Abstract
Study Objectives:
Sleep after learning promotes the quantitative strengthening of new memories. Less is known about the impact of sleep on the qualitative reorganization of memory content. This study tested the hypothesis that sleep facilitates both memory strengthening and reorganization as indexed by a verbal creativity task.
Methods:
Sixty healthy university students (30 female, 30 male, 20–30 years) were investigated in a randomized, controlled parallel-group study with three experimental groups (sleep, sleep deprivation, daytime wakefulness). At baseline, 60 items of the Compound Remote Associate (CRA) task were presented. At retest after the experimental conditions, the same items were presented again together with 20 new control items to disentangle off-line incubation from online performance effects.
Results:
Sleep significantly strengthened formerly encoded memories in comparison to both wake conditions (improvement in speed of correctly resolved items). Offline reorganization was not enhanced following sleep, but was enhanced following sleep-deprivation in comparison to sleep and daytime wakefulness (solution time of previously incubated, newly solved items). Online performance did not differ between the groups (solution time of new control items).
Conclusions:
The results support the notion that sleep promotes the strengthening, but not the reorganization, of newly encoded memory traces in a verbal creativity task. Future studies are needed to further determine the impact of sleep on different types of memory reorganization, such as associative thinking, creativity and emotional memory processing, and potential clinical translations, such as the augmentation of psychotherapy through sleep interventions.
Citation:
Landmann N, Kuhn M, Maier JG, Feige B, Spiegelhalder K, Riemann D, Nissen C. Sleep strengthens but does not reorganize memory traces in a verbal creativity task. SLEEP 2016;39(3):705–713.
Keywords: sleep, learning, memory, consolidation, strengthening, reorganization, creativity, CRA, RAT, sleep deprivation
Significance.
This study identifies as an important function of sleep the veridical strengthening of newly acquired memories, but not their creative reorganization. Further characterization of memory formation across the 24 h sleep-wake cycle is essential for healthy learning, as well as for the identification of novel treatment options targeting highly prevalent sleep and memory problems.
INTRODUCTION
Sleep has been shown to facilitate the quantitative strengthening of different types of new memories.1 In contrast, the impact of sleep on the qualitative reorganization of memories that can result in a change of memory content and the emergence of new memories, e.g., in the form of creative solutions, remains to be further characterized.2
In the declarative memory system, memory strengthening refers to the veridical preservation of explicit information, with sleep promoting this strengthening of, e.g., word-pairs.3,4 Besides, declarative memories are also constructive in nature. This means that memories are not always stored as exact copies of the past. Rather, they can be transformed in a process of memory reorganization and the new representation can substantially differ from what has originally been encoded. Examples include erroneous autobiographical memories, inconsistencies in eyewitness testimonies, and memory distortions in mental disorders.5–7
Initial evidence suggests that sleep facilitates some forms of memory reorganization,2,8 including the extraction of rules, their generalization to novel situations, the integration of recent and remote memories, and relational memory formation.9–12
The present study was designed to further characterize the impact of sleep on the highest form of memory reorganization, i.e., the recombination of given information that allow for associative thinking and creativity. In this context, creativity refers to the “the forming of associative elements into new combinations which […] are in some way useful.”13
The idea that sleep can support creativity has a long history.14,15 Anecdotal reports reach back to the 19th century, as Dmitri Mendeleyev (1834–1907) invented the periodic table following sleep or August Kekulé (1829–1896) conceptualized the structure of benzene after a dream.16 Some first experimental, yet indirect evidence for the promotion of creativity by sleep was provided by studies showing facilitated associative thinking following nighttime sleep compared to sleep deprivation.17–19 Subsequent studies investigated the performance after experimental awakenings from different sleep stages and pointed to a particular effect of REM sleep. Specifically, the performance in solving anagram word puzzles20 and the acquisition of weak (e.g., thief – wrong) but not strong primes (e.g., hot – cold)21 were enhanced after awakenings from REM sleep compared to awakenings from NREM sleep or periods of sustained wakefulness. This indirect set-up is based on the idea that stage-specific brain activity patterns persist for a certain period of time after awakening.22
Some recent studies have begun using pre-post sleep assessments to more directly examine the impact of sleep on associative thinking and creativity. To this end, the Remote Associates Task (RAT), the first reliable task to systematically investigate creativity in the declarative memory system,13,23 was translated to sleep research. The RAT comprises a series of three unrelated stimulus words (RAT items) that are related to one solution word in different semantic ways (e.g., stimulus words: same – tennis – head; solution word: match; explanation: same = match, tennis match, match head). Again, REM sleep enhanced the ability to find creative solutions in RAT items that were primed prior to a daytime nap.24 In contrast, improvements in performance were independent of the brain state during the incubation phase (nap, wake or quiet rest), if the RAT items have not been primed in advance.
Critically, the individual RAT items are difficult to compare due to heterogeneous solution strategies (e.g., synonym, association, compound, and others). This is why Bowden and Jung Beeman25 developed the more homogenous Compound Remote Associates (CRA) task. CRA problems require participants to find a solution noun that produces a meaningful compound in combination with three unrelated stimulus nouns (e.g., stimulus nouns: dream – break – light; solution noun: day; explanation: daydream, daybreak, daylight). CRA problems have become an established task to investigate associative thinking and creativity in the Anglo-American language area and has been used in a variety of studies on neuropsychological processes underlying creativity.26 In a recent nighttime sleep study, a sleep-specific advantage in solving CRA items has been observed in a secondary analysis, yet only for difficult items.27 Together, preliminary evidence suggests that sleep, and maybe particularly REM sleep, can facilitate creative problem solving, with the exact prerequisites and mechanisms remaining to be characterized.28
The aim of this study was to further determine the impact of sleep on the strengthening and reorganization of memory traces based on the CRA task. In the development of this study, we constructed a German version of the task.29 Specifically, we hypothesized that nighttime sleep would both strengthen new memories and facilitate the reorganization of memory traces in comparison to both sleep deprivation and daytime wakefulness in healthy adults.
