Abstract
Study Objectives:
To evaluate the prevalence of obstructive sleep apnea (OSA) in a large cohort of children with Down syndrome (DS), and to investigate which patient- related factors correlate with disease severity.
Methods:
We performed a retrospective, cross-sectional study in children with DS referred for full overnight polysomnography in a tertiary care center.
Results:
Polysomnographic data are available for 122 children (70 boys), age 5.0 y (2.8–10.5), and body mass index (BMI) z-score 0.7 (−0.3 to 1.7). The overall prevalence of OSA was 66.4%. In almost half of these children severe OSA was diagnosed (obstructive AHI [oAHI] ≥ 10/h). In children with parental reports of snoring or witnessed apneas (group A), OSA was significantly more common (75.7%) than in those without these symptoms (group B) 53.8% (P = 0.019). Children in group A had more severe OSA, oAHI 5.7/h (1.7–13.8) compared to those in group B 2.2/h (0.8–8.0) (P = 0.018). A significant inverse correlation between age and oAHI (P = 0.028) was found. Sex and BMI z-score were not significantly correlated to oAHI.
Conclusions:
Based upon full night polysomnography, an overall 66.4% prevalence of OSA was found in children with Down syndrome. Even in those with a negative history for OSA, the prevalence was 53.8%. Younger age was associated with more severe disease.
Citation:
Maris M, Verhulst S, Wojciechowski M, Van de Heyning P, Boudewyns A. Prevalence of obstructive sleep apnea in children with Down syndrome. SLEEP 2016;39(3):699–704.
Keywords: Down syndrome, obstructive sleep apnea, polysomnography
Significance.
More than half of the children with Down syndrome have obstructive sleep apnea, documented by full night polysomnography. This high prevalence was found across all age groups between 0–18 years. It is strongly recommended to perform polysomnography to exclude obstructive sleep apnea in children with Down syndrome, irrespective of age or parental reports of snoring and witnessed apneas.
INTRODUCTION
Down syndrome (DS) is the most common chromosomal disorder in live-born children, with an incidence of 1 in 700. Children with DS are predisposed to a number of health problems affecting their development and quality of life as described in several reports.1–3 Obstructive sleep apnea (OSA) is frequently diagnosed in children with DS, with estimated prevalences between 30% and 60%, compared to 0.7% to 2% in a general pediatric population.4 OSA in children with DS is often a complex and multifactorial condition due to a combination of pre-disposing risk factors to develop airway obstruction. Factors contributing to OSA in children with DS are midfacial and mandibular hypoplasia, a narrow nasopharynx, relative macroglossia (due to crowding of the oropharynx), adenotonsillar hypertrophy, and a shortened palate. These anatomical abnormalities along with a generalized hypotonia, an immature immune system (more respiratory infections), thyroid dysfunction, and a propensity for obesity predispose DS children to upper airway obstruction.2 Furthermore, gastroesophageal reflux and chronic lung disease are also common and may worsen airway problems.5 In children younger than 2 years, laryngomalacia is more frequently reported as cause of upper airway obstruction.5
Potential negative health effects of OSA in children include failure to thrive, pulmonary hypertension (more prevalent than expected only due to cardiac anomalies), behavior problems, and sleep fragmentation affecting daytime and cognitive functioning.2,6,7 Children with DS have preexisting medical, cognitive, emotional, and behavioral deficits, and may be more susceptible to the negative health consequences of disturbed sleep and OSA.
Because there is a poor correlation between parental reports of OSA and polysomnography (PSG) findings, the American Academy of Pediatrics recommends a polysomnogram in all children with DS at the age of 4 y, or earlier when there is a positive history for upper airway obstruction during sleep.1,4
This study aims to evaluate the prevalence of OSA assessed by full-night PSG in a large cohort of nonselected children with DS and to investigate which patient-related factors are correlated with disease severity.
METHODS
All children in this study are enrolled in our multidisciplinary Down team (Antwerp University Hospital, Belgium) and seen at the ear, nose, and throat (ENT) department between November 2008 and January 2015. At each visit, parents were questioned regarding OSA related symptoms (group A). When the parents reported habitual snoring (> 3 nights/w) or witnessed apneas, the child was referred for PSG, independent of age. Asymptomatic children younger than 4 y and those younger than 4 y who snored occasionally were not sent for PSG. All children older than 4 y who never had a PSG were scheduled for a sleep study independent of symptoms or a previous history of upper airway surgery. This is in accordance with international guidelines1 and these (by history) asymptomatic children constitute group B.
