Abstract
Background
Triple-negative breast cancer (TNBC) is known for aggressive biologic features and poor prognosis. Epidermal growth factor receptor (EGFR) overexpression in TNBC indicates poor prognosis. However, there is no previous study of the relationship between expression of the entire human epidermal growth factor receptor (HER) family genes and patient prognosis in TNBC. Accordingly, we investigated the expression profiles of HER family genes in patients with TNBC to determine the prognostic value and clinical implications of HER family expression.
Methods
We used the nCounter expression assay (NanoString®) to measure the expression of EGFR, erb-B2 receptor tyrosine kinase 2 (ERBB2), ERBB3, ERBB4, and estrogen receptor 1 (ESR1) genes using mRNA extracted from paraffin-embedded tumor tissues from 203 patients diagnosed with TNBC. Our data were validated using a separate cohort of 84 TNBC patients.
Results
A total of 203 TNBC patients who received adjuvant chemotherapy after curative surgery from 2000 to 2004 formed the training set. The 84 TNBC patients in the validation consort were selected from breast cancer patients who received curative surgery since 2005 to 2010. Analysis of the expression profiles of the HER family genes in TNBC tissue specimens revealed that increased expression of ERBB4 was associated with poor prognosis according to survival analysis (5-year distant relapse free survival [5Y DRFS], low vs. high expression [cut-off: median]: 90.1 % vs. 80.2 %; p = 0.022). This trend was also observed in the validation set of TNBC patients (5Y DRFS, low vs. high: 69.4 % vs. 44.7 %; p = 0.053). In a multivariate Cox regression model, ERBB4 expression was identified as a indicator of long-term prognosis in patients with TNBC.
Conclusions
The expression profile of ERBB4, a member of the HER family, might serve as a prognostic marker in patients with TNBC.
Electronic supplementary material
The online version of this article (doi:10.1186/s12885-016-2195-3) contains supplementary material, which is available to authorized users.
Keywords: ERBB4, Triple negative breast cancer, nCounter expression assay
Background
Triple negative breast cancer (TNBC), defined as the absence of both hormone receptor expression and erb-B2 receptor tyrosine kinase 2 (ERBB2) overexpression, accounts for approximately 15-20 % of all breast cancers [1]. In general, TNBC is diagnosed at a higher stage and has more aggressive biologic features and worse prognosis than other subtypes [2, 3].
Overexpression of the human epidermal growth factor receptor (HER) family members, consisting of epidermal growth factor receptor (EGFR), ERBB2, ERBB3, and ERBB4, is frequently observed in many kinds of human epithelial malignancies [4]. Of the four HER family members, ERBB2 overexpression is known to induce carcinogenesis in mammalian cells [5, 6]. ERBB2 overexpression is found in 15-20 % of breast cancers and defines a unique subtype of breast cancer [7]. Indeed, ERBB2 overexpression is the therapeutic target for the monoclonal antibodies trastuzumab and pertuzumab and the tyrosine kinase inhibitor lapatinib [8–11].
In addition to the known role of ERBB2, research on other HER family genes in breast cancer is now ongoing. According to previous research, overexpression of EGFR, ERBB2, and ERBB3 is associated with poor prognosis and negatively correlated with estrogen receptor (ER) expression in breast cancer [12, 13].
In terms of ERBB4, intracellular domain 4ICD of ERBB4 promotes apoptosis of breast cancer cells and cytosolic expression of 4ICD is associated with good prognosis [14, 15]. Moreover, ERBB4 expression is significantly related to levels of phospho-AKT and ERK in TNBC as a good prognostic factor [16]. Another study reported that ERBB4 expression is positively related to ER-positive breast cancer [17–19]. Patients with breast cancer showing co-expression of ERBB4 and ER have fewer recurrences and improved survival compared to patients diagnosed with ER-positive breast cancer without ERBB4 expression [20, 21].
Some studies have found that EGFR overexpression indicates poor prognosis in TNBC [22, 23]. In preclinical studies, EGFR overexpression was detected more frequently and at higher levels in TNBC cell lines than in other subtypes and the combination of an EGFR targeting agent and cytotoxic agent inhibited cell growth more effectively than cytotoxic chemotherapy alone [24]. The results of phase I/II clinical trials of cetuximab, a monoclonal antibody targeting EGFR overexpression, demonstrated clinical benefit in TNBC with EGFR overexpression [25–27]. However, there are no clear data supporting the clinical significance of expression of the entire HER family genes in TNBC.
Accordingly, we determined how the mRNA expression levels of HER family genes affect the prognosis of patients with TNBC.
Methods
Patients
This study was a retrospective analysis of the clinical records of patients with invasive breast cancer who received adjuvant chemotherapy after curative surgery at Samsung Medical Center between 2000 and 2004. Women who were diagnosed with breast cancer at stage I to IIIC by diagnostic examination (breast magnetic resonance imaging [MRI], abdominal computed tomography [CT] scan, bone scan, and/or positron emission tomography [PET]-CT scans if indicated) were included in the training cohort. To validate our data, we retrospectively reviewed clinical records of breast cancer patients who received curative surgery at Samsung Medical Center from 2005 to 2010.
The institutional review board of Samsung Medical Center, Seoul, Korea approved our study protocol and waived the need for informed consent due to this study was conducted using archival tissues with retrospective clinical data (IRB No: 2012-08-065).
