Some mammals (whales, dolphins, fur seals, sea lions) sleep with one hemisphere of the brain being asleep while the other is awake.1,2 This is referred to as unihemispheric slow wave sleep (USWS) and contrasts with the bihemispheric slow-wave sleep (BSWS) exhibited by humans and other mammals. Whales (Delphinapterus leucas) and dolphins (Tursiops truncates) show only USWS.3,4 Northern fur seals and sea lions (family Otariidae) are aquatic and terrestrial. While in water these animals have USWS, like cetaceans, but on land they have both USWS and BSWS.3 It is unclear whether cetaceans have REM sleep, whereas Otariidae have REM sleep on land, and it is always bilateral. Some birds also have USWS,5,6 but neurochemicals related to USWS have only been measured in the fur seal. The evolutionary basis of USWS is unclear.7,8
Polysomnography studies have conclusively shown that USWS is indeed sleep because one hemisphere has high-amplitude slow wave activity (1.2–4 Hz), while the other hemisphere has desynchronized EEG activity.5,6,9 The daily quota of sleep is equally distributed between the hemispheres.10 If the hemisphere with USWS is repeatedly interrupted, then that hemisphere will have rebound USWS indicating a homeostatic need for USWS in that hemisphere.5,6,9 In such unihemispheric sleep deprivation studies, there is no compensatory increase in sleep in the non-deprived hemisphere, indicating that the homeostatic need for sleep accumulates independently in each hemisphere. These data indicate that USWS is actively generated and there is a compensatory need for it.
The discovery of unihemispheric sleep is a boon to sleep research as it provides a unique opportunity to empirically test neural circuit models of sleep-wake regulation. Lyamin, Mukhametov, Siegel and colleagues have clearly recognized this and have conducted elegant studies in northern fur seals (Callorthinus ursinus) to test the hypothesis that neurochemicals linked to wakefulness are elevated in the awake hemisphere compared to the hemisphere with USWS.11–13 In these studies the seals were instrumented to record the EEG, EMG, and EOG, and also implanted with pairs of guide cannulas targeted in symmetrical positions in each hemisphere. Microdialysis probes collected extracellular fluid from each site every 10 minutes and the levels of acetylcholine, histamine, norepinephrine (NE), and serotonin in the sample were assessed. The seals were maintained on a 12–12h light-dark cycle and samples were collected in active waking, quiet waking, BSWS, USWS, and REM sleep. Acetylcholine was measured in the first study,12 serotonin in the second study,11 and in a study published in the current issue of SLEEP, Lyamin and colleagues13 measured levels of histamine, norepinephrine, and serotonin.
They found that cortical levels of histamine, NE, and serotonin were highest during active waking, declined in BSWS, and were lowest in REM sleep. Subcortical levels of NE (hypothalamus) and serotonin (caudate and thalamus) showed a pattern similar to that seen in the cortex. In their previous study they had found that acetylcholine levels were maximal during active wake, declined during quiet waking and REM sleep, and were minimal in BSWS.12 Thus, in BSWS, the levels of these neurochemicals are consistent with the pattern seen in other terrestrial mammals and correlates very well with the pattern of activity of their respective neurons (Table 1).
Table 1.
Summary of changes in neuronal activity and neurotransmitters in wake, BSWS, USWS, and REM sleep.
