Abstract
Study Objectives:
To assess sleep inertia following 10-min and 30-min naps during a simulated night shift.
Methods:
Thirty-one healthy adults (aged 21–35 y; 18 females) participated in a 3-day laboratory study that included one baseline (BL) sleep (22:00–07:00) and one experimental night involving randomization to either: total sleep deprivation (NO-NAP), a 10-min nap (10-NAP) or a 30-min nap (30-NAP). Nap opportunities ended at 04:00. A 3-min psychomotor vigilance task (PVT-B), digit-symbol substitution task (DSST), fatigue scale, sleepiness scale, and self-rated performance scale were undertaken pre-nap (03:00) and at 2, 17, 32, and 47 min post-nap.
Results:
The 30-NAP (14.7 ± 5.7 min) had more slow wave sleep than the 10-NAP (0.8 ± 1.5 min; P < 0.001) condition. In the NO-NAP condition, PVT-B performance was worse than pre-nap (4.6 ± 0.3 1/sec) at 47 min post-nap (4.1 ± 0.4 1/sec; P < 0.001). There was no change across time in the 10-NAP condition. In the 30-NAP condition, performance immediately deteriorated from pre-nap (4.3 ± 0.3 1/sec) and was still worse at 47 min post-nap (4.0 ± 0.5 1/sec; P < 0.015). DSST performance deteriorated in the NO-NAP (worse than pre-nap from 17 to 47 min; P < 0.008), did not change in the 10-NAP, and was impaired 2 min post-nap in the 30-NAP condition (P = 0.028). All conditions self-rated performance as better than pre-nap for all post-nap test points (P < 0.001).
Conclusions:
This study is the first to show that a 10-min (but not a 30-min) nighttime nap had minimal sleep inertia and helped to mitigate short-term performance impairment during a simulated night shift. Self-rated performance did not reflect objective performance following a nap.
Citation:
Hilditch CJ, Centofanti SA, Dorrian J, Banks S. A 30-minute, but not a 10-minute nighttime nap is associated with sleep inertia. SLEEP 2016;39(3):675–685.
Keywords: alertness, fatigue countermeasure, nap sleep, night shift work, psychomotor vigilance task, sleep deprivation, sleep inertia, subjective performance
Significance.
Short, 10-min naps taken in the afternoon have been shown to produce immediate benefits to performance without the side effect of sleep inertia. This study is the first to investigate the effectiveness of a 10-min nap taken at night during a simulated night shift. The findings from this study are important for informing recommendations for napping on-shift. The key messages include: a 10-min nap at night is not as effective as during the day; a 30-min nap at night can result in sleep inertia lasting nearly an hour; and workers may not be aware of their own performance impairment following a nap at night. Future research should look to trial 10-min naps in a workplace setting.
INTRODUCTION
Napping is an important fatigue management strategy for shift workers. This countermeasure is often used on night shifts, which are associated with increased sleepiness, performance impairment, and risk of workplace injury and errors.1–10 Napping can alleviate the buildup of homeostatic sleep pressure across the night11,12 and, depending on timing, remove workers from safety-critical tasks during a period of low circadian alertness (i.e., 02:00–06:00).13,14 Although the methodology and operational settings vary, most napping studies from the laboratory,11,15–19 and field,12,20–26 have observed benefits to performance and subjective alertness following night shift naps.27
A potential downside to on-shift napping, however, is the possibility of sleep inertia following the nap. Sleep inertia refers to the brief period of reduced alertness and impaired cognitive performance experienced immediately after waking.28 Human error attributed to sleep inertia has been a causal factor in several major incidents across many industries including aviation, military, and maritime.29–32
Some studies suggest that sleep inertia can be exacerbated by the amount of slow wave sleep (SWS) in the prior sleep period, or by waking from SWS.33–35 In order to minimize sleep inertia, studies have investigated brief naps (≤ 30 min) to reduce the chance of entering, and waking from, SWS.36,37 Brief naps are also more operationally feasible for night shift workers who only have short breaks.20,25,38 A series of studies investigating brief naps during the afternoon showed that although a 30-min nap resulted in sleep inertia lasting between 5–35 min, a 10-min nap did not cause sleep inertia.36,37 Instead, a 10-min nap afforded immediate performance benefits (5 min post-nap), and these benefits were observed for at least 35 min after waking.36,37
Despite the demonstrated benefits of a 10-min nap in the afternoon, no studies have investigated a 10-min nap at night. It is unknown whether 10-min naps will behave the same way during a simulated night shift when the homeostatic and circadian pressure for sleep is greater.13 Evidence suggests that under these conditions, sleep inertia is likely to be more severe due to time of day34,39,40 and prior sleep loss.34,41 The sleep architecture of the nap may also differ from afternoon studies, which report little to no SWS in 10-min naps.36,42 Naps on night shift often follow periods of extended prior wakefulness and high homeostatic pressure.43 Therefore, a shorter onset latency of SWS in night naps may be expected,44,45 and this may, in turn, affect sleep inertia.33–35
Studies of 30-min naps during the night are also scarce. Fewer still have comprehensively measured performance and sleepiness during the first hour after waking, focusing instead on the potential for the nap to sustain performance toward the end of the shift.12,22 Previous studies of 30-min nighttime naps therefore offer limited insight into the effects of sleep inertia due to methodological issues such as only having one post-nap testing point,12,22,26 or not testing until at least 10–15 min after waking.11,26 From the limited data available from these studies, it appears that sleep inertia associated with 30-min nighttime naps tends to be of short duration (< 15 min) and any improvements in performance relative to no-nap conditions are typically delayed until at least 60 min after the nap.11,12,26 These observations might suggest that there are longer-lasting inertia effects in the intermediary period (15–60 min post-nap), which mask the eventual benefits from the nap. The current study has been designed to address this gap by including multiple, frequent testing points to determine the time course of any potential inertia during the first hour after waking.
The aim of this study is to systematically investigate, for the first time, performance and subjective sleepiness immediately following a 10-min and a 30-min nap ending at 04:00. We hypothesized that under the conditions of biological night and extended wakefulness, a 10-min nighttime nap would lead to short-lasting sleep inertia. Further, we predicted that a 30-min nap would lead to sleep inertia of a greater magnitude and duration than the 10-min nap.
METHODS
Participants
Thirty-two healthy adult volunteers were recruited for the study. One participant withdrew due to illness part-way through the study and was not included in the analyses. The mean age (± standard deviation) of the 31 participants analyzed was 24.3 ± 3.4 y (range: 21–35 y; 18 female). The average body mass index (BMI) of the group was 22.2 ± 3.0 kg/m2.
Participants habitually obtained a minimum of 7 h of sleep per night, with bedtime before midnight and rise time before 09:00. This was assessed during a telephone screening interview and confirmed with a sleep diary, actigraphy, and timestamped messages in the week before the study. During this week, participants were not allowed to nap or consume caffeine nor alcohol.
The following exclusion criteria were applied (as assessed by self-report, as well as other measures where indicated): smoking; drinking more than two cups of caffeinated drinks or two standard drinks of alcohol per day; transmeridian travel in the past 3 mo; shift work in the past 2 y; regularly taking more than one nap per week; BMI above 30 kg/m2; current medication or recreational drug use (other than oral contraception); absence of illicit drugs confirmed by urine test; and any medical disorders, psychological disorders (Beck Depression Inventory46), or sleep disorders (Pittsburgh Sleep Quality Index47). Blood chemistry was conducted to confirm general health. Participants were intermediate type on the Composite Scale of Morningness-Eveningness.48
The study was approved by the University of South Australia Human Research Ethics Committee. Participants gave written, informed consent and were financially compensated for their time.