Immunohistochemistry and RNA extraction
We obtained all available hematoxylin and eosin (H&E)-stained slides of archival formalin-fixed, paraffin-embedded (FFPE) primary breast tumor tissue samples. Two independent pathologists reviewed all pathology specimens to determine tumor histologic characteristics (histological grade [28] and nuclear grade) and immunohistochemical (IHC) findings (ER and progesterone receptor [PgR] expression and ERBB2 overexpression). ER and PgR positivity were defined using Allred scores ranging from 3 to 8 based on IHC using antibodies to ER (Immunotech, Marseille, France) and PgR (Novocastra Laboratories Ltd., Newcastle upon Tyne, UK). HER2 status was evaluated using a specific antibody (Dako, Glostrop, Denmark) and/or silver in situ hybridization (SISH). Grades 0 and 1 for ERBB2, as assessed by IHC, were defined as a negative result, and grade 3 was defined as a positive result. Amplification of ERBB2 was confirmed by SISH if ERBB2 was rated as 2+ by IHC. TNBC was defined as a negative result for ER/PgR and ERBB2.
RNA was extracted from 2–4 sections of 4-μm thick FFPE sections containing more than 75 percent of tumor cells in tumor tissue using the High Pure RNA Paraffin kit (Roche Diagnostic, Mannheim, Germany). RNA yield and purity were assessed using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Rockland, DE, USA). Samples with total RNA concentration < 50 ng/μL were excluded from analysis because 200 ng of input RNA in a 5 μL volume was needed for hybridization with 20 μL of probe set master mix.
nCounter expression assay (NanoString®)
The NanoString nCounter Analysis System (NanoString Technologies, Seattle, WA, USA) was used to measure gene expression. This system measures the relative abundance of each mRNA transcript via a multiplexed hybridization assay and digital readouts of fluorescent probes [29]. We used an nCounter CodeSet (NanoString Technologies) containing biotinylated capture probes for the EGFR, ERBB2, ERBB3, ERBB4, and ESR1 genes and five housekeeping genes and reporter probes attached to color barcode tags, according to the nCounterTM code-set design. These were hybridized in solution to 200 ng of total RNA for 18 h at 65 °C according to the manufacturer’s instructions.
Hybridized samples were loaded into the nCounter Prep Station for post-hybridization processing. Hybridized samples were purified and immobilized on the deck of the Prep Station in a sample cartridge for data collection, and target mRNA was quantified in each sample using the nCounterTM Digital Analyzer. Quantified expression data were analyzed using NanoString nSolver Analysis Software.
After performing image quality control using a predefined cutoff value, we excluded outlier samples using a normalization factor based on the sum of positive control counts greater than threefold. The counts of the probes were then normalized using the geometric mean of the five housekeeping genes and log2 transformed for further analysis.
Statistical analysis
Differences in clinicopathologic characteristics were analyzed using Student’s t-test for continuous variables and Pearson chi-square test for categorical variables. Distant relapse-free survival (DRFS) was defined as the elapsed time from the date of curative surgery to the detection of distant relapse of breast cancer. DRFS was analyzed by the Kaplan-Meier (KM) method. Univariate and multivariate analyses of DRFS were performed using Cox’s proportional hazards regression tests. To evaluate relationships among the expression levels of the five genes, we used Pearson correlation analysis. Finally, receiver operating characteristic (ROC) analysis was performed to evaluate the prognostic value of the level of gene expression. ROC analysis was conducted using weighted variables that were significantly associated with prognosis in previous univariate and multivariate analysis. Weighting of variables was performed using the hazard ratio in multivariate analysis. Two-tailed p values <0.05 were considered statistically significant, and IBM SPSS Statistics 21 for Windows (IBM Corp., Armonk, NY, USA) was used to analyze all data.
Remark guidelines
In reporting our study, we have adhered to the guidelines of an important methodological paper from 2005 titled “Reporting recommendations for tumor marker prognostic studies (REMARK guidelines) [30].” To decrease any potential bias arising from a review of the medical records, we included “Patient Cohort” analysis to fulfill these criteria (Fig. 1).
Fig. 1.
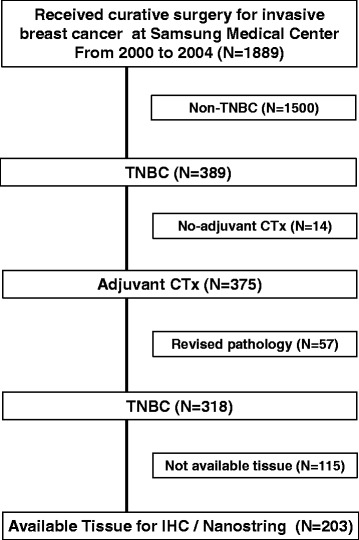
Patient cohort (N = 203)
Results
Baseline characteristics of patients
A total of 203 patients with TNBC were included in the training cohort (Fig. 1). In addition, 84 patients with TNBC were retrospectively included in a validation cohort. This validation cohort was composed of patients diagnosed with invasive breast cancer who received curative surgery from 2005 to 2010, regardless of chemotherapy status.
The baseline characteristics of patients in the training and validation cohorts are presented in Table 1. In the training cohort, the median age at diagnosis of breast cancer was 46.4 years (range, 23.5-74.1). Most patients were diagnosed with invasive ductal carcinoma (IDC) (88.7 %) and stage I-II disease (27.1 % as stage I and 62.6 % as stage II).
Table 1.