METHODS
Participants
Sixty university students participated in the study (30 female, 30 male, mean age = 23.4 years, standard deviation = 2.1, range 20–31 years). All participants were in good physical and mental health, nonsmokers, and did not take any medication, as assured by clinical examinations and the Composite International Diagnostic Interview.30 All participants were right handed (Edinburgh Handedness Inventory)31 and native German speakers. Female participants were in the follicular phase of their menstrual cycle at the time of the study. All participants followed a regular sleep-wake pattern within 2 weeks prior to the study, as determined by sleep diaries and actigraphy. Subjective sleep and sleepiness were assessed by the Pittsburgh Sleep Quality Index (PSQI)32 and the Epworth Sleepiness Scale (ESS).33 Participants were further characterized by the Beck Depression Inventory (BDI),34 the Perceived Stress Questionnaire (PSQ-20),35 and the Multiple Choice Verbal Intelligence Test.36 Participants received financial reimbursement for participation. Written informed consent was obtained from all participants prior to the study. The procedures had been approved by the local Ethics Committee (N° 297/11) and were carried out in accordance with the Declaration of Helsinki.
Study Design
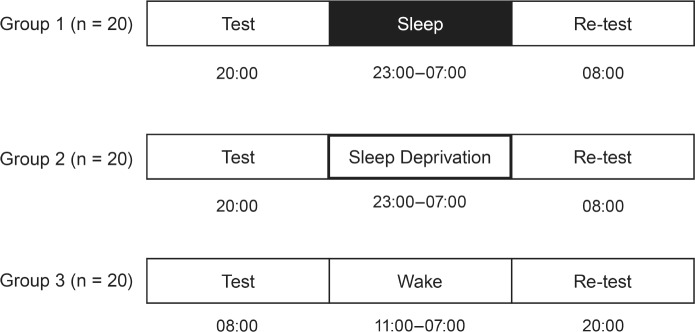
The study design is depicted in Figure 1. Participants were randomized to 1 of 3 experimental groups (parallel group design) and tested prior to and after the experimental interventions (sleep, sleep deprivation, daytime wakefulness) on the CRA task.
Figure 1.
Study design. The sleep group (group 1) completed a baseline session in the evening (20:00–20:15) prior to a sleep laboratory night with polysomnographic monitoring (23:00–07:00). The sleep deprivation group (group 2) completed the baseline session in the evening (20:00–20:15) prior to a night of total sleep deprivation with supervised and standardized activities (cooking, dinner, card games, movie, and walks). The wake group (group 3) completed the baseline session in the morning (08:00–08:15) and then followed their daily routines. In all groups, a re-test session followed after an incubation period of 12 h referred to the baseline session.
Compound Remote Associates Task
The Compound Remote Associates (CRA) task represents an important paradigm to systematically examine verbal-associative processes as an index for creativity. In the development of this study, we recently constructed a German version of this task.29
In the CRA task, participants are required to find a solution noun that produces a meaning in combination with 3 unrelated stimulus nouns (e.g., stimulus nouns: dream – break – light; solution noun: day). In contrast to a systematic solution process, such as, for example, required for simple arithmetic problems, participants have to freely associate in a creative process, requiring a reorganization of memory traces. In contrast to classical insight problems, CRA items can be presented in a standardized manner on a computer screen, and a high number of items with defined levels of difficulty and comparability of the solution strategies can be solved within a short period of time.37,38
In the current study, 80 CRA items of 2 levels of difficulty (easy, difficult) were randomly selected from the German version of the CRA task.29 Every CRA item had only one solution noun and each stimulus and solution noun was only used once. The task was presented on a computer screen using the Presentation software. Each participant was tested individually. A standardized instruction printed on a DIN A4 paper was provided. Subsequently, 3 practice CRA items were presented and potential questions were answered prior to testing.
At the beginning of each trial, a black screen appeared. Each trial had to be started by the participant by pressing the SPACE button. The 3 stimulus nouns of each CRA item appeared in the middle of the screen (Arial, font size 50 pt, black on white screen, separated by dashes). Participants were instructed to press the SPACE button and verbalize the solution word as quickly as possible, with trained staff noting if the answer was correct or not. The interval between the onset of the presentation of the CRA items and the SPACE signal served as the outcome parameter for solution time.
At baseline, 60 CRA items (30 easy, 30 difficult) were randomly presented for a maximum duration of 10 s. This relatively short maximal presentation time was selected based on pilot trials to avoid ceiling effects in solution. At re-test, the 60 items were randomly presented again, now for a maximum duration of 30 s, together with 20 novel control items to control for state dependent-effects following the experimental conditions.
Sleep Recordings
Polysomnography was recorded during two sleep laboratory nights in the sleep group (adaptation and experimental night) according to procedures described previously.39 Sleep recordings were scored visually by experienced raters using standard criteria.40 The setup included the EEG electrodes C3-A2 and C4-A1, submental electromyogram (EMG), vertical and horizontal electrooculogram (EOG) and electrocardiogram (ECG). The following variables of sleep continuity and architecture were assessed: sleep latency (defined as the period between lights-off until the onset of stage 2 sleep), sleep period time (defined as the period between sleep onset and final awakening), total sleep time, sleep efficiency (defined as the percentage of total sleep time referred to the time in bed), time spent awake as well as in sleep stages 1, 2, slow-wave sleep (SWS; combined stages 3 and 4), and REM sleep (as percentage of sleep period time). REM sleep variables included REM sleep latency (time from sleep onset until the first epoch of REM sleep) and REM density (calculated as the ratio of 3 s REM sleep mini-epochs including REMs to the total amount of REM sleep mini-epochs).
