Abstract
Study Objectives:
Men with sleep disordered breathing (SDB) may be at increased stroke risk, due to nocturnal hypoxemia, sleep loss or fragmentation, or other mechanisms. We examined the association of SDB with risk of incident stroke in a large cohort of older men.
Methods:
Participants were 2,872 community-dwelling men (mean age 76 years) enrolled in the MrOS Sleep Study, which gathered data from 2003 to 2005 at six clinical sites in the Unites States. SDB predictors (obstructive apnea-hypopnea index, apnea-hypopnea index, central apnea index, and nocturnal hypoxemia) were measured using overnight polysomnography. Incident stroke over an average follow-up of 7.3 years was centrally adjudicated by physician review of medical records.
Results:
One hundred fifty-six men (5.4%) had a stroke during follow-up. After adjustment for age, clinic site, race, body mass index, and smoking status, older men with severe nocturnal hypoxemia (≥ 10% of the night with SpO2 levels below 90%) had a 1.8-fold increased risk of incident stroke compared to those without nocturnal hypoxemia (relative hazard = 1.83; 95% confidence interval 1.12–2.98; P trend = 0.02). Results were similar after further adjustment for other potential covariates and after excluding men with a history of stroke. Other indices of SDB were not associated with incident stroke.
Conclusions:
Older men with severe nocturnal hypoxemia are at significantly increased risk of incident stroke. Measures of overnight oxygen saturation may better identify older men at risk for stroke than measures of apnea frequency.
Citation:
Stone KL, Blackwell TL, Ancoli-Israel S, Barrett-Connor E, Bauer DC, Cauley JA, Ensrud KE, Hoffman AR, Mehra R, Stefanick ML, Varosy PD, Yaffe K, Redline S; Osteoporotic Fractures in Men Study Research Group. Sleep disordered breathing and risk of stroke in older community-dwelling men. SLEEP 2016;39(3):531–540.
Keywords: stroke, sleep disordered breathing, nocturnal hypoxemia
Significance.
Previous studies have reported increased risk of stroke among adults with sleep disordered breathing (SDB), but this association is not well established in the elderly. Using data from the MrOS Sleep Study, we examined associations between traditional indices of SDB and nocturnal hypoxemia with risk of incident stroke, and incident fatal stroke, among community-dwelling older men. Findings showed that nocturnal hypoxemia, but not traditional count-based indicators of SDB, predicted incident stroke and strongly predicted fatal stroke among older men. Associations were independent of traditional risk factors as well as potential mediators including sleep disturbance, atrial fibrillation, inflammation, and glucose metabolism. Future studies should explore if treatment of SDB in older men prevents risk of stroke, and unnecessary death from stroke.
INTRODUCTION
Each year, approximately 800,000 individuals in the United States will experience a stroke.1 Stroke is a leading cause of death and is a significant cause of disability and healthcare costs.2,3 Major risk factors for stroke include age, ethnicity, hypertension, high blood cholesterol levels, obesity and physical inactivity, diabetes, and smoking.4
Evidence has identified sleep disordered breathing (SDB), and in particular obstructive sleep apnea (OSA), as a risk factor for stroke. However, the majority of studies have been limited by either cross-sectional design,5 small number of stroke cases and/or lack of adequate control for potential confounding factors,6 or inclusion of a composite endpoint (e.g., stroke or death).7 However, data from the Sleep Heart Health Study (SHHS), a large population-based prospective study of middle-aged to older adults, showed a nearly 3-fold significant increase in ischemic stroke risk among men in the highest quartile of obstructive apnea-hypopnea index (OAHI; OAHI > 19) compared to those in the lowest quartile (OAHI ≤ 4).8 Similarly, in a community-based cohort of middle-aged Australian adults, moderate to severe OSA was associated with a 3.7-fold increase in risk of incident stroke over a 20-year follow-up period.9 However, these findings were based on relatively few stroke cases (31 cases among 397 people without prior history of stroke), therefore confidence intervals were wide. In addition, the stroke outcomes were based on International Classification of Diseases (ICD)-10 codes from hospital admissions and death records rather than adjudicated events. The association between SDB and risk of stroke among community dwelling older men therefore remains uncertain. Furthermore, no prior study has reported the association between OSA and risk of fatal stroke.
The mechanisms linking OSA to incident stroke are also uncertain. Potential pathways may include atrial fibrillation (AF) or other hemodynamic, neural, circadian, vascular, metabolic, inflammatory, or thrombotic mechanisms.10 In addition, sleep disturbance (e.g., reduced sleep duration or increased sleep fragmentation) also results from OSA and may contribute to metabolic abnormalities and risk for diabetes, which are risk factors for stroke.11–13
Using the Outcomes of Sleep Disorders in Men (MrOS Sleep) Study data, we examined the association between indices of SDB with incident stroke and fatal stroke in a large cohort of community-dwelling older men. We further extended results from prior studies by testing whether increased risk of stroke associated with SDB was independent of potential mediators such as sleep disturbance, AF, inflammation, or glucose metabolism.
METHODS
Participants
During the baseline examination from 2000 to 2002, 5,994 community-dwelling men 65 years or older were enrolled for the MrOS Study at 6 clinical centers in the United States: Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; the Monongahela Valley near Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California.14,15 In order to participate, men needed to be able to walk without assistance and must not have had a bilateral hip replacement.
The MrOS Sleep Study, an ancillary study of the parent MrOS Study conducted between December 2003 and March 2005, recruited 3,135 of these participants for a comprehensive sleep assessment.16 Men were screened for use of mechanical devices during sleep and were excluded if they could not OK use of these devices during a polysomnography recording. Of the 2,859 men who did not participate, 349 died before the sleep visit, 39 had already terminated the study, 324 were not asked because recruitment goals had already been met, 150 were not eligible, and 1,997 refused. Of the 3,135 men enrolled, 2,911 had in-home overnight polysomnography. Of these, 39 were missing data on incident stroke, leaving 2,872 in the analytic cohort (Figure 1).
Figure 1.
