Abstract
Study Objectives:
In addition to enhancing sleep onset and maintenance, a desirable insomnia therapeutic agent would preserve healthy sleep's ability to wake and respond to salient situations while maintaining sleep during irrelevant noise. Dual orexin receptor antagonists (DORAs) promote sleep by selectively inhibiting wake-promoting neuropeptide signaling, unlike global inhibition of central nervous system excitation by gamma-aminobutyric acid (GABA)-A receptor (GABAaR) modulators. We evaluated the effect of DORA versus GABAaR modulators on underlying sleep architecture, ability to waken to emotionally relevant stimuli versus neutral auditory cues, and performance on a sleepiness-sensitive cognitive task upon awakening.
Methods:
DORA-22 and GABAaR modulators (eszopiclone, diazepam) were evaluated in adult male rhesus monkeys (n = 34) with continuous polysomnography recordings in crossover studies of sleep architecture, arousability to a classically conditioned salient versus neutral acoustical stimulus, and psychomotor vigilance task (PVT) performance if awakened.
Results:
All compounds decreased wakefulness, but only DORA-22 sleep resembled unmedicated sleep in terms of underlying sleep architecture, preserved ability to awaken to salient-conditioned acoustic stimuli while maintaining sleep during neutral acoustic stimuli, and no congnitive impairment in PVT performance. Although GABAaR modulators induced lighter sleep, monkeys rarely woke to salient stimuli and PVT performance was impaired if monkeys were awakened.
Conclusions:
In nonhuman primates, DORAs' targeted mechanism for promoting sleep protects the ability to selectively arouse to salient stimuli and perform attentional tasks unimpaired, suggesting meaningful differentiation between a hypnotic agent that works through antagonizing orexin wake signaling versus the sedative hypnotic effects of the GABAaR modulator mechanism of action.
Citation:
Tannenbaum PL, Tye SJ, Stevens J, Gotter AL, Fox SV, Savitz AT, Coleman PJ, Uslaner JM, Kuduk SD, Hargreaves R, Winrow CJ, Renger JJ. Inhibition of orexin signaling promotes sleep yet preserves salient arousability in monkeys. SLEEP 2016;39(3):603–612.
Keywords: arousal, auditory discrimination, nonhuman primates, orexin receptor antagonist, psychomotor vigilance task, sleep
Significance.
Dual receptor antagonists (DORAs) enhance sleep onset and sleep maintenance by antagonizing wake signaling mechanisms whereas GABAaR insomnia agents induce sedation with global CNS depressant action. In this study of nonhuman primates, DORA sleep retained unmedicated healthy sleep's desired capacity to appropriately awaken from deep sleep in response to emotionally salient situations and perform attentional tasks unimpaired, yet preserved uninterrupted sleep with irrelevant noise exposure. In contrast, such salience-gated arousability and psychomotor performance retention were lost during GABAaR modulator sleep in monkeys. The translational similarities between nonhuman primate and human sleep architecture suggest a DORA wake-antagonizing mechanism of action may also provide meaningful differentiation from existing sedating insomnia agents for patients under similar middle of the night situations.
INTRODUCTION
Insomnia affects up to 22% of the US population and is associated with substantial decrements in perceived health.1 In addition to effectively promoting sleep onset and maintenance, an ideal insomnia medication would also preserve the ability to awaken and respond appropriately to emotionally important stimuli, yet maintain sleep when presented with irrelevant background noise. This capacity to differentiate emotionally relevant (e.g., crying infant, intruder, smoke alarm) from irrelevant stimuli (e.g., traffic, construction, aircraft noise, snoring partner) during sleep, or salience discrimination, is an important feature of natural sleep yet one that is underevaluated among insomnia pharmacologic treatment options.
The mechanism of action of the most widely used standard-of-care pharmacologic treatments for insomnia promotes sleep by increasing the action of the neurotransmitter gamma-aminobutyric acid (GABA)-A to inhibit brain arousal systems.2–5 GABA-A receptors (GABAaR), however, are widely distributed throughout the central nervous system (CNS) and have roles in numerous other brain functions extraneous to sleep induction, but important for respiratory, cognitive, emotional, and attentional functions.6
The orexin signaling pathway plays a central role in maintaining wakefulness and has attracted interest as a target for insomnia treatment.7–9 The orexin neuropeptides are produced by a focused cluster of orexin neurons located in the lateral hypothalamus with projections to nuclei involved in regulating arousal and vigilance state.10
Antagonism of both orexin receptors (dual orexin receptor antagonists [DORAs]) safely promotes sleep in preclinical animal and clinical patient studies.11–15 These agents have potential for more specific targeting of sleep/wake systems compared with GABAaR modulators, which act as global CNS inhibitors of excitatory activity.
Classic experiments in preclinical models and humans find the threshold for awakening to an emotionally salient acoustical stimulus is much lower than for a neutral acoustical stimulus.16 Building on our previous work evaluating the lack of impairment with DORAs on arousability in dogs17 and on cognitive function in monkeys,18 we hypothesized that DORAs' targeted wake-modulating mechanism would differentiate from GABAaR modulators' general global CNS depressant action on discrimination and functional arousability to emotionally salient stimuli during sleep in nonhuman primates.
To address our hypothesis, we evaluated the impact of DORA-22 and two GABAaR modulators, eszopiclone (Lunesta, a nonbenzodiazepine) and diazepam (a classic benzodiaze-pine) on underlying sleep architecture and their relative ability to affect wakening to an emotionally relevant conditioned stimulus versus neutral auditory cue in monkeys. To evaluate performance that would be necessary for responding to a salient middle-of-the-night awakening, the effect of DORA-22 and GABAaR modulator administration on the psychomotor vigilance task, a cognitive task sensitive to sleepiness19 that requires sustained attention and alertness, was also tested in rhesus monkeys.
METHODS
Research Objectives and Generalized Design
A series of studies conducted with adult, male rhesus monkeys compared sleep architecture, salient stimulus arousability, and psychomotor vigilance task (PVT) testing among comparable cross-mechanism, standardized sleep inducing doses of DORA-22, eszopiclone, and diazepam based on previously published data in monkeys from our laboratory.18
Salient-stimulus arousability and PVT were evaluated during the early lights-out period 2 h or 3 h after vehicle or hypnotic dosing. This time frame was selected to both maximize hypnotic drug exposure levels20 and unmedicated sleep propensity. In rhesus monkeys, as in humans, deeper Delta Sleep II and underlying low frequency power bands (qEEG 0.5–4Hz) peak during the first part of the night.21 For each crossover study, animals were tested on vehicles and on all doses of compounds in a randomized, counterbalanced order.
