coronary heart disease among women. Circulation. 2012;126:1689–94. doi: 10.1161/CIRCULATIONAHA.112.112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin CH, Sy HN, Chang HW, et al. Restless legs syndrome is associated with cardio/cerebrovascular events and mortality in end-stage renal disease. Eur J Neurol. 2015;22:142–9. doi: 10.1111/ene.12545. [DOI] [PubMed] [Google Scholar]
- 3.Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008;70:35–42. doi: 10.1212/01.wnl.0000287072.93277.c9. [DOI] [PubMed] [Google Scholar]
- 4.Winter AC, Berger K, Glynn RJ, et al. Vascular risk factors, cardiovascular disease, and restless legs syndrome in men. Am J Med. 2013;126:228–35. doi: 10.1016/j.amjmed.2012.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winter AC, Schurks M, Glynn RJ, et al. Vascular risk factors, cardiovascular disease, and restless legs syndrome in women. Am J Med. 2013;126:220–7. doi: 10.1016/j.amjmed.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winter AC, Schurks M, Glynn RJ, et al. Restless legs syndrome and risk of incident cardiovascular disease in women and men: prospective cohort study. BMJ Open. 2012;2:e000866. doi: 10.1136/bmjopen-2012-000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winkelman JW, Finn L, Young T. Prevalence and correlates of restless legs syndrome symptoms in the Wisconsin Sleep Cohort. Sleep Med. 2006;7:545–52. doi: 10.1016/j.sleep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–30. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–8. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 10.Winkelman JW. The evoked heart rate response to periodic leg movements of sleep. Sleep. 1999;22:575–80. doi: 10.1093/sleep/22.5.575. [DOI] [PubMed] [Google Scholar]
- 11.Manconi M, Ferri R, Zucconi M, et al. Effects of acute dopamineagonist treatment in restless legs syndrome on heart rate variability during sleep. Sleep Med. 2011;12:47–55. doi: 10.1016/j.sleep.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Cikrikcioglu MA, Hursitoglu M, Erkal H, et al. Oxidative stress and autonomic nervous system functions in restless legs syndrome. Eur J Clin Invest. 2011;41:734–42. doi: 10.1111/j.1365-2362.2010.02461.x. [DOI] [PubMed] [Google Scholar]
- 13.Izzi F, Placidi F, Romigi A, et al. Is autonomic nervous system involved in restless legs syndrome during wakefulness? Sleep Med. 2014;15:1392–7. doi: 10.1016/j.sleep.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 14.Kotrly KJ, Ebert TJ, Vucins E, Igler FO, Barney JA, Kampine JP. Baroreceptor reflex control of heart rate during isoflurane anesthesia in humans. Anesthesiology. 1984;60:173–9. doi: 10.1097/00000542-198403000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Ebert TJ, Muzi M, Berens R, Goff D, Kampine JP. Sympathetic responses to induction of anesthesia in humans with propofol or etomidate. Anesthesiology. 1992;76:725–33. doi: 10.1097/00000542-199205000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Seals DR. Sympathetic neural discharge and vascular resistance during exercise in humans. J Appl Physiol. 1989;66:2472–8. doi: 10.1152/jappl.1989.66.5.2472. [DOI] [PubMed] [Google Scholar]
- 17.Taylor JA, Studinger P. Counterpoint: cardiovascular variability is not an index of autonomic control of the circulation. J Appl Physiol. 2006;101:678–81. doi: 10.1152/japplphysiol.00446.2006. discussion 81. [DOI] [PubMed] [Google Scholar]
- 18.Eckberg DL. The human respiratory gate. J Physiol. 2003;548:339–52. doi: 10.1113/jphysiol.2003.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 22.Sforza E, Johannes M, Claudio B. The PAM-RL ambulatory device for detection of periodic leg movements: a validation study. Sleep Med. 2005;6:407–13. doi: 10.1016/j.sleep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Studinger P, Goldstein R, Taylor JA. Mechanical and neural contributions to hysteresis in the cardiac vagal limb of the arterial baroreflex. J Physiol. 2007;583:1041–8. doi: 10.1113/jphysiol.2007.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funderburk CF, Hipskind SG, Welton RC, Lind AR. Development of and recovery from fatigue induced by static effort at various tensions. J Appl Physiol. 1974;37:392–6. doi: 10.1152/jappl.1974.37.3.392. [DOI] [PubMed] [Google Scholar]
- 25.Taylor JA, Hand GA, Johnson DG, Seals DR. Sympathoadrenalcirculatory regulation during sustained isometric exercise in young and older men. Am J Physiol. 1991;261:R1061–9. doi: 10.1152/ajpregu.1991.261.5.R1061. [DOI] [PubMed] [Google Scholar]
- 26.Taylor JA, Eckberg DL. Fundamental relations between short-term RR interval and arterial pressure oscillations in humans. Circulation. 1996;93:1527–32. doi: 10.1161/01.cir.93.8.1527. [DOI] [PubMed] [Google Scholar]