Baseline characteristics of patient cohorts
| Training N = 203 (%) | Validation N = 84 (%) | p-value | |
|---|---|---|---|
| Age (median) | 46.4 ± 10.2 | 46.1 ± 11.0 | 0.308 |
| Range | 23.5-74.1 | 22.4 – 74.0 | |
| < 40 years | 48 (23.6) | 28 (33.3) | |
| ≥ 40 years | 155 (76.4) | 56 (66.7) | |
| Histology | 0.654 | ||
| IDCa | 180 (88.7) | 76 (90.5) | |
| Others | 23 (11.3) | 8 (9.5) | |
| Stage | <0.001 | ||
| I | 55 (27.1) | 15 (17.9) | |
| IIA | 94 (46.3) | 18 (21.4) | |
| IIB | 33 (16.3) | 9 (11.9) | |
| IIIA | 13 (6.4) | 26 (31.0) | |
| IIIB | 0 (0) | 1 (1.2) | |
| IIIC | 8 (3.9) | 14 (16.7) | |
| Unknown | 0 (0) | 1 (1.2) | |
| Nuclear grade | 0.191 | ||
| 1 | 2 (1.0) | 0 (0) | |
| 2 | 47 (23.2) | 18 (21.4) | |
| 3 | 145 (71.4) | 57 (67.9) | |
| Unknown | 9 (4.4) | 9 (10.7) | |
| Histologic grade | 0.411 | ||
| 1 | 3 (1.5) | 1 (1.2) | |
| 2 | 45 (22.2) | 20 (23.8) | |
| 3 | 144 (70.9) | 54 (64.3) | |
| Unknown | 11 (5.4) | 9 (10.7) | |
| RNA expression (log2 scale, median) | |||
| EGFR | 7.0 ± 1.1 | 7.6 ± 1.2 | 0.456 |
| ERBB2 | 9.0 ± 1.0 | 9.4 ± 1.6 | 0.439 |
| ERBB3 | 7.2 ± 1.0 | 7.7 ± 1.0 | 0.503 |
| ERBB4 | 1.3 ± 1.4 | 2.3 ± 2.2 | 0.302 |
| ESR1 | 4.4 ± 0.1 | 4.5 ± 2.0 | 0.425 |
| Chemotherapy | NA | ||
| Adjuvant | 203 (100) | 60 (71.4) | |
| Neoadjuvant | 0 (0) | 24 (28.6) | |
| Unknown | 0 (0) | 0 (0) | |
| Regimen | NA | ||
| CMF1 | 86 (42.4) | 10 (11.9) | |
| FAC2 | 58 (28.6) | 16 (19.0) | |
| AC3 | 17 (8.4) | 5 (6.0) | |
| AC –T(H)4 | 41 (20.2) | 46 (54.8) | |
| Hormone | 0 (0) | 0 (0) | |
| Unknown | 1 (0.5) | 7 (8.3) | |
| Adjuvant RTx5 | 0.161 | ||
| Yes | 130 (64.0) | 61 (72.6) | |
| No | 73 (36.0) | 23 (27.4) |
aInvasive ductal carcinoma, 1Cyclophosphamide/Methotrexate/Fluorouracil, 2Fluorouracil/Adriamycin/Cyclophosphamide, 3Adriamycin/Cyclophosphamide, 4Taxane (Herceptin), 5Radiotherapy
The baseline characteristics were similar in training and validation cohorts. However, the patients in the validation cohort had a higher stage of breast cancer than those in the training cohort (p < 0.001).
Gene expression profile of HER family genes and the ESR1 gene
The expression profiles of HER family genes and the ESR1 gene are presented in Fig. 2. Even within TNBC, each tumor sample had a distinct expression profile. However, the results of the nCounter expression assay showed that the level of EGFR, ERBB2, ERBB3, ERBB4, and ESR1 expression was similar in the training and validation cohorts (Table 1).
Fig. 2.
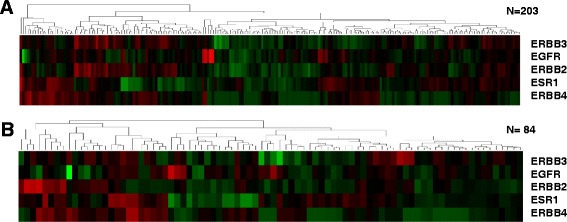
Heatmap for HER family and ESR1 gene expression. a Training set (N = 203); b Validation set (N = 84)
We also found that TNBC in the training and validation cohorts had lower expression of ERBB2, ERBB3, ERBB4, and ESR1 than non-TNBC subtypes with statistical significance (Additional file 1: Table S1 and Additional file 2: Figure S1). However, EGFR expression did not differ between TNBC and non-TNBC subtypes (p = 0.825). For further survival analysis, we set the median expression score of the five genes as the cut-off value to divide patients into low expression and high expression groups.
The association among the expression levels of the five genes was analyzed by Pearson correlation analysis. We found that ERBB2 expression in TNBC was positively correlated with ERBB3 expression (Pearson r = 0.651, p < 0.001), as well as with ERBB4 and ESR1 expression (Pearson r = 0.414, p < 0.001) (Additional file 2: Figure S2 and Additional file 1: Table S2).
Effect of ERBB4 expression on distant relapse-free survival: univariate and multivariate analysis
Univariate analysis was conducted to investigate the effect of baseline characteristics on distant relapse-free survival (Table 2). The 5-year DRFS rate in patients with stage I and IIA disease was 90.9 % and 91.5 %, respectively, in contrast to patients with stage IIB, IIIA, and IIIC disease, who had 5Y DRFS of 78.8 %, 67.7 %, and 25.0 %, respectively (p < 0.001 by KM survival analysis) (Table 2 and Fig. 3a). Of gene expressions, patients with high-ERBB4 TNBC had 5Y DRFS of 80.2 %, compared with 90.1 % for those with low ERBB4 expression (p = 0.022) (Table 2 and Fig. 3b). We also found that patients who received taxane-containing chemotherapy had poor prognosis. However, we removed the variable of chemotherapy regimen for further statistical analysis due to the chemotherapy regimen was highly related to the stage of disease (p < 0.001 by chi-square test) (Additional file 1: Table S3).
Table 2.