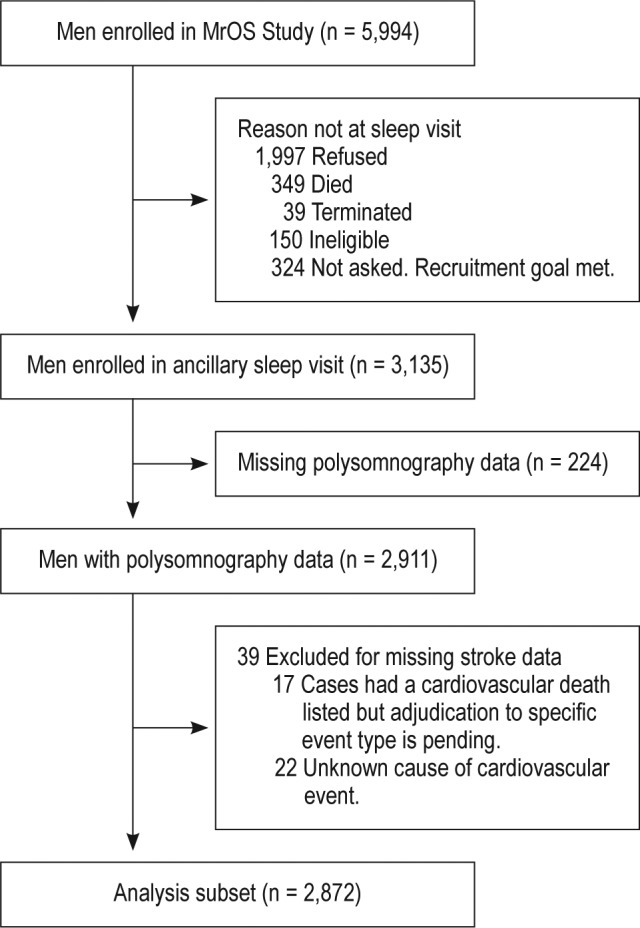
Progression of participants through the MrOS and MrOS Sleep Studies.
All men provided written informed consent, and the study was approved by the institutional review board at each site.
Polysomnography Parameters
In-home sleep studies were completed using unattended, in-home polysomnography (Safiro, Compumedics, Inc., Melbourne, Australia). The PSG recordings were to be gathered within one month of the clinic visit (mean 6.9 ± 15.8 days from visit), with 78% of recordings gathered within one week of the clinic visit. The recording montage included: C3/A2 and C4/A1 electroencephalograms, bilateral electroculograms and a bipolar submental electromyogram to determine sleep status; thoracic and abdominal respiratory inductance plethysmography to determine respiratory effort; airflow (by nasal-oral thermo-couple and nasal pressure cannula); finger pulse oximetry; lead I electrocardiogram; body position (mercury switch sensor); and bilateral tibialis leg movements (piezoelectric sensors). Staff who performed home visits were centrally trained and used standardized protocols similar to those in the SHHS.17,18 Scoring was performed by certified research polysomnologists. The polysomnography failure rate was < 4%, and > 70% of studies were rated as excellent or outstanding quality.
Parameters of SDB included measures prespecified as possible predictors of incident stroke in the aims of the study grant: the apnea-hypopnea index (AHI, number of apneas plus hypopneas/h of sleep associated with a desaturation ≥ 3%) and nocturnal hypoxemia which was expressed as the percent of time during overnight sleep in which arterial oxygen saturation (SpO2) was < 90% (% of sleep time with SpO2 < 90%). The predictors of OAHI (number of obstructive apneas plus hypopneas per hour of sleep associated with a desaturation ≥ 3%) and the central apnea index (CAI, number of central apneas at any desaturation level per hour of sleep) were also included to examine if associations were primarily driven by obstructive or central apnea. Apnea was defined as complete or near complete cessation of airflow for > 10 sec. The event was categorized as obstructive if effort persisted on thoraco-abdominal inductance channels or as central if there was no effort detected. Hypopneas were scored if clear reductions in breathing amplitude (≥ 30% below baseline breathing) occurred, and lasted > 10 sec with a drop in arterial saturation of 3% or more.19 The inter-scorer reliability for apnea and hypopnea indices was high (interclass correlation coefficient = 0.99).16,18
Sleep duration and sleep fragmentation (sleep efficiency, the percent of time scored as sleep during the sleep period) were also measured by polysomnography. Resting SpO2 level was determined just prior to sleep using the polysomnography recorder's finger pulse oximeter.
Incident Stroke Events
Participants were surveyed for potential incident cardiovascular events by postcard and/or phone contact every 4 months. Of the men expected to return the tri-annual postcards (those still alive and had not terminated the study), the response rate was over 99%. Follow-up time was 7.3 ± 1.9 years, with 2,089 (73%) alive and active at the end of follow-up. Participants reported any emergency room visits or hospital admissions for conditions or surgeries related to the heart. Conditions included chest pain, heart attack, heart failure, shortness of breath, stroke, blood clots or blockages in the arteries of the legs, or related conditions. Surgeries included angioplasty, coronary or peripheral bypass surgery, carotid surgery, or other surgical procedures. Hospital records regarding these potential events were collected. Death certificates were also examined for a primary or underlying cause of death indicating potential cardiovascular cause. Records were reviewed and events classified by a centrally trained physician adjudicator.
Two physician adjudicators are responsible for adjudication of incident events. The primary physician adjudicator reviews and classifies all events reported. The expert adjudicator independently reviews a subset of reported events and is also available to assist the physician adjudicator when classification is uncertain. The expert adjudicator independently adjudicates a random set of events each year. Any disagreements between the expert adjudicator and physician adjudicator are discussed and resolved to ensure the validity of the protocol. Overall agreement has been excellent, and no protocol adjustments have been necessary. Stroke events were defined as the rapid onset of a persistent neurologic deficit attributed to an obstruction or rupture of the arterial system which was not known to be secondary to brain trauma, tumor, infection or other cause. The deficit must have lasted more than 24 hours unless death intervened or there was a demonstrable lesion compatible with an acute stroke on computerized tomography or magnetic resonance imaging. Stroke events were further adjudicated as fatal or nonfatal.