Animal Subjects
Monkeys (Macaca mulatta; adult male, 9–15 kg) were housed under standard laboratory conditions of controlled temperature, humidity, and lighting (12 h light:12 h dark; lights on at 04:30). Monkeys were fed a calorie-controlled diet of laboratory chow supplemented with fruits and vegetables to achieve a Clingerman body condition score of 2.5–3.22 Rhesus monkeys are considered an excellent translational biomedical model for human sleep because of similar circadian sleep architecture patterns.23 The studies, surgical procedures, and animal husbandry were approved by and conducted in accordance with standards of the Merck Institutional Animal Care and Use Committee and the United States Department of Agriculture.
Compounds
DORA-22 was synthesized at Merck Research Laboratories. Eszopiclone and diazepam were purchased commercially from Sigma-Aldrich Corporation (St. Louis, MO, USA) and Myoderm (Norristown, PA, USA), respectively.
Doses of DORA-22 (3, 10, 30 mg/kg orally [PO] in 6% sucrose), eszopiclone (1, 3, 10 mg/kg PO in 20% vitamin E TPGS [d-alpha-tocopheryl polyethylene glycol 1000 succinate]), or diazepam (1, 5, 10 mg/kg PO in 0.5% carboxymethylcellulose) were based on previously published data on monkeys from our laboratory.18
The doses were titrated to decrease Active Wake by a similar amount when administered during the daytime active circadian phase: the highest doses used decreased Active Wake by approximately 40–50 min per 2 h and the mid-doses by approximately 20–35 min per 2 h, as described previously.18 The lowest 1 mg/kg dose of DORA-22 decreased Active Wake by approximately 20 min per 2 h, but the lowest doses of eszopi-clone or diazepam did not significantly decrease wake during the daytime.18
DORA-22 (3, 10, 30 mg/kg), diazepam (1, 5, 10 mg/kg), or eszopiclone (1, 3, 10 mg/kg) were administered either 2 h (sleep architecture study) or 1 h (salience arousability and PVT studies) prior to the onset of the inactive phase. The arousability and PVT test time was aligned after 2 h of lights-out and approximately within the drugs' peak exposure times, as described previously.20 Testing with either active compounds or vehicle alone was performed 1 to 2 times per week, with a minimum of 3 washout days; all subjects received the same dose of compound on the same day.
Monkey Polysomnography and Sleep Architecture Staging
Thirty-four adult male rhesus monkeys implanted with subcutaneous telemetric devices (D70; Data Sciences International, Arden Hills, MN, USA) were used to record electrocortico-gram (ECoG), electrooculogram (EOG), electromyogram (EMG), and locomotor activity simultaneously. Monkeys typically underwent surgery more than 3 mo before the current study, as described previously.13,14,18 Monkeys were free from any drug testing for more than 2 w prior to the start of the current study, and baseline measures were similar to historical baseline data with telemetry signal integrity confirmed.
Sleep stages were evaluated based on a combination of continuous ECoG, EMG, and EOG activity and movement within the field of the radiofrequency receiver using both a validated, customized version of the sleep algorithm Somnologica (Embla Systems; for 24-h sleep architecture studies) and hand-scoring (1 h total before, during, and after each stimulus arousability test and PVT session), as described previously.13,14,18 Briefly, ECoG, EMG, EOG, and locomotion data were used to characterize five sleep-wake stages in the monkeys: Active Wake, Light Sleep, Slow Wave Sleep I (lighter nonrapid eye movement [non-REM] sleep), Delta Sleep II (deep non-REM sleep), and REM sleep. Sleep architecture data were evaluated in 30-sec epochs and then averaged into 30-min bins across 24 h (sleep-wake studies only). Occasionally, a monkey exhibited poor ECoG/EMG/ EOG signal quality that impacted the accuracy of sleep-stage scoring; such individuals were eliminated from the sleep-wake analyses in that particular study arm.
Comparative Nighttime Sleep Architecture Studies
Comparable cross-mechanism standardized sleep inducing doses of DORA-22 (30 mg/kg PO), diazepam (10 mg/kg PO), and eszopiclone (10 mg/kg PO) were evaluated for nighttime sleep architecture compared with their respective vehicles.18 All sleep architecture studies used a 2-d, within-subject cross-over design (vehicle × drug). Monkeys (n = 16) were dosed 2 h prior to lights out (Zeitgeber time: 10:00), and 24-h telemetric polysomnography (PSG) recordings began 1 h before dose administration.
Salient Stimulus Arousability Testing
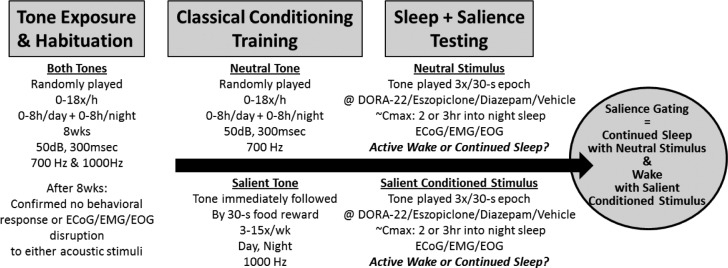
To evaluate arousability to a classically conditioned salient acoustical versus a neutral acoustical stimulus, monkeys were presented with each of two different acoustic stimuli (700 or 1000 Hz, sine-wave, 3 dBA above residual noise, presented for 3 sec three times during a 30-sec interval, with an approximately 7-sec interstimulus interval) via space-balanced, in-room speakers (Figure 1; also see Tannenbaum et al., 201417).
Figure 1.
Salience-gated arousability paradigm in monkeys. After baseline habituation to novel electronic tones, rhesus monkeys (n = 12) were classically conditioned to anticipate nothing (Neutral) or food reward (Salient) following distinct 300 msec acoustical stimulus tones. During salience testing, only the Neutral or Salient Conditioned Stimulus tone was presented to sleeping monkeys (two tests/monkey/condition); no room entry or reward was given. Telemeterized ECoG/EMG/EOG was used to quantify Active Wake or continued sleep in response to the differently salienced stimuli.
For 8 w of habituation, an equivalent number of both (novel, unconditioned) tones were randomly presented according to a Poisson distribution in 3-sec/3×/30-sec random clusters 18 times per hour for up to 8 h per day or night. ECoG/EMG/EOG recordings at the end of the habituation period confirmed that neither stimuli interrupted sleep (Figure 1).
After the habituation period confirming that neither tone interrupted sleep (ECoG/EMG/EOG, closed-circuit video) the 700-Hz tone was maintained randomly as previously mentioned, but the 1000-Hz tone was presented in a three-tone per 30-sec cluster for repeated classic conditioning stimulus paired with a salient food reward. For salience conditioning, an experimenter entered the room wearing novel laboratory clothing (e.g., not associated with any animal procedures or cleaning) after the first tone of the cluster and gave the monkeys a food reward (M&Ms, Mars, Inc.) as the other two cluster tones played. Conditioning associated with the salient tone continued three to 15 times per week for 4 w (Figure 1), followed by three to five times per week for maintenance throughout the study. The learned classically conditioned association of the salient tone with reward was confirmed by video-monitored behavioral and food vocalization responses. Thirty percent of the stimulus-food conditioning sessions were randomly preceded by oral dosing of sterile water 3 h prior to prevent the animals from learning a predictive association with oral dose days and stimulus-only sessions without food reward.