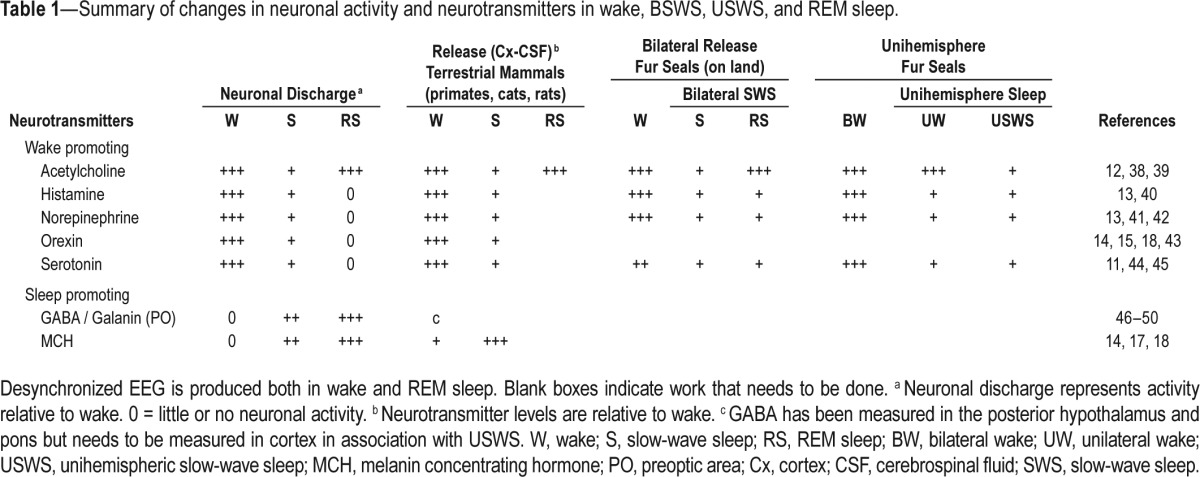
The surprise was that during USWS levels of histamine, NE, and serotonin were not higher in the desynchronized (awake) hemisphere compared to the contralateral hemisphere with USWS.13 On the other hand, acetylcholine release in the cortex was lateralized and tightly linked to the hemisphere that was awake.12 Therefore, the current circuit models cannot explain how activity of histamine, NE, and serotonin neurons drives cortical waking in one hemisphere and not in the other. These models also fail to explain accumulation of sleep drive in one hemisphere and not the other.
We agree that more data are needed. For instance, the next step would be to measure orexin levels in the USWS model system since orexin neurons are active in waking but silent in REM sleep.14,15 However, it is possible that orexin may also not be lateralized to the awake hemisphere since orexin levels are minimal in humans with an activated EEG during pain.16 Moreover, neurochemicals associated with sleep, such as GABA and melanin concentrating hormone (MCH) should be measured.17,18 It is important to measure GABA since it is strongly linked to sleep. Moreover, new data in mice (who only have BSWS) indicate that histamine neurons also release GABA.19 Is GABA lateralized in USWS? Peptides associated with activity, such as prokineticin20 and neuropeptide S21 should also be measured. Moreover, the effects of sleep deprivation in the USWS model on levels of neurotransmitters and peptides should also be assessed.
The current widely accepted circuit model of sleep-wake regulation is based on studies in animals with BSWS.22 The underlying premise of this model is that separate populations of neurons are responsible for wakefulness, slow wave sleep, and REM sleep (summarized in Table 1 and Figure 1A). Although stimulation of specific phenotypes of neurons can robustly influence state17,23 lesions (genetic or neurotoxin) of one or multiple arousal populations does not change daily levels of sleep.24 Moreover, areas of the cortex can be “offline” during waking in humans and rats, suggesting that parts of the brain can be asleep even in waking.25,26 Other data indicate that activating local neurons in the barrel cortex produces a homeo-static load at that site, which is then dissipated by increased sleep at the site.27 Thus, sleep may represent a collective output of small networks of neurons and sleep homeostasis is a reflection of local use.28 This is in contrast with the currently accepted model that sleep and wake result from activity of phenotype-specific subcortical neurons.22
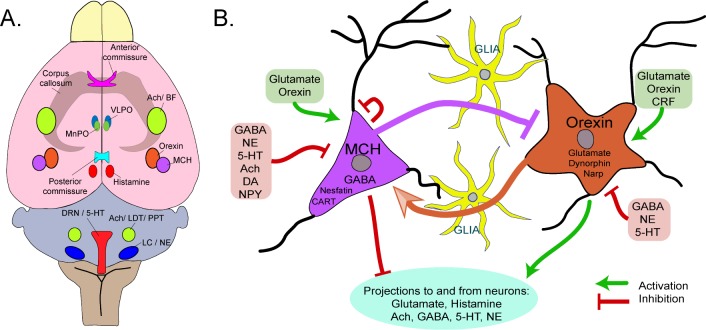
Figure 1.