Study Design
Participants resided in a windowless and sound-insulated sleep laboratory for 3 consecutive days (2 nights). Ambient room temperature was maintained at 22 (± 1)°C. Light intensity was set to < 50 lux at head height (dim light) during all wake periods of the protocol, and < 0.03 lux (darkness) during all scheduled sleep periods.
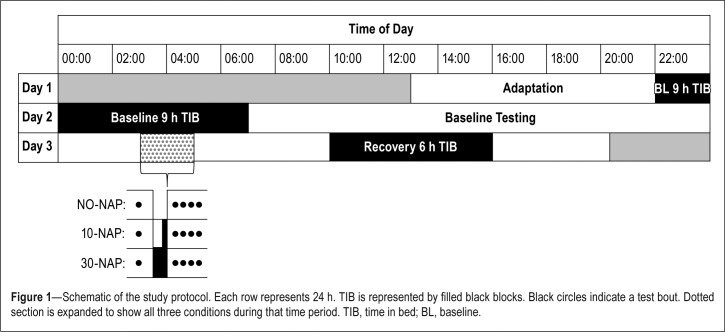
Figure 1 illustrates the study protocol. Participants spent 2 nights and 3 days in the sleep laboratory: 1 day for adaptation and training, 1 baseline day and night, 1 experimental night of sleep deprivation, and 1 recovery day. Participants arrived at the laboratory at 13:00 and spent the adaptation day practicing various performance tasks. They had a 9-h sleep opportunity between 22:00 and 07:00 on the baseline night. The second night involved a simulated night shift in which participants were randomly assigned, in a between-subjects design, to one of three conditions: a control condition (NO-NAP; n = 11, 7 females); a 10-min “on-shift” nap (10-NAP; n = 10, 5 females); or a 30-min “on-shift” nap (30-NAP; n = 10, 6 females). Both naps ended at 04:00. On the final day of the study, participants were allowed a 6-h daytime recovery sleep opportunity between 10:00 and 16:00.
Figure 1.
Schematic of the study protocol. Each row represents 24 h. TIB is represented by filled black blocks. Black circles indicate a test bout. Dotted section is expanded to show all three conditions during that time period. TIB, time in bed; BL, baseline.
During wake periods, participants performed neurobehavioral test batteries approximately every 2 h and were permitted to read books, play card/board games, watch DVDs, interact with each other and study staff, or listen to music between test sessions. Participants did not have access to clock-bearing or telecommunication devices, but were allowed access to their mobile phones for 10 min at the start of a 90-min free-time period (09:30) on the baseline day to make a short call or send a text. They were not allowed to access the Internet. Participants were not allowed to perform any vigorous activities during the study.
Neurobehavioral Testing
In order to capture the time course of the dissipation of sleep inertia, an 8-min inertia test battery was performed pre-nap (03:00) and 2 min, 17 min, 32 min, and 47 min after the nap ending at 04:00 (lights on).
The inertia test battery was conducted on a computer positioned next to the bed in each participant's room. At the scheduled time of awakening, the lights were turned on, and participants immediately moved from bed to a computer chair adjacent to their bed. Throughout the 1-h period after scheduled awakening, participants remained seated at the computer; they were not allowed to stand or leave the room. During the 7-min intervals between test bouts, participants sat quietly and were not allowed to chat, read, or listen to music.
The sleep inertia test battery included the following objective tasks and subjective scales, in order of presentation: a brief psychomotor vigilance task (PVT-B, 3 min); the Samn-Perelli Fatigue Scale (SP-Fatigue); the Karolinska Sleepiness Scale (KSS); a digit-symbol substitution task (DSST, 3 min), and a Likert-type scale of self-rated performance.
The PVT-B is a validated,49 3-min version of the well-established 10-min PVT, a measure of behavioral alertness50 that has no significant learning curve.7 The PVT-B requires participants to press a button on a handheld device as soon as a visual stimulus is presented. Participants are instructed to react as quickly as possible without making false starts. The interstimulus interval was randomized and varied between 1 sec and 4 sec. The outcome measure from the PVT-B presented here is response speed (the mean of reciprocal reaction times).
The DSST is a 3-min computerized version of a task included in the Wechsler Adult Intelligence Scale.51 It is a self-paced task that requires participants to match numbers 1–9 to a series of randomly presented symbols (e.g., #, *, = ) based on a code displayed at the top of the screen. The pairings of the digits and symbols were randomized for each test. At the start of each test, 10 symbols were presented with feedback to the participant; these pairings were not included in the analysis. Participants were instructed to respond accurately and quickly to correctly match as many symbols as possible. The outcome measure from the DSST presented here is number of correct responses.
The Samn-Perelli Fatigue Scale (SP-Fatigue) is a 7-point Likert-type scale with scores ranging from 1 (“fully alert, wide awake”) to 7 (“completely exhausted, unable to function effectively”).52 The Karolinska Sleepiness Scale (KSS) is a 9-point Likert-type scale with scores ranging from 1 (“extremely alert”) to 9 (“extremely sleepy, fighting sleep”).53 Participants were also asked to self-rate their performance after each test bout on a 7-point Likert-type scale ranging from 1 (“extremely good”) to 7 (“extremely poor”).
Polysomnography
Sleep was recorded using polysomnography (PSG). The electrode montage included derivations C3/A2, C4/A1, F3/A2, F4/ A1, O1/A2, two-channel electrooculogram (EOG), electro-myogram (EMG), and electrocardiogram (ECG). PSG was recorded to a data acquisition, storage, and analysis system (Grael; Compumedics, Melbourne, Australia). A trained sleep scorer, blinded to the experimental aims, used Rechtshaffen and Kales rules54 to score all sleep periods. Sleep variables analyzed include: total sleep time (TST), sleep efficiency (SE; TST / time in bed * 100), sleep onset latency (SOL), wake after sleep onset (WASO), amount of stage 1, 2, 3, 4 and rapid eye movement. Sleep stage at lights on was defined as the sleep stage (or wakefulness) scored in the 30-sec epoch immediately prior to lights on.
Statistical Analyses
One-way analysis of variance tests (ANOVAs) were used to compare demographics between conditions.
In order to test the effect of a nap on neurobehavioral outcomes during the sleep inertia measurement period, a fully saturated, linear mixed-effects ANOVA55 with a between-subjects fixed effect of condition (NO-NAP, 10-NAP, 30-NAP) and a within-subject fixed effect of time (pre-nap, 2, 17, 32, 47 min) and a random intercept over participants was used. Simple planned contrasts (pre-nap versus each post-nap test point) were conducted to further investigate significant interaction effects within conditions. Within-condition comparisons were chosen in order to minimize the influence of individual differences. As a secondary analysis, between-conditions comparisons were also assessed for each time point.
To compare baseline sleep variables between groups, a oneway ANOVA was used with a between-subjects fixed effect of condition (NO-NAP, 10-NAP, 30-NAP). Sleep variables during the nap opportunity were analyzed using a one-way ANOVA with a between-subjects fixed effect of condition (10-NAP, 30-NAP).
Correlations were used to assess the relationship between sleep and neurobehavioral variables. The neurobehavioral outcome measure used in the analysis was the change from pre-nap to the first test point (2 min after waking). The sleep variables of interest were TST and SWS.
A Satterthwaite correction was applied to the denominator degrees of freedom. However, these values have been reported to the nearest whole number.
RESULTS
There were no significant differences between conditions in terms of age (F2,30 = 0.297; P = 0.745), BMI (F2,30 = 2.932; P = 0.07), or morningness-eveningness scores (F2,30 = 0.9; P = 0.418).