An all-night spectral power analysis was performed as described previously.41,42 In brief, the analysis was performed on the C3-A2 derivation in 30-s epochs for which sleep stages had been determined. Data were recorded with a sampling rate of 200 Hz and a resolution of 16 bits. Signals were recorded with a time constant of 0.3 s and low-pass filtered at 70 Hz. Spectral estimates for each epoch were obtained as the average of 22 overlapping fast Fourier transform (FFT) windows (512 data points, 2.56 s) covering a 30-s epoch to obtain the spectral power within that epoch, resulting in a spectral resolution of 0.39 Hz. A Welch taper was applied to each FFT window after demeaning and detrending the data in that window. The spectral power values were then log-transformed (base e) and continuously stored on disk. All subsequent steps including the statistical analysis were performed on these logarithmic values, which have more symmetrically distributed errors than raw spectral power. Artifact rejection used an automatic method discarding epochs due to abnormal total power or gamma-band power values relative to a 10-min moving window.43 The log spectra of the remaining epochs were averaged across all NREM sleep epochs. Spectral band power was calculated for the following frequency ranges: delta (0.1–3.5 Hz), theta (3.5–8 Hz), alpha (8–12 Hz), slow sigma (12–14 Hz), fast sigma (14–16 Hz), beta (16–24 Hz), slow beta (16–20 Hz), fast beta (20–24 Hz), and gamma (24–50 Hz).
Operationalization of Variables
The selected design allows investigating the strengthening of newly encoded memories, the reorganization of formerly primed memories and state-dependent effects after the experimental conditions. The operationalization of the outcome parameters is visualized in Figure 2.
Figure 2.
Operationalization of variables. The strengthening of memory traces was calculated as the percentage of the number of resolved items at re-test referred to baseline (%) and as the percentage of improvement in the solution time of resolved items at re-test referred to baseline (%). The reorganization of memory traces was calculated as the absolute number and solution time (s) of newly solved items at re-test that had already been presented and primed, but not solved, at baseline. State-dependent effects after the experimental conditions were operationalized as the absolute number and solution time (s) of newly presented and solved control items at re-test.
Cognitive Assessments
Cognitive performance, including visual and auditory alertness (Test for Attentional Performance, TAP44; working memory, Digit span45) were assessed at baseline and re-test to control for potentially confounding effects.
Data Analysis
Data were analyzed using the R Statistical Software.46 Descriptive values are given as means and standard deviations. Analyses of variance (ANOVAs) with the between-subject factor Group (sleep, sleep deprivation, daytime wakefulness) were used to test for group differences in baseline measures of demographic/clinical characteristics and parameters of baseline performance in the CRA task. To detect group differences for memory strengthening, memory reorganization, and state-dependent effects, ANOVAs with the factor Group (sleep, sleep deprivation, daytime wakefulness) and the dependent variables as defined in Figure 2 were calculated. For exploratory correlational analyses, Pearson correlation coefficients for the parameters of memory strengthening/reorganization and sleep parameters in the sleep group were calculated. The level of significance was set at P < 0.05 (two-tailed).
RESULTS
Demographic and Clinical Characteristics
The three experimental groups did not significantly differ in relevant demographic and clinical characteristics (Table 1). The difference in age almost reached significance.
Table 1.
Study sample.

Compound Remote Associates Task
Baseline Performance
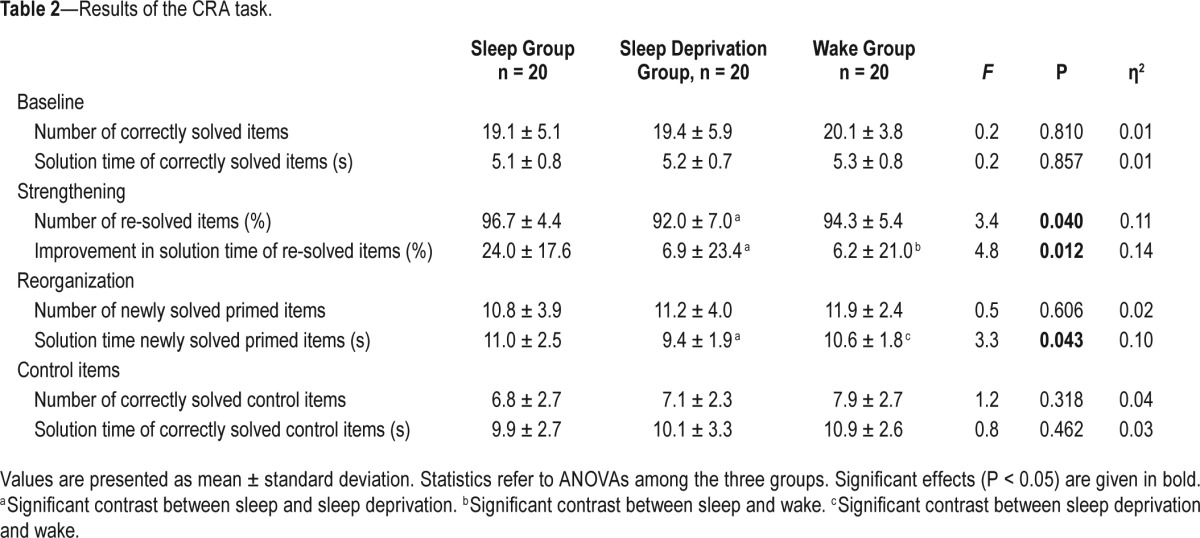
All three groups (sleep, sleep deprivation, daytime wakefulness) demonstrated a highly similar number and solution time of correctly solved items at baseline (Table 2), indicative for similar task performance at baseline and, particularly, similar task performance at different point in times (circadian phases) of the day.
Table 2.
Results of the CRA task.
Strengthening of Memory Traces
The ANOVA with the factor Group showed a significant effect for the percentage of re-solved items at re-test (Table 2). Follow-up pairwise t-tests indicated that participants of the sleep group resolved significantly more items correctly than those in the sleep deprivation group (t38 = 2.6, P = 0.015, d = 0.8). The other group comparisons did not reach statistical significance (sleep versus wake: t38 = 1.5, P = 0.131, d = 0.5; sleep deprivation versus wake: t38 = −1.2, P = 0.248, d = 0.4). All groups correctly re-solved over 90% of the items they had solved at baseline.
The ANOVA with the factor Group showed a significant effect for the improvement in solution time of resolved items from baseline to re-test. Follow-up pairwise t-tests demonstrated that the sleep group showed a significantly higher improvement in speed from baseline to re-test than participants of the sleep deprivation group (t38 = 2.6, P = 0.012, d = 0.8) and participants of the daytime wakefulness group (t38 = 2.9, P = 0.006, d = 0.9). The group comparison of sleep deprivation versus wake did not reach statistical significance (t38 = 0.1, P = 0.920, d = 0.03).