Other Measurements
All participants completed questionnaires at the clinic visit including items about demographics, medical history, physical activity, smoking, and alcohol use. All prescription and non-prescription medications used in the preceding 30 days were entered into an electronic database. Each medication was matched to its ingredient(s) based on the Iowa Drug Information Service Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA).20 Physical activity was assessed using the Physical Activity Scale for the Elderly.21 The Ep-worth Sleepiness Scale was used to classify subjective daytime sleepiness.22,23 The Geriatric Depression Scale was used to assess depressive symptoms.24 The Modified Mini-Mental State examination was administered to assess cognitive function.25
Resting blood pressure, body weight and height were measured in clinic. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Prevalent hypertension was defined either self-report of hyper-tension, antihypertensive medications usage, or systolic or diastolic pressure ≥ 140 or 90 mm Hg. Cholesterol was measured approximately 3 years earlier using a Roche COBAS Integra 800 automated analyzer that was calibrated daily (Roche Diagnostics Cbmiorp, Indianapolis, IN). Total cholesterol (mg/ dL) was calculated as: high-density lipoprotein (mg/dL) + low-density lipoprotein (mg/dL) + 0.5*(triglycerides, mg/dL). C-reactive protein was measured using the ELISA assay kit from ALPCO (CRP sensitive ELISA), Interleukin-6 and tumor necrosis factor-α were assayed using the Human ProInflammatory I 4-Plex Ultra-Sensitive Kit by MSD (catalog #K15009C-4). Analyses of fasting glucose were performed enzymically on a YSI 2300 STAT PLUS Analyzer. Insulin concentrations were measured with a Linco Human-Insulin Specific RIA Kit.
Every 4 months, participants were asked about treatment for SDB. Twenty-four men reported using continuous positive airway pressure device (CPAP) at the sleep examination but were able to OK it during the polysomnography recording, and 290 started CPAP therapy sometime during follow-up.
Statistical Analysis
The polysomnography parameters were expressed as both continuous and categorical variables (AHI, OAHI as quartiles; CAI ≥ 5 vs. < 5; percent of sleep time with SpO2 < 90% as < 1%, 1 to < 3.5%, 3.5 to < 10%, ≥ 10%).
Characteristics of participants were compared across categories of incident stroke status and category of nocturnal hypoxemia using χ2 tests for categorical variables, t-tests or ANOVA for normally distributed continuous variables, and Wilcoxon rank sum or Kruskal-Wallis tests for continuous variables with skewed distributions. Multivariable adjusted cumulative incidence curves show the events across categories of nocturnal hypoxemia.
Cox proportional hazards regression was used to assess the association between SDB and risk of stroke, and results are presented as hazard ratios (HR) with 95% confidence intervals (CI). Models were minimally adjusted for age, clinic, race, body mass index, and smoking. Fully adjusted models were also adjusted for history of diabetes mellitus, congestive heart failure, hypertension, chronic obstructive pulmonary disease (COPD), total cholesterol, high-density lipoprotein cholesterol, and statin use. To assess whether results were explained by underlying lung impairment, models were further adjusted for resting SpO2 levels.