Sleep
There were no significant differences between conditions for any sleep variables at baseline (Table 1).
Table 1.
Baseline sleep variables.
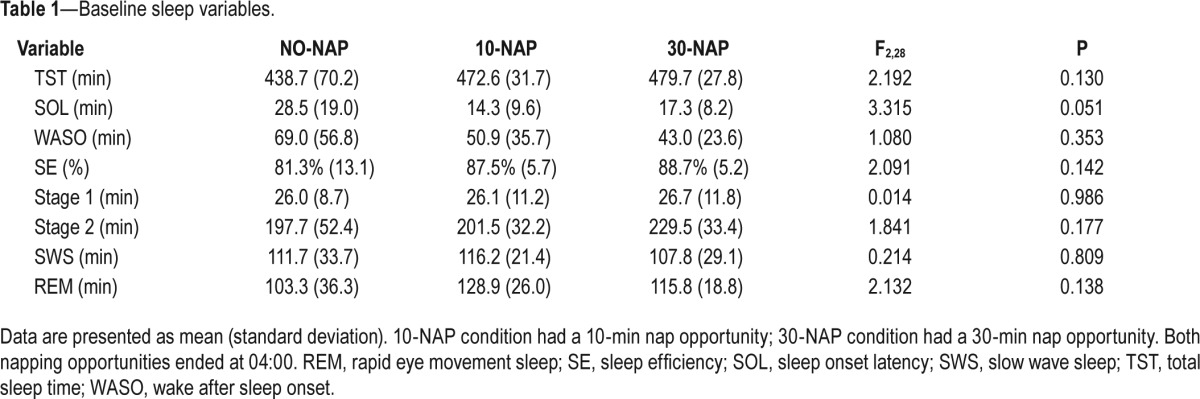
Table 2 shows the distribution of sleep stages for the 10-min and 30-min nap. All participants obtained physiologically defined sleep during the napping opportunity according to stan-dardised criteria.54 The 10-NAP group had significantly less TST (8 min versus 26.4 min in the 30-NAP group; P < 0.001), Stage 2 (4.8 min versus 8.4 min; P = 0.002), and SWS (0.8 min versus 14.7 min; P < 0.001) than the 30-NAP condition.
Table 2.
Nap sleep variables.
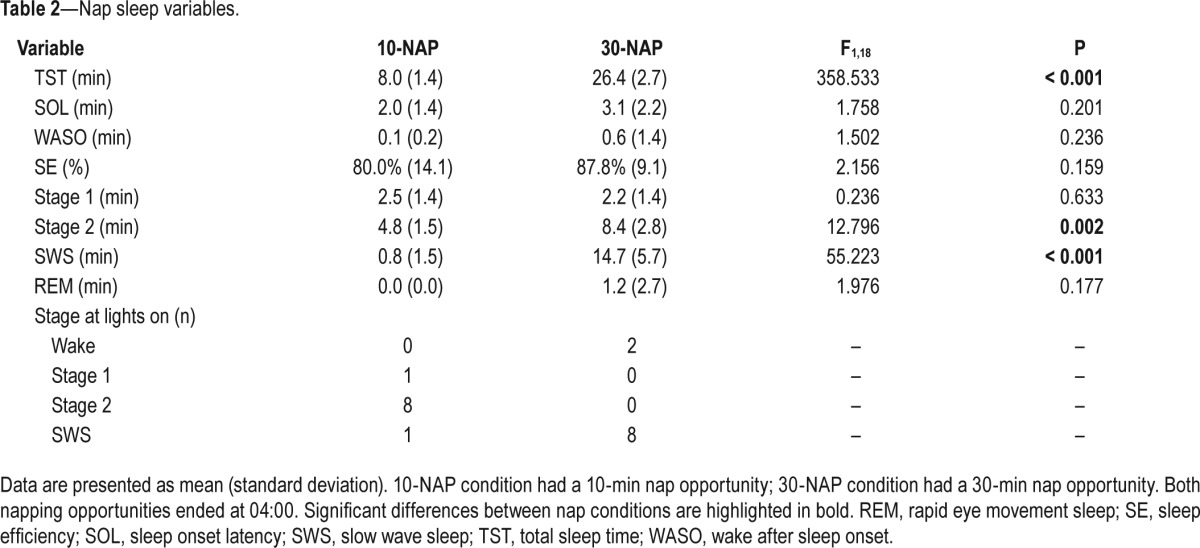
With regard to sleep stage at lights on, the majority of participants in the 10-NAP condition woke from Stage 2 (80%); the majority of participants in the 30-NAP condition woke from SWS (80%) (Table 2). Two participants in the 30-NAP condition were awake at lights on, for a maximum of 60 sec. One participant in the 10-NAP condition woke from SWS. Three participants in the 10-NAP condition entered SWS during the nap; all participants (n = 10) in the 30-NAP condition entered SWS.
Neurobehavioral Measures
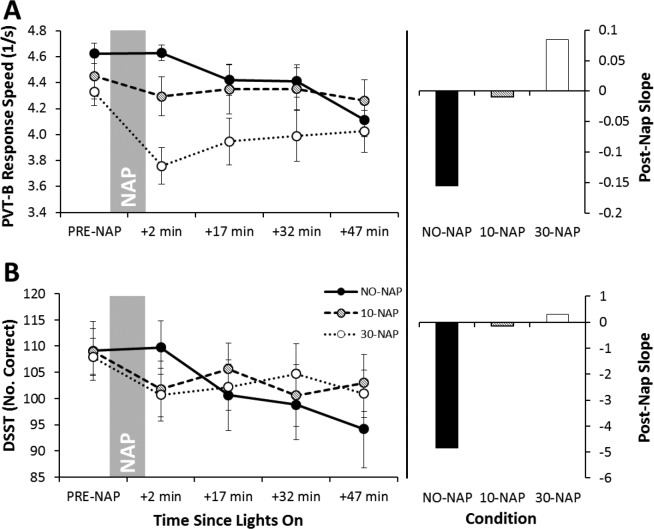
Figure 2A shows response speed data for the PVT-B. There was a significant main effect of condition (P = 0.032), time (P < 0.001) and their interaction (P = 0.002; Table 3). In the NO-NAP condition, performance deteriorated across time and was significantly worse than pre-nap at 47 min post-nap (P < 0.001). There was no change across time in the 10-NAP condition. In the 30-NAP condition, all post-nap testing points were significantly worse than pre-nap performance (P < 0.015). There was no difference between conditions at pre-nap. The 30-NAP condition was significantly worse than NO-NAP and 10-NAP at 2, 17, and 32 min after waking (P < 0.04). The right-side panel of Figure 2A highlights the slope of the post-nap testing points for each condition. PVT-B performance in the NO-NAP condition continued to deteriorate across time, whereas there was no change in the 10-NAP condition. The 30-NAP condition improved across testing points following the initial decrease in performance immediately after waking.
Figure 2.
Mean (± standard error of the mean) for cognitive performance tasks. (A) Psychomotor Vigilance Task-B Response Speed; (B) Digit-Symbol Substitution Task Number of Correct Responses. Higher values represent better performance. Left-side panels display data per condition across pre- and post-nap testing points. Marker shading in the left-side panels matches column shading in the right-side panels for each condition. To assist the reader in clearly identifying the post-nap slopes for each group, the bars on the right simply represent the slope of the mean points as displayed in the left panel.
Table 3.
Results from the linear mixed-effects analysis of variance for neurobehavioral outcomes.