This pattern of results is in line with the notion that sleep fosters the strengthening of newly encoded memory traces. Figure 3A illustrates the main findings with regard to memory strengthening, as indexed by the solution time of resolved items.
Figure 3.
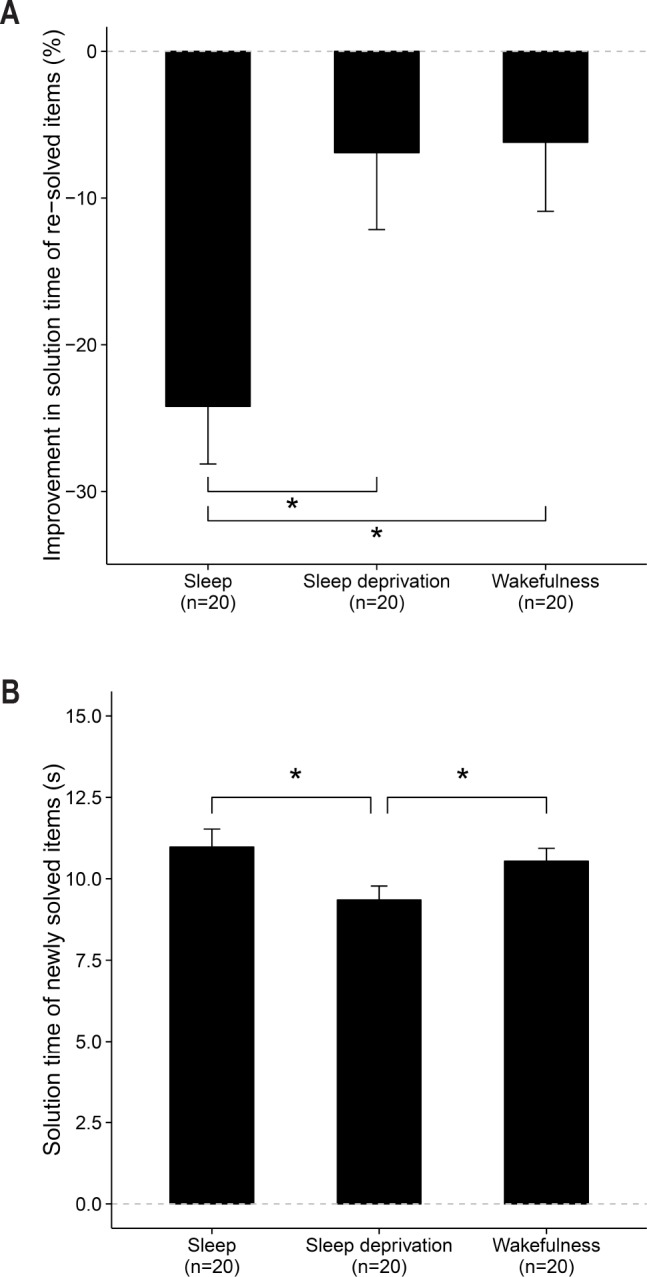
Effects of sleep, sleep deprivation and wake on the strengthening and reorganization of memories. (A) Memory strengthening. The sleep group showed a significantly higher improvement in solution time (%) for resolved items than the sleep deprivation or the daytime wakefulness group. (B) Memory reorganization. The sleep deprivation group showed significantly shorter solution times (s) for newly solved items than the sleep or the daytime wakefulness group. Bars represent means ± SEM. Significant effects are highlighted by an asterisk.
Reorganization of Memory Traces
The ANOVA with the factor Group and absolute number of newly solved items as a dependent variable revealed no differences between the experimental groups (Table 2). Participants of all three groups solved about 12 new items at re-test that they had not solved at baseline.
An ANOVA with solution time of newly solved items as an outcome variable revealed a significant group difference (Table 2). Of particular note and in contrast to the hypothesis that sleep fosters the reorganization of memories, pairwise t-tests indicated that the sleep deprivation group showed a significantly higher speed in solution time of primed items (presented but not solved prior to the intervention) than participants of the sleep group (t38 = 2.3, P = 0.025, d = 0.7) and participants of the wake group (t38 = 2.0, P = 0.047, d = 0.6). The group comparison of sleep versus wake did not reach statistical significance (t38 = 0.6, P = 0.523, d = 0.2).
These findings indicate that sleep, in contrast to our hypothesis, did not facilitate the solution of formerly primed items as an index of offline reorganization. Rather, sleep deprivation facilitated this process. Figure 3B illustrates the group differences for the reorganization of memory traces, indexed by the solution time of newly solved items.
Control Items
We presented 20 new control items at re-test that had not been presented at baseline in order to examine whether the observed effects at re-test were driven by offline-effects of the sleep/wake intervention on the incubation period after training at baseline and not by state-dependent effects after the experimental conditions. Here, an ANOVA revealed no differences between the experimental groups for the absolute number or the speed of correctly solved control items (Table 2). The highly similar levels of performance for the control items characterize the described group effects on memory strengthening and reorganization as effects of the incubation period after training, rather than simple state-dependent effects at re-test.