All children underwent full-night PSG. The following variables were continuously measured and recorded by a computerized polysomnogram (Brain RT, OSG, Rumst, Belgium): electroencephalogram (C4/Al and C3/A2); electro-oculogram; electromyogram of anterior tibialis and chin muscles; and electrocardiogram. Respiratory effort was measured by respiratory inductance plethysmography and oxygen saturation by a finger probe connected to a pulse oximeter. Airflow was measured with a nasal pressure cannula and thermistor, and snoring was detected by means of a microphone at the suprasternal notch. Children were also monitored on audiotape/videotape using an infrared camera. Polysomnograms were manually scored by certified technicians according to international guidelines.8 A diagnosis of OSA is established with an obstructive apneahypopnea index (oAHI) ≥ 2/h.9 OSA severity was defined as mild with an oAHI > 2/h and < 5/h, moderate when oAHI ≥ 5/h and < 10/h and severe OSA with an oAHI ≥ 10/h. All children with DS and available PSG data were included in this study, irrespective of previous upper airway surgery or use of medication that might influence breathing during sleep. There were no specific exclusion criteria.
In each child a clinical examination was performed, and tonsil size was scored according to the Brodsky score.10 Body mass index (BMI) z-scores were calculated according to Flemish growth curves for boys and girls.
Children were divided in three different age groups, group 1; 0–4.9 y, group 2; 5–11.9 y and group 3; 12–18 y. All results are presented as median and 25th –75th percentile. Correlation between disease severity (oAHI) and patient risk factors (age, BMI z-score, sex) were evaluated with SPSS 22.0, IBM Corp with level of significance defined as P < 0.05 (Spearman correlation, Mann-Whitney U test).
RESULTS
During the study period, 216 children with DS and followed at the multidisciplinary Down team were seen at the ENT department. In 147 children (68.1%), PSG was recommended based on the aforementioned criteria (in the Methods section). In 25 children (17%), PSG was not performed for various reasons: cancellation of PSG by the parents (n = 10); lost to follow-up at the ENT department (n = 8); and PSG deemed not feasible because of behavioral problems (n = 6). For one child, full-night PSG data were not available because electrodes and sensors were pulled of during the registration and the child refused a replacement. Overall, for the 147 children in whom PSG was recommended, PSG data were obtained in 122. Children for whom no PSG data were available had a median age of 10.3 y (7.5–14.0); 52% of them were boys and in 36% snoring was reported by the parents. The demographics of those with/without PSG are presented in Table 1.
Table 1.
Demographics for the children with Down syndrome referred for polysomnography (n = 147).
Full polysomnographic data were available for 122 children with DS and they represent the study population for the current report. The study group consisted of 70 boys and 52 girls, age 5.0 y (2.8–10.5), BMI z-score 0.7 (−0.3 to 1.7) and oAHI 4.3/h (1.3–11.5). OSA was diagnosed in 66.4% of these children with a median oAHI of 8.2/h (4.3–16.7). The overall mean oxygen saturation (mean saturation of oxygen) was 96.5% (95.6–97.1) and the minimum oxygen saturation (minimum saturation of oxygen) 89.0% (84.8–92.0). Seventy children (57.9%) had a positive history for OSA (group A). The distribution of OSA according to severity is displayed in Figure 1. About half of the children were found to have severe disease.
Figure 1.