- 27.Eckberg DL, Harkins SW, Fritsch JM, Musgrave GE, Gardner DF. Baroreflex control of plasma norepinephrine and heart period in healthy subjects and diabetic patients. J Clin Invest. 1986;78:366–74. doi: 10.1172/JCI112586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White M, Fourney A, Mikes E, Leenen FH. Effects of age and hypertension on cardiac responses to the alpha1-agonist phenylephrine in humans. Am J Hypertens. 1999;12:151–8. doi: 10.1016/s0895-7061(98)00220-9. [DOI] [PubMed] [Google Scholar]
- 29.Simula S, Laitinen T, Vanninen E, et al. Baroreflex sensitivity in asymptomatic coronary atherosclerosis. Clin Physiol Funct Imaging. 2013;33:70–4. doi: 10.1111/j.1475-097X.2012.01165.x. [DOI] [PubMed] [Google Scholar]
- 30.La Rovere MT, Bigger JT, Jr., Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–84. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 31.De Ferrari GM, Sanzo A, Bertoletti A, Specchia G, Vanoli E, Schwartz PJ. Baroreflex sensitivity predicts long-term cardiovascular mortality after myocardial infarction even in patients with preserved left ventricular function. J Am Coll Cardiol. 2007;50:2285–90. doi: 10.1016/j.jacc.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 32.La Rovere MT, Specchia G, Mortara A, Schwartz PJ. Baroreflex sensitivity, clinical correlates, and cardiovascular mortality among patients with a first myocardial infarction. A prospective study. Circulation. 1988;78:816–24. doi: 10.1161/01.cir.78.4.816. [DOI] [PubMed] [Google Scholar]
- 33.Seals DR, Chase PB, Taylor JA. Autonomic mediation of the pressor responses to isometric exercise in humans. J Appl Physiol. 1988;64:2190–6. doi: 10.1152/jappl.1988.64.5.2190. [DOI] [PubMed] [Google Scholar]
- 34.Baskol G, Korkmaz S, Erdem F, Caniklioglu A, Kocyigit M, Aksu M. Assessment of nitric oxide, advanced oxidation protein products, malondialdehyde, and thiol levels in patients with restless legs syndrome. Sleep Med. 2012;13:414–8. doi: 10.1016/j.sleep.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Winkelmann J, Lichtner P, Schormair B, et al. Variants in the neuronal nitric oxide synthase (nNOS, NOS1) gene are associated with restless legs syndrome. Mov Disord. 2008;23:350–8. doi: 10.1002/mds.21647. [DOI] [PubMed] [Google Scholar]
- 36.Shukla G, Goyal V, Srivastava A, Behari M. Quantitative thermal sensory testing and sympathetic skin response in primary Restless legs syndrome - A prospective study on 57 Indian patients. Ann Indian Acad Neurol. 2012;15:260–2. doi: 10.4103/0972-2327.104332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salminen AV, Rimpila V, Polo O. Peripheral hypoxia in restless legs syndrome (Willis-Ekbom disease) Neurology. 2014;82:1856–61. doi: 10.1212/WNL.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 38.Grassi G, Colombo M, Seravalle G, Spaziani D, Mancia G. Dissociation between muscle and skin sympathetic nerve activity in essential hypertension, obesity, and congestive heart failure. Hypertension. 1998;31:64–7. doi: 10.1161/01.hyp.31.1.64. [DOI] [PubMed] [Google Scholar]
- 39.Ancoli-Israel S, Seifert AR, Lemon M. Thermal biofeedback and periodic movements in sleep: patients' subjective reports and a case study. Biofeedback and self-regulation. 1986;11:177–88. doi: 10.1007/BF01003477. [DOI] [PubMed] [Google Scholar]
- 40.Ware JC, Blumoff R, Pittard JT. Peripheral vasoconstriction in patients with sleep related periodic leg movements. Sleep. 1988;11:182–6. doi: 10.1093/sleep/11.2.182. [DOI] [PubMed] [Google Scholar]
- 41.Smith ML, Beightol LA, Fritsch-Yelle JM, Ellenbogen KA, Porter TR, Eckberg DL. Valsalva's maneuver revisited: a quantitative method yielding insights into human autonomic control. Am J Physiol. 1996;271:H1240–9. doi: 10.1152/ajpheart.1996.271.3.H1240. [DOI] [PubMed] [Google Scholar]
- 42.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32:589–97. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferini-Strambi L, Walters AS, Sica D. The relationship among restless legs syndrome (Willis-Ekbom Disease), hypertension, cardiovascular disease, and cerebrovascular disease. J Neurol. 2014;261:1051–68. doi: 10.1007/s00415-013-7065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fantini ML, Michaud M, Gosselin N, Lavigne G, Montplaisir J. Periodic leg movements in REM sleep behavior disorder and related autonomic and EEG activation. Neurology. 2002;59:1889–94. doi: 10.1212/01.wnl.0000038348.94399.f6. [DOI] [PubMed] [Google Scholar]
- 45.Pennestri MH, Montplaisir J, Fradette L, Lavigne G, Colombo R, Lanfranchi PA. Blood pressure changes associated with periodic leg movements during sleep in healthy subjects. Sleep Med. 2013;14:555–61. doi: 10.1016/j.sleep.2013.02.005. [DOI] [PubMed] [Google Scholar]