Impact of baseline characteristics on patient prognosis in the training cohort (N = 203)
| Training N = 203 (%) | 5-year disease relapse-free survival (%) |
p-value (Log-rank) |
|
|---|---|---|---|
| Age (median) | 46.4 ± 10.2 | ||
| Range | 23.5-74.1 | 0.633 | |
| < 40 years | 48 (23.6) | 81.2 | |
| ≥ 40 years | 155 (76.4) | 86.4 | |
| Histology | 0.507 | ||
| IDCa | 180 (88.7) | 85.5 | |
| Others | 23 (11.3) | 82.9 | |
| Stage | <0.001 | ||
| I | 55 (27.1) | 90.9 | |
| IIA | 94 (46.3) | 91.5 | |
| IIB | 33 (16.3) | 78.8 | |
| IIIA | 13 (6.4) | 67.7 | |
| IIIB | 0 (0) | ||
| IIIC | 8 (3.9) | 25.0 | |
| Unknown | 0 (0) | ||
| Nuclear grade | 0.258 | ||
| 1 | 2 (1.0) | 50.0 | |
| 2 | 47 (23.2) | 82.8 | |
| 3 | 145 (71.4) | 86.9 | |
| Unknown | 9 (4.4) | 77.8 | |
| Histologic grade | 0.704 | ||
| 1 | 3 (1.5) | 100.0 | |
| 2 | 45 (22.2) | 84.4 | |
| 3 | 144 (70.9) | 86.0 | |
| Unknown | 11 (5.4) | 72.7 | |
| EGFR (median: 7.0) | 0.084 | ||
| Low | 104 (51.2) | 90.3 | |
| High | 99 (48.8) | 79.8 | |
| ERBB2 (median: 9.0) | 0.402 | ||
| Low | 102 (50.2) | 88.2 | |
| High | 101 (49.8) | 82.1 | |
| ERBB3 (median: 7.2) | 0.106 | ||
| Low | 102 (50.2) | 90.2 | |
| High | 101 (49.8) | 80.1 | |
| ERBB4 (median: 1.3) | 0.022 | ||
| Low | 102 (50.2) | 90.1 | |
| High | 101 (49.8) | 80.2 | |
| ESR1 (median: 4.4) | 0.689 | ||
| Low | 102 (50.2) | 84.2 | |
| High | 101 (49.8) | 86.1 | |
| Adjuvant chemotherapy | 0.001 | ||
| CMF1 | 86 (42.4) | 90.7 | |
| FAC2 | 58 (28.6) | 86.0 | |
| AC3 | 17 (8.4) | 100.0 | |
| AC –T4 | 41 (20.2) | 65.9 | |
| Unknown | 1 (0.5) | 100.0 | |
| Adjuvant RTx5 | 0.093 | ||
| Yes | 130 (64.0) | 83.0 | |
| No | 73 (36.0) | 89.0 |
aInvasive ductal carcinoma, 1Cyclophosphamide /Methotrexate/Fluorouracil, 2Fluorouracil/Adriamycin/Cyclophosphamide, 3Adriamycin/Cyclophosphamide, 4Taxane, 5Radiotherapy
Fig. 3.
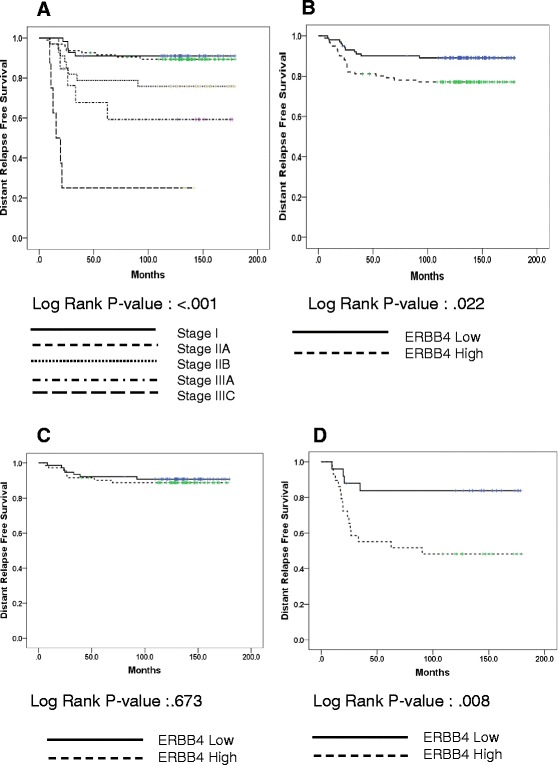
Survival analysis in the training set (N = 203). a Kaplan-Meier survival curve for stage at diagnosis. b Kaplan-Meier survival curve for level of ERBB4 expression. c Kaplan-Meier survival curve for level of ERBB4 expression in stage I/IIA (N = 149). d Kaplan-Meier survival curve for level of ERBB4 expression in stage IIB/IIIA/IIIC (N = 54)
In multivariate analysis using stage and ERBB4 gene expression, both variables remained statistically significant prognostic factors for DRFS: hazard ratio (HR) of DRFS 1.37 (95 % confidence interval [CI] 0.47-4.01 for stage IIA; HR 3.28 (95 % CI 1.07-10.04) for stage IIB; HR 4.81, (95 % CI 1.39-16.65), for stage IIIA, and HR 35.12, (95 % CI 9.62-128.27) for IIIC; HR 3.12 (95 % CI 1.42-6.87) for high expression of ERBB4 (Table 3A).
Table 3.
