Abstract
Background
In solid state structures of organic molecules, identical sets of H-bond donor and acceptor functions can result in a range of distinct H-bond connectivity modes. Specifically, competing H-bond structures (HBSs) may differ in the quantitative proportion between one-point and multiple-point H-bond connections. For an assessment of such HBSs, the effects of their internal as well as external (packing) interactions need to be taken into consideration. The semi-classical density sums (SCDS-PIXEL) method, which enables the calculation of interaction energies for molecule–molecule pairs, was used to investigate six polymorphs of phenobarbital (Pbtl) with different quantitative proportions of one-point and two-point H-bond connections.
Results
The structures of polymorphs V and VI of Pbtl were determined from single crystal data. Two-point H-bond connections are inherently inflexible in their geometry and lie within a small PIXEL energy range (−45.7 to −49.7 kJ mol−1). One-point H-bond connections are geometrically less restricted and subsequently show large variations in their dispersion terms and total energies (−23.1 to −40.5 kJ mol−1). The comparison of sums of interaction energies in small clusters containing only the strongest intermolecular interactions showed an advantage for compact HBSs with multiple-point connections, whereas alternative HBSs based on one-point connections may enable more favourable overall packing interactions (i.e. V vs. III). Energy penalties associated with experimental intramolecular geometries relative to the global conformational energy minimum were calculated and used to correct total PIXEL energies. The estimated order of stabilities (based on PIXEL energies) is III > I > II > VI > X > V, with a difference of just 1.7 kJ mol−1 between the three most stable forms.
Conclusions
For an analysis of competing HBSs, one has to consider the contributions from internal H-bond and non-H-bond interactions, from the packing of multiple HBS instances and intramolecular energy penalties. A compact HBS based on multiple-point H-bond connections should typically lead to more packing alternatives and ultimately to a larger number of viable low-energy structures than a competing one-point HBS (i.e. dimer vs. catemer). Coulombic interaction energies associated with typical short intermolecular C–H···O contact geometries are small in comparison with dispersion effects associated with the packing complementary molecular shapes.
Graphical abstract.
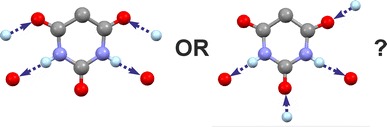
Competing H-bond motifs can differ markedly in their energy contributions
Electronic supplementary material
The online version of this article (doi:10.1186/s13065-016-0152-5) contains supplementary material, which is available to authorized users.
Background
The competition between alternative H-bonded structures (HBSs) is an important aspect of crystal polymorphism. The polymorphic forms of an organic compound may contain different HBSs which are based on the same set of (conventional [1]) H-bond donor (D-H) and acceptor (A) functions. Similarly, chemically distinct molecules with identical H-bond functions may form different HBSs, leading to the question of how molecular structure and H-bond preferences are correlated with one another.
The dimer versus catemer competition (Fig. 1) in small carboxylic acids [2, 3] is an example for two HBSs which are based on identical D-H and A sites but differ in the multiplicity of their H-bond connections (two-point vs. one-point). The stabilisation contribution from a molecule–molecule interaction involving two H-bonds exceeds that from each of two alternative one-point interactions significantly. Polymorphs differing in the multiplicity of their H-bond connections therefore also differ substantially in the relative distribution of energy contributions from individual molecule–molecule interactions, whereas the lattice energy differences for polymorph pairs of small organic molecules are typically very small [4–6] (<2 kJ mol−1 for 50 % of pairs and >7.2 kJ mol−1 for only 5 % of pairs [7]). This means that compensation effects arising from the packing of multiple HBS instances may be critical for the competition between one-point and multiple-point HBSs. In order to gain a better understanding of the nature of this competition, the molecule–molecule interactions in the corresponding crystals need to be examined in their entirety.
Fig. 1.