(A) Horizontal view of the rat brain depicts the regional location of neurons implicated in regulating wake and slow wave sleep (SWS). Neurons regulating REM sleep are located in the brainstem and not depicted here since REM sleep only occurs in both hemispheres. All of these neurons innervate cortical and subcortical regions and release their respective neurotransmitters onto downstream targets. The serotonin neurons are located in the midline, while the other neurotransmitter/peptide containing neurons are located laterally. The extent to which these neurons crossover to the contralateral hemisphere in animals with USWS is not known, and analysis of the decussation pathways (corpus callosum, anterior commissure, and posterior commissure) are inconclusive.36,37 (B) Schematic illustration that local interactions between glia and neurons change local excitability. Wake-induced adenosine released from glia dampens excitability of the wake-active neurons (orexin), which diminishes local wake drive. This small network is affected by local levels of glucose. This network is based upon the local use dependent model.32 Abbreviations: ventrolateral preoptic nucleus (VLPO); median preoptic nucleus (MnPO); basal forebrain (BF); dorsal raphe nucleus (DRN); laterodorsal tegmental nucleus (LDT); posterior pretectal nucleus (PPT); Locus ceruleus (LC); Melanin concentrating hormone (MCH) γ-aminobutyric acid (GABA); norepinephrine (NE); serotonin (5-HT); acetylcholine (Ach); dopamine (DA); neuropeptide Y (NPY); corticotropin releasing factor (CRF); cocaine-amphetamine-regulated transcript (CART); neuronal activity regulated pentraxin (Narp).
In the small network model, glia are included (see Figure 1B).28 Glia outnumber neurons in the brain and a growing body of evidence now considers glia to be partners with neurons in regulating sleep.29 Emergent sleep-like states are evident in small neuronal-glial networks grown in vitro.30 In wildtype C57BL/6J mice optogenetic activation of local astrocytes in the posterior hypothalamus increases sleep.31 Glio-transmitters such as adenosine likely impart the homeostatic load locally.29 This supports the idea of “local use dependent sleep.”32 The interaction between glia and neurons in small networks efficiently regulates synaptic strength.33,34 The local use dependent model nicely explains the accumulation of sleep in one hemisphere and not in the other in response to USWS deprivation.
We are grateful to Lyamin and colleagues for pursuing very difficult studies to identify the cellular correlates of USWS and BSWS in Otariidae. We recognize that research in other animal models will expedite discovery. We suggest harnessing the power of genetics to identify unihemispheric sleep in roundworms (C. elegans), fruit flies (D. melanogoster), and zebrafish (D. danio). For instance, in these model systems developmental cell fate mapping approach could target cells in one hemisphere and not the other.35 We also suggest the use of new methods such as optogenetics and pharmacogenetics in birds. Because the size of the bird brain is small, it could be probed by new tissue clearing methods such as CLARITY to identify difference in connectivity between hemispheres and comparisons can be made with the mouse brain. We believe that a collective effort is necessary to identify the cellular basis of sleep.
CITATION
Konadhode RR, Pelluru D, Shiromani PJ. Unihemispheric Sleep: An enigma for current models of sleep-wake regulation. SLEEP 2016;39(3):491–494.
DISCLOSURE STATEMENT
Supported by the Medical Research Service of the Department of Veterans Affairs and NIH grants NS084477, NS052287, and NS079940. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. Carlos Blanco-Centurion and Dr. Meng Liu for their helpful comments during the writing of the commentary.