Similar models were performed for the outcome of incident fatal stroke.
Sensitivity analyses were performed excluding those with a self-report of a prior stroke, and truncating follow-up to the start of CPAP therapy for those 314 men who reported treatment for SDB. This analysis was performed to exclude time men were on CPAP to avoid confounding by treatment. We also tested whether associations were similar after excluding men with a history of COPD. Those men with the highest nocturnal hypoxemia (highest 5% of values for percent of sleep time with SpO2 < 90%, > 20%) were excluded in sensitivity analyses to examine if associations were primarily driven by those with extreme values.
The effects of possible mediators were explored by adding one at a time to the multivariable model. These mediators included a history of AF, sleep duration, sleep efficiency, inflammation markers (C-reactive protein, Interleukin-6, and tumor necrosis factor-alpha) and fasting glucose and insulin. The covariates hypertension and history of diabetes mellitus were selected to allow results to be comparable to other prior work examining the association of SDB and stroke.5,7–9 However, we also examined the possibility of these covariates acting as possible mediators by removing them from the multivariable models to examine if the results were similar in effect size or statistical significance.
All significance levels reported were two-sided and all analyses were conducted using SAS version 9.2 (SAS Institute Inc, Cary, NC).
RESULTS
Characteristics
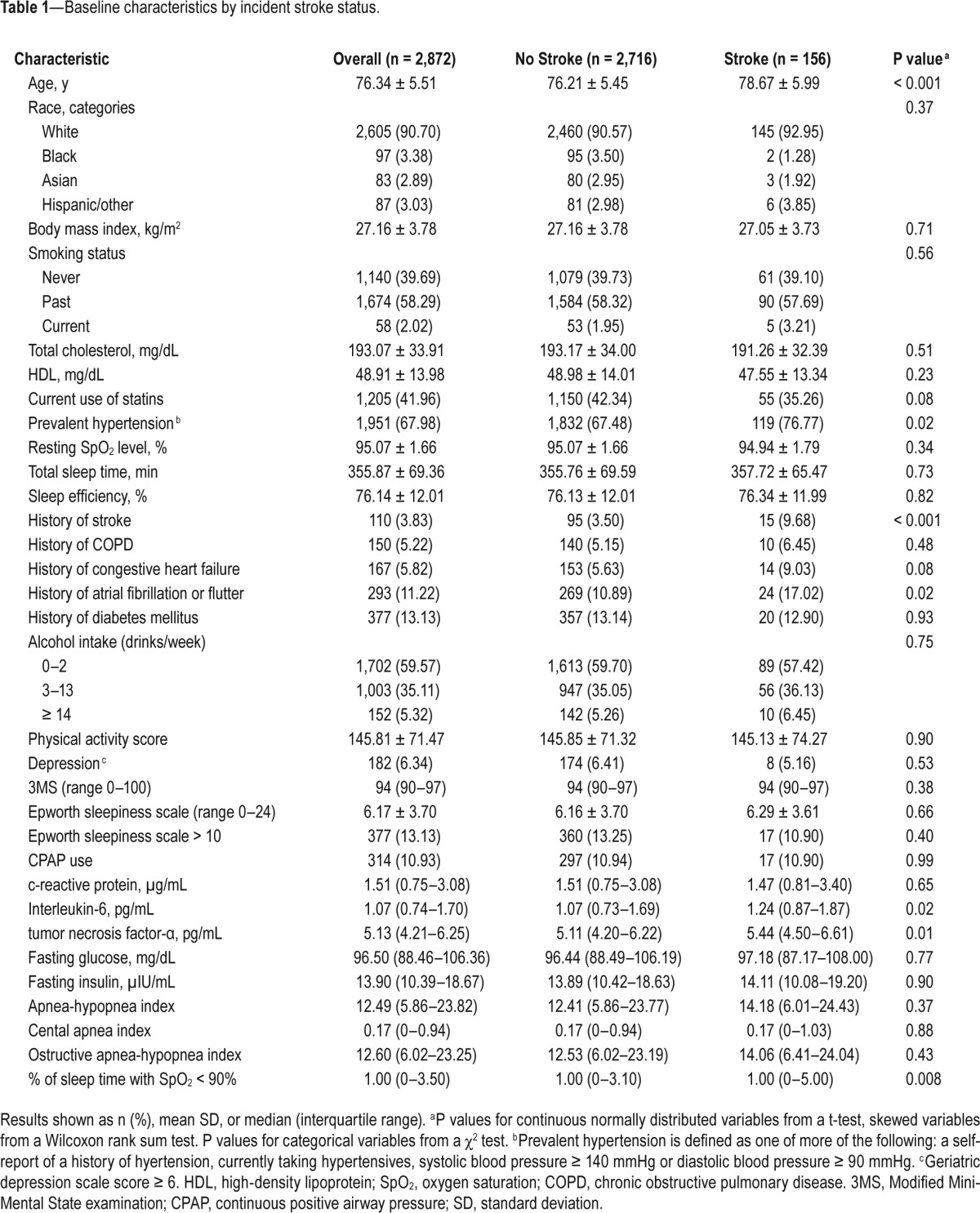
Among the 2,872 men, 156 (5.4%) had an incident stroke (including 31 fatal) during a mean of 7.3 ± 1.9 years following the sleep exam. Characteristics of participants are shown in Table 1. The mean age of the analytic cohort was 76 years, and approximately 91% were white. The median (interquartile range = IQR) AHI was 12.5 (5.9–23.8) events per hour, and median (IQR) % of sleep time with SpO2 < 90% was 1.0 (0–3.5)%. Compared to men without a stroke during follow-up, those with a stroke on average tended to be older, were more likely to have hypertension and history of AF, had higher levels of inflammation, and greater % time spent with SpO2 < 90% during sleep. In addition, men with stroke events were more likely to have reported a prevalent stroke.
Table 1.
Baseline characteristics by incident stroke status.
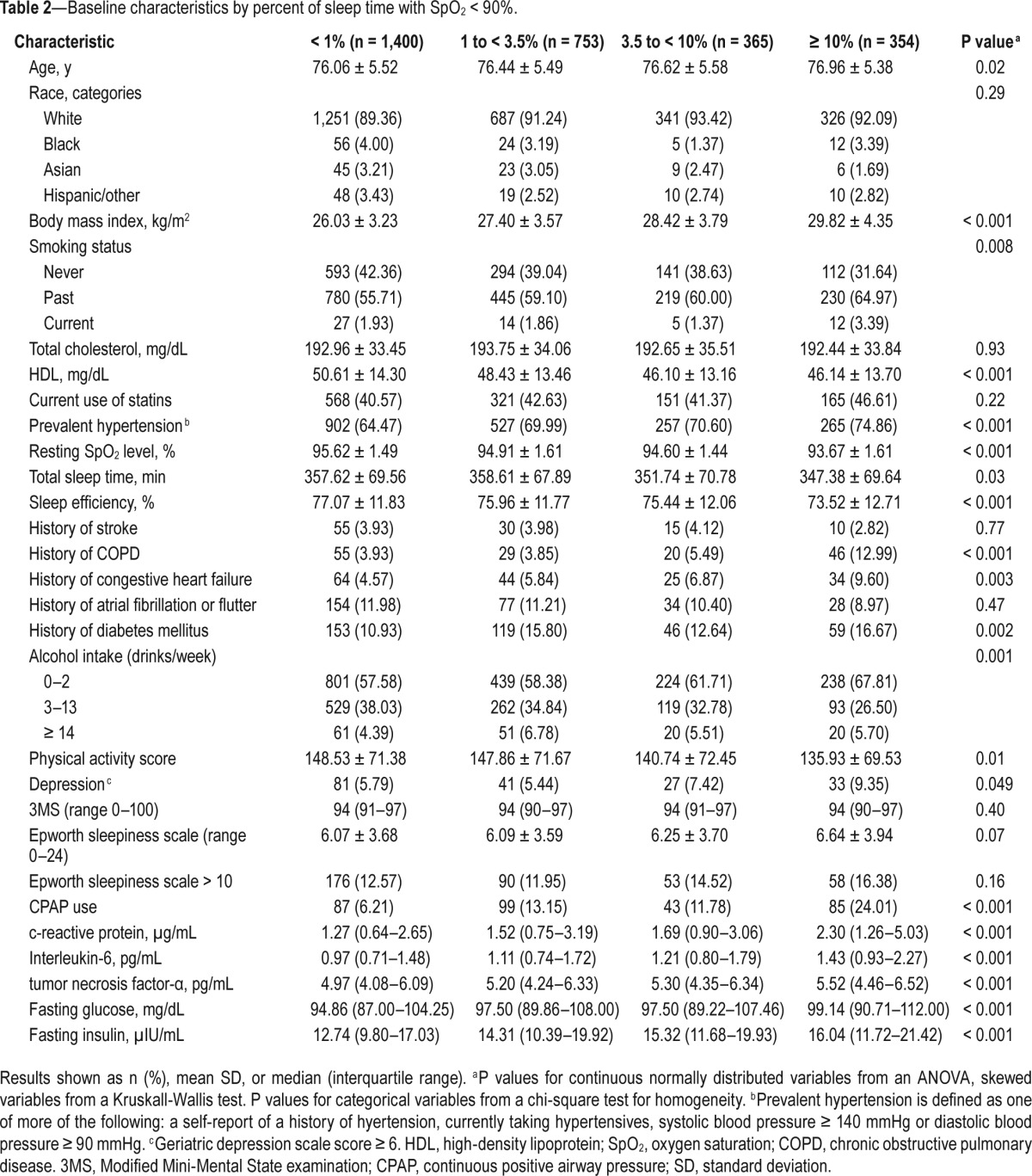
We also examined characteristics of participants across categories of % time spent with SpO2 < 90% during sleep (Table 2). Those with more severe hypoxemia were on average older and less active, had higher BMI, lower high-density lipoprotein cholesterol levels, higher rates of depression, were more likely to have smoked, and were more likely to be non-drinkers. In addition, higher hypoxemia levels were associated with a history of diabetes mellitus, hypertension, and history of COPD and congestive heart failure. Those who experienced greater hypoxemia during sleep also had shorter nocturnal sleep duration and worse sleep efficiency, and were more likely to have reported initiating use of CPAP during the follow-up period. Those with more hypoxemia also had higher levels of inflammation and greater levels of impairment in glucose metabolism.