Competing H-bonded dimer (t-connection) and catemer (o-connection) structures composed of molecules with one H-bond donor (D-H) and one acceptor group (A)
Aside from small carboxylic acids [2, 3, 8] and aromatic urea dicarboxylic acids [9], competing one-point/multiple-point H-bond motifs occur for example in uracils [10], carbamazepine and its analogues [11–14], compound DB7 [15], aripiprazole [16–18], sulfonamides [19–21] and in barbiturates [22–24]. The 5,5-disubstituted derivatives of barbituric acid display a rigid 2,4,6-pyrimidinetrione skeleton whose two N–H and three carbonyl groups can serve as donor and acceptor sites, respectively, of N–H···O=C bonds. The rigid geometry of the 2,4,6-pyrimidinetrione fragment predetermines the geometries of intermolecular N–H···O=C bonds (Fig. 2) within the ensuing 1-, 2- or 3-periodic HBSs (chains, layers and frameworks). As a result of these restrictions, only a limited number of experimental HBSs are found in this set of barbiturates [23] (see Table 1), and these HBSs are based on different combinations of one-point and two-point N–H···O=C-bond connections (o- and t-connections).
Fig. 2.

Schematic representation according to Ref. [23] of selected N–H···O=C bonded chain and layer HBSs found in derivatives of barbituric acid
Table 1.
N–H···O=C bonded chain (C-1 to C-5), layer (L-1 to L-6) and framework (F-1, F-2) structures found in solid forms of barbituric acid and its 5-substituted derivatives
| R5 | R5′ | Common name(s) | Form | Motif | CSD refcode | References |
|---|---|---|---|---|---|---|
| Methyl | Methyl | C-1 | NUXTAC | [63] | ||
| Ethyl | Isopropyl | Ipral | I | C-1 | FUFTAC | [25] |
| Ethyl | Butyl | Soneryl, butobarbital | RT-Form | C-1 | ETBBAR | [64] |
| Ethyl | Butyl | Soneryl, butobarbital | LT-Form | C-1 | ETBBAR01 | [65] |
| Ethyl | Butyl | Soneryl, butobarbital | C-1 | ETBBAR02 | [66] | |
| Allyl | Isobutyl | Sandoptal | C-1 | FUFTIK | [25] | |
| Ethyl | Pentan-2-yl | Pentobarbital, nembutal | I | C-1 | FUFTEG01 | [48] |
| Ethyl | Pentan-2-yl | Pentobarbital, nembutal | II | C-1 | FUFTEG04 | [48] |
| Ethyl | Pentan-2-yl or phenyl | a | co-crystal | C-1 | LATMEA | [48] |
| Ethyl | n-pentyl | C-1 | ENPBAR | [67] | ||
| Ethyl | Isopentyl | Amobarbital | IIb | C-1 | AMYTAL10 | [68] |
| Ethyl | Isopentyl | Amobarbital | Ib | C-1 | AMYTAL11 | [68] |
| Ethyl | But-2-enyl | C-1 | BEBWUA | [69] | ||
| Ethyl | 3-Methylbut-2-enyl | C-1 | BECLIE | [70] | ||
| Ethyl | 1,3-Dimethylbut-1-enyl | C-1 | BEBWOU | [71] | ||
| Ethyl | 1,3-Dimethylbut-2-enyl | C-1 | JIFRIZ | [72] | ||
| Ethyl | 1,3-Dimethylbutyl | α-Methylamobarbital | C-1 | MAOBAR | [73] | |
| Ethyl | Phenyl | Phenobarbital | CH3CN solvate | C-1 | – | [35] |
| Ethyl | Phenyl | Phenobarbital | CH3NO2 solvate | C-1 | – | [35] |
| Ethyl | 1-Cyclohexen-1-yl | Phanodorm | C-1 | ETCYBA01 | [25] | |
| Ethyl | Cyclohexyl | II | C-1 | YOZJUU01 | [49] | |
| Allyl | Allyl | Dial | C-1 | DALLBA | [74] | |