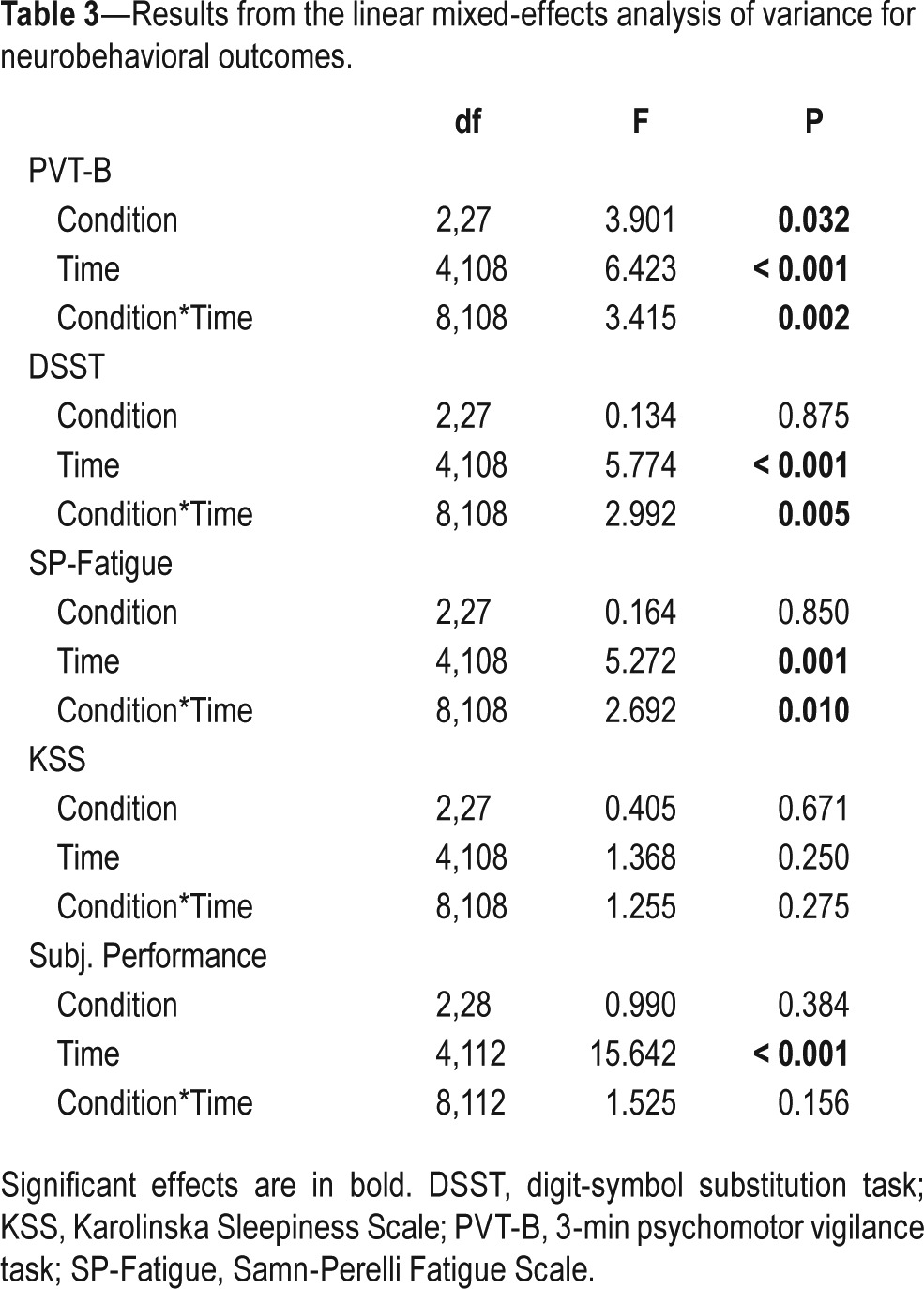
Figure 2B shows number of correct responses on the DSST. There was a significant main effect of time (P < 0.001) and a significant condition*time interaction (P = 0.005; Table 3). In the NO-NAP condition, performance was significantly worse than pre-nap at 17 min, 32 min, and 47 min post-nap (P < 0.008), with a continual decline across post-nap testing points (see post-nap slope). Despite a downward trend, there was no significant change pre-nap to post-nap for the 10-NAP condition. The 30-NAP condition was significantly worse than pre-nap at 2 min and 47 min post-nap (P < 0.034). There were no differences between conditions at pre-nap, or any post-nap testing points.
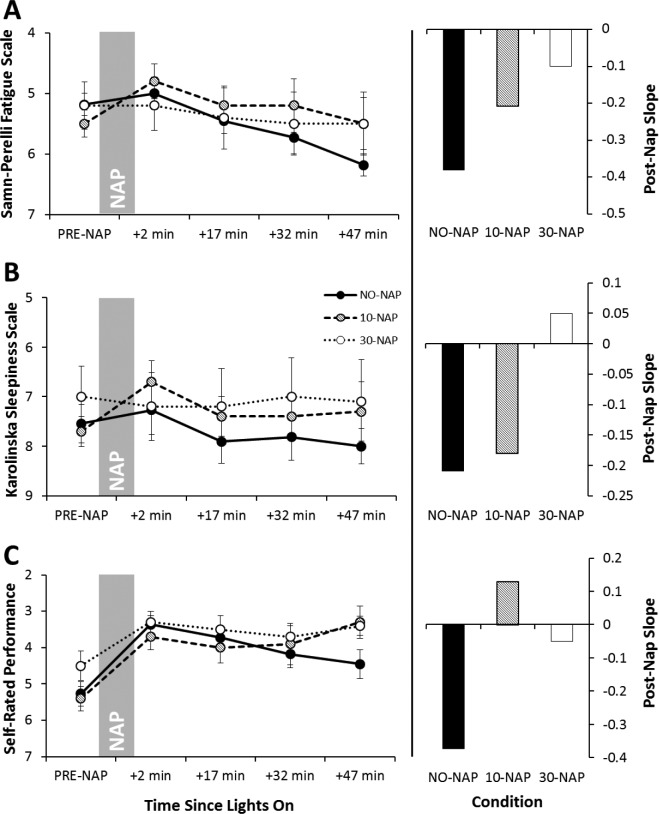
Figure 3 shows the scores for each subjective scale. For SP-Fatigue there was a significant main effect of time (P = 0.001) and a significant condition*time interaction (P = 0.010; Table 3). SP-Fatigue in the NO-NAP condition was significantly worse than pre-nap at 32 min and 47 min post-nap (P < 0.023). In the 10-NAP condition, SP-Fatigue was significantly better at 2 min and 32 min post-nap compared to pre-nap (P < 0.035). There was no change across time for the 30-NAP condition. There was no difference between conditions at any time point. After the nap, SP-Fatigue worsened across testing points in all conditions. The NO-NAP condition had the steepest slope, followed by the 10-NAP and 30-NAP conditions, respectively (Figure 3A).
Figure 3.
Mean (± standard error of the mean) for subjective scales. (A) Samn-Perelli Fatigue Scale; (B) Karolinska Sleepiness Scale; (C) Self-Rated Performance Scale. Scales are reversed for presentation, with better ratings higher on the y-axis. Left-side panels display data per condition across preand post-nap testing points. Marker shading in the left-side panels matches column shading in the right-side panels for each condition. To assist the reader in clearly identifying the post-nap slopes for each group, the bars on the right simply represent the slope of the mean points as displayed in the left panel.
The pattern of KSS ratings resembled SP-Fatigue; however, there were no significant main or interaction effects (Table 3). After the nap, sleepiness worsened across testing points in the NO-NAP and 10-NAP conditions, but was relatively stable in the 30-NAP condition (Figure 3B).