Effect of mRNA expression levels of ERBB4 and stage on DRFS (multivariate analysis, Cox-regression)
| Clinical variables | HR | 95% CI | p-value |
|---|---|---|---|
| (A) All stages | |||
| Stage | <0.001 | ||
| I | 1.0 | NA | |
| IIA | 1.37 | 0.47 – 4.01 | |
| IIB | 3.28 | 1.07 – 10.04 | |
| IIIA | 4.81 | 1.39 – 16.65 | |
| IIIC | 35.12 | 9.62 – 128.27 | |
| ERBB4 (median: 1.3) | 0.005 | ||
| Low | 1.0 | NA | |
| High | 3.12 | 1.42 – 6.87 | |
| (B) Early stage (N = 149) | |||
| Stage | 0.669 | ||
| I | 1.0 | NA | |
| IIA | 1.37 | 0.43 – 3.77 | |
| ERBB4 (median: 1.3) | 0.728 | ||
| Low | 1.0 | NA | |
| High | 1.21 | 0.41 – 3.55 | |
| (C) Advanced stage (N = 54) | |||
| Stage | <0.001 | ||
| IIB | 1.0 | NA | |
| IIIA | 1.12 | 0.33 – 3.83 | |
| IIIC | 35.71 | 6.73 – 189.65 | |
| ERBB4 (median: 1.3) | 0.004 | ||
| Low | 1.0 | NA | |
| High | 7.79 | 1.96 – 31.01 |
Furthermore, we analyzed the relationship between ERBB4 expression and DRFS according to stage. Because previous analysis showed that patients with stages I or IIA had similar survival rates whereas those with more advanced stage had poor prognosis, we divided patients into an early-stage group (stage I/IIA) and an advanced-stage group (stage IIB/IIIA/IIIC). In early-stage breast cancer, ERBB4 expression did not affect patient survival (5Y DRFS, low vs. high: 90.9 % vs. 90.2 %, p = 0.672) (Fig. 3c and Table 3B). However, for patients diagnosed at advanced stage, those with high ERBB4 expression had significantly poorer survival outcomes than those with low ERBB4 expression (5Y DRFS, low vs. high: 83.8 % vs. 55.2 %, p = 0.008) (Fig. 3d and Table 3C).
We performed survival analysis on the validation set. Patients in the validation set with high ERBB4-expressing breast cancer (cut-off value (log2 scale) = 1.3, the same as that used for the training set) had worse DRFS rates than those with low expression (5Y DRFS, low vs. high: 69.4 % vs. 44.7 %, p = 0.053) (Fig. 4b). We also observed this trend after dividing the patients into subgroups of early stage and advanced stage, although it lacked statistical significance (Fig. 4c and 4d). In addition, we analyzed relationship between ERBB4 expression and patients’ prognosis in the control group. In contrast of TNBC, this analysis showed that breast cancer with high ERBB4 expression had better prognosis than that with low ERBB4 expression (p = 0.003), as like as previous research presenting that ERBB4 overexpression was good prognostic indicator of ER positive and/or HER2 positive breast cancer.
Fig. 4.
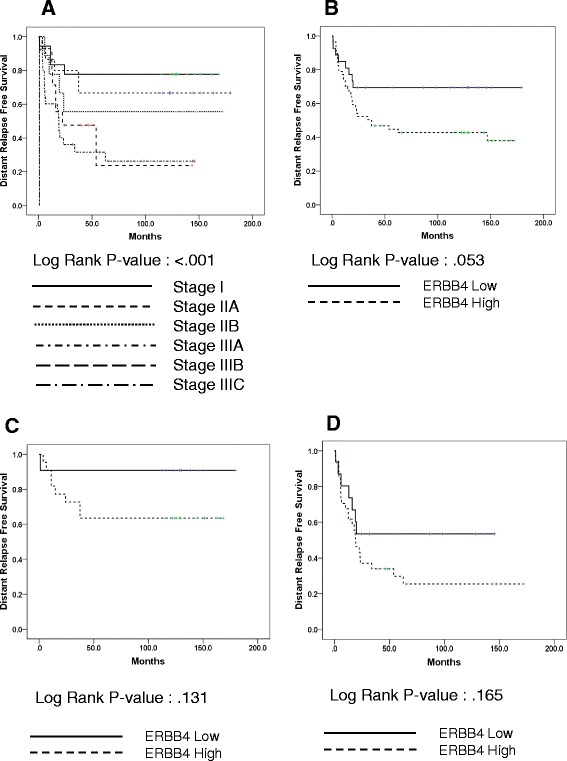
Survival analysis in the validation set (N = 84). a Kaplan-Meier survival curve for stage at diagnosis. b Kaplan-Meier survival curve for level of ERBB4 expression. c Kaplan-Meier survival curve for level of ERBB4 expression in stage I/IIA (N = 33). d Kaplan-Meier survival curve for level of ERBB4 expression in stage IIB/IIIA/IIIC (N = 51)
Effect of interaction between ERBB4 and ESR1 gene expression on distant relapse-free survival in TNBC
To elucidate the interaction between ERBB4 and ESR1 gene expression, we analyzed the impact of ERBB4 and ESR1 expression on DRFS in patients with TNBC.
We found that the group of high ERBB4 and low ESR1 expression had the worst DRFS duration whereas patients with low ERBB4 and low ESR1 expression had the longest DRFS (p = 0.002) (Fig. 5a). This trend remained but without statistical significance in the validation set (Fig. 5b) and in subgroup analyses according to early-stage and advanced-stage breast cancer (Additional file 2: Figure S3A and 3B).
Fig. 5.