| Allyl | Isopropyl | Aprobarbital | I | C-1 | AIPBAR | [75] |
| F | Phenyl | C-2 | HEKTOG | [47] | ||
| Ethyl | Ethyl | Barbital | II | C-2 | DETBAA02 | [76] |
| Ethyl | Pentan-2-yl | Pentobarbital, nembutal | III | C-2 | FUFTEG02 | [48] |
| Ethyl | Phenyl | Phenobarbital | III | C-2 | PHBARB09 | [26] |
| Ethyl | Phenyl | Phenobarbital | CH2Cl2 solvate | C-2 | – | [35] |
| Ethyl | 6-Oxocyclohexenyl | 6-Oxocyclobarbital | C-2 | OXCBAR | [77] | |
| Cl | Cl | III | C-3 | UXIYOQ02 | [78] | |
| Ethyl | 3,3-Dimethyl-n-butyl | γ-Methylamobarbital | C-3 | EMBBAR20 | [79] | |
| Ethyl | Phenyl | Phenobarbital | V | C-3 | – | This work |
| Allyl | Phenyl | Alphenal | C-3 | FUFSOP | [25] | |
| Propenyl | 1-Methylbutyl | Quinal barbitone | C-3 | TICFER | [80] | |
| H | H | Barbituric acid | I | C-4 | BARBAC01 | [46] |
| H | Ethyl | I | C-4 | ETBARB | [81] | |
| Methyl | Phenyl | Rutonal, heptobarbital | I | C-4 | MPBRBL01 | [25] |
| Methyl | Phenyl | Rutonal, heptobarbital | II | C-4 | MPBRBL | [82] |
| Ethyl | Ethyl | Barbital | I | C-4 | DETBAA01 | [76] |
| Allyl | Cyclopent-2-en-1-yl | Cyclopal | I | C-4 | FUFSUV | [25] |
| Ethyl | Butyl | Soneryl, butobarbital | C-4 + C-3 | ETBBAR03 | [83] | |
| Ethyl | Phenyl | Phenobarbital | VI | C-5 | – | This work |
| Ethyl | Ethyl | Barbital | IV | L-1 | DETBAA03 | [84] |
| Ethyl | Pentan-2-yl | Pentobarbital, nembutal | IV | L-1 | FUFTEG03 | [48] |
| Ethyl | 1-Methylbutenyl | Vinbarbital | L-1 | VINBAR | [85] | |
| Ethyl | 1-Cyclohepten-1-yl | Medomin | L-1 | CHEBAR01 | [25] | |
| H | H | Barbituric acid | II | L-2 | BARBAC02 | [46] |
| Ethyl | Phenyl | Phenobarbital | I | L-3 + C-2 | PHBARB07 | [26] |
| Ethyl | Phenyl | Phenobarbital | II | L-3 + C-2 | PHBARB08 | [26] |
| Ethyl | Cyclohexyl | I | L-4 | YOZJUU | [49] | |
| Isopropyl | 2-Bromoallyl | Noctal | II | L-4 | UXIYIK | [23] |
| Cl | Cl | I | L-5 | UXIYOQ | [23] | |
| Cl | Cl | II | L-6 | UXIYOQ01 | [23] | |
| Br | Br | I | L-6 | UXIZAD | [23] | |
| F | F | F-1 | HEKTIA | [47] | ||
| Br | Br | II | F-2 | UXIZAD01 | [23] |
A prototypical barbiturate is phenobarbital [Pbtl, 5-ethyl-5-phenyl-2,4,6(1H, 3H, 5H)-pyrimidinetrione, Scheme 1] which is a sedative and anticonvulsant agent, applied as an anaesthetic and in the treatment of epilepsy and neonatal seizures. The polymorphism of Pbtl has been studied extensively [25–27] and eleven polymorphic forms, denoted by I–XI, are known [28–31]. Forms I–VI are relatively stable at ambient conditions. Their experimental order of stability at 20 °C is I > II > III > IV > V/VI [26], and they can be produced by sublimation (I–VI) or crystallisation from solution (I–III; IV only as an intermediate [32]) or from the melt (IV–VI). Each of the modifications VII–XI can be obtained only in a melt film preparation and only in the presence of a specific second barbiturate as a structural template (“isomorphic seeding”) [25]. Crystal structure reports exist for I–III (Table 2) [26, 33, 34], several solvates [35] and a monohydrate [36] of Pbtl.