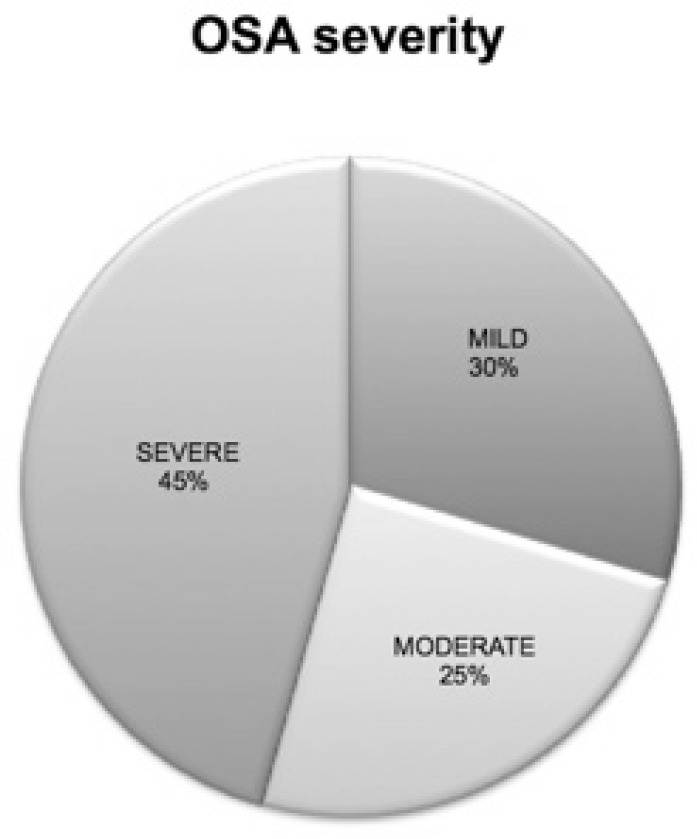
Distribution of obstructive sleep apnea severity.
Table 2 summarizes the results of the main variables for both groups.
Table 2.
Demographics and polysomnographic findings for children with a positive history for OSA (group A) and children with a negative history for OSA (group B).
OSA was significantly more common in group A (75.7%) compared to group B (53.8%) (P = 0.019) and children in group A had more severe disease compared those in group B (P = 0.018). However, even in those with a negative history of OSA (group B), more than half of the patients received a diagnosis of OSA. No significant difference was found between both groups concerning sleep stage, total sleep time, and sleep efficiency. The mean oxygen saturation was significantly lower in group B.
Twenty children had upper airway surgery before their first PSG (adenoidectomy n = 4; tonsillectomy n = 4; adenotonsillectomy n = 12). However, 75% of them had persistent symptoms and PSG was performed because of suspected OSA (group A). A diagnosis of OSA was confirmed in 12 children (60%).
Half of the children included in this study were younger than 5 y (50.0%). The percentage of children for each age group is presented in Figure 2.
Figure 2.
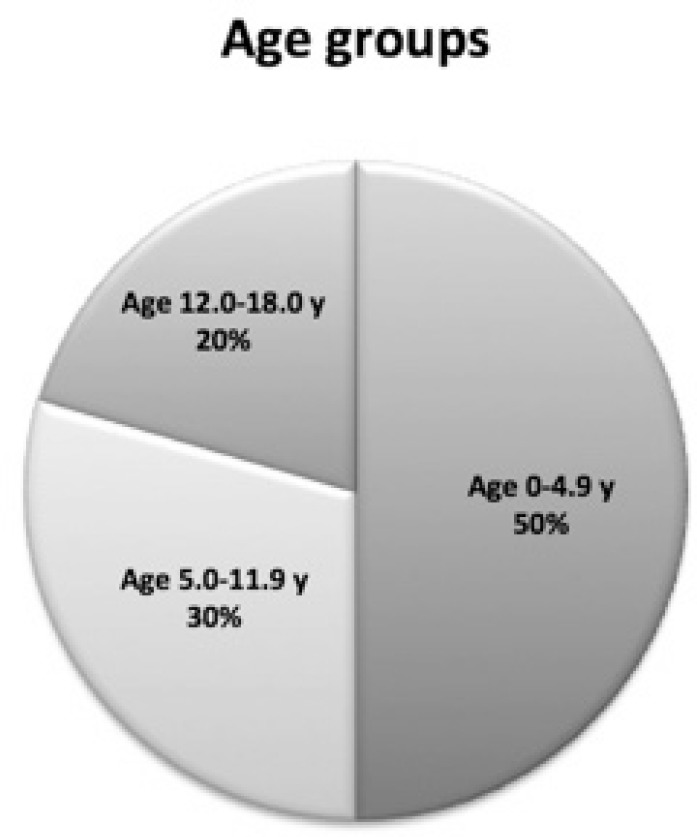
Percentages of children for each age group.