Once conditioned, test sessions (Figure 1; two sessions per vehicle or compound dose per tone type per animal) consisted of three 3-sec per 30-sec clusters of either the neutral tone or the conditioned salient tone alone (no room entry, no reward) presented 2 and 3 h into the monkey's nighttime inactive phase in a Latin Square, counterbalanced tone presentation order. All monkeys received the same randomized counterbalanced drug and dose per test day.
Data from individual tests were excluded if the monkey's PSG recordings did not confirm sleep during the 2 min immediately preceding the stimulus tone. Arousal was defined as at least a single 30-sec epoch of PSG-defined “Active Wake” beginning with the epoch containing the three stimulus tones. Each animal had a percentage awakened score averaged between the two tests per dose or vehicle.
Psychomotor Vigilance Task
Twelve additional telemetry-implanted monkeys familiar with a variety of tasks in a cognitive battery, including the simple PVT, were evaluated on psychomotor parameters using a counter-balanced Latin Square design for all compound doses and vehicles. Prior to PVT testing, water was restricted for up to 3 h prior to and during cognitive testing. PVT testing was conducted in the monkey's home cage via a stainless-steel, touch-screen, computerized cognition task box built into the cage wall; a networked computer presented stimuli and recorded responses (as described previously18). All monkeys were successfully trained on the PVT prior to the study.
A 30-min PVT was presented 2 h into the lights-out resting phase. At the onset of each trial, a single red square (R180, G0, B0), the “target” (150 × 150 pixels), was presented at the center of the screen. To obtain a liquid juice reward, the monkey was required to touch the target, at which point the touch screen became blank and an interstimulus interval (0.1–30 sec) was initiated prior to the next trial. Responses not directed at the target were recorded as misses but had no other contingencies. Failure to respond to the presentation of the target image within 60 sec resulted in the screen becoming blank for a 5-sec timeout prior to the start of the next trial. Test sessions were terminated after 30 min.
The PVT primary dependent measures reported are: (1) response omissions, in which subjects failed to respond within 60 sec of stimulus presentation, and (2) latency to touch the target (on trials in which no missed response occurred). Individual animals were excluded from the latency analyses if they completed fewer than 10 responses in 30 min (90 responses is typical with vehicle treatment, unpublished data). ECoG/EMG/EOG recording and sleep staging was performed 15 min prior to testing, during the 30-min testing session, and for 15 min following the PVT.
Data Analysis
An independent mixed-model repeated-measures analysis was applied for the 24-h sleep architecture studies for each 30-min time bin versus vehicle averaged per animal, as described previously.13
Repeated-measures analysis of variance (ANOVA) with appropriate post hoc comparisons assessed the arousability (2-way ANOVA) and PVT (one-way ANOVA) tests; the PVT studies also applied a Greenhouse-Geisser correction. All data are reported as the mean per animal (across days or tests) with standard deviation (note: previously published data from our laboratory were expressed using standard error of the mean13,14,17,18,21). For the vehicle-only baseline arousability assessment, all vehicle trials (6% sucrose, 0.5% carboxymethylcellulose, and vitamin E TPGS) were combined into a single vehicle mean per animal per condition, and evaluated by paired two-tailed t-test.
RESULTS
Decreased Wake but Differing Underlying Sleep Architecture Induced by DORA-22 and GABAaR Modulators
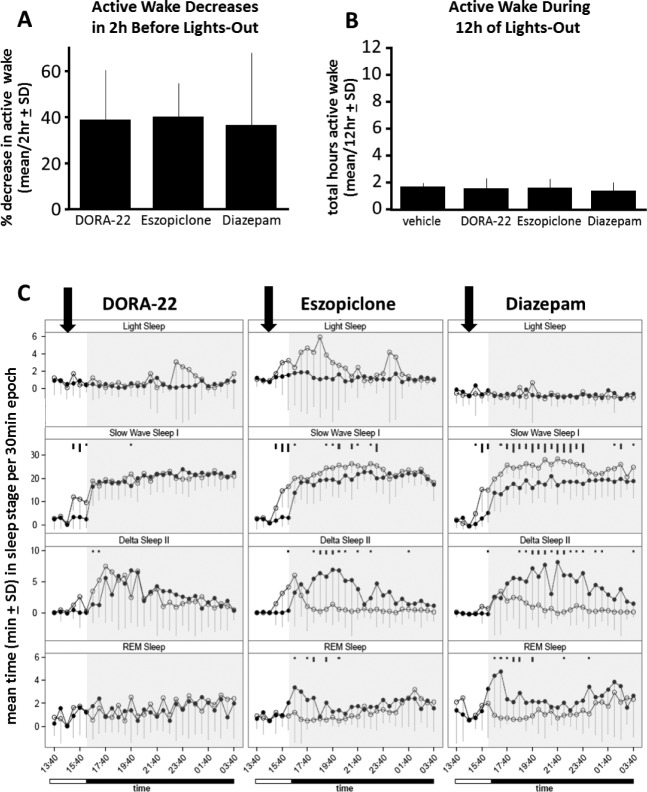
To compare sleep architecture induced by DORA-22 and GABAaR modulators, sleep stages were evaluated in rhesus monkeys using PSG, a combination of ECoG, EOG, and EMG recordings, and locomotor activity, as described previously.13,18 When administered 2 h prior to the lights-out period, DORA-22 (30 mg/kg; t(10) = 5.82, P = 0.0002), eszopi -clone (10 mg/kg; t(12) = 9.56, P < 0.0001), and diazepam (10 mg/kg; t(12) = 4.12, P = 0.0014) each significantly attenuated Active Wake prior to lights out by 36% to 39% when compared to vehicle treatment (Figure 2A). There was no difference in the decrease in Active Wake among drugs during this 2-h postdose period (Figure 2A; F(2,34) = 0.08, P = 0.93) or the amount of time the monkeys were not awake on drug or vehicle during the subsequent 12-h lights-out nighttime period (Figure 2B, F(3,39) = 0.37, P = 0.77).
Figure 2.
Monkeys decrease Active Wake with different underlying sleep architecture with DORA-22 and GABAaR modulators. (A) DORA-22 (30 mg/kg), eszopiclone (10 mg/kg), and diazepam (10 mg/kg) all significantly decreased Active Wake 2 h (P < 0.0002) after dosing prior to lights out; there was no difference in the decrease in Active Wake among drug treatments. (B) There was no difference between treatments in the total time spent in Active Wake during the 12 h nighttime lights-out period. (C) Rhesus monkey sleep stage architecture when dosed with DORA-22, eszopiclone, and diazepam (open circles) or respective vehicles (closed circles) 2 h prior to lights-out nighttime (inactive) period. DORA-22 (10 mg/kg) time spent in sleep stages recapitulates physiologic nighttime (vehicle) sleep, but eszopiclone (10 mg/kg) and diazepam (10 mg/kg) sleep architecture is skewed toward lighter sleep and away from Delta and REM sleep relative to vehicle sleep. Arrows indicate the timing of dose administration, open ‘Time’ bar is lights-on, black ‘Time’ bar and shading are lights-out. Significant differences are noted by black tick marks indicating significance level (short, medium, long; P < 0.05, 0.01, 0.001, respectively). DORA, dual orexin receptor antagonist; GABA, gamma-aminobutyric acid; GABAaR, GABA-A receptor; REM, rapid eye movement; SD, standard deviation.