There was a significant main effect of time (P < 0.001) for self-rated performance such that all post-nap time points were significantly better than pre-nap (P < 0.001; Table 3). Participants rated their performance as worsening across post-nap testing points in the NO-NAP condition. The 10-NAP condition group thought they had slightly improved, and the 30-NAP condition group reported no change across the post-nap testing period (Figure 3C).
Sleep and Neurobehavioral Measures
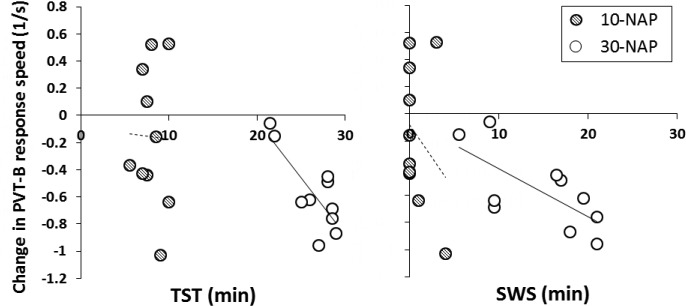
Table 4 displays the results of the correlational analyses between each neurobehavioral variable and sleep (TST and SWS). Significant relationships were found for PVT-B response speed in the 30-NAP condition with better performance associated with less TST (r = −0.784, P = 0.007) and less SWS (r = −0.689, P = 0.027) (Figure 4).
Table 4.
Correlations between the change in neurobehavioral outcomes from pre-nap to 2 min post-nap and sleep.
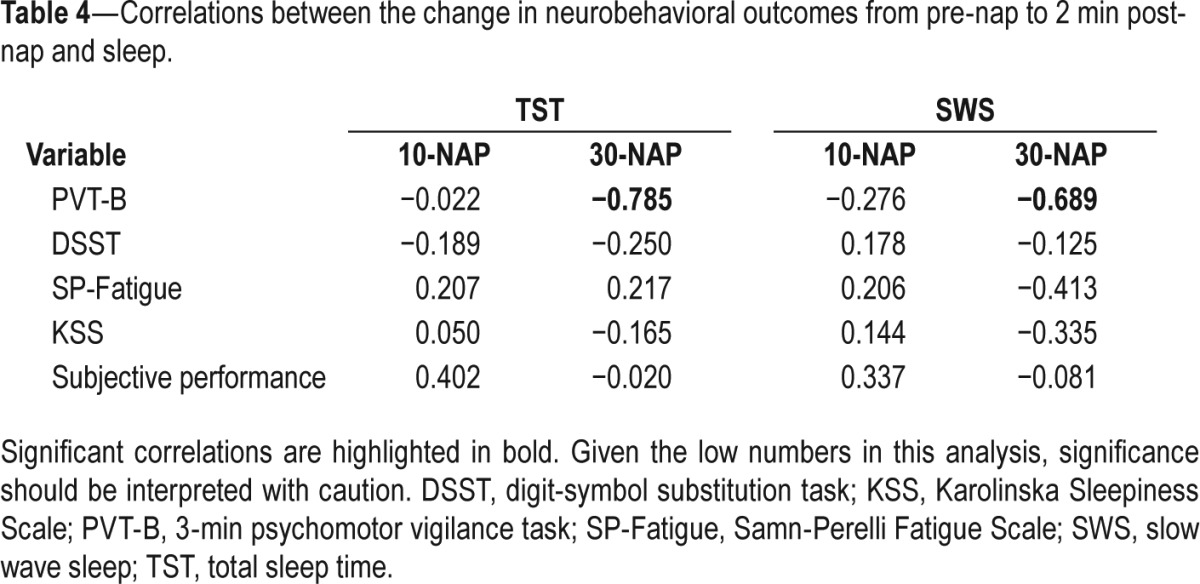
Figure 4.
Scatterplots for change in psychomotor vigilance task-B response speed at first wake-up test point (+2 min) compared to pre-nap against total sleep (left panel) and SWS (right panel). Trend lines are shown for each condition (10-NAP: striped circles, dashed lines; 30-NAP: white circles, solid lines). SWS, slow wave sleep; TST, total sleep time.
DISCUSSION
This is the first study to have investigated whether brief naps ending at 04:00 result in sleep inertia. Results suggested that a 10-min nap was associated with minimal sleep inertia, and mitigated the decline in performance observed in the NONAP condition at 47 min post-nap. The 30-min nap, however, was associated with substantial sleep inertia as measured by a decrease in performance after waking. Performance on the PVT-B was also slow to recover across the 47-min post-nap period. Based on these results, if safety critical tasks requiring rapid responses to stimuli are scheduled between 04:00–05:00 during a night shift, a 10-min nap ending at 04:00 would be recommended over a 30-min nap. The 10-min nap attenuated continuing performance impairment from cumulative hours of wakefulness, while avoiding the pitfall of sleep inertia following the nap.
In workplace scenarios where there are long rest break opportunities (> 1 h), a longer nap may provide immediate and long lasting benefits. For example, Kubo et al.15 found minimal sleep inertia following 2-h naps taken at night. However, long breaks are often not available in many workplaces.38,56 The costs and benefits of short naps are therefore relevant to many operational situations.
Based on previous studies,11,12,22,26 we hypothesized that sleep inertia would dissipate within half an hour following the 30-min nap. Response speed, however, did not recover to pre-nap levels after 47 min post-nap. Furthermore, long-term benefits of the 30-min nap were only observed for subjective measures of alertness, with no benefits to objective performance.57 Previous studies of 30-min naps during the night reported that objective measures of sleep inertia dissipated within 15 min of waking,12 or were not significantly different to a no-nap condition up to 45 min post-nap.11 However, in the first case, no further testing points were used to determine whether performance continued to improve, and there was no pre-nap test for reference.12 In the latter case, the first testing point was not until 15 min post-nap and a 2-h prophylactic nap was taken in the afternoon, which may have affected nap sleep and subsequent performance.11
Afternoon studies of 10-min naps reported immediate and sustained improvement relative to pre-nap values.36,37 Our results, however, suggest that at night, under greater homeostatic and circadian sleep pressures, a 10-min nap was only able to maintain pre-nap performance levels and attenuate the performance impairment seen in the no-nap condition. This still shows a value for 10-min naps at this time, although their efficacy appears to be reduced compared to when taken in the afternoon.
Similar patterns in performance were seen for both PVT-B and DSST. On both tasks, the no-nap condition showed continued deterioration of performance as hours of wakefulness extended, the 10-min nap condition showed no change across time, and the 30-min nap resulted in an immediate decrease in performance. However, following the 30-min nap, performance impairment observed for the DSST was shorter and less severe than on the PVT-B.
Different findings between these tasks may be explained by learning effects, which are much stronger for DSST.7,58 However, Lovato and colleagues11 measured both PVT and DSST after a 30-min nap ending at 03:00, and reported no inertia effects for either task. This may have been due to reduced sleep pressure from an afternoon nap opportunity prior to the simulated night shift. The PVT-B used in this study, however, may be a more sensitive measure of sleep inertia than the standard 10-min PVT or the DSST. The PVT-B is a 3-min version of the 10-min PVT but has shorter interstimulus intervals (1–4 sec compared to 2–10 sec) to increase the number of stimuli presented in the shorter time frame.49 Therefore, it could be argued that this task has a higher attentional load as there are more stimuli presented per minute, which consequently requires a greater number of responses, with less recovery time in between.59 The DSST, however, is a self-paced task so the workload is set by the participant. Therefore, during the inertia period, participants may be better able to maintain a steady pace, or respond to stimuli with enough time in between, but are unable to handle rapid processing of information. Santhi et al.60 found that lower order tasks including the 10-min PVT were most sensitive to sleep inertia, whereas Burke et al.61 reported low effect sizes for the 10-min PVT and high effect sizes for higher order tasks such as spatial configuration. Given this contrast in the literature, further research is needed to determine the relative sensitivity of different performance tasks (including the 3-min and 10-min PVT) to sleep inertia effects.