Table 2.
Baseline characteristics by percent of sleep time with SpO2 < 90%.
SDB and Incident Stroke
In minimally adjusted models (adjusted for age, clinic site, race, BMI, and smoking status), there was a significant 1.8-fold increase in risk of stroke among those who spent ≥ 10% of sleep time with SpO2 < 90% (HR = 1.83; 95% CI 1.12–2.98) compared to those with little or no hypoxemia (< 1% of time spent with SpO2 < 90%) (Table 3). The effect size remained similar, though significance was attenuated after further adjustment for comorbidities, cholesterol levels, and statin use (HR = 1.66; 95% CI = 0.99–2.79, P = 0.055). Results were un -changed after further adjustment for resting SpO2 levels. There were no significant associations between AHI, CAI, or OAHI and risk of stroke. Figure 2A shows multivariable adjusted cumulative incidence across categories of nocturnal hypoxemia.
Table 3.
The association of sleep disordered breathing and incident stroke.
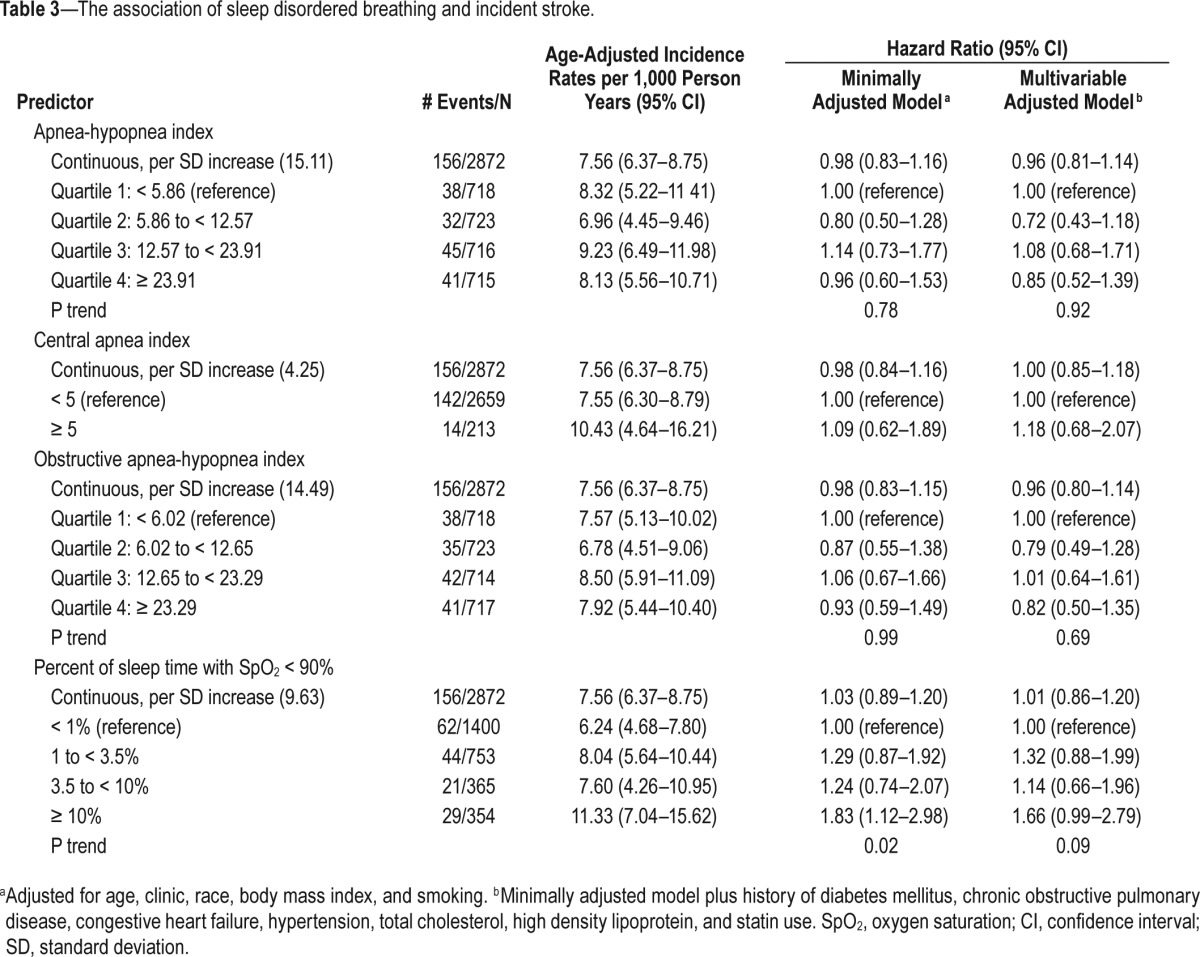
Figure 2.