To qualitatively compare the underlying sleep architecture induced by DORA-22 and GABAaR modulators, comparable sleep inducing doses (DORA-22 30 mg/kg, eszopiclone 10 mg/kg, and diazepam 10 mg/kg)18 were evaluated for nighttime sleep architecture versus their respective vehicles. Despite the similar effects on Active Wake prior to lights out and throughout the night (Figures 2A and 2B), the underlying nighttime sleep architecture with DORA-22 versus the GABAaR modulators differed. Following DORA-22 treatment, night time Light Sleep, Slow Wave Sleep I, and Delta Sleep II resembled that seen during physiologic (vehicle-treated) sleep (Figure 2C). In contrast, eszopiclone and diazepam substantially altered nighttime sleep architecture relative to vehicle: sleep architecture was skewed toward more time in lighter sleep with increases in Light Sleep and Slow Wave Sleep I but decreases in time spent in Delta Sleep II and REM sleep compared to vehicle-treated sleep (Figure 2C). Some between-group variation did exist in REM. Figure S1 (supplemental material) shows parallel frequency of sleep stage bout entry data for the same conditions.
Salience-Gated Arousal to Acoustic Stimuli Maintained with DORA-22 Sleep, Impaired with GABAaR Modulator Sleep
To assess the ability to selectively awaken to an emotionally salient conditioned stimulus versus an irrelevant stimulus, sleeping monkeys were presented with either a series of salience-conditioned acoustic-only stimuli or neutral acoustic-only stimuli during a 30-sec epoch of vehicle, DORA-22, or GABAaR modulator nighttime sleep. PSG defined the animals' arousal (at least one 30-sec epoch of Active Wake) or continued sleep in response to the stimuli. Figure 3A shows unmedicated vehicle-treated monkeys woke significantly more from nighttime sleep to the salience-conditioned acoustic stimulus than to the neutral stimulus (t(11) = 4.52, P = 0.0009).
Figure 3.
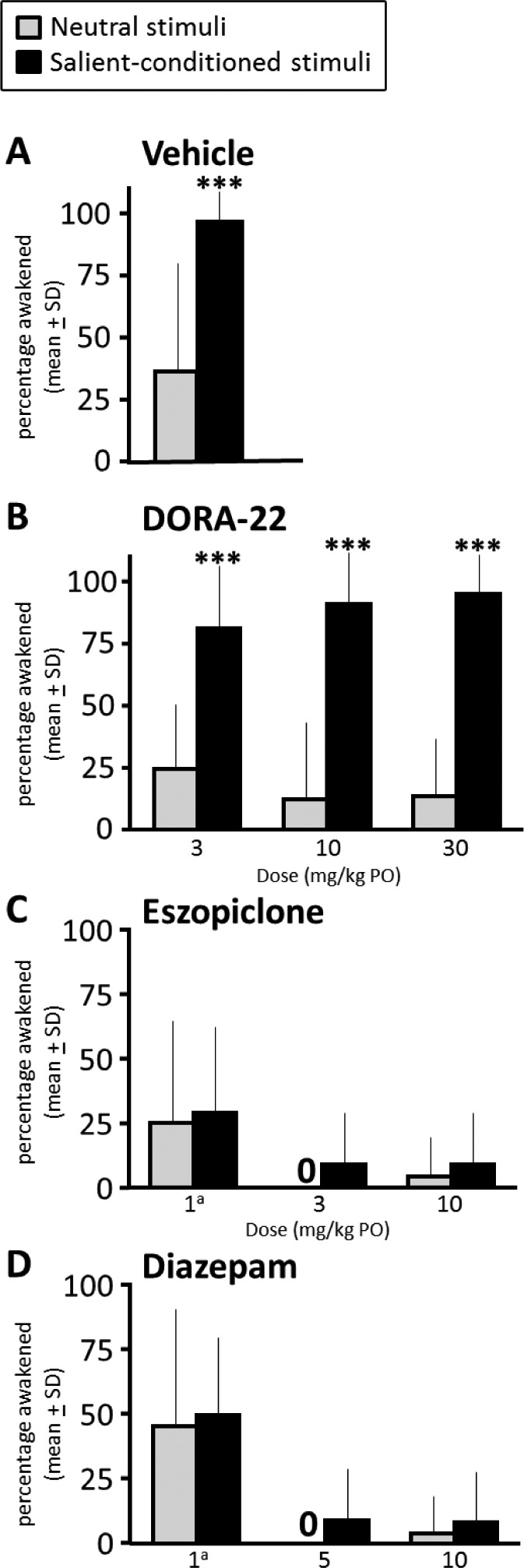
Monkeys wake from sleep preferentially to emotionally salient stimuli with physiological nighttime sleep and at maximal drug exposure of DORA-22, but do not distinguish salience and barely wake on GABAaR modulators. (A) During vehicle (physiological) sleep at night, rhesus monkeys (n = 12, two tests/condition, counterbalanced order) woke to salient-conditioned acoustical stimuli significantly more often than they woke to neutral stimuli. (B) During all doses of DORA-22 sleep, rhesus monkeys woke to salient-conditioned stimuli significantly more often than they woke to neutral stimuli. (C) During eszopiclone treatment, monkeys did not discriminate between salient and neutral stimuli; monkeys tended to sleep through both stimuli. (D) During diazepam treatment, monkeys did not discriminate between salient and neutral stimuli; at doses that induce daytime sleep (5 and 10 mg/kg), monkeys typically slept through both stimuli. ***P < 0.001. aNonsedating daytime dose. DORA, dual orexin receptor antagonist; GABA, gamma-aminobutyric acid; GABAaR, GABA-A receptor; PO, by mouth; SD, standard deviation.
The treatment with DORA-22 did not impair arousal from sleep to the salient-conditioned stimulus (Figure 3B). Similar to vehicle-treated sleep, monkeys treated with DORA-22 at all doses tested woke significantly more to the salient stimulus than to the neutral stimulus (main effect salience F(1,20) = 75.98, P < 0.0001). There was no main effect of DORA-22 dose on salience arousability.