The current study is the first to have reported a scale of self-rated performance during a sleep inertia period. Our results show that despite significant impairment following the 30-min nap, and no change in performance after the 10-min nap, both groups rated their performance as significantly better following the naps. Similarly, subjective ratings of sleepiness and fatigue did not match objective performance. This disconnection between subjective perceptions of performance or alertness and actual performance has been found in other studies of sleep loss,17,62–64 and highlights the need to educate safety-critical workers about the potential for overestimating performance following nighttime naps.
This study was not designed to differentiate between the independent influences of SWS and nap duration. Nevertheless, our results are in line with previous studies that show that a greater amount of SWS in the prior sleep bout is associated with greater sleep inertia.33–35 In the 30-min nap condition, which resulted in significant sleep inertia, participants slept for an average of 26.4 min, with 14.7 min spent in SWS. This duration is in keeping with previous studies of 30-min nighttime naps between 03:00 and 05:00, which have reported TST in the range of 16.2–33.2 min, with SWS ranging from 0.5–17.8 min.11,12,22,26 In our 10-min nap opportunity, participants slept for 8 min with only 0.8 min spent in SWS, and no sleep inertia was observed. Neither napping condition negatively affected daytime recovery sleep.65 Thus, although it is unclear from our data whether the amount of SWS per se was the primary influencing factor,18 30-min naps containing more SWS resulted in greater sleep inertia than 10-min naps. Correlations investigating the association between TST, SWS, and neurobehavioral outcomes revealed that in the 30-min nap condition, response times slowed with both increasing SWS and TST. A clear effect of SWS independent of TST was therefore not observed. It should also be noted that our small sample size limits the interpretation of these findings.
In further support of the idea that waking from a deeper stage of sleep leads to greater sleep inertia,33–35 eight participants in the 30-NAP condition woke from SWS, compared to only one in the 10-NAP condition. However, given the low numbers in this study, we were unable to statistically analyse this relationship. Lovato et al.'s study11 found no objectively measured sleep inertia following a 30-min nighttime nap despite 91% of participants waking from SWS, and Signal et al.18 found no relationship between sleep stage at waking and the degree of sleep inertia. This suggests that the relationship between sleep stage at waking and sleep inertia is not straightforward and requires further investigation.
In contrast to studies of brief afternoon naps,36,42 in our nighttime study, 30% of participants entered SWS within 10 min of lights off. This is likely due to increased homeostatic sleep pressure from an extended period of wakefulness before the nap.44,45 However, our participants were otherwise well rested before the simulated night shift, as confirmed by actigraphy and sleep diaries the week before the study, and an average baseline night sleep of 7.7 h. In a real shift work population, workers are likely to have a sleep debt.43,66 Therefore, the homeostatic drive for sleep before a nighttime nap may be greater in the shift work population. Shift workers may be more likely to enter SWS within 10 min of lights off, spend more time in SWS, and wake from SWS. As discussed previously, changes in SWS may increase the potential for sleep inertia following the nap, although the relationships between these factors have not been consistently demonstrated.18,34,44,45
There is greater heterogeneity in the workforce compared to our thoroughly screened participant sample of healthy, young adults. Individual differences such as age or illness may influence sleep inertia by altering the total sleep time and proportion of SWS obtained in a nap opportunity.67 Shift workers also often develop strategies to manage fatigue that are unavailable in the laboratory, for example, the use of caffeine or face washing.68,69 Hence, the results presented here may be exacerbated, or mitigated, in real-world situations.
Our study was conducted in a controlled sleep laboratory with nap opportunities taken in an environment conducive to sleep. It is unknown how our findings would translate to the real world in which napping environments on shift can be noisy, hot, brightly lit, or uncomfortable.38 Another consideration is that under conditions of reduced homeostatic sleep pressure (e.g., on a night shift following daytime sleep), longer sleep onset latencies may limit the amount of sleep in a 10-min nap opportunity. However, if the nap is taken during the period low in circadian alertness, then sleep propensity is still likely to be high.70 In addition, SOL in the afternoon following sleep restriction similar to that experienced by night shift workers between shifts,71 is only 2–4 min.36,37,72 Therefore, as we observed in our study, provided the sleeping environment is suitable, a 10-min nighttime nap opportunity should allow for at least 6–10 min of sleep.
Our results add to the current debate in the literature as to which performance tasks are most sensitive to sleep inertia. However, although these laboratory tasks are often validated as objective measures of sleepiness, they do not necessarily reflect performance on real-world tasks. This presents a case for future research to employ workplace tasks, preferably in operational settings, in order to understand the effect of sleep inertia in real-world scenarios.
The current research is the first to contribute to our understanding of sleep inertia associated with 10-min naps during a simulated night shift. This study found that a 10-min nap ending at 04:00 can help, in the short term, to ameliorate the effects of extended wakefulness without significant sleep inertia effects. A 30-min nap at this time, however, resulted in significant, sustained sleep inertia on a simple task. A greater amount of, and higher rate of waking from, SWS in the 30-min nap is likely to have contributed to this difference, although the influence of SWS independent of nap length, is unknown. Finally, it is important to note that participants were unaware of their performance impairment and consequently overestimated performance during this period. These findings can be used to inform fatigue management guidelines for napping on shift to promote optimal cognitive performance and safety.
DISCLOSURE STATEMENT
This was not an industry supported study. Support was provided by a University of South Australia DPRF Seeding Grant. The research was conducted at the Centre for Sleep Research, University of South Australia, Adelaide, South Australia. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Prof. Hans P.A. Van Dongen for his assistance and mentorship.