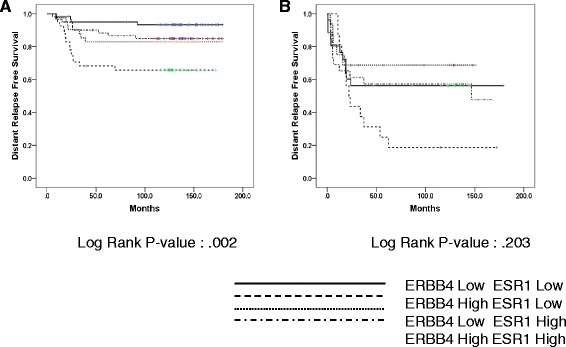
Survival analysis according to the level of ESR1 and ERBB4 expression. a Training set (N = 203). b Validation set (N = 84)
Prognostic value of the level of ERBB4 expression in TNBC
ROC analysis was performed to evaluate the prognostic value of ERBB4 expression level. According to multivariate analysis, addition of ERBB4 expression level to TNM stage enabled prediction of DRFS in both the training and the validation set (Fig. 6). The results of ROC analysis revealed that the value of ERBB4 expression strengthened the predictive efficacy of TNM stage at diagnosis in both the training and the validation set. In the training set, the AUC of the expression level of ERBB4 with TNM stage was 0.732 (p < 0.001), which is superior to the individual predictive values of ERBB4 expression and TNM stage (TNM stage: AUC 0.703, p < 0.001; ERBB4 expression: AUC 0.607, p = 0.048) (Fig. 6a). We confirmed that this trend was also evident in the validation set (TNM stage: AUC 0.677, p = 0.005; ERBB4 expression: AUC 0.611, p = 0.079; ERBB4 expression + TNM stage: AUC 0.711, p < 0.001; Fig. 6b).
Fig. 6.
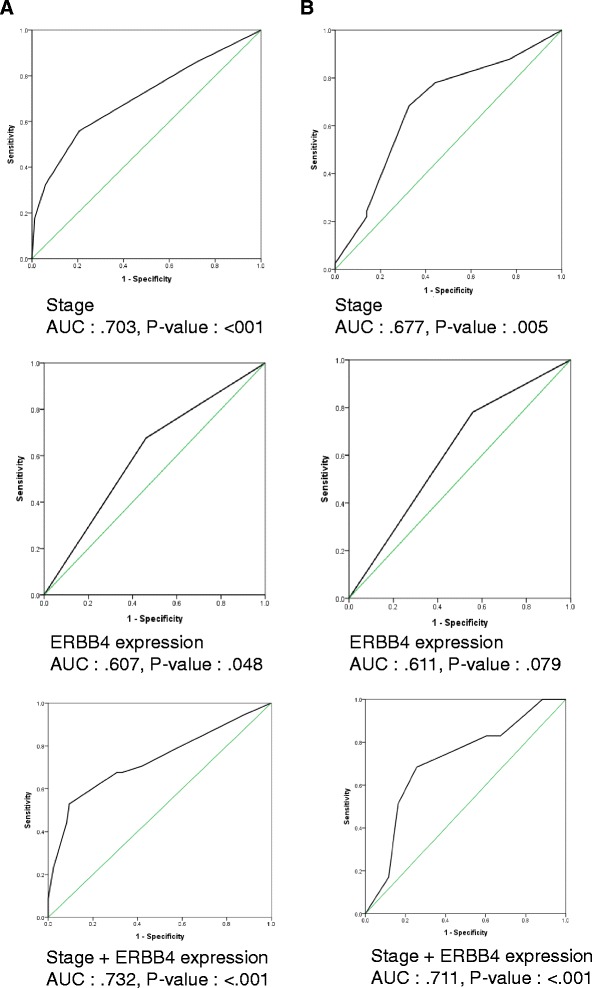
ROC analysis of predictive accuracy of stage and ERBB4 expression for DRFS. a Training set (N = 203). b Validation set (N = 84)
Discussion
In this study, we demonstrated the role of HER family genes in TNBC and we suggested that the level of ERBB4 expression had potential prognostic value in TNBC.
Many studies on HER family gene expression in breast cancer have previously been performed. Although most of these studies involved breast cancer with EGFR or ERBB2 overexpression, some researchers have conducted studies on the other HER family genes, including ERBB4. These studies revealed that ERBB4 overexpression in breast cancer is correlated with hormone receptor positivity [13, 20] and/or ERBB3 overexpression [31]. In addition, ERBB4 overexpression was reported to be associated with favorable prognosis in breast cancer patients [14], especially in cases of ER-positive [18, 31] and/or ERBB2-amplified breast cancer [20, 32]. In contrast, Bieche et al. showed that ERBB4-expression of breast cancer more than that of normal breast tissue had extremely poor prognosis compared with ERBB4-underexpressing breast cancer [17]. This study also included subgroup analysis that identified ERBB4 overexpression as a marker for poor prognosis in ER-negative breast cancer.
In our study, HER family gene expression was measured by nCounter expression assay. Because we used formalin fixed paraffin embedded (FFPE) tissues which collected 10 years ago, we needed a more sensitive technology for detection of gene expression to overcome the weakness of IHC using old FFPE samples. Moreover, in contrast to EGFR and ERBB2, immunohistochemistry of ERBB3 and ERBB4 is not well established. Accordingly, we chose to use the nCounter expression assay as the method to measure gene expression rather than IHC. Therefore, although this study was conducted on triple negative breast cancer, we were able to detect RNA expression of ERBB2 and ESR1, and found a complex expression pattern among HER family members and the ESR1 gene in TNBC. Moreover, our research showed that ERBB4 expression could serve as a potential prognostic factor when combined with pathologic stage in TNBC, and confirmed this result in the validation set. In a subgroup analysis, we found that the significance of ERBB4 expression was more prominent in advanced-stage TNBC.