Sleep Parameters
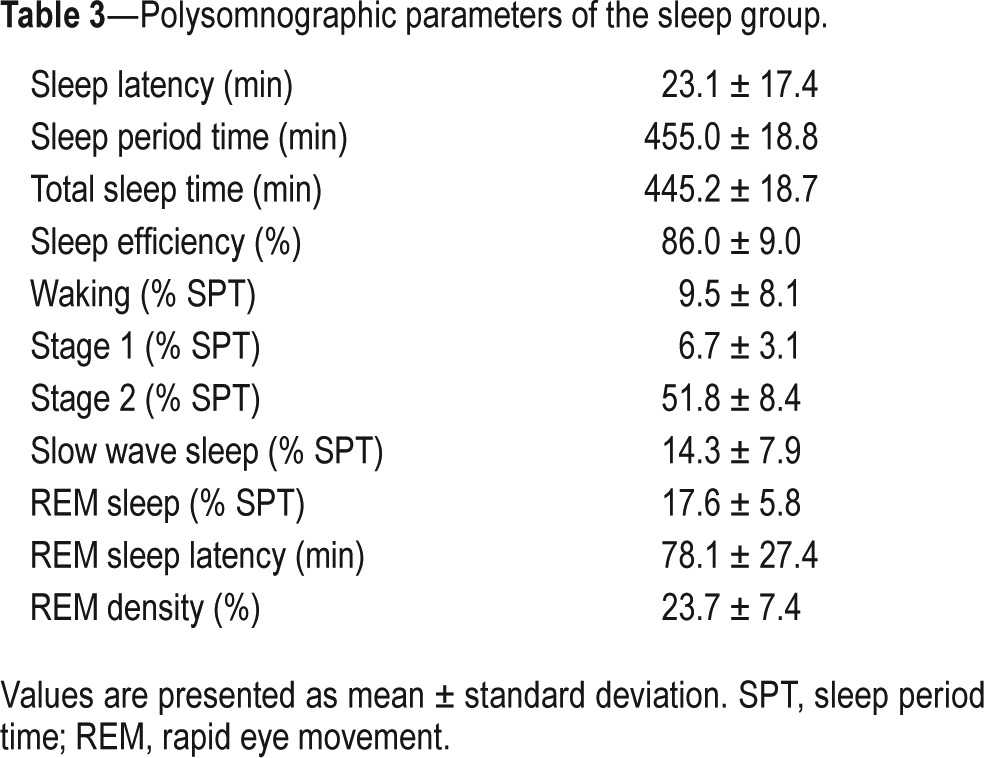
Polysomnographic parameters of the experimental night of the sleep group are listed in Table 3 and demonstrate a healthy sleep profile. Exploratory Pearson correlations did not show any significant correlations between the sleep parameters (polysomnography and EEG spectral analysis parameters, not shown) and parameters of memory strengthening or reorganization in the sleep group (P > 0.1 for all analyses).
Table 3.
Polysomnographic parameters of the sleep group.
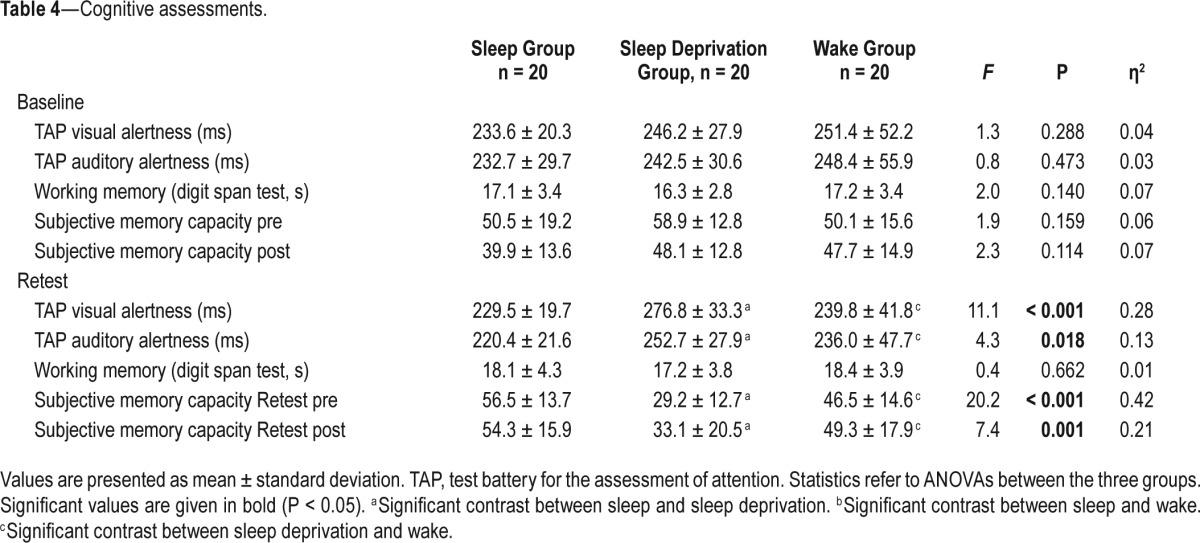
Cognitive Assessments
As listed in Table 4, the groups did not differ in visual and auditory alertness at baseline prior to the sleep/wake intervention. An ANOVA with the factor Group and the cognitive parameters for visual and auditory alertness revealed significant group differences for both dependent variables at re-test. Pairwise t-tests indicated that the sleep deprivation group showed significantly lower values for visual and auditory alertness in comparison to the other two groups (P < 0.05 for all post hoc t-tests).
Table 4.
Cognitive assessments.
Secondary Analyses on the Impact of Sex and Age
When including sex and age as additional factors into the ANOVAs, the significant effects for the factor Group on the outcome parameters persisted (percentage of re-solved items at re-test: F2,51 = 3.6, P = 0.034, η2 = 0.12; improvement in solution time of resolved items from baseline to re-test: F2,51 = 4.7, P = 0.013, η2 = 0.16; solution time (s) of newly solved items, F 2,51 = 3.3, P = 0.047, η2 = 0.11). No significant main effects for the variables age and sex were observed. Only for the dependent variable of percentage of re-solved items at re-test, a significant Group × Sex interaction effect was observed (F2,51 = 3.9, P = 0.027, η2 = 0.15.). Follow-up analyses showed that this interaction was driven by a selective Group effect in male participants (ANOVA with factor Group for female participants: F2,28 = 0.2, P = 0.801, η2 = 0.02; ANOVA with factor Group for male participants: F2,26 = 7.8, P = 0.002, η2 = 0.38). Subsequent pairwise t-tests showed that male participants forgot significantly less items following sleep in comparison to sleep deprivation (t17 = −3.6, P = 0.002, d = 1.7) and wake (t17 = −2.1, P = 0.047, d = 1.0). Furthermore, male participants forgot significantly less items following wake in comparison to sleep deprivation (t18 = −2.1, P = 0.048, d = 0.6).