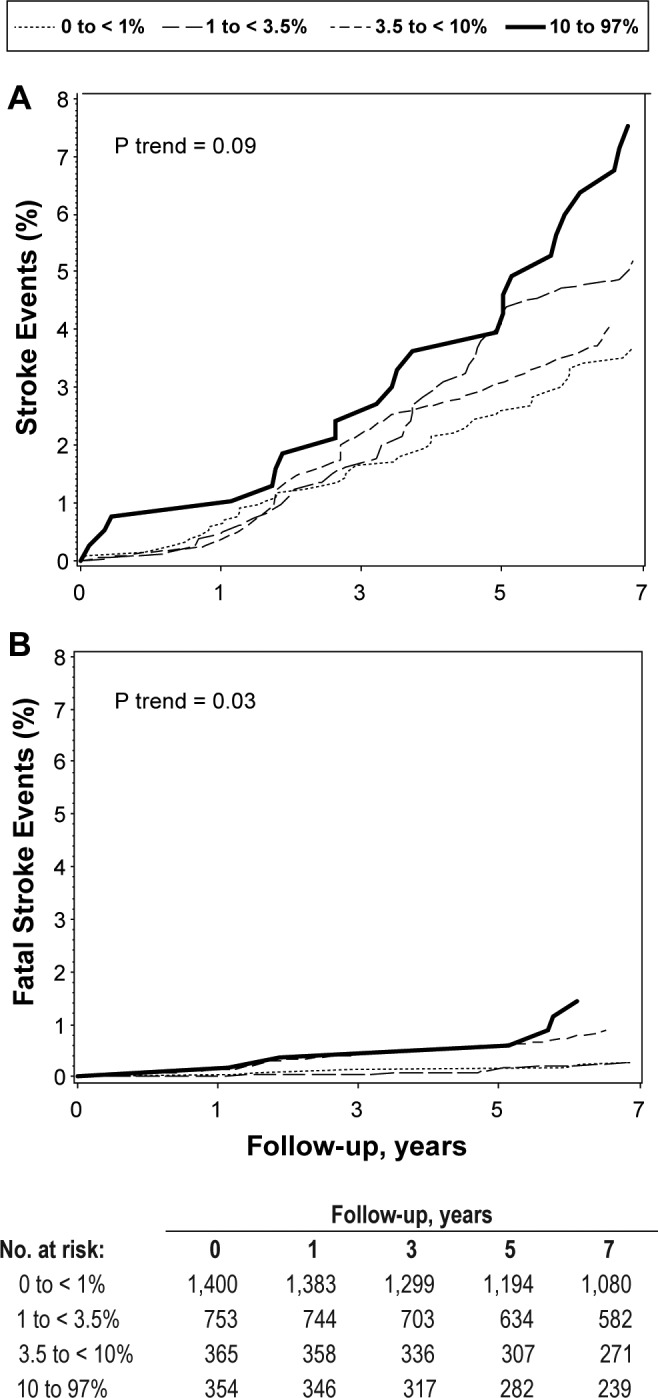
Cumulative Stroke Incidence by percent of sleep time with SpO2 < 90%, adjusted for age, race, clinic, body mass index, smoking status, history of diabetes, chronic obstructive pulmonary disease, congestive heart failure, total cholesterol, high density lipoprotein, and statin use.
SDB and Incident Fatal Stroke
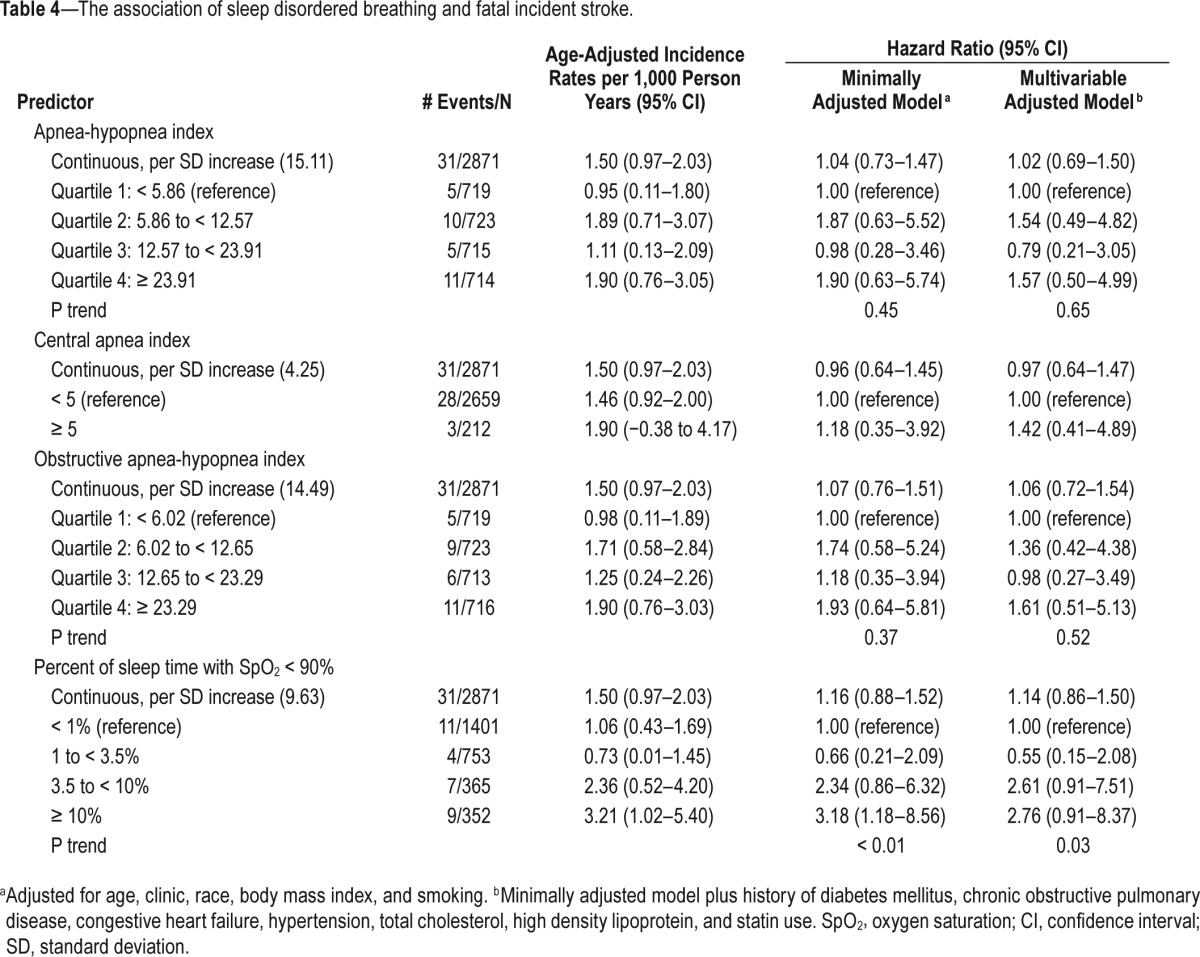
In secondary analyses, we examined the association between SDB and incident fatal stroke (Table 4). In minimally adjusted models, those who spent ≥ 10% of sleep time with SpO2 < 90% experienced a significant 3.2-fold increase in risk of fatal stroke compared to those in the lowest category of nocturnal hypoxemia (HR = 3.18; 95% CI 1.18–8.56; P-trend < 0.01). Although the effect size was attenuated somewhat in the fully adjusted model, the trend remained significant (RH = 2.76; 95% CI 0.91–8.37; P-trend = 0.03). Figure 2B shows multivariable adjusted cumulative incidence across categories of nocturnal hypoxemia. Results were similar after adjustment for resting SpO2 levels. Similar patterns of association were observed for AHI, CAI, and OAHI, but these trends were not statistically significant.