Scheme 1.
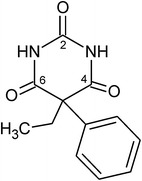
Structural formula of Pbtl
Table 2.
Descriptors for HBS types found in barbiturates: short HBS symbol [19] and number of o- and t-connections [N o, N t]
| Type | Short HBS symbol | [N o, N t] | [N o, N t]A [N o, N t]B … | Pbtl form(s) |
|---|---|---|---|---|
| C-1 | C42[0] | [0, 2] | X | |
| C-2 | C42[0] | [0, 2] | I, II, III | |
| C-3 | C44[33.42.5] | [4, 0] | V | |
| C-4 | C43[42.6] | [2, 1] | ||
| C-5 | C54.32[(53.62.7)(5)] | [2, 1] | [3, 1][1, 1] | VI |
| L-1 | L43[63-hcb] | [2, 1] | ||
| L-2 | L44[44.62-sql] | [4, 0] | ||
| L-3 | L64.22[(64.8.10)(6)] | [2, 1] | [2, 2][2, 0] | I, II |
| L-4 | L43[63-hcb] | [2, 1] | ||
| L-5 | L43[63-hcb] | [2, 1] | ||
| L-6 | L32.54.43[(10)(63.103)(63)] | [2, 1] | [1, 1][3, 1][2, 1] | |
| F-1 | F44[66-dia] | [4, 0] | ||
| F-2 | F43[103-ths] | [2, 1] |
Herein we report single crystal structure determinations for forms IV and V. A structure model for polymorph X was derived from an isostructural co-crystal. The polymorphs I–V and X contain five distinct N–H···O=C-bond motifs (or combinations of such motifs) with different quantitative proportions of o- and t-connections. Interaction energies associated with these HBSs were systematically compared using specific energy contributions of molecule–molecule interactions obtained from semi-classical density sums (SCDS-PIXEL) calculations [37–40]. An optimisation of molecular geometry was carried out and the intramolecular energy penalties of the experimental molecular geometries were determined. Using the XPac method [41], the new crystal data for V, VI and X were compared to theoretical Pbtl structures from a previous study [42].
Results
Hydrogen-bonded structures
The Cambridge Structural Database (version 5.35) [43] and recent literature contain the 53 unique crystal structures of barbituric acid and its 5-substituted derivatives listed in Table 1. These crystals have in common that each of the two N–H groups per molecule is engaged in a single intermolecular N–H···O=C interaction. The availability of three carbonyl groups per molecule enables various H-bond connectivity modes, whereas the inflexible arrangement of the D and A functionalities within the 2,4,6(1H,3H)-pyrimidinetrione unit predetermines the geometry of the resulting H-bonded structures. Altogether, 13 distinct H-bonded chain, layer or framework structures have been identified so far (Table 2), with one-dimensional structures, specifically the loop chains C-1 and C-2, dominating this set of barbiturates (Table 1). For the purpose of classification, one has to distinguish between the carbonyl group at C2 on the one hand and the two topologically equivalent carbonyl groups at C4 and C6 on the other (Fig. 2).1 The observed HBSs contain different quantitative proportions of o- and t-connections, but as each NH donor function is employed exactly once, the condition
| 1 |
applies throughout, where No and Nt is the number of o- and t-connections, respectively. Each [No, Nt] combination of [0, 2], [4, 0] and [2, 1] is permitted for uninodal nets. The structures C-5 (form VI) and L-3 (forms I and II) are both binodal, i.e. they feature two sets of topologically distinct molecules, whereas the layer L-6 [23] contains three molecule types with distinct H-bond connectivity modes. In these cases, condition (1) applies for No and Nt parameters averaged over the HBS (Table 2).