Many tyrosine kinase inhibitors and monoclonal antibodies against EGFR and ERBB2 RTK have successfully been used as cancer drugs, for example cetuximab [33] and panitumumab [34] for EGFR overexpression, and afatinib [35] and dacomitinib as pan-HER inhibitors. In the clinic, trastuzumab [8], pertuzumab [10], lapatinib [11], and TDM-1[36] have been used in anti-HER2 therapy for ERBB2-overexpressing breast cancer. However, the biologic role of ERBB4 and its potential as a target for cancer drugs has not been clearly identified. Some studies have reported that ERBB4 regulated cell differentiation and cell survival via the Mek/Erk pathway [37, 38] and lapatinib inhibited the interaction of ERBB4 with EGFR and ERBB2 [39]. Therefore, lapatinib or a MEK inhibitor might be an effective therapeutic option in TNBC with high ERBB4 expression.
This study is the first study to demonstrate the impact of ERBB4 expression on patient prognosis in TNBC. Our study revealed that high expression of ERBB4 is an independent prognostic factor in TNBC. Because this study was performed in two independent groups, a training set and a validation set, the results of this study are of high validity and reliability.
Our study did not include analysis of patient overall survival. Because overall survival is influenced by patient’s characteristics like age, comorbidities as well as breast cancer characteristics, DRFS might be more reliable indicator for breast cancer-specific survival rather than overall survival.
In conclusion, our research suggests that ERBB4 expression is a valuable prognostic marker and may be useful to predict response to therapy for triple negative breast cancer. Moreover, we expect that further clinical trials on RTKs would benefit patients suffering from refractory triple negative breast cancer with high ERBB4 expression.
Conclusions
Our research suggests that ERBB4 expression is a useful prognostic marker and may be useful to predict response to therapy for triple negative breast cancer.
Acknowledgements
This research was funded by the Samsung Biomedical Research Institute (SMO 11311841).
Abbreviations
- HER
Human epidermal growth factor receptor
- TNBC
Triple negative breast cancer
- ESR1
estrogen receptor 1
- EGFR
Epidermal growth factor receptor
- ERBB2
Erb-B2 Receptor Tyrosine Kinase 2
- ERBB3
Erb-B2 Receptor Tyrosine Kinase 3
- ERBB4
Erb-B2 Receptor Tyrosine Kinase 4
- ER
Estrogen receptor
- PgR
Progesterone receptor
- FFPE
Formalin-fixed paraffin-embedded
- DRFS
Distant relapse-free survival
- KM
Kaplan-Meier
- ROC
Receiver operating characteristic
- IDC
Invasive ductal carcinoma
- HR
Hazard ratio
Additional files
Baseline characteristics of patients’ cohort. Table S2. The relationship among expression levels of ERBB family RTKs in TNBC. Table S3. elationships between stage of disease and chemotherapeutic agent. Table S4. Univariate analysis of baseline characteristics affecting ERBB4 expression (N=203). (DOCX 29 kb)
Box plots for the expression level of HER family genes and ESR1 gene. (A) Training set (N = 203). (B) Validation set (N = 84). (C) Group consisting of non-triple negative breast cancer (N = 52). Figure S2. Relationship among expression of the ERBB family genes and ESR1 gene in TNBC. Figure S3. Survival analysis according to the expression level of ESR1 and ERBB4 expression in the training set. (A) Kaplan-Meier survival curve for stage I/IIA (N = 149). (B) Kaplan-Meier survival curve for stage IIB/IIIA/IIIC (N = 54). (PPTX 266 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
J-K performed statistical analyses of genetic and clinical data and drafted and revised the manuscript. HHJ carried out sample preparation and the genetic study. J-K and HHJ participated in interpreting the genetic and clinical data. I-D carried out the tissue preparation and pathologic review. SYB, SKL, SWK, JEL, SJN participated in TNBC sample preparation and coordination to analyze clinical data. JSA, YHP, and Y-I carried out clinical data analyses and helped to draft the manuscript. YHP and Y-I conceived and designed this study. All authors read and approved the final manuscript.
Contributor Information
Ji-Yeon Kim, Email: jyeon25.kim@samsung.com.
Hae Hyun Jung, Email: haehyun.jung@sbri.co.kr.
In-Gu Do, Email: ingu.do@samsung.com.
SooYoun Bae, Email: sooyoun.bae@samsung.com.
Se Kyung Lee, Email: sekyung.lee@samsung.com.
Seok Won Kim, Email: seokwon1.kim@samsung.com.
Jeong Eon Lee, Email: jeongeon.lee@samsung.com.
Seok Jin Nam, Email: seokjin.nam@samsung.com.
Jin Seok Ahn, Email: jinseok.ahn@samsung.com.
Yeon Hee Park, Phone: 82-2-3410-1780, Email: yhparkhmo@skku.edu.
Young-Hyuck Im, Phone: 82-2-3410-3445, Email: imyh00@skku.edu.