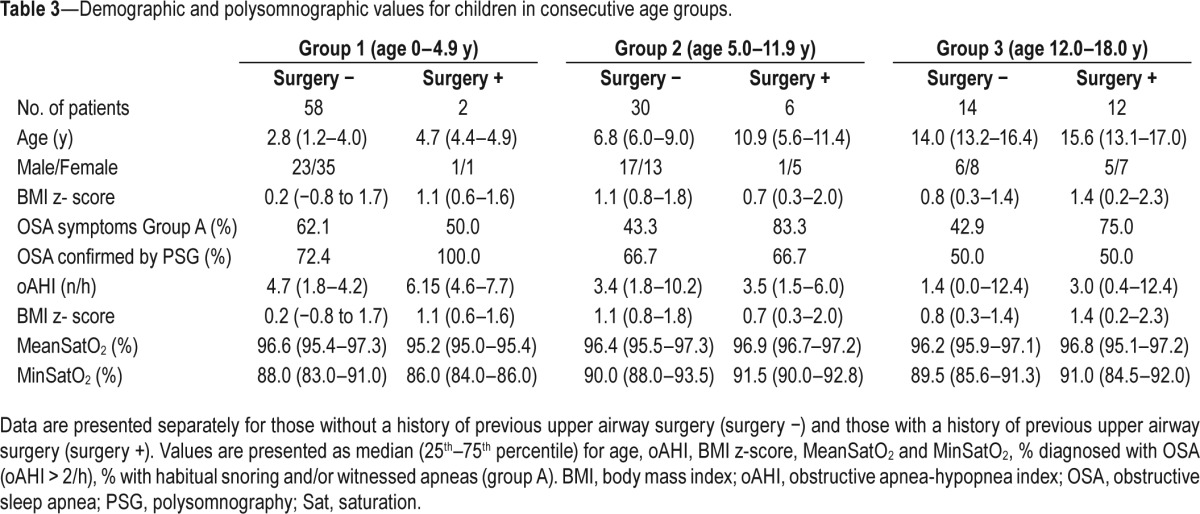
The main results for each age group are depicted in Table 3. In every age group, OSA was diagnosed in more than 50% of the children. Even in the youngest age group with a negative history for snoring or witnessed apneas (group B), still 56.5% received a diagnosis of OSA. In the older age groups, a larger number of children had a history of previous upper airway surgery. There were no significant differences in oAHI, mean saturation, or minimum saturation of oxygen between the three different age groups. Older children had a similar prevalence of OSA compared to the younger age group. Among children with previous upper airway surgery, persistent OSA was found in 50% of those between 12 and 18 y. This number increased up to 100% in the youngest children (age 0–4.9 y) with previous upper airway surgery. The small number of patients with previous upper airway surgery in age group 1 and 2 precluded meaningful statistical comparisons between those with/without upper airway surgery within each age group. In group 3, there were no significant differences between children with/without a history of upper airway surgery.
Table 3.
Demographic and polysomnographic values for children in consecutive age groups.
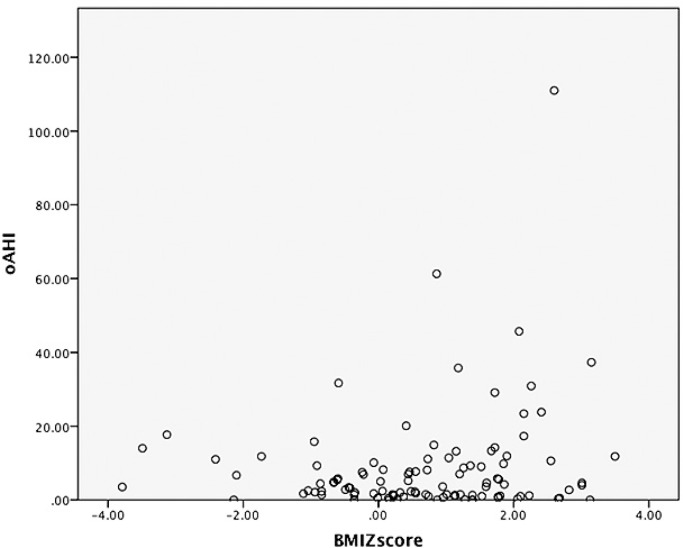
In the overall study population, correlation analysis showed a significant inverse correlation between age and oAHI (r = −0.199; P = 0.028). More severe OSA (higher oAHI values) significantly correlated with less stage 2 sleep (r = −0.205, P = 0.026), more stage 3 sleep (R = 0.269, P = 0.003) and more rapid eye movement (REM) sleep (r = 0.204, P = 0.027). Children with OSA had also significantly more REM sleep (P = 0.022). Sex, tonsil score, and BMI z-scores were not significantly correlated to oAHI. Individual data for oAHI versus BMI z-scores are displayed in Figure 3.