REFERENCES
- 1.Lyamin OI, Manger PR, Ridgway SH, Mukhametov LM, Siegel JM. Cetacean sleep: an unusual form of mammalian sleep. Neurosci Biobehav Rev. 2008;32:1451–84. doi: 10.1016/j.neubiorev.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–71. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyamin OI, Kosenko PO, Lapierre JL, Mukhametov LM, Siegel JM. Fur seals display a strong drive for bilateral slow-wave sleep while on land. J Neurosci. 2008;28:12614–21. doi: 10.1523/JNEUROSCI.2306-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukhametov LM, Supin AY, Polyakova IG. Interhemispheric asymmetry of the electroencephalographic sleep patterns in dolphins. Brain Res. 1977;134:581–4. doi: 10.1016/0006-8993(77)90835-6. [DOI] [PubMed] [Google Scholar]
- 5.Roth TC, Jr, Lesku JA, Amlaner CJ, Lima SL. A phylogenetic analysis of the correlates of sleep in birds. J Sleep Res. 2006;15:395–402. doi: 10.1111/j.1365-2869.2006.00559.x. [DOI] [PubMed] [Google Scholar]
- 6.Rattenborg NC. Do birds sleep in flight? Naturwissenschaften. 2006;93:413–25. doi: 10.1007/s00114-006-0120-3. [DOI] [PubMed] [Google Scholar]
- 7.Lesku JA, Rattenborg NC, Amlaner CJ. The evolution of sleep: a phylogenetic approach. In: Lee-Chiong TL, editor. Sleep: a comprehensive handbook. Hoboken, NJ: J. Wiley; 2005. [Google Scholar]
- 8.Manger PR. An examination of cetacean brain structure with a novel hypothesis correlating thermogenesis to the evolution of a big brain. Biol Rev Camb Philos Soc. 2006;81:293–338. doi: 10.1017/S1464793106007019. [DOI] [PubMed] [Google Scholar]
- 9.Oleksenko AI, Mukhametov LM, Polyakova IG, Supin AY, Kovalzon VM. Unihemispheric sleep deprivation in bottlenose dolphins. J Sleep Res. 1992;1:40–4. doi: 10.1111/j.1365-2869.1992.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 10.Mukhametov LM, Oleksenko AI, Polyakova IG. Quantification of ECoG stages of sleep in the bottlenose dolphin. Neurophysiology. 1988;20:398–403. [Google Scholar]
- 11.Lapierre JL, Kosenko PO, Kodama T, et al. Symmetrical serotonin release during asymmetrical slow-wave sleep: implications for the neurochemistry of sleep-waking states. J Neurosci. 2013;33:2555–61. doi: 10.1523/JNEUROSCI.2603-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lapierre JL, Kosenko PO, Lyamin OI, Kodama T, Mukhametov LM, Siegel JM. Cortical acetylcholine release is lateralized during asymmetrical slow-wave sleep in northern fur seals. J Neurosci. 2007;27:11999–2006. doi: 10.1523/JNEUROSCI.2968-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyamin OI, Lapierre JL, Kosenko PO, et al. Monoamine release during unihemispheric sleep and unihemispheric waking in the fur seal. Sleep. 2016;39:625–36. doi: 10.5665/sleep.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci U S A. 2009;106:2418–22. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–98. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blouin AM, Fried I, Wilson CL, et al. Human hypocretin and melanin-concentrating hormone levels are linked to emotion and social interaction. Nat Commun. 2013;4:1547. doi: 10.1038/ncomms2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konadhode RR, Pelluru D, Blanco-Centurion C, et al. Optogenetic stimulation of MCH neurons increases sleep. J Neurosci. 2013;33:10257–63. doi: 10.1523/JNEUROSCI.1225-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelluru D, Konadhode R, Shiromani PJ. MCH neurons are the primary sleep-promoting group. Sleep. 2013;36:1779–81. doi: 10.5665/sleep.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu X, Ye Z, Houston CM, et al. Wakefulness is governed by GABA and histamine cotransmission. Neuron. 2015;87:164–78. doi: 10.1016/j.neuron.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu WP, Li XM, Chen JG, Li ZW, et al. Altered circadian and homeostatic sleep regulation in prokineticin 2-deficient mice. Sleep. 2007;30:247–56. [PMC free article] [PubMed] [Google Scholar]
- 21.Ahnaou A, Drinkenburg WH. Neuropeptide-S evoked arousal with electroencephalogram slow-wave compensatory drive in rats. Neuropsychobiology. 2012;65:195–205. doi: 10.1159/000336998. [DOI] [PubMed] [Google Scholar]
- 22.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–42. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter ME, de Lecea L. Optogenetic investigation of neural circuits in vivo. Trends Mol Med. 2011;17:197–206. doi: 10.1016/j.molmed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanco-Centurion C, Gerashchenko D, Shiromani PJ. Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake. J Neurosci. 2007;27:14041–8. doi: 10.1523/JNEUROSCI.3217-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vyazovskiy VV, Olcese U, Lazimy YM, et al. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–78. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Local sleep in awake rats. Nature. 2011;472:443–7. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandt JA, Churchill L, Guan Z, Fang J, Chen L, Krueger JM. Sleep deprivation but not a whisker trim increases nerve growth factor within barrel cortical neurons. Brain Res. 2001;898:105–12. doi: 10.1016/s0006-8993(01)02149-7. [DOI] [PubMed] [Google Scholar]