ABBREVIATIONS
- 10-NAP
10-min nap condition
- 30-NAP
30-min nap condition
- ANOVA
analysis of variance
- DSST
digit-symbol substitution task
- ECG
electrocardiogram
- EMG
electromyogram
- EOG
electrooculogram
- KSS
Karolinska sleepiness scale
- NO-NAP
total sleep deprivation condition
- NSD
no significant difference
- PRE
pre-nap test bout
- PSG
polysomnography
- PVT-B
3-min psychomotor vigilance test
- REM
rapid eye movement sleep
- SD
standard deviation
- SE
sleep efficiency
- SEM
standard error of the mean
- SP-Fatigue
Samn-Perelli fatigue scale
- SOL
sleep onset latency
- SWS
slow wave sleep
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1.Folkard S, Lombardi DA, Tucker PT. Shiftwork: Safety, Sleepiness and Sleep. Ind Health. 2005;43:20–3. doi: 10.2486/indhealth.43.20. [DOI] [PubMed] [Google Scholar]
- 2.Folkard S, Tucker P. Shift work, safety and productivity. Occup Med. 2003;53:95–101. doi: 10.1093/occmed/kqg047. [DOI] [PubMed] [Google Scholar]
- 3.Wagstaff AS, Sigstad Lie JA. Shift and night work and long working hours--a systematic review of safety implications. Scand J Work Env Hea. 2011;37:173–85. doi: 10.5271/sjweh.3146. [DOI] [PubMed] [Google Scholar]
- 4.Wong IS, McLeod CB, Demers PA. Shift work trends and risk of work injury among Canadian workers. Scand J Work Env Hea. 2011;37:54–61. doi: 10.5271/sjweh.3124. [DOI] [PubMed] [Google Scholar]
- 5.Chimamise C, Gombe NT, Tshimanga M, Chadambuka A, Shambira G, Chimusoro A. Factors associated with severe occupational injuries at mining company in Zimbabwe, 2010: a cross-sectional study. Pan Afr Med J. 2013;14:5. doi: 10.11604/pamj.2013.14.5.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillberg M, Kecklund G, Åkerstedt T. Relations between performance and subjective ratings of sleepiness during a night awake. Sleep. 1994;17:236–41. doi: 10.1093/sleep/17.3.236. [DOI] [PubMed] [Google Scholar]
- 7.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 8.Carskadon MA, Dement WC. Effects of total sleep loss on sleep tendency. Percept Motor Skill. 1979;48:495–506. doi: 10.2466/pms.1979.48.2.495. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds AC, Banks S. Total sleep deprivation, chronic sleep restriction and sleep disruption. Prog Brain Res. 2010;185:91–103. doi: 10.1016/B978-0-444-53702-7.00006-3. [DOI] [PubMed] [Google Scholar]
- 10.Baulk SD, Fletcher A, Kandelaars KJ, Dawson D, Roach GD. A field study of sleep and fatigue in a regular rotating 12-h shift system. Appl Ergon. 2009;40:694–8. doi: 10.1016/j.apergo.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Lovato N, Lack L, Ferguson S, Tremaine R. The effects of a 30-min nap during night shift following a prophylactic sleep in the afternoon. Sleep Biol Rhythms. 2009;7:34–42. [Google Scholar]
- 12.Sallinen M, Harma M, Åkerstedt T, Rosa R, Lillqvist O. Promoting alertness with a short nap during a night shift. J Sleep Res. 1998;7:240–7. doi: 10.1046/j.1365-2869.1998.00121.x. [DOI] [PubMed] [Google Scholar]
- 13.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 14.Lack LC, Lushington K. The rhythms of human sleep propensity and core body temperature. J Sleep Res. 1996;5:1–11. doi: 10.1046/j.1365-2869.1996.00005.x. [DOI] [PubMed] [Google Scholar]
- 15.Kubo T, Takahashi M, Takeyama H, et al. How do the timing and length of a night-shift nap affect sleep inertia? Chronobiol Int. 2010;27:1031–44. doi: 10.3109/07420528.2010.489502. [DOI] [PubMed] [Google Scholar]
- 16.Kubo T, Takeyama H, Matsumoto S, et al. Impact of nap length, nap timing and sleep quality on sustaining early morning performance. Ind Health. 2007;45:552–63. doi: 10.2486/indhealth.45.552. [DOI] [PubMed] [Google Scholar]
- 17.Tremaine R, Dorrian J, Lack L, et al. The relationship between subjective and objective sleepiness and performance during a simulated night-shift with a nap countermeasure. Appl Ergon. 2010;42:52–61. doi: 10.1016/j.apergo.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Signal TL, van den Berg MJ, Mulrine HM, Gander PH. Duration of sleep inertia after napping during simulated night work and in extended operations. Chronobiol Int. 2012;29:769–79. doi: 10.3109/07420528.2012.686547. [DOI] [PubMed] [Google Scholar]
- 19.Mulrine HM, Signal TL, van den Berg MJ, Gander PH. Post-sleep inertia performance benefits of longer naps in simulated nightwork and extended operations. Chronobiol Int. 2012;29:1249–57. doi: 10.3109/07420528.2012.719957. [DOI] [PubMed] [Google Scholar]
- 20.Arora V, Dunphy C, Chang VY, Ahmad F, Humphrey HJ, Meltzer D. The effects of on-duty napping on intern sleep time and fatigue. Ann Intern Med. 2006;144:792–8. doi: 10.7326/0003-4819-144-11-200606060-00005. [DOI] [PubMed] [Google Scholar]
- 21.Daurat A, Foret J. Sleep strategies of 12-hour shift nurses with emphasis on night sleep episodes. Scand J Work Env Hea. 2004;30:299–305. doi: 10.5271/sjweh.798. [DOI] [PubMed] [Google Scholar]
- 22.Howard ME, Radford L, Jackson ML, Swann P, Kennedy GA. The effects of a 30-minute napping opportunity during an actual night shift on performance and sleepiness in shift workers. Biol Rhythm Res. 2009;41:137–48. [Google Scholar]
- 23.Oriyama S, Miyakoshi Y, Kobayashi T. Effects of two 15-min naps on the subjective sleepiness, fatigue and heart rate variability of night shift nurses. Ind Health. 2014;52:25–35. doi: 10.2486/indhealth.2013-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purnell MT, Feyer AM, Herbison GP. The impact of a nap opportunity during the night shift on the performance and alertness of 12-h shift workers. J Sleep Res. 2002;11:219–27. doi: 10.1046/j.1365-2869.2002.00309.x. [DOI] [PubMed] [Google Scholar]
- 25.Shea JA, Dinges DF, Small DS, et al. A randomized trial of a three-hour protected nap period in a medicine training program: sleep, alertness, and patient outcomes. Acad Med. 2014;89:452–9. doi: 10.1097/ACM.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 26.Smith SS, Kilby S, Jorgensen G, Douglas JA. Napping and nightshift work: effects of a short nap on psychomotor vigilance and subjective sleepiness in health workers. Sleep Biol Rhythms. 2007;5:117–25. [Google Scholar]
- 27.Ruggiero JS, Redeker NS. Effects of napping on sleepiness and sleep-related performance deficits in night-shift workers: a systematic review. Biol Research Nurs. 2014;16:134–42. doi: 10.1177/1099800413476571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tassi P, Muzet A. Sleep inertia. Sleep Med Rev. 2000;4:341–53. doi: 10.1053/smrv.2000.0098. [DOI] [PubMed] [Google Scholar]
- 29.Transportation Safety Board of Canada. Gatineau: Government of Canada; 2011. Aviation investigation report A11F0012. [Google Scholar]
- 30.Government of India Ministry of Civil Aviation. New Delhi: Government of India; 2010. Report on accident to Air India Express Boeing 737-800 Aircraft VT-AXV on 22nd May 2010 at Mangalore. [Google Scholar]
- 31.Marine Accident Investigation Branch. London, UK: Department for Transport; 2011. Heavy contact by Skandi Foula with OMS Resolution, Aberdeen Harbour 29 May 2010. [Google Scholar]
- 32.Armentrout JJ, Holland DA, O'Toole KJ, Ercoline WR. Fatigue and related human factors in the near crash of a large military aircraft. Aviat Space Envir Med. 2006;77:963–70. [PubMed] [Google Scholar]
- 33.Tassi P, Bonnefond A, Engasser O, Hoeft A, Eschenlauer R, Muzet A. EEG spectral power and cognitive performance during sleep inertia: the effect of normal sleep duration and partial sleep deprivation. Physiol Behav. 2006;87:177–84. doi: 10.1016/j.physbeh.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Dinges D, Orne M, Orne E. Assessing performance upon abrupt awakening from naps during quasi-continuous operations. Behav Res Methods. 1985;17:37–45. [Google Scholar]