Figure 3.
Scatterplot with individual data for obstructive apnea-hypopnea index (oAHI) and body mass index (BMI) z-score.
No statistical differences could be found in mean and minimum oxygen saturation between those with or without OSA.
DISCUSSION
This study shows a high prevalence of OSA in children with DS (66.4%) with about half of these patients presenting with severe disease. To our best knowledge, this is the first report on a large group of Down syndrome children (n > 120), evaluated by full-night PSG. Our prevalence figures are in line with previous reports. However, the number of children undergoing full-night PSG in our current paper is much larger than in previous studies. In addition, there are methodological differences. In earlier studies, different thresholds and criteria for defining OSA were used. Stebbens et al.11 found a rather low prevalence of 22% of upper airway obstruction in a cohort of 34 children with DS. This study monitored oxygen saturation in combination with chest wall movements to detect upper airway obstruction, which could underestimate the problem of airway obstruction. Shott et al.4 reported a prevalence of 57% in children with DS aged 2 to 4 y. Marcus et al.12 found a high prevalence of OSA (63%) in a cohort of 53 subjects with DS, age range 2 w to 51 y, based on data from daytime nap polysomnograms. Only a small number of their study population (16 subjects) underwent overnight PSG. Dyken et al.13 found a high prevalence of OSA (79%) in a pilot study of 19 children with DS aged 3–18 y.13
Most previous studies didn't specify a classification of OSA severity (mild, moderate, or severe). In our study, severe OSA (oAHI ≥ 10/h) was diagnosed in 52% of the children with DS and OSA. Moderate OSA was found in 28%. We found a negative correlation between OSA severity and age, regardless of previous upper airway surgery. This could be important because it is not only the presence of OSA but also its severity that should guide treatment decisions.
Even in those with a negative history of snoring or witnessed apneas, we found an overall high prevalence of OSA (53.8%). Although we found a high prevalence of OSA in children with a negative history of snoring or apneas, children with a positive history for OSA had significantly more severe disease. Parental reports may be unreliable to predict OSA in DS children. Ng et al.14 found the absence of snoring in 61.5% of patients with DS and OSA. Shott et al.4 showed a poor relation between parental impressions of sleep problems and PSG results. These findings underscore the necessity to perform an overnight PSG in all children with DS, regardless of history, recommended at the age of 3 to 4 y or earlier when OSA is suspected.1,4,8 In this study, however, OSA was diagnosed in more than half of the children in the youngest age group (younger than 5 y) even with a negative history of snoring or apneas. These findings challenge current clinical practice and the question arises whether PSG should be recommended at even younger ages to exclude OSA in DS children? More studies including a larger group of young children with DS are required to answer this question. We recommend questioning the parents of DS children at all ages for sleep disturbances. OSA is linked to multiple morbidities, of which pulmonary hypertension and cor pulmonale could be life threatening, especially in children with DS who are already at high risk for these conditions. OSA may contribute to neurocognitive impairment in children with DS,6 and treatment of OSA may have a positive effect on sleep quality, neurocognitive behavior symptoms, and quality of life as shown in typically developing children.15
We found no correlation between OSA and typical risk factors for pediatric OSA such as tonsil score and BMI z-score. In contrast to an adult OSA population where males are at higher risk to develop OSA, in prepubertal children the risk of OSA is equal between both sexes.16 In the literature there are conflicting data on the relationship between OSA and BMI (z-scores) in children with DS.16 Weight control is part of the health supervision program in DS children and should derive special attention in those with OSA. Children with DS and OSA showed a significant increased percentage of REM sleep, accompanied with less stage 2 sleep and more stage 3 sleep. OSA severity (oAHI) correlated with more REM sleep.
Our study has some limitations, as the studied population represents a broad age range (11 w–17.6 y), whereas 50% of the children are aged 5 y or younger (16.4% younger than 2 y), and 79.5% younger than 12 y. Younger children presented with more severe OSA. As discussed before, more studies are needed to evaluate the need to screen for OSA in younger children with DS.