Molecules forming the loop chains C-1 and C-2 (Fig. 2) are linked by two antiparallel t-connections so that [No, Nt] = [0, 2]. The underlying topology of each of C-1 and C-2 is that of a simple chain. In an alternative graph-set description according to Etter [44, 45], their “loops” represent rings. The C-1 type (form X) contains two topologically distinct rings in which either two O2 or two O4/6 sites are employed, whereas in a C-2 chain (forms I, II and III) only O4/6 acceptor sites are employed, and all its rings are topologically equivalent.
The molecules in a C-3 tape (form V) possess four o-connections so that [No, Nt] = [4, 0] (Fig. 2). Via C4/6 carbonyl groups, they form two parallel N–H···O=C bonded strands which are offset against one another by one half of a period along the translation vector. N–H···O=C bonding between the strands via C2 carbonyl groups results in fused rings. Four o-connections per molecule are also present in the layer structure L-2 [46] which has the topology of the (4,4) net and in the dia framework F-1 [47].
In an L-3 layer (forms I and II), molecules of type A are linked into C-2 chains and B-type molecules serve as N–H···O=C bonded bridges between these chains (Fig. 2). In molecule A, the H-bond acceptor functions of the carbonyl groups at C4 and C6 are each employed twice, whereas none of the carbonyl groups of molecule B is involved in hydrogen bonding. Each molecule A forms two t-connections to A molecules and o-connections to two B molecules. There are no H-bonds between B molecules. The [No, Nt] parameters for molecules A and B are [2, 2] and [2, 0], respectively, and the overall [No, Nt] parameter combination for the L-3 layer is [2, 1].
The binodal tape C-5 (Fig. 2) is a novel structure found exclusively in the Pbtl polymorph VI. Molecules of type A are linked, by o-connections via C4 carbonyl groups, into two parallel strands. Additionally, the C4 and C2 carbonyl groups of molecules A and B, respectively, are employed in an asymmetrical and antiparallel t-connection. Molecule A forms also an o-connection to a second B molecule via its C2 carbonyl group. There are no H-bonds between B molecules, which serve as H-bridges between two strands. The molecule types A and B have the parameters [No, Nt]A = [3, 1] and [No, Nt]B = [1, 1] and the overall [No, Nt] combination for the C-5 tape is [2, 1]. Five uninodal HBSs with [No, Nt] = [2, 1] are known, namely the C-4 ladder, three distinct layer structures (L-1, L-4, L-5), each having the topology of the (6,3) net, and the ths framework F-2 [23]. The connectivity and topology characteristics of the barbiturate HBSs are listed in Table 2 and an illustration of the variations in No and Nt is given in Fig. 3.
Fig. 3.

The parameters [N o, N t] for the HBS types formed by barbiturates and for two combinations of HBS types (L-3 + C-2 and C-3 + C-4). Roman numerals indicate the relevant data points for Pbtl polymorphs
SCDS-PIXEL calculations
Total PIXEL energies of individual molecule–molecule interactions (ET) can be divided into contributions from Coulombic (EC), polarisation (EP), dispersion (ED) and repulsion (ER) terms. The polarisation energy is not pairwise additive (many-body effect) so that the total PIXEL energy for the crystal, ET,Cry, differs slightly from the sum of all individual PIXEL interaction energies ET,Σ. For the Pbtl polymorphs, this difference is 2–3 kJ mol−1 (<2.5 % of ET,Cry; see Table 3).
Table 3.