Table 4.
The association of sleep disordered breathing and fatal incident stroke.
Additional Analyses
In sensitivity analyses, we re-analyzed the fully adjusted models after truncating follow-up to the time of initiation of CPAP for those who reported beginning CPAP therapy during follow-up. In addition, we repeated analyses after excluding men who had reported a history of stroke at the sleep baseline visit. All results were similar in effect size, although significance was diminished in some cases given reduced power. After exclusion of men with nocturnal hypoxemia values in the top 5%, results were largely similar for the fatal stroke outcome, and were actually stronger and statistically signifi-cant for the any incident stroke outcome. For example, after excluding these men, those who spent ≥ 10% of sleep with SpO2 levels < 90% had a 2-fold increase in risk of all incident stroke (multivariable adjusted RH = 2.00; 95% CI 1.13–1.82, P-trend = 0.04).
We also performed further adjustment of the multivariable models for the possible mediators (history of AF, sleep duration, sleep efficiency, inflammation markers, and markers of glucose metabolism) and removed hypertension and history of diabetes mellitus from multivariable models. Results were similar. For example, after adjustment for history of AF, older men who spent ≥ 10% of sleep with SpO2 levels < 90% had a 1.8-fold increase in risk of all incident stroke (RH = 1.78; 95% CI 1.04–3.06).
DISCUSSION
We identified nocturnal hypoxemia as a robust risk factor for incident stroke among older men, conferring a 1.7 to 1.8-fold increase in risk among those with the most severe hypoxemia. Nocturnal hypoxemia was also associated with a significant 2.8 to 3.2-fold increase in risk for fatal stroke. Although there was a higher prevalence of COPD and CHF among those with more percent of sleep time with SpO2 > 90%, our findings remained significant after adjusting for resting SpO2 levels and after excluding those with self-reported COPD, suggesting that this result is not explained by underlying lung disease. Furthermore, results remained significant after excluding those with a history of stroke at the initial sleep exam. Sleep duration and sleep efficiency did not explain the increased risk of stroke associated with hypoxemia, nor did other potential mediators such as AF and markers of inflammation and glucose metabolism.
In contrast to the consistent findings observed with nocturnal hypoxemia and stroke, in our study measures of obstructive or central apnea were not significantly associated with stroke incidence. This finding differs from results reported based on the SHHS dataset.8 Among men in the SHHS, the OAHI was identified as the strongest risk factor for incident ischemic stroke, with those in the highest quartile (OAHI > 19) experiencing a nearly 3-fold significant increase in risk of ischemic stroke, whereas the association between nocturnal hypoxemia and incident stroke was no longer significant after multivariable adjustment. However, only 85 stroke cases occurred among men in SHHS, and the distribution of SDB exposures was different given the younger age of the men (mean age approximately 63 years). Our results also differ from those that observed an association of AHI and incident stroke.5–7 Of note, there are differences in study design making direct comparison difficult, including population differences, differences in the outcome, and differences in adjustment for confounders. The study by Yaggi and colleagues was comprised of patients referred to a sleep clinic for evaluation of SDB and combined stroke and death in one outcome, rather than examining incident stroke independently.7 The study by Arzt and colleagues did find and association of AHI and incident stroke after adjustment for age and sex, but the association was attenuated after further adjustment for BMI.5 This study was comprised of both men and women who were much younger in age than the current study (47 ± 8 vs. 76 ± 6, respectively).5 Munoz and colleagues observed an association in older men and women, but results were only adjusted for gender.6
Older men have a higher prevalence of OSA and also more variable levels of lung function. Our findings suggest that among older men, traditional SDB metrics that are comprised of counts may be less informative than more direct measures of nocturnal hypoxemia in predicting cerebrovascular disease risk. There are several possible explanations. Physiological stresses associated with SDB are likely mediated through surges in sympathetic activation that result from hypoxemia, arousal, or changes in intrathoracic pressure. Levels of such physiological stresses may not be consistently measured by count-based exposures that may result in variable degrees of desaturation depending on apneic duration, baseline saturation levels and other factors. Thus, a direct measure of overnight hypoxemia in an elderly population may more directly reflect the relevant physiological stresses. Nocturnal hypoxemia could also reflect general pulmonary impairment rather than SDB, however our findings persisted after accounting for self-reported COPD and resting SpO2 levels, and excluding men with COPD. We also found a high frequency of CPAP use in those with more nocturnal hypoxemia.
The importance of nocturnal hypoxemia as a marker of adverse health in older adults is also supported by prior research showing its association with cognitive impairment among older men,26 and incident mild cognitive impairment and dementia among older women.27 These prior findings are consistent with our findings of an association between hypoxemia and incident stroke because dementia shares common vascular pathology with stroke.