However, we cannot exclude a bias in this finding because the largest number of children for whom PSG was performed because of a positive history (62.3%) was part of the youngest age group. In this age group, especially younger than 4 y, guidelines do not recommend PSG, unless OSA is suspected. In the older children enrolled in our multidisciplinary Down team, a PSG was performed on indication in 50% in age group 2 and in 57.6% in age group 3. In addition to a heterogeneous age distribution, all children with DS, regardless of previous upper airway surgery, were included in this study. Unfortunately the study groups were too small to compare children with and without previous upper airway surgery in different age groups.
Although our study consisted of a large number of children with DS, this study population does not represent a community sample of children with DS. Rather, it is a selected group of children who are enrolled in the multidisciplinary Down team at our institution. However, this team had the largest number of children with Down syndrome in Flanders (Belgium). Although selection bias could not be excluded, we believe that this study population could represent a cohort of DS children in Flanders.
CONCLUSION
The high prevalence of OSA in children with DS, even with a negative history of snoring, supports the notion that PSG should be part of the routine care for children with DS. Even at a young age (younger than 5 y) and with a negative history for OSA, a high prevalence of OSA is found. Most DS children were found to have moderate to severe disease, negatively correlated to age but not to BMI z-score or sex.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Bull MJ Committee on G. Health supervision for children with Down syndrome. Pediatrics. 2011;128:393–406. doi: 10.1542/peds.2011-1605. [DOI] [PubMed] [Google Scholar]
- 2.Shott SR. Down syndrome: common otolaryngologic manifestations. Am J Med Genet C Semin Med Genet. 2006;142C:131–40. doi: 10.1002/ajmg.c.30095. [DOI] [PubMed] [Google Scholar]
- 3.Chin CJ, Khami MM, Husein M. A general review of the otolaryngologic manifestations of Down syndrome. Int J Pediatr Otorhinolaryngol. 2014;78:899–904. doi: 10.1016/j.ijporl.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Shott SR, Amin R, Chini B, Heubi C, Hotze S, Akers R. Obstructive sleep apnea: should all children with Down syndrome be tested? Arch Otolaryngol Head Neck Surg. 2006;132:432–6. doi: 10.1001/archotol.132.4.432. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell RB, Call E, Kelly J. Ear, nose and throat disorders in children with Down syndrome. Laryngoscope. 2003;113:259–63. doi: 10.1097/00005537-200302000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Breslin J, Spano G, Bootzin R, Anand P, Nadel L, Edgin J. Obstructive sleep apnea syndrome and cognition in Down syndrome. Dev Med Child Neurol. 2014;56:657–64. doi: 10.1111/dmcn.12376. [DOI] [PubMed] [Google Scholar]
- 7.Baumer N, Davidson EJ. Supporting a happy, healthy adolescence for young people with Down syndrome and other intellectual disabilities: recommendations for clinicians. Curr Opin Pediatr. 2014;26:428–34. doi: 10.1097/MOP.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 8.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–76. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am. 1989;36:1551–69. doi: 10.1016/s0031-3955(16)36806-7. [DOI] [PubMed] [Google Scholar]
- 11.Stebbens VA, Dennis J, Samuels MP, Croft CB, Southall DP. Sleep related upper airway obstruction in a cohort with Down's syndrome. Arch Dis Child. 1991;66:1333–8. doi: 10.1136/adc.66.11.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcus CL, Keens TG, Bautista DB, von Pechmann WS, Ward SL. Obstructive sleep apnea in children with Down syndrome. Pediatrics. 1991;88:132–9. [PubMed] [Google Scholar]
- 13.Dyken ME, Lin-Dyken DC, Poulton S, Zimmerman MB, Sedars E. Prospective polysomnographic analysis of obstructive sleep apnea in down syndrome. Arch Pediatr Adolesc Med. 2003;157:655–60. doi: 10.1001/archpedi.157.7.655. [DOI] [PubMed] [Google Scholar]
- 14.Ng DK, Hui HN, Chan CH, et al. Obstructive sleep apnoea in children with Down syndrome. Singapore Med J. 2006;47:774–9. [PubMed] [Google Scholar]
- 15.Austeng ME, Overland B, Kvaerner KJ, et al. Obstructive sleep apnea in younger school children with Down syndrome. Int J Pediatr Otorhinolaryngol. 2014;78:1026–9. doi: 10.1016/j.ijporl.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Churchill SS, Kieckhefer GM, Landis CA, Ward TM. Sleep measurement and monitoring in children with Down syndrome: a review of the literature, 1960-2010. Sleep Med Rev. 2012;16:477–88. doi: 10.1016/j.smrv.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]