Crystal data and PIXEL energies of polymorphs of Pbtl
| Form | I | II | III | V a | VI | X b |
|---|---|---|---|---|---|---|
| References | [26] | [26] | [26] | This work | This work | [25, 48] |
| CCDC refcode | PHBARB07 | PHBARB08 | PHBARB09 | – | – | LATMEA |
| Space group | P21/n | P | P21/c | P21/n | P21/n | C2/c |
| Z′ | 3 | 3 | 1 | 2 | 2 | 1 |
| a (Å) | 10.70 | 10.74 | 9.55 | 12.76 | 14.67 | 12.67 |
| b (Å) | 47.26 | 23.40 | 11.85 | 6.76 | 6.90 | 20.69 |
| c (Å) | 6.80 | 6.72 | 10.81 | 26.85 | 23.03 | 10.25 |
| α (°) | 90 | 91.0 | 90 | 90 | 90 | 90 |
| β (°) | 94.2 | 94.5 | 111.6 | 98.8 | 94.1 | 118.5 |
| γ (°) | 90 | 88.4 | 90 | 90 | 90 | 90 |
| T exp (K) | 298 | 173 | 298 | 173 | 173 | 173 |
| D (g cm−3) | 1.349 | 1.376 | 1.357 | 1.348 | 1.327 | d |
| HBS | C-2 + L-3 | C-2 + L-3 | C-2 | C-3 | C-5 | C-1 |
| [N o, N t] | [4/3, 4/3] | [4/3, 4/3] | [0, 2] | [4, 0] | [2, 1] | [0, 2] |
| m.p. (°C) [26] | 176 | 174 | 168 | 160 | 156 | 126 |
| E T,Cry/ΔE intra (kJ mol−1) | c/7.3 | c/7.5 | −118.3/3.9 | −122.4/13.1 | −114.9/3.7 | −118.3/8.0 |
| E T,Σ (kJ mol−1) | −123.3 | −122.4 | −120.5 | −124.1 | −117.9 | −121.1 |
| E T,Σ(A)/ΔE intra (kJ mol−1) | −143.1/8.9 | −141.4/8.7 | – | −120.9/8.5 | −128.3/0.3 | – |
| E T,Σ(B)/ΔE intra (kJ mol−1) | −103.8/6.9 | −104.0/8.2 | – | −127.4/17.6 | −107.5/7.1 | – |
| E T,Σ(C)/ΔE intra (kJ mol−1) | −122.9/6.0 | −121.9/5.5 | – | – | – | – |
| Density order | 3rd | 1st | 2nd | 4th | 5th | d |
| Stability order (RT) [26] | 1st | 2nd | 3rd | 4/5th | 4/5th | e |
| Stability order (calc.)f | 2nd | 3rd | 1st | 6th | 4th | 5th |
aThe matrix () transforms the room temperature data reported by Williams [36] (a = 12.66, b = 6.75, c = 27.69 Å; β = 106.9°; P21/c) into a unit cell (a′ = 12.66, b′ = 6.75, c′ = 26.89 Å; β’ = 99.9°; P21/n) which matches our data
bThe structure model for form X (Additional file 1: Section 8) was derived from the isostructural co-crystal of Pbtl with pentobarbital (the quoted CCDC refcode, unit cell data and T exp all refer to the co-crystal)
c E T,Cry not determined because of Z′ > 2
dNot applicable
eExists only in a melt-film preparation and in the presence of a structurally analogous second barbiturate
fBased on the results of SCDS-PIXEL calculations, corrected for ΔE intra
Various aspects of the PIXEL calculation for each polymorph will be visualised in a special kind of diagram whose data points represent molecule–molecule interactions energies accounting for at least 95 % of ET,Cry, with internal HBS interactions separated from contacts between different instances of the HBS (labelled @1, @2,…). Moreover, sums of PIXEL energies will be compared in order to assess relative contributions from certain groups of interactions. The molecule–molecule interactions in each crystal structure will be ranked in descending order of their stability contributions (#1, #2, #3…), with symmetry equivalence indicated by a prime (e.g. #1/1′).
Polymorphs containing exclusively or predominantly t-connections, i.e. X (C-1), III (C-2), I and II (C-2 + L-3), will be discussed first, followed by forms V (C-3) and VI (C-5). PIXEL energies do not account for differences in molecular conformation, and this topic will be discussed in a separate section. Detailed results of SCDS-PIXEL calculations are given in Additional file 1: Fig. S7 and Tables S1–S12.