Maximal arousal to the salient stimuli with GABAaR modulators (eszopiclone, Figure 3C; diazepam, Figure 3D), at even nonsedating-in-daytime low doses,18 was always significantly less than salient arousal during vehicle sleep (t(11) > 7.42, P < 0.0001) or less than any DORA-22 dose (Figure 3B, t(11) > 6.17, P < 0.0001). With the nonsedating-in-daytime low diazepam dose (1 mg/kg), monkeys did wake at night more often than to higher sedating doses (F(2,40) = 28.2, P < 0.001); any nighttime eszopiclone arousal from sleep was not dose dependent (Figure 3C).
At sedating daytime doses of eszopiclone (3 and 10 mg/kg) and diazepam (5 and 10 mg/kg), monkeys rarely woke to either type of stimuli (0–9.1% ± 6). At all doses tested for eszopiclone (Figure 3C) and diazepam (Figure 3D), the arousal salience discrimination hallmark of physiologic vehicle sleep was completely impaired, as there was no difference in the arousal response to the salient versus neutral stimuli.
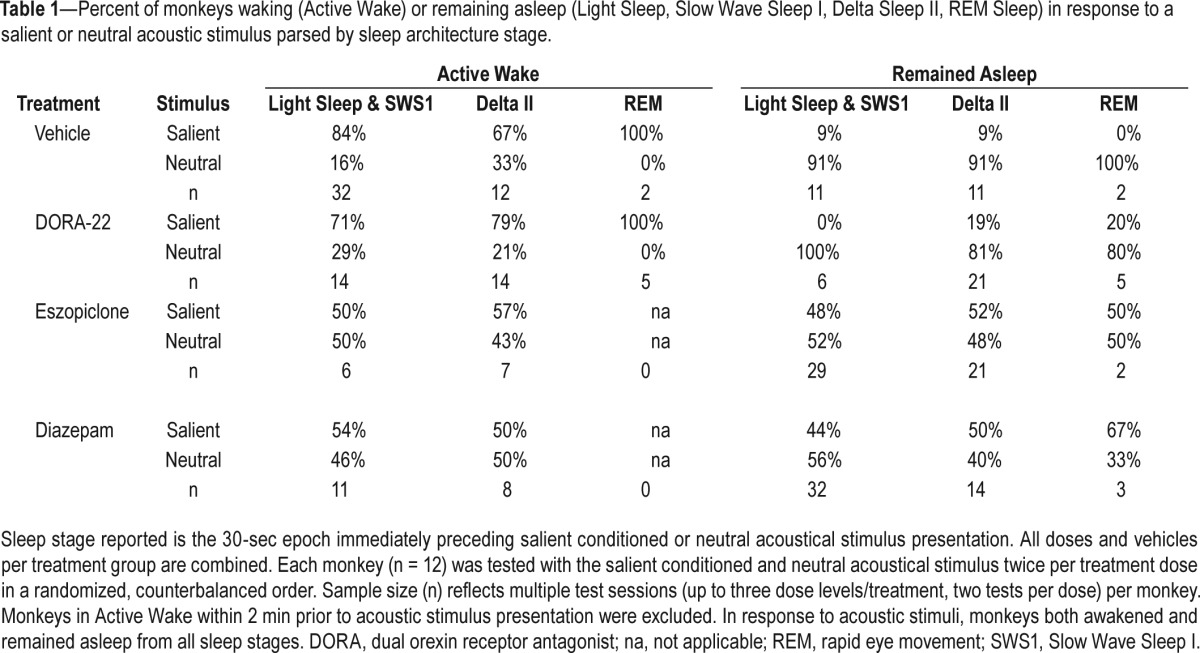
The sleep architecture stages in the epoch immediately preceding a salience-gated arousal trial are described in Table 1. Under vehicle baseline conditions, monkeys appeared to wake more easily from lighter sleep stages (Light Sleep, Slow Wave Sleep I) than Delta Sleep II. However, the ability of monkeys to wake to a salient stimulus or remain sleeping through a neutral stimulus with hypnotic treatment occurred in all sleep architecture stages.
Table 1.
Percent of monkeys waking (Active Wake) or remaining asleep (Light Sleep, Slow Wave Sleep I, Delta Sleep II, REM Sleep) in response to a salient or neutral acoustic stimulus parsed by sleep architecture stage.
If Awakened, PVT Performance is Not Impaired with DORA-22 but is Impaired with GABAaR Modulators
To characterize the effect of DORA-22 and GABAaR modulator administration on psychomotor performance with nighttime awakening, performance on the sleepiness-sensitive PVT was determined during nighttime lights-out vehicle, DORA-22, or GABAaR sleep. Monkey performance was assessed in terms of response omissions and the latency to touch the target on trials in which no missed response occurred. Figure 4 shows that when awoken during the night around the time of peak drug exposure, all 12 DORA-22-treated monkeys engaged in the PVT tasks and had no deficits in either omissions or response latency at any dose (Figure 4).
Figure 4.
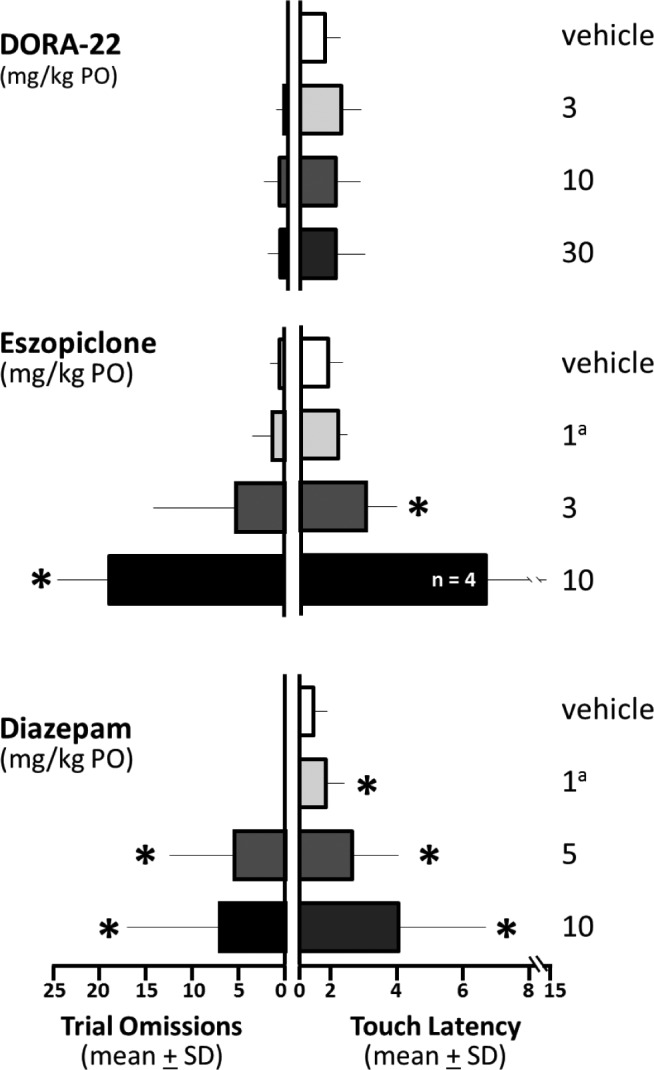
PVT performance if awakened was not impaired on DORA-22 but was impaired on GABAaR modulators. All DORA-22-dosed monkeys (n = 12) met PVT performance criteria, but fewer GABAaR modulator-treated monkeys were able to engage in the PVT at all (eszopiclone 10 mg/kg, n = 4 of 12; diazepam 5 mg/kg, n = 9 of 12; diazepam10 mg/kg, n = 7 of 12). (Left panels) Trial omissions: trials on which the monkeys failed to respond during the 60-sec limit. DORA-22-treated monkey performance did not vary from vehicle performance. With increasing doses, monkeys treated with eszopiclone and diazepam had more omission errors. (Right panels) Touch latency: mean latency to touch the target following presentation (in seconds) in monkeys making at least 10 responses. DORA-22-treated monkey performance did not vary from vehicle performance. Touch latency was increased with eszopiclone and diazepam. *P < 0.05. aNonsedating daytime dose. DORA, dual orexin receptor antagonist; GABA, gamma-aminobutyric acid; GABAaR, GABA-A receptor; PO, by mouth; PVT, psychomotor vigilance task; SD, standard deviation.