Secondary Analyses on the Impact of the Factor Difficulty of the CRA Items
A significant effect for the factor difficulty was observed for both dependent variables associated with memory strengthening (percentage of re-solved items: F1,54 = 18.6, P < 0.001, η2 = 0.26; improvement in speed of re-solved items: F1,51 = 7.5, P = 0.008, η2 = 0.13). Subsequent analyses revealed that group differences of the main findings can be attributed to the percentage of re-solved easy items (easy items: F2,57 = 6.0, P = 0.004, η2 = 0.17; difficult items, F2,54 = 0.3, P = 0.772, η2 = 0.01) and to the improvement in speed of re-solved easy items (easy items: F2,57 = 3.2, P = 0.046, η2 = 0.10; difficult items, F2,51 = 1.3, P = 0.283, η2 = 0.05).
Moreover, a significant effect for the factor Difficulty was observed for the solution time of newly solved items (F1,55 = 10.5, P = 0.002, η2 = 0.16). However, the Group effect of the main analysis could not be attributed to one of the two levels of difficulty (easy items: F2,57 = 2.1, P = 0.137, η2 = 0.07; difficult items: F2,55 = 1.4, P = 0.258, η2 = 0.05).
DISCUSSION
The results of this study indicate that sleep fosters the strengthening, but not the reorganization, of newly encoded memories in a verbal creativity task. More specifically, nighttime sleep, in line with our first hypothesis, significantly strengthened new memories, as indicated by an improved solution time at recall of CRA items that had already been solved at initial training in comparison to sleep deprivation and daytime wakefulness. However, contrary to our second hypothesis, sleep did not promote memory reorganization. Rather, we even observed a significant reorganization advantage for sleep deprivation, as indexed by a significantly improved solution time at recall for primed, newly solved items following sleep deprivation in comparison to nighttime sleep and daytime wakefulness.
The observed strengthening of memories across sleep corroborates a multitude of studies on declarative and non-declarative memory.1 Sleep-related strengthening of declarative memory has been demonstrated for various tasks, including nonsense syllables,47 word-pair associates,3,48 and word lists.49 In comparison to these studies, in which the learning material was directly presented to the participants at acquisition, our experimental design went one step further: the participants had to create the memory traces themselves during initial training by generating the correct solution. This resembles various situations in everyday life in which memories evolve in the process of acquiring new knowledge. Exploratory analyses demonstrated that only male, but not female, participants were detrimentally affected by sleep deprivation with regard to the strengthening of newly encoded memories.
In contrast to the robust effect on memory strengthening, the impact of sleep on memory reorganization appears to be more fragile and complex, with sleep enhancing memory reorganization under some, but not other, conditions.2 Particularly in our study, emotional tagging of CRA items that had successfully been solved at initial training (positive “Aha” experience after successful solution25,50,51) might have shifted the information processing during sleep towards the processing of these solved and tagged items (strengthening) over the processing of initially unsolved and non-tagged items (reorganization). This interpretation corroborates prior work indicating that sleep preferentially consolidates memories that are of particular relevance, including emotionally intense,52 motivationally salient,53 and future action-related memories.54 Particularly in our study, the observed strengthening after sleep might be evolutionarily adaptive when considering that the veridical consolidation is in most conditions more critical to the organism than complex and creative solutions.
Sleep-related memory reorganization has been most robustly observed for schema formation (extraction of rules and their generalization to other situations) and schema integration (integration of recent and remote memories).2,8–11 The highest form of memory reorganization, i.e., schema disintegration and recombination, might be more fragile and might less benefit from sleep. This notion is in line with previous studies that did not find a general sleep-related advantage in the same or similar task.24,27 Rather, schema disintegration and recombination during sleep might only evolve under specific conditions. Specifically, creative performance (Unusual Uses Task) was enhanced after reactivating the task during sleep with previously cued odors. In contrast, no beneficial effect of sleep on creativity was observed without odor-induced reactivation.59
The sleep deprivation advantage on creativity might represent a false positive finding. Alternatively, the advantage might fit to activity changes in neural networks and related information processing during prolonged wakefulness. Particularly, prolonged wakefulness resulted in reduced activity in frontal lobe structures, including the prefrontal cortex,55,56 which has been related to reduced cognitive control.57,58 The functional decoupling of cognitive control areas from cortical association areas might facilitate the disintegration of familiar associations and the flexible recombination of separate components across schemas, which enables creative solutions in the CRA task.28 Yet we implemented a design investigating not only primed and newly solved items, but also unprimed control items to disentangle an incubation from a mere state effect after sleep deprivation. A mere state-dependent effect explaining the faster solution times for newly solved items at re-test could be excluded, demonstrated by similar performance levels in control items at re-test in all experimental groups.
Previous models proposed that memory reorganization, emerging from the creation of novel combinations of preexisting memories, is facilitated during REM sleep through a functional decoupling of the hippocampus from the cortex.61 Particularly, high cholinergic in combination with low aminergic neurotransmission during REM sleep might offer favorable conditions for intracortical spreading activation and memory reorganization.62,63 Our data do not support this concept for the current task, showing no REM sleep-related advantage for the creation of new combinations.
Together, our results support the notion that sleep promotes the strengthening, but not the reorganization, of newly encoded memory traces in a verbal creativity task. Future studies are needed to further determine the potential impact of sleep on different types of memory reorganization, such as associative thinking, creativity, and emotional memory processing, as well as on potential clinical translations, such as the augmentation of psychotherapy through sleep interventions.
DISCLOSURE STATEMENT
This was not an industry supported study. The work has been supported by a PhD grant to Nina Landmann (Cusanus Foundation). Financial support was provided by the Scientific Society Freiburg and intramural funds of the University Medical Center Freiburg. Christoph Nissen has received speaker honoraria from Servier. Dieter Riemann has received an honorarium from Abbvie. Marion Kuhn and Jonathan G. Maier have been supported by PhD grants provided by the FAZIT Foundation. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to thank the technical staff and the students Stefan Kowark, Hanna Giselbrecht and Anita Knezevic at the Department of Psychiatry and Psychotherapy, University of Freiburg Medical Center, for their help in conducting the study.