- 35.Takahashi M, Arito H. Sleep inertia and autonomic effects on post-nap P300 event-related potential. Ind Health. 1998;36:347–53. doi: 10.2486/indhealth.36.347. [DOI] [PubMed] [Google Scholar]
- 36.Brooks A, Lack L. A brief afternoon nap following nocturnal sleep restriction: which nap duration is most recuperative? Sleep. 2006;29:831–40. doi: 10.1093/sleep/29.6.831. [DOI] [PubMed] [Google Scholar]
- 37.Tietzel AJ, Lack LC. The short-term benefits of brief and long naps following nocturnal sleep restriction. Sleep. 2001;24:293–300. doi: 10.1093/sleep/24.3.293. [DOI] [PubMed] [Google Scholar]
- 38.Fallis WM, McMillan DE, Edwards MP. Napping during night shift: practices, preferences, and perceptions of critical care and emergency department nurses. Crit Care Nurse. 2011;31:e1–11. doi: 10.4037/ccn2011710. [DOI] [PubMed] [Google Scholar]
- 39.Scheer FA, Shea TJ, Hilton MF, Shea SA. An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. J Biol Rhythm. 2008;23:353–61. doi: 10.1177/0748730408318081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva EJ, Duffy JF. Sleep inertia varies with circadian phase and sleep stage in older adults. Behav Neurosci. 2008;122:928–35. doi: 10.1037/0735-7044.122.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miccoli L, Versace F, Koterle S, Cavallero C. Comparing sleep-loss sleepiness and sleep inertia: lapses make the difference. Chronobiol Int. 2008;25:725–44. doi: 10.1080/07420520802397228. [DOI] [PubMed] [Google Scholar]
- 42.Fushimi A, Hayashi M. Pattern of slow-wave sleep in afternoon naps. Sleep Biol Rhythms. 2008;6:187–9. [Google Scholar]
- 43.Rajaratnam SM, Arendt J. Health in a 24-h society. Lancet. 2001;358:999–1005. doi: 10.1016/S0140-6736(01)06108-6. [DOI] [PubMed] [Google Scholar]
- 44.Ferrara M, De Gennaro L, Bertini M. The effects of slow-wave sleep (SWS) deprivation and time of night on behavioral performance upon awakening. Physiol Behav. 1999;68:55–61. doi: 10.1016/s0031-9384(99)00150-x. [DOI] [PubMed] [Google Scholar]
- 45.Tilley A, Donohoe F, Hensby S. Homeostatic changes in slow wave sleep during recovery sleep following restricted nocturnal sleep and partial slow wave sleep recovery during an afternoon nap. Sleep. 1987;10:600–5. [PubMed] [Google Scholar]
- 46.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiat. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 47.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiat Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 48.Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74:728–38. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- 49.Basner M, Mollicone D, Dinges DF. Validity and sensitivity of a brief psychomotor vigilance test (PVT-B) to total and partial sleep deprivation. Acta Astronaut. 2011;69:949–59. doi: 10.1016/j.actaastro.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim J, Dinges DF. Sleep Deprivation and Vigilant Attention. Ann NY Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 51.Wechsler D. New York, NY: Psychological Corporation; 1981. Manual for the Wechsler Adult Intelligence Scale -Revised. [Google Scholar]
- 52.Samn SW, Perelli LP. Brooks Air Force Base, TX: USAF School of Aerospace Medicine; 1982. Estimating aircrew fatigue: a technique with implications to airlift operations. Report No. SAM-TR-82-21. [Google Scholar]
- 53.Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 54.Rechtschaffen A, Kales A. Los Angeles: Brain Information Service/Brain Research Institute, University of California; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 55.Van Dongen HP, Maislin G, Dinges DF. Dealing with inter-individual differences in the temporal dynamics of fatigue and performance: importance and techniques. Aviat Space Envir Med. 2004;75:A147–54. [PubMed] [Google Scholar]
- 56.Jackson EJ, Moreton A. Safety during night shifts: a cross-sectional survey of junior doctors' preparation and practice. BMJ Open. 2013;3:e003567. doi: 10.1136/bmjopen-2013-003567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Centofanti S, Hilditch C, Dorrian J, Van Dongen H, Banks S. Napping on the night-shift: do recovery benefits extend to the commute home? (Abstract P613) J Sleep Res. 2014;23(Suppl 1):189. [Google Scholar]
- 58.Banks S, Van Dongen HP, Maislin G, Dinges DF. Neurobehavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery. Sleep. 2010;33:1013–26. doi: 10.1093/sleep/33.8.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferrara M, De Gennaro L. The sleep inertia phenomenon during the sleep-wake transition: theoretical and operational issues. Aviat Space Envir Med. 2000;71:843–8. [PubMed] [Google Scholar]
- 60.Santhi N, Groeger JA, Archer SN, Giménez M, Schlangen LJ, Dijk DJ. Morning sleep inertia in alertness and performance: effect of cognitive domain and white light conditions. PloS One. 2013;8:e79688. doi: 10.1371/journal.pone.0079688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burke TM, Scheer FAJL, Ronda JM, Czeisler CA, Wright KP. Sleep inertia, sleep homeostatic and circadian influences on higher-order cognitive functions. J Sleep Res. 2015;24:364–71. doi: 10.1111/jsr.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dorrian J, Lamond N, Dawson D. The ability to self-monitor performance when fatigued. J Sleep Res. 2000;9:137–44. doi: 10.1046/j.1365-2869.2000.00195.x. [DOI] [PubMed] [Google Scholar]
- 63.Dorrian J, Lamond N, Holmes AL, et al. The ability to self-monitor performance during a week of simulated night shifts. Sleep. 2003;26:871–7. doi: 10.1093/sleep/26.7.871. [DOI] [PubMed] [Google Scholar]
- 64.Dorrian J, Roach GD, Fletcher A, Dawson D. Simulated train driving: fatigue, self-awareness and cognitive disengagement. Appl Ergon. 2007;38:155–66. doi: 10.1016/j.apergo.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 65.Centofanti SA, Dorrian J, Hilditch CJ, Banks S. Do night naps impact driving performance and daytime recovery sleep? Accid Anal Prev. 2015 Nov 23; doi: 10.1016/j.aap.2015.11.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66.Åkerstedt T. Sleepiness as a consequence of shift work. Sleep. 1988;11:17–34. doi: 10.1093/sleep/11.1.17. [DOI] [PubMed] [Google Scholar]
- 67.Bliwise DL. Normal aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. St. Louis. MO: Elsevier Saunders; 2011. pp. 27–41. [Google Scholar]
- 68.Van Dongen HPA, Price NJ, Mullington JM, Szuba MP, Kapoor SC, Dinges DF. Caffeine eliminates psychomotor vigilance deficits from sleep inertia. Sleep. 2001;24:813–9. doi: 10.1093/sleep/24.7.813. [DOI] [PubMed] [Google Scholar]
- 69.Hayashi M, Masuda A, Hori T. The alerting effects of caffeine, bright light and face washing after a short daytime nap. Clin Neurophysiol. 2003;114:2268–78. doi: 10.1016/s1388-2457(03)00255-4. [DOI] [PubMed] [Google Scholar]
- 70.Sargent C, Darwent D, Ferguson SA, Kennaway DJ, Roach GD. Sleep restriction masks the influence of the circadian process on sleep propensity. Chronobiol Int. 2012;29:565–71. doi: 10.3109/07420528.2012.675256. [DOI] [PubMed] [Google Scholar]
- 71.Åkerstedt T. Shift work and disturbed sleep/wakefulness. Occup Med. 2003;53:89–94. doi: 10.1093/occmed/kqg046. [DOI] [PubMed] [Google Scholar]
- 72.Tietzel AJ, Lack LC. The recuperative value of brief and ultra-brief naps on alertness and cognitive performance. J Sleep Res. 2002;11:213–8. doi: 10.1046/j.1365-2869.2002.00299.x. [DOI] [PubMed] [Google Scholar]