Fewer GABAaR modulator-treated monkeys were able to engage in the PVT at all (eszopiclone 10 mg/kg, n = 4 of 12; diazepam 5 mg/kg, n = 9 of 12; diazepam 10 mg/kg, n = 7 of 12). Overall, Figure 4 shows that with increasing doses of GABAaR modulators, the monkeys able to meet minimum response criteria (i.e., make at least 10 responses in 30 min) had more lapses in attention (omission errors: eszopiclone F(1.68,15.10) = 31.97, P < 0.0001; diazepam F(1.42,14.20) = 5.13, P < 0.05) and took longer to respond to test stimuli (latency: eszopiclone F(1.33,9.29) = 10.64, P < 0.05 with 10 mg/kg removed from repeated-measures ANOVA because only four monkeys met criteria; diazepam F(1.07,6.42) = 7.58, P < 0.05).
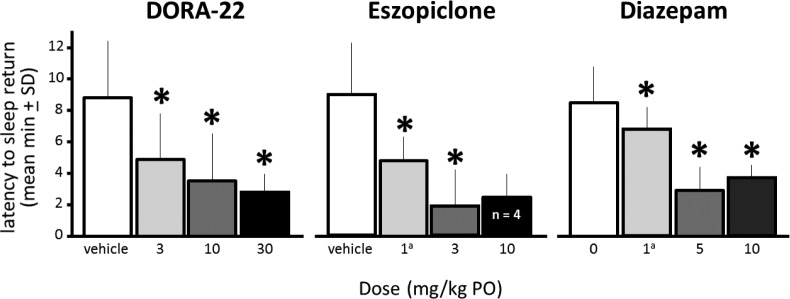
DORA-22 and GABAaR Modulators All Facilitate Return to Sleep after PVT Testing
The ability to return to sleep following awakening and engagement in a cognitive task, the latency to return to Slow Wave Sleep I following PVT testing, is depicted in Figure 5. Although all DORA-22-treated monkeys were completely engaged during the PVT, they returned to slow wave sleep faster after 30 min of PVT at all DORA-22 doses relative to vehicle (P < 0.05). Monkeys that were able to engage in the PVT with eszopiclone and diazepam exhibited a similar decreased latency to return to slow wave sleep relative to vehicle treatment (P < 0.05).
Figure 5.
DORA-22 and GABAaR modulators all facilitated return to sleep after PVT testing. Mean latency to return to slow wave sleep (in minutes) after testing in monkeys awake at the end of the test. Latency was decreased at all DORA-22 and with eszopiclone and diazepam in those monkeys that engaged in the PVT. *P < 0.05 compared with vehicle. aNonsedating daytime dose. DORA, dual orexin receptor antagonist; GABA, gamma-aminobutyric acid; GABAaR, GABA-A receptor; PO, by mouth; PVT, psychomotor vigilance task; SD, standard deviation.
DISCUSSION
DORAs promote sleep onset and maintenance9 by selectively targeting wake-promoting regions in the brain, differing from the classic GABAaR modulator hypnotics that act globally to inhibit CNS activity.4,24 The current study further differentiates the DORA from the GABA-A mechanism of action of sleep promotion, revealing that DORA-treated monkeys preserve the ability to selectively wake from deep sleep and respond to salient stimuli yet remain sleeping through irrelevant noise. In contrast, the CNS-sedating effects of GABAaR modulators skewed sleep architecture toward lighter sleep and suppressed REM, abolished the ability of monkeys to wake preferentially to a salient-conditioned stimulus, and impaired their psychomotor performance if they did wake.
Although DORA-22 and the benzodiazepine and nonbenzodiazepine GABAaR modulators all promoted sleep in monkeys,18,20,21 our current assessment of the underlying sleep architecture revealed their mechanisms of action affected sleep-stage parameters differently. DORA-22 sleep architecture resembled unmedicated baseline (vehicle-treated) overnight sleep, but sleep with eszopiclone and diazepam was biased towards lighter sleep stages (Light Sleep, Slow Wave Sleep I) and reduced potentially more restorative sleep stages (Delta Sleep II, REM). These findings are consistent with previous observations in mice, rats, and dogs, demonstrating that although DORAs (almorexant, DORA-22) had minimal effects on healthy sleep architecture, standard-of-care GABAaR modulators (eszopiclone, zolpidem) tended to reduce the amount of REM sleep.11,21,25 Eszopiclone and zolpidem also markedly disrupted EEG power spectra within sleep stages in rats, even at low doses that did not induce sleep.25 Meanwhile, in healthy human subjects, no consistent changes in sleep architecture or EEG power spectra were observed following nighttime administration of the DORA, suvorexant, relative to placebo sleep.26
In human subjects exposed to a traffic noise model of situational insomnia, the DORA SB-649868 and the GABAaR modulator, zolpidem, both increased total sleep time.27 Again, zolpidem significantly reduced the proportion of the sleep period spent in REM sleep and disrupted EEG power spectra during non-REM sleep, whereas the DORA SB-649868 did not alter these parameters compared with unmedicated sleep.27 Although the effect of the observed alterations in sleep architecture with GABA-A modulators remains unclear, our current findings in rhesus monkeys and healthy human subjects26 support the conclusion that DORA-induced sleep resembles normal baseline sleep in architecture and EEG power spectra profile. These data likely reflect the difference of mechanism of action of these classes of therapeutic agents at the level of altering local cortical neural circuitry underlying the EEG signal recorded by the EEG electrodes.