HBS type C-1: polymorph X
The structure of polymorph X has not been determined from single crystal data. Melt film experiments [25] indicated it to be isostructural with the co-crystal of Pbtl with 5-ethyl-5-(pentan-2-yl)barbituric acid (pentobarbital). The asymmetric unit of this co-crystal (space group C2/c) consists of a single barbiturate molecule whose R5′ substituent is disordered between the pentan-2-yl and phenyl groups of the two chemical components [48]. An approximate structure model for polymorph X was derived by removing the pentan-2-yl disorder fragment from the co-crystal structure (Additional file 1: Section 8).
The C-1 structure (Fig. 2) is defined by two independent t-connections with very similar interaction energies (#1: −47.5 kJ mol−1; A: O4) and (#2: −47.2 kJ mol−1; A: O2), with a crystallographic two-fold axis passing through the centre of the respective ring. As expected, these interactions are dominated by the EC term and the C-1 tape contains no significant non-H-bonded interactions (Fig. 4a).
Fig. 4.

Results of SCDS-PIXEL calculations for polymorph X. a Interaction energies, represented by balls, are separated into internal C-1 interactions (blue) and chain–chain contacts (highlighted @1, red; @2, orange; @3, green). The horizontal bars indicate cumulative PIXEL energies (summation from left to right) relative to E T,Cry (scale on the right-hand side). b The eight most important pairwise interactions involving a central molecule (orange). The mean plane of the pyrimidine ring of the central molecule is drawn, H atoms are omitted for clarity and H-bonds are indicated by blue lines
Each Pbtl molecule interacts with eight other molecules belonging to four different C-1 chains, i.e. @1 (#3, #4, #9), @2 (#6/6′, #8), @3 (#5) and @4 (#9). Each of the eight interactions (PIXEL energies −19.7 to −12.1 kJ mol−1) is dominated by the ED term (Additional file 1: Table S12). The chain–chain contact @1 involves the mutual interdigitation of phenyl groups (#3, #4) and contact @2 the interdigitation of ethyl groups (#6/6′) (Figs. 4b, 5). Internal C-1 interactions contribute 39 % to the ET,Cry value of −121.1 kJ mol−1, whilst @1 and @2 account for 21 and 18 %, respectively, of ET,Cry. A number of 2D and 3D packing relationships between barbiturates are based on the packing motif of the centrosymmetric chain pair @2 [25, 49].
Fig. 5.

Packing diagram of polymorph X, showing interactions of a selected Pbtl molecule (drawn in ball-and-sticks-style) within the same C-1 chain (blue) and with molecules belonging to three neighbouring chains (@1–@3; see Fig. 4). Together, hydrogen bonding and the …@1 @2 @1 @2… stacking of chain pairs account for 78 % of E T,Cry
Each of the molecule–molecule interactions #3, #5 and #8 involves a pair of symmetry-related C–H···O contacts (H···O = 2.51–2.68 Å and CHO = 140°–170° and a significant EC contribution (−9.1 to −9.8 kJ mol−1), which is however still considerably lower than the respective ED contribution (−15.1 to −21.4 kJ mol−1). These C–H···O contacts are formed between the phenyl group (#3) or the CH2 group (#5) and the C4/6 carbonyl group not involved in classical H-bonds or between the methyl and the C2 carbonyl group (#8; for details, see Additional file 1: Table S12).
HBS type C-2: polymorph III
The structure of III (space group P21/c) contains one independent molecule. Its C-2 chain (Fig. 2) possesses 21 symmetry. The interaction energy of its t-connections (#1/1′) of −45.4 kJ mol−1 is similar to the corresponding values in X. The energies of the next four strongest interactions (#3, #4, #5/5′) lie between −22.1 and −19.7 kJ mol−1 and each of them is dominated by the ED term (Additional file 1: Table S7). They result mainly from the pairwise antiparallel alignment of ethyl-C5-phenyl fragments in the case of #3 and from the pairwise stacking of ethyl groups with phenyl groups in the case of #5/5′. The relatively large EC term (−13.2 kJ mol−1) for interaction #4 coincides with the presence of two symmetry-related (phenyl)C–H···O=C contacts (H···O = 2.53 Å, CHO = 139°) involving the C2 carbonyl group, which is not engaged in classical hydrogen bonding. However, the stabilisation contribution from ED (−17.3 kJ mol−1) is still higher than EC for interaction #4. A similar (phenyl)C–H···O=C contact geometry (H···O 2.61 Å, CHO = 151°), also involving the C2 carbonyl group, is associated with interaction #10/10′, but here the EC contribution is just −5.5 kJ mol−1.