- 28.Krueger JM, Huang YH, Rector DM, Buysse DJ. Sleep: a synchrony of cell activity-driven small network states. Eur J Neurosci. 2013;38:199–209. doi: 10.1111/ejn.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank MG. Astroglial regulation of sleep homeostasis. Curr Opin Neurobiol. 2013;23:812–8. doi: 10.1016/j.conb.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Jewett KA, Taishi P, Sengupta P, Roy S, Davis CJ, Krueger JM. Tumor necrosis factor enhances the sleep-like state and electrical stimulation induces a wake-like state in co-cultures of neurons and glia. Eur J Neurosci. 2015;42:2078–90. doi: 10.1111/ejn.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelluru D, Konadhode RR, Bhat NR, Shiromani PJ. Optogenetic stimulation of astrocytes in the posterior hypothalamus increases sleep at night in C57BL/6J mice. Eur J Neurosci. 2015 Sep 15; doi: 10.1111/ejn.13074. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krueger JM, Frank MG, Wisor JP, Roy S. Sleep function: toward elucidating an enigma. Sleep Med Rev. 2015;28:42–50. doi: 10.1016/j.smrv.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krueger JM, Obal F. A neuronal group theory of sleep function. J Sleep Res. 1993;2:63–9. doi: 10.1111/j.1365-2869.1993.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 34.Rattenborg NC, Lima SL, Lesku JA. Sleep locally, act globally. Neuroscientist. 2012;18:533–46. doi: 10.1177/1073858412441086. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi Y, Kashiwagi M, Yasuda K, et al. Cells of a common developmental origin regulate REM/non-REM sleep and wakefulness in mice. Science. 2015;350:957–61. doi: 10.1126/science.aad1023. [DOI] [PubMed] [Google Scholar]
- 36.Suarez R, Gobius I, Richards LJ. Evolution and development of interhemispheric connections in the vertebrate forebrain. Front Hum Neurosci. 2014;8:497. doi: 10.3389/fnhum.2014.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rattenborg NC, Amlaner CJ, Lima SL. Behavioral, neurophysiological and evolutionary perspectives on unihemispheric sleep. Neurosci Biobehav Rev. 2000;24:817–42. doi: 10.1016/s0149-7634(00)00039-7. [DOI] [PubMed] [Google Scholar]
- 38.Steriade M, Datta S, Paré D, Oakson G, Curró Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10:2541–59. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Celesia GG, Jasper HH. Acetylcholine released from cerebral cortex in relation to state of activation. Neurology. 1966;16:1053–63. doi: 10.1212/wnl.16.11.1053. [DOI] [PubMed] [Google Scholar]
- 40.John J, Wu MF, Boehmer LN, Siegel JM. Cataplexy-active neurons in the hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron. 2004;42:619–34. doi: 10.1016/s0896-6273(04)00247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–86. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu N, Bloom FE. Norepinephrine-containing neurons: changes in spontaneous discharge patterns during sleeping and waking. Science. 1973;179:908–10. doi: 10.1126/science.179.4076.908. [DOI] [PubMed] [Google Scholar]
- 43.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–20. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–75. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- 45.Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology. 1999;21(2 Suppl):9S–15S. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 46.Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–9. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 47.Szymusiak R, McGinty D. Sleep-related neuronal discharge in the basal forebrain of cats. Brain Res. 1986;370:82–92. doi: 10.1016/0006-8993(86)91107-8. [DOI] [PubMed] [Google Scholar]
- 48.Nitz D, Siegel J. GABA release in the dorsal raphe nucleus: role in the control of REM sleep. Am J Physiol. 1997;273(1 Pt 2):R451–5. doi: 10.1152/ajpregu.1997.273.1.R451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nitz D, Siegel JM. GABA release in posterior hypothalamus across sleep-wake cycle. Am J Physiol. 1996;271(6 Pt 2):R1707–12. doi: 10.1152/ajpregu.1996.271.6.R1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nitz D, Siegel JM. GABA release in the locus coeruleus as a function of sleep/wake state. Neuroscience. 1997;78:795–801. doi: 10.1016/s0306-4522(96)00549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]