Anecdotal and experimental evidence in humans indicates that environmental noise can disturb sleep, which may have detrimental effects on health and quality of life.27 An ideal insomnia therapeutic agent would promote the ability to sleep undisturbed by ambient background noise (e.g., traffic, construction or airport noise, partner's snoring). Maintaining the ability to awaken to important acoustic stimuli, however, is also clearly important (e.g., crying infant, intruder, smoke alarm). In humans, classic GABAaR modulators appear to increase the threshold for salient auditory arousal as well as for benign noise. For example, the benzodiazepine, flurazepam, significantly increased the threshold for awakening to both a neutral tone and calling of a subject's name,28 even though hearing one's name is associated with a lower arousal threshold during unmedicated sleep.29,30 Moreover, alcohol and GABA modulators, including the benzodiazepine triazolam, may impair the ability to awaken to smoke-detector alarm signals,31,32 highlighting the importance of preserved salience discrimination during sleep.
Despite spending more time in deeper sleep stages than during GABAaR-modulated sleep, sleep induced by a DORA-22 maintained the ability to differentiate between salient versus irrelevant auditory stimuli in monkeys. Salience-gated arousability with DORA sleep was also maintained in dogs, a species where GABAaR modulators cause paradoxical hyperarousal.17 In the current study with monkeys, however, GABAaR hypnotic agents could also be evaluated alongside a DORA, demonstrating that the classic benzodiazepine, diazepam, and the nonbenzodiazepine GABAaR modulator, eszopiclone, impaired salience discrimination.
Although the relative time spent in lighter sleep with GABAaR modulators increased, almost no monkeys awoke to either the salient-conditioned or neutral acoustic stimuli at sleep-promoting doses. Even administration of low doses of GABAaR modulators, insufficient to promote sleep during the daytime, impaired salience-gated discrimination. Salience discrimination was preserved with DORAs, but not GABAaR modulators, even though the DORA-22 monkeys were in deeper (Delta II) sleep stages.
When awakened during PVT testing, a vigilance reaction-time task that requires sustained attention and alertness, the DORA-22-treated monkeys did not exhibit cognitive or performance impairments. This test is sensitive to sleepiness and has established ecologic validity in reflecting real-world risks on task performance.19 The GABAaR modulator-treated monkeys that were able to awaken and engage in at least some PVT trials during the 30 min of testing exhibited significant deficits in their ability to respond, and lapses in cognitive engagement and attention. These results are consistent with impaired monkey performance in the delayed match to sample and choice reaction time cognition tests with administration of GABAaR modulators.18 Thus, this study expands the number of cognitive tasks demonstrating unimpaired DORA-related performance versus impaired GABAaR-related performance in nonhuman primates. Our results are also consistent with previous observations in rats that sleep-promoting doses of the DORA, almorexant,11 did not impair Morris water maze task acquisition or passive avoidance learning.33 The minimal cognitive changes with DORAs in preclinical models of middle-of-the-night awakening suggest that these agents may better preserve cognitive and psychomotor capacity during unexpected awakenings at night when a response is required.
It is of note that more GABAaR modulator-treated monkeys were able to wake during some part of prolonged PVT testing than awakened to the briefer acoustic stimuli testing. The PVT testing involved 30 min of continued arousing disturbances with multimodal acoustic stimulation (PVT and food-reward pump noise, sounds, and vocalizations of other monkeys engaged in task and consuming food reward), vibrations (awake and performing monkeys in adjacent caging), and lighting (in-cage brightly lit computer screen in a dark testing room). Although the ECoG data determined that some nonspecific arousal can occur with GABAaR modulator sleep, it is also clear that merely waking up was not sufficient for performing psychomotor tasks in GABAaR modulator-treated monkeys.
In humans, the broad distribution of the GABAaRs targeted by the current standard-of-care class of sedative hypnotic agents is associated with some undesirable side effects, including unsteady gait, next-day sedation, cognition deficits, lost balance, somnambulism, and confusion.34–37 Previous pre-clinical studies demonstrated that targeting the highly conserved orexin pathway, which appears to be involved primarily with regulating wakefulness, may potentially result in fewer side effects compared with GABAaR modulators, including less cognitive impairment at clinically meaningful doses and arousal to Active Wake from DORA-medicated sleep if needed.
Of course, whereas the DORA and GABAaR modulator sleep promotion characteristics translate well between rodent and primate preclinical and human clinical studies, experimental data are needed in patients to complete the understanding of the translational nature of differential salience-gated arousability, cognitive performance, and somnambulism among insomnia medication mechanisms.
Our current findings indicate that DORA-mediated sleep maintains the capacity to awaken specifically in response to emotionally salient stimuli in nonhuman primates. When these animals are awake, cognitive and psychomotor performance is not impaired, and animals rapidly return to sleep after accurately completing even a sustained task. The features of salient arousability and retention of psychomotor performance were lost during eszopiclone and diazepam sleep, suggesting a potentially meaningful differentiation between a hypnotic agent that works through antagonizing orexin wake signaling versus the sedative hypnotic effects of the GABAaR modulator's mechanism of action.
DISCLOSURE STATEMENT
This study was funded by Merck & Co., Inc., Kenilworth, NJ. All authors are current or former full-time employees of Merck & Co., Inc. and own or owned stock or stock options in Merck & Co., Inc. The work was carried out at Merck Research Laboratories, Merck & Co., Inc., West Point, PA.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the assistance of the following colleagues in completing the studies described herein: D. Ryan, D. Wright and K. Jackson for animal care; P. Mathers and J. Binns for study assistance; and C. Johnson, A. Bone, and T. Montgomery for surgical support. Medical writing assistance was provided by E. Bekes, PhD, of Complete Medical Communications, Hackensack, NJ. This assistance was funded by Merck & Co., Inc., Kenilworth, NJ.