References
- 1.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 2.Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52(1):108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 3.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 4.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5(5):341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 5.van de Vijver MJ, Peterse JL, Mooi WJ, Wisman P, Lomans J, Dalesio O, Nusse R. Neu-protein overexpression in breast cancer. Association with comedo-type ductal carcinoma in situ and limited prognostic value in stage II breast cancer. N Engl J Med. 1988;319(19):1239–1245. doi: 10.1056/NEJM198811103191902. [DOI] [PubMed] [Google Scholar]
- 6.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100(14):8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 9.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 10.Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 12.Tsutsui S, Ohno S, Murakami S, Hachitanda Y, Oda S. Prognostic value of epidermal growth factor receptor (EGFR) and its relationship to the estrogen receptor status in 1029 patients with breast cancer. Breast Cancer Res Treat. 2002;71(1):67–75. doi: 10.1023/A:1013397232011. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara S, Ibusuki M, Yamamoto S, Yamamoto Y, Iwase H. Association of ErbB1-4 expression in invasive breast cancer with clinicopathological characteristics and prognosis. Breast Cancer. 2014;21(4):472–481. doi: 10.1007/s12282-012-0415-5. [DOI] [PubMed] [Google Scholar]
- 14.Thor AD, Edgerton SM, Jones FE. Subcellular localization of the HER4 intracellular domain, 4ICD, identifies distinct prognostic outcomes for breast cancer patients. The American journal of pathology. 2009;175(5):1802–1809. doi: 10.2353/ajpath.2009.090204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naresh A, Long W, Vidal GA, Wimley WC, Marrero L, Sartor CI, Tovey S, Cooke TG, Bartlett JM, Jones FE. The ERBB4/HER4 intracellular domain 4ICD is a BH3-only protein promoting apoptosis of breast cancer cells. Cancer Res. 2006;66(12):6412–6420. doi: 10.1158/0008-5472.CAN-05-2368. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto K, Tsuda H, Koizumi F, Shimizu C, Yonemori K, Ando M, Kodaira M, Yunokawa M, Fujiwara Y, Tamura K. Activated PI3K/AKT and MAPK pathways are potential good prognostic markers in node-positive, triple-negative breast cancer. Ann Oncol. 2014;25(10):1973–1979. doi: 10.1093/annonc/mdu247. [DOI] [PubMed] [Google Scholar]
- 17.Bieche I, Onody P, Tozlu S, Driouch K, Vidaud M, Lidereau R. Prognostic value of ERBB family mRNA expression in breast carcinomas. Int J Cancer. 2003;106(5):758–765. doi: 10.1002/ijc.11273. [DOI] [PubMed] [Google Scholar]
- 18.Junttila TT, Sundvall M, Lundin M, Lundin J, Tanner M, Harkonen P, Joensuu H, Isola J, Elenius K. Cleavable ErbB4 isoform in estrogen receptor-regulated growth of breast cancer cells. Cancer Res. 2005;65(4):1384–1393. doi: 10.1158/0008-5472.CAN-04-3150. [DOI] [PubMed] [Google Scholar]
- 19.Knowlden JM, Gee JM, Seery LT, Farrow L, Gullick WJ, Ellis IO, Blamey RW, Robertson JF, Nicholson RI. c-erbB3 and c-erbB4 expression is a feature of the endocrine responsive phenotype in clinical breast cancer. Oncogene. 1998;17(15):1949–1957. doi: 10.1038/sj.onc.1202107. [DOI] [PubMed] [Google Scholar]
- 20.Barnes NL, Khavari S, Boland GP, Cramer A, Knox WF, Bundred NJ. Absence of HER4 expression predicts recurrence of ductal carcinoma in situ of the breast. Clin Cancer Res. 2005;11(6):2163–2168. doi: 10.1158/1078-0432.CCR-04-1633. [DOI] [PubMed] [Google Scholar]
- 21.Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200(3):290–297. doi: 10.1002/path.1370. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10(16):5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 23.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 24.Corkery B, Crown J, Clynes M, O'Donovan N. Epidermal growth factor receptor as a potential therapeutic target in triple-negative breast cancer. Ann Oncol. 2009;20(5):862–867. doi: 10.1093/annonc/mdn710. [DOI] [PubMed] [Google Scholar]
- 25.Baselga J, Gomez P, Greil R, Braga S, Climent MA, Wardley AM, Kaufman B, Stemmer SM, Pego A, Chan A, et al. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2013;31(20):2586–2592. doi: 10.1200/JCO.2012.46.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nechushtan H, Vainer G, Stainberg H, Salmon AY, Hamburger T, Peretz T. A phase 1/2 of a combination of cetuximab and taxane for "triple negative" breast cancer patients. Breast. 2014;23(4):435–438. doi: 10.1016/j.breast.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Tredan O, Campone M, Jassem J, Vyzula R, Coudert B, Pacilio C, Prausova J, Hardy-Bessard AC, Arance A, Mukhopadhyay P, et al. Ixabepilone alone or with cetuximab as first-line treatment for advanced/metastatic triple-negative breast cancer. Clin Breast Cancer. 2015;15(1):8–15. doi: 10.1016/j.clbc.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11(3):359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26(3):317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 30.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, Statistics Subcommittee of the NCIEWGoCD Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23(36):9067–9072. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
- 31.Sundvall M, Iljin K, Kilpinen S, Sara H, Kallioniemi OP, Elenius K. Role of ErbB4 in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13(2):259–268. doi: 10.1007/s10911-008-9079-3. [DOI] [PubMed] [Google Scholar]
- 32.Suo Z, Risberg B, Kalsson MG, Willman K, Tierens A, Skovlund E, Nesland JM. EGFR family expression in breast carcinomas. c-erbB-2 and c-erbB-4 receptors have different effects on survival. J Pathol. 2002;196(1):17–25. doi: 10.1002/path.1003. [DOI] [PubMed] [Google Scholar]
- 33.Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357(20):2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 34.Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 35.Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372(18):1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 36.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Dieras V, Guardino E, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundvall M, Veikkolainen V, Kurppa K, Salah Z, Tvorogov D, van Zoelen EJ, Aqeilan R, Elenius K. Cell death or survival promoted by alternative isoforms of ErbB4. Mol Biol Cell. 2010;21(23):4275–4286. doi: 10.1091/mbc.E10-04-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Z, Wang Y, Nayak PS, Dammann CE, Sanchez-Esteban J. Stretch-induced fetal type II cell differentiation is mediated via ErbB1-ErbB4 interactions. J Biol Chem. 2012;287(22):18091–18102. doi: 10.1074/jbc.M111.313163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu C, Tarrant MK, Choi SH, Sathyamurthy A, Bose R, Banjade S, Pal A, Bornmann WG, Lemmon MA, Cole PA, et al. Mechanism of activation and inhibition of the HER4/ErbB4 kinase. Structure. 2008;16(3):460–467. doi: 10.1016/j.str.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