REFERENCES
- 1.Rasch B, Born J. About sleep's role in memory. Physiol Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landmann N, Kuhn M, Piosczyk H, et al. The reorganisation of memory during sleep. Sleep Med Rev. 2014;18:531–41. doi: 10.1016/j.smrv.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Curr Biol. 2006;16:1290–4. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 5.Brown AS, Marsh EJ. Evoking false beliefs about autobiographical experience. Psychol Bull Rev. 2008;15:186–90. doi: 10.3758/pbr.15.1.186. [DOI] [PubMed] [Google Scholar]
- 6.Marsh EJ, Tversky B, Hutson M. How eyewitnesses talk about events: implications for memory. Appl Cogn Psychol. 2005;19:531–44. [Google Scholar]
- 7.Halberstadt L, Haeffel GJ, Abramson LY, Mukherji BR, Metalsky GI, Dykman BM. Schematic processing: a comparison of clinically depressed, dysphoric, and nondepressed college students. Cogn Ther Res. 2008:843–55. [Google Scholar]
- 8.Stickgold R, Walker MP. Sleep-dependent memory triage: evolving generalization through selective processing. Nat Neurosci. 2013;16:139–45. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427:352–5. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- 10.Fenn KM, Nusbaum HC, Margoliash D. Consolidation during sleep of perceptual learning of spoken language. Nature. 2003;425:614–6. doi: 10.1038/nature01951. [DOI] [PubMed] [Google Scholar]
- 11.Tamminen J, Payne JD, Stickgold R, Wamsley EJ, Gaskell MG. Sleep spindle activity is associated with the integration of new memories and existing knowledge. J Neurosci. 2010;30:14356–60. doi: 10.1523/JNEUROSCI.3028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellenbogen JM, Hu PT, Payne JD, Titone D, Walker MP. Human relational memory requires time and sleep. Proc Natl Acad Sci U S A. 2007;104:7723–28. doi: 10.1073/pnas.0700094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mednick SA. The associative basis of the creative process. Psychol Rev. 1962;69:220–32. doi: 10.1037/h0048850. [DOI] [PubMed] [Google Scholar]
- 14.Cartwright RD. Problem solving: waking and dreaming. J Abnorm Psychol. 1974;83:451–55. doi: 10.1037/h0036811. [DOI] [PubMed] [Google Scholar]
- 15.Mazzarello P. What dreams may come? Nature. 2000;408:523. doi: 10.1038/35046170. [DOI] [PubMed] [Google Scholar]
- 16.Hubert A. Kekulé von Stradonitz, Friedrich August. In: Halsey WD, Friedman E, editors. Collier's Encyclopedia. Vol. 14. New York, NY: Macmillan; 1985. [Google Scholar]
- 17.Babkoff H, Genser SG, Sing HC, Thorne DR, Hegge FW. The effects of progressive sleep loss on a lexical decision task: response lapses and response accuracy. Behav Res Methods Instrum Comput. 1985;17:614–22. [Google Scholar]
- 18.Babkoff H, Thorne DR, Sing HC, et al. Dynamic changes in work/rest duty cycles in a study of sleep deprivation. Behav Res Methods Instrum Comput. 1985;17:604–13. [Google Scholar]
- 19.Horne JA. Sleep loss and “divergent” thinking ability. Sleep. 1988;11:528–36. doi: 10.1093/sleep/11.6.528. [DOI] [PubMed] [Google Scholar]
- 20.Walker MP, Liston C, Hobson JA, Stickgold R. Cognitive flexibility across the sleep-wake cycle: REM-sleep enhancement of anagram problem solving. Brain Res Cogn Brain Res. 2002;14:317–24. doi: 10.1016/s0926-6410(02)00134-9. [DOI] [PubMed] [Google Scholar]
- 21.Stickgold R, Scott L, Rittenhouse C, Hobson JA. Sleep-induced changes in associative memory. J Cogn Neurosci. 1999;11:182–93. doi: 10.1162/089892999563319. [DOI] [PubMed] [Google Scholar]
- 22.Dinges DF. Are you awake? Cognitive performance and reverie during the hypnopompic state. In: Bootzin R, Kihlstrom J, Schacter D, editors. Sleep and cognition. Washington, DC: American Psychological Association; 1990. [Google Scholar]
- 23.Mednick SA. The Remote Associates Test. J Creat Behav. 1968;2:213–4. [Google Scholar]
- 24.Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC. REM, not incubation, improves creativity by priming associative networks. Proc Natl Acad Sci U S A. 2009;106:10130–4. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowden EM, Jung-Beeman M. Aha! Insight experience correlates with solution activation in the right hemisphere. Psychon Bull Rev. 2003;10:730–7. doi: 10.3758/bf03196539. [DOI] [PubMed] [Google Scholar]
- 26.Bowden EM, Jung-Beeman M, Fleck J, Kounios J. New approaches to demystifying insight. Trends Cogn Sci. 2005;9:322–8. doi: 10.1016/j.tics.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Sio UN, Monaghan P, Ormerod T. Sleep on it, but only if it is difficult: effects of sleep on problem solving. Mem Cognit. 2013;41:159–66. doi: 10.3758/s13421-012-0256-7. [DOI] [PubMed] [Google Scholar]
- 28.Landmann N, Kuhn M, Maier J, et al. REM sleep and memory reorganization: potential relevance for psychiatry and psychotherapy. Neurobiol Learn Mem. 2015;122:28–40. doi: 10.1016/j.nlm.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Landmann N, Kuhn M, Piosczyk H, Feige B, Riemann D, Nissen C. Entwicklung von 130 deutschsprachigen Compound Remote Associate (CRA)-Worträtseln zur Untersuchung kreativer Prozesse im deutschen Sprachraum. Psychologische Rundschau. 2014;65:200–11. [Google Scholar]
- 30.Kessler RC, Andrews G, Mroczek D, Ustun B, Wittchen H. The World Health Organization Composite International Diagnostic Interview short-form (CIDI-SF) Int J Methods Psychiatr Res. 1998;7:171–85. [Google Scholar]