ABBREVIATIONS
- ANOVA
analysis of variance
- CNS
central nervous system
- DORA
dual orexin receptor antagonist
- EcoG
electrocorticogram
- EEG
electroencephalography
- EMG
electromyogram
- EOG
electrooculogram
- GABA
gamma-aminobutyric acid
- GABAaR
GABA-A receptor
- PO
orally
- PSG
polysomnography
- PVT
psychomotor vigilance task
- REM
rapid eye movement
- SD
standard deviation
REFERENCES
- 1.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Benca RM. Diagnosis and treatment of chronic insomnia: a review. Psychiatr Serv. 2005;56:332–43. doi: 10.1176/appi.ps.56.3.332. [DOI] [PubMed] [Google Scholar]
- 3.Wilson SJ, Nutt DJ, Alford C, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol. 2010;24:1577–601. doi: 10.1177/0269881110379307. [DOI] [PubMed] [Google Scholar]
- 4.Sanger DJ. The pharmacology and mechanisms of action of new generation, non-benzodiazepine hypnotic agents. CNS Drugs. 2004;18:9–15. doi: 10.2165/00023210-200418001-00004. [DOI] [PubMed] [Google Scholar]
- 5.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- 6.Sieghart W, Sperk G. Subunit composition, distribution and function of GABA-A receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 7.Scammell TE, Winrow CJ. Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51:243–66. doi: 10.1146/annurev-pharmtox-010510-100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsujino N, Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci. 2013;7:28. doi: 10.3389/fnbeh.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winrow CJ, Renger JJ. Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol. 2014;171:283–93. doi: 10.1111/bph.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brisbare-Roch C, Dingemanse J, Koberstein R, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13:150–5. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- 12.Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79:2265–74. doi: 10.1212/WNL.0b013e31827688ee. [DOI] [PubMed] [Google Scholar]
- 13.Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexant - a novel dual orexin receptor antagonist. J Neurogenet. 2011;25:52–61. doi: 10.3109/01677063.2011.566953. [DOI] [PubMed] [Google Scholar]
- 14.Winrow CJ, Gotter AL, Cox CD, et al. Pharmacological characterization of MK-6096 - a dual orexin receptor antagonist for insomnia. Neuropharmacology. 2012;62:978–87. doi: 10.1016/j.neuropharm.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Bettica P, Squassante L, Zamuner S, Nucci G, Danker-Hopfe H, Ratti E. The orexin antagonist SB-649868 promotes and maintains sleep in men with primary insomnia. Sleep. 2012;35:1097–104. doi: 10.5665/sleep.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coenen A. Subconscious stimulus recognition and processing during sleep. Psyche. 2010;16:90–7. [Google Scholar]
- 17.Tannenbaum PL, Stevens J, Binns J, et al. Orexin receptor antagonist-induced sleep does not impair the ability to wake in response to emotionally salient acoustic stimuli in dogs. Front Behav Neurosci. 2014;8:182. doi: 10.3389/fnbeh.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uslaner JM, Tye SJ, Eddins DM, et al. Orexin receptor antagonists differ from standard sleep drugs by promoting sleep at doses that do not disrupt cognition. Sci Transl Med. 2013;5:179ra44. doi: 10.1126/scitranslmed.3005213. [DOI] [PubMed] [Google Scholar]
- 19.Dorrian J, Rogers N, Dinges D. Psychomotor vigilance performance: neurocognitive assay sensitive to sleep loss. In: Kushida C, editor. Sleep deprivation: clinical issues, pharmacology and sleep loss effects. New York, NY: Marcel Dekker Inc; 2005. pp. 39–70. [Google Scholar]
- 20.Gotter AL, Winrow CJ, Brunner J, et al. The duration of sleep promoting efficacy by dual orexin receptor antagonists is dependent upon receptor occupancy threshold. BMC Neurosci. 2013;14:90. doi: 10.1186/1471-2202-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gotter AL, Garson SL, Stevens J, et al. Differential sleep-promoting effects of dual orexin receptor antagonists and GABAA receptor modulators. BMC Neurosci. 2014;15:109. doi: 10.1186/1471-2202-15-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clingerman KJ, Summers L. Development of a body condition scoring system for nonhuman primates using Macaca mulatta as a model. Lab Anim (NY) 2005;34:31–6. doi: 10.1038/laban0505-31. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh KC, Robinson EL, Fuller CA. Sleep architecture in unrestrained rhesus monkeys (Macaca mulatta) synchronized to 24-hour light-dark cycles. Sleep. 2008;31:1239–50. [PMC free article] [PubMed] [Google Scholar]
- 24.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 25.Fox SV, Gotter AL, Tye SJ, et al. Quantitative electroencephalography within sleep/wake states differentiates GABA-A modulators eszopiclone and zolpidem from dual orexin receptor antagonists in rats. Neuropsychopharmacology. 2013;38:2401–8. doi: 10.1038/npp.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun H, Kennedy WP, Wilbraham D, et al. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. Sleep. 2013;36:259–67. doi: 10.5665/sleep.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bettica P, Squassante L, Groeger JA, Gennery B, Winsky-Sommerer R, Dijk DJ. Differential effects of a dual orexin receptor antagonist (SB-649868) and zolpidem on sleep initiation and consolidation, SWS, REM sleep, and EEG power spectra in a model of situational insomnia. Neuropsychopharmacology. 2012;37:1224–33. doi: 10.1038/npp.2011.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muzet A. Environmental noise, sleep and health. Sleep Med Rev. 2007;11:135–42. doi: 10.1016/j.smrv.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Mendelson WB, Martin JV, Stephens H, Giesen H, James SP. Effects of flurazepam on sleep, arousal threshold, and the perception of being asleep. Psychopharmacology (Berl) 1988;95:258–62. doi: 10.1007/BF00174520. [DOI] [PubMed] [Google Scholar]
- 30.Langford GW, Meddis R, Pearson AJ. Awakening latency from sleep for meaningful and non-meaningful stimuli. Psychophysiology. 1974;11:1–5. doi: 10.1111/j.1469-8986.1974.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 31.Oswald I, Taylor AM, Treisman M. Discriminative responses to stimulation during human sleep. Brain. 1960;83:440–53. doi: 10.1093/brain/83.3.440. [DOI] [PubMed] [Google Scholar]
- 32.Ball M, Bruck D. The effect of alcohol upon response to fire alarm signals in sleeping young adults. In: Shields J, editor. Proceedings of the Third International Symposium on Human Behaviour in Fire, Interscience Communications; 2004; London. pp. 291–302. [Google Scholar]
- 33.Johnson LC, Spinweber CL, Webb SC, Muzet AG. Dose level effects of triazolam on sleep and response to a smoke detector alarm. Psychopharmacology (Berl) 1987;91:397–402. doi: 10.1007/BF00216003. [DOI] [PubMed] [Google Scholar]
- 34.Dietrich H, Jenck F. Intact learning and memory in rats following treatment with the dual orexin receptor antagonist almorexant. Psychopharmacology (Berl) 2010;212:145–54. doi: 10.1007/s00213-010-1933-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barker MJ, Greenwood KM, Jackson M, Crowe SF. Cognitive effects of long-term benzodiazepine use: a meta-analysis. CNS Drugs. 2004;18:37–48. doi: 10.2165/00023210-200418010-00004. [DOI] [PubMed] [Google Scholar]
- 36.Hindmarch I, Legangneux E, Stanley N, Emegbo S, Dawson J. A double-blind, placebo-controlled investigation of the residual psychomotor and cognitive effects of zolpidem-MR in healthy elderly volunteers. Br J Clin Pharmacol. 2006;62:538–45. doi: 10.1111/j.1365-2125.2006.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otmani S, Demazières A, Staner C, et al. Effects of prolonged-release melatonin, zolpidem, and their combination on psychomotor functions, memory recall, and driving skills in healthy middle aged and elderly volunteers. Hum Psychopharmacol. 2008;23:693–705. doi: 10.1002/hup.980. [DOI] [PubMed] [Google Scholar]
- 38.Vermeeren A, Coenen AM. Effects of the use of hypnotics on cognition. Prog Brain Res. 2011;190:89–103. doi: 10.1016/B978-0-444-53817-8.00005-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.