Ours is the first population-based study of older men to examine the association of SDB with incident fatal stroke. We report a significant approximate 3-fold increase in risk of fatal stroke among those with severe hypoxemia, suggesting that the presence of nocturnal hypoxemia may increase susceptibility to death in those experiencing an incident stroke, and that successful treatment of SDB may prevent unnecessary death from stroke in older men.
The mechanisms for the association of SDB with incident stroke remain uncertain. SDB is associated with nocturnal hypoxemia, arousals from sleep, and hemodynamic changes. In addition, activation of the sympathetic nervous system occurs, leading to increased blood pressure and heart rate. SDB is also associated with increased inflammation and metabolic abnormalities, both of which are associated with stroke risk. In our study, we found that the associations of nocturnal hypoxemia and incident stroke were not explained by inflammation, glucose metabolism, AF, or the duration or fragmentation of sleep. Future studies are needed to further investigate mechanisms linking SDB to risk of stroke.
Our study has several strengths, including the prospective design, large sample size, the older age of the cohort, and rich characterization for potential confounding factors and mediators. Nonetheless, there are some limitations. Results cannot be generalized to younger or middle-aged adults or to women. In addition, we did not sub-classify strokes as ischemic or hemorrhagic, although the vast majority of strokes (at least 85%) are ischemic.28 Exposure information on SDB and nocturnal hypoxemia was based on a single overnight assessment, which may have resulted in some misclassification. Although there is rationale to support our findings that nocturnal hypoxemia (and not AHI) may be the most relevant physiological stress among older men, these results contradict those of other studies showing the AHI strongly predicted incident stroke in somewhat younger adults. Therefore, these findings require confirmation in other cohorts of older adults. COPD was gathered by self-report and may have recall bias, although agreement of self-reported and physician diagnosed lung disease has been shown to have a high concordance in community dwelling elderly (92%).29 Finally, power was limited for analyses of fatal stroke and for subsets of older men without history of stroke and COPD.
In conclusion, nocturnal hypoxemia (independent of resting saturation levels and COPD) is associated incident stroke, and strongly predicts incident fatal stroke among older men. Results from this study, and our previous findings, suggest that hypoxemia during sleep may represent a more sensitive indicator for severity of SDB among older adults relative to the traditional count-based indices of SDB. Treatment of SDB in older men may prove effective in reducing the risk of stroke, and preventing unnecessary death from stroke.
DISCLOSURE STATEMENT
This was not an industry supported study. The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. Dr Yaffe received funding from K 24 grant AG031155. The measurement of inflammation markers was funded by the NHLBI, grant number R01 HL089467. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or the decision to submit the manuscript for publication. Dr. Stone has consulted for Merck. Dr. Ancoli-Israel is a consultant for Merck and Purdue University. Dr. Redline's institution has received grant funding from ResMed, Inc, Philips Respironics, and ResMed Foundation and equipment from them for use in NIH studies. The other authors have indicated no financial conflicts of interest. Analysis was performed at California Pacific Medical Center, Research Institute.
ABBREVIATIONS
- AF
atrial fibrillation
- AHI
apnea-hypopnea index
- BMI
body mass index
- CAI
central apnea index
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CPAP
continuous positive airway pressure device
- HR
hazard ratio
- ICD
International Classifications of Diseases
- IQR
interquartile range
- MrOS Sleep Study
the Outcomes of Sleep Disorders in Men Study
- OAHI
obstructive apnea-hypopnea index
- OSA
obstructive sleep apnea
- SpO2
arterial oxygen saturation
- SDB
sleep disordered breathing
- SHHS
Sleep Heart Health Study
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown DL, Boden-Albala B, Langa KM, et al. Projected costs of ischemic stroke in the United States. Neurology. 2006;67:1390–5. doi: 10.1212/01.wnl.0000237024.16438.20. [DOI] [PubMed] [Google Scholar]
- 3.Hoyert DL, Xu JQ. Hyattsville, MD: National Center for Health Statistics; 2011. Deaths: preliminary data for 2011. National vital statistics reports. [PubMed] [Google Scholar]
- 4.Stroke: Risk Factors. Mayo Clinic. 2013. http://www.mayoclinic.com/health/stroke/DS00150/DSECTION=risk-factors.
- 5.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munoz R, Duran-Cantolla J, Martínez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–21. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 7.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 8.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apneahypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10:355–62. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barone DA, Krieger AC. Stroke and obstructive sleep apnea: a review. Curr Atheroscler Rep. 2013;15:334. doi: 10.1007/s11883-013-0334-8. [DOI] [PubMed] [Google Scholar]
- 11.Spiegel K. Sleep loss as a risk factor for obesity and diabetes. Int J Pediatr Obes. 2008;3(Suppl 2):27–8. doi: 10.1080/17477160802404681. [DOI] [PubMed] [Google Scholar]
- 12.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 13.Tasali E, Leproult R, Spiegel K. Reduced sleep duration or quality: relationships with insulin resistance and type 2 diabetes. Prog Cardiovasc Dis. 2009;51:381–91. doi: 10.1016/j.pcad.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Mehra R, Stone KL, Blackwell T, et al. Prevalence and correlates of sleep-disordered breathing in older men: osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2007;55:1356–64. doi: 10.1111/j.1532-5415.2007.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rechtschaffen A, Kales A. Washington DC: National Institutes of Health; 1968. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. [Google Scholar]
- 18.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–67. [PubMed] [Google Scholar]
- 19.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 21.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 22.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 23.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 24.Sheikh J, Yesavage J. Geriatric Depression Scale: recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–73. [Google Scholar]
- 25.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 26.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2011;59:2217–25. doi: 10.1111/j.1532-5415.2011.03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–9. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics - 2013 update: a report from the American Heart Association. Circulation. 2012:e2–241. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kriegsman DM, Penninx BW, van Eijk JT, Boeke AJ, Deeg DJ. Self-reports and general practitioner information on the presence of chronic diseases in community dwelling elderly. A study on the accuracy of patients' self-reports and on determinants of inaccuracy. J Clin Epidemiol. 1996;49:1407–17. doi: 10.1016/s0895-4356(96)00274-0. [DOI] [PubMed] [Google Scholar]