- 31.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 32.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 33.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 34.Beck AT, Steer RA, Brown G. San Antonio, TX: Psychological Corporation; 1996. Manual for the Beck Depression Inventory-II. [Google Scholar]
- 35.Levenstein S, Prantera C, Varvo V, et al. Development of the perceived stress questionnaire: a new tool for psychosomatic research. J Psychosom Res. 1993;37:19–32. doi: 10.1016/0022-3999(93)90120-5. [DOI] [PubMed] [Google Scholar]
- 36.Lehrl S. Erlangen: Perimed-Verlagsgesellschaft; 1989. Mehrfachwahl-Wortschatz-Intelligenztest: MWT-B. (2. überarbeitete Auflage) [Google Scholar]
- 37.Bowden EM, Jung-Beeman M. Aha! Insight experience correlates with solution activation in the right hemisphere. Psychol Bull Rev. 2003;10:730–7. doi: 10.3758/bf03196539. [DOI] [PubMed] [Google Scholar]
- 38.Bowden EM, Jung-Beeman M. Methods for investigating the neural components of insight. Methods. 2007;42:87–99. doi: 10.1016/j.ymeth.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Nissen C, Kloepfer C, Feige B, et al. Sleep-related memory consolidation in primary insomnia. J Sleep Res. 2011;20:129–36. doi: 10.1111/j.1365-2869.2010.00872.x. [DOI] [PubMed] [Google Scholar]
- 40.Berry RB, Brooks R, Gamaldo CE, et al. Darien, IL: American Academy of Sleep Medicine; 2015. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, Version 2.2. www.aasmnet.org. [Google Scholar]
- 41.Feige B. Berlin, Germany: Waxmann; 1999. Oscillatory brain activity and its analysis on the basis of MEG and EEG. [Google Scholar]
- 42.Holz J, Piosczyk H, Feige B, et al. EEG Sigma and slow-wave activity during NREM sleep correlate with overnight declarative and procedural memory consolidation. J Sleep Res. 2012;21:612–9. doi: 10.1111/j.1365-2869.2012.01017.x. [DOI] [PubMed] [Google Scholar]
- 43.Feige B, Voderholzer U, Riemann D, Hohagen F, Berger M. Independent sleep EEG slow-wave and spindle band dynamics associated with 4 weeks of continuous application of short-half-life hypnotics in healthy subjects. Clin Neurophysiol. 1999;110:1965–74. doi: 10.1016/s1388-2457(99)00147-9. [DOI] [PubMed] [Google Scholar]
- 44.Fimm B, Zimmermann P. Freiburg, Germany: Psytest; 1993. Testbatterie zur Aufmerksamkeitsprüfung (TAP). Version 1.02, Handbuch Teil 1. [Google Scholar]
- 45.Tewes U, Wechsler D. Bern, Switzerland: Verlag Hans Huber; 1994. Hamburg-Wechsler-Intelligenztest für Erwachsene Revision 1991 (HAWIE-R) [Google Scholar]
- 46.R Development Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2008. R: A language and environment for statistical computing. [Google Scholar]
- 47.Jenkins JG, Dallenbach KM. Obliviscence during sleep and waking. Am J Psychol. 1924;35:605–12. [Google Scholar]
- 48.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9:534–47. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 49.Lahl O, Wispel C, Willigens B, Pietrowsky R. An ultra short episode of sleep is sufficient to promote declarative memory performance. J Sleep Res. 2008;17:3–10. doi: 10.1111/j.1365-2869.2008.00622.x. [DOI] [PubMed] [Google Scholar]
- 50.Topolinski S, Reber R. Gaining insight into the “aha” experience. Curr Dir Psychol Sci. 2010;19:402–5. [Google Scholar]
- 51.Subramaniam K, Kounios J, Parrish TB, Jung-Beeman M. A brain mechanism for facilitation of insight by positive affect. J Cogn Neurosci. 2009;21:415–32. doi: 10.1162/jocn.2009.21057. [DOI] [PubMed] [Google Scholar]
- 52.Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19:1158–66. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischer S, Born J. Anticipated reward enhances offline learning during sleep. J Exp Psychol Learn Mem Cogn. 2009;35:1586–93. doi: 10.1037/a0017256. [DOI] [PubMed] [Google Scholar]
- 54.Wilhelm I, Diekelmann S, Molzow I, Ayoub A, Molle M, Born J. Sleep selectively enhances memory expected to be of future relevance. J Neurosci. 2011;31:1563–9. doi: 10.1523/JNEUROSCI.3575-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu JC, Gillin JC, Buchsbaum MS, et al. The effect of sleep deprivation on cerebral glucose metabolic rate in normal humans assessed with positron emission tomography. Sleep. 1991;14:155–62. [PubMed] [Google Scholar]
- 56.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 57.Nilsson JP, Soderstrom M, Karlsson AU, et al. Less effective executive functioning after one night's sleep deprivation. J Sleep Res. 2005;14:1–6. doi: 10.1111/j.1365-2869.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 58.Kerkhof G, van Dongen H. Effects of sleep deprivation on cognition. Hum Sleep Cogn Basic Res. 2010;185:205. [Google Scholar]
- 59.Ritter SM, Strick M, Bos MW, van Baaren, Rick B, Dijksterhuis A. Good morning creativity: task reactivation during sleep enhances beneficial effect of sleep on creative performance. J Sleep Res. 2012;21:643–47. doi: 10.1111/j.1365-2869.2012.01006.x. [DOI] [PubMed] [Google Scholar]
- 60.Harrison Y, Horne JA. One night of sleep loss impairs innovative thinking and flexible decision making. Organ Behav Hum Decis Process. 1999;78:128–45. doi: 10.1006/obhd.1999.2827. [DOI] [PubMed] [Google Scholar]
- 61.McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–57. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 62.Power AE. Slow-wave sleep, acetylcholine, and memory consolidation. Proc Natl Acad Sci U S A. 2004;101:1795–6. doi: 10.1073/pnas.0400237101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson JR. A spreading activation theory of memory. J Verbal Learning Verbal Behav. 1983;22:261–95. [Google Scholar]