The two internal C-2 interactions account for approximately 38 % of ET,Cry of −118.3 kJ mol−1, and the interactions with molecules belonging to four neighbouring chains @1 (2 pairwise interactions), @2 (2), @3 (2) and @4 (3) account for 17, 13, 12 and 11 %, respectively, of ET,Cry (Figs. 6, 7). This situation differs somewhat from the packing of C-1 chains in X which is dominated by just two chain–chain interactions (@1, @2) which contribute 40 % of ET,Cry.
Fig. 6.

Results of SCDS-PIXEL calculations for polymorph III. a Interaction energies, represented by balls, are separated into internal C-2 interactions (blue) and chain–chain interactions (highlighted @1, red; @2, orange; @3, green). The horizontal bars indicate cumulative PIXEL energies (summation from left to right) relative to the E T,Cry (scale on the right-hand side). b The six most important pairwise interactions involving a central molecule (orange). The mean plane of the pyrimidine ring of the central molecule is drawn, H atoms are omitted for clarity and H-bonds are indicated by blue lines
Fig. 7.

Packing diagram of polymorph III, showing interactions of a selected Pbtl molecule (drawn in ball-and-sticks-style) within the same C-2 chain (blue) and with molecules belonging to four neighbouring chains (@1–@4; see Fig. 6). Together, these interactions account for 91 % of E T,Cry
HBS types L-3 + C-2: polymorph I
The crystal structure of form I (space group P21/c) contains three independent molecules, labelled A–C. A and B molecules are linked into an L-3 layer (Fig. 2). This layer consists of C-2 chains, formed exclusively by A molecules, and bridging B molecules. The L-3 structures lie parallel to (010) and alternate with stacks of C-2 chains composed of C molecules (Additional file 1: Fig. S4). The two distinct C-2 chains formed by A and C molecules differ in that the former (as part of a L-3 layer) possess glide symmetry, whereas the latter contain inversion centres (Additional file 1: Fig. S5).
The energy associated with the centrosymmetric t-interaction between A molecules is −49.2 kJ mol−1 (#2/2′) and energies of −40.5 and −34.0 kJ mol−1 (5/5′ and 7/7′) are calculated for the o-interactions between A and B molecules (Fig. 8). Within an L-3 layer, the strongest non-H-bonded AA interactions of −17.2 kJ mol−1 (#10/10′), between neighbouring C-2 subunits (related by a [001] translation), and the strongest BB interactions of −15.5 kJ mol−1 (#14/14′) each involve relatively large ED contributions. There are another eight intra-L-3 contacts with energies between −11.1 and −8.4 kJ mol−1. The energies for the t-connections of the C-2 chain of molecule C, −49.7 and −48.1 kJ mol−1, are very similar to the corresponding values for the C-2 chains formed by A molecules and in polymorph III.
Fig. 8.

Results of SCDS-PIXEL calculations for polymorph I. a Interaction energies, represented by balls, are separated into internal L-3 (blue) interactions, internal C-2 (red) interactions, interactions between a L-3 layer and a stack of C-2 chains (@1, orange) and interactions between neighbouring C-2 (@2, green; @3, beige). The horizontal bars indicate cumulative PIXEL energies (summation from left to right) relative to the E T,Cry (scale on the right-hand side). b–d A central molecule A, B or C (coloured orange) and neighbouring molecules involved in six (b, c) or seven (d) pairwise interactions (see Additional file 1: Tables S1–S3). The mean plane of the pyrimidine ring of the central molecule is drawn, H atoms are omitted for clarity and H-bonds are indicated by blue lines
Internal H-bond and non-H-bond interactions of the L-3 layer account for 54 % and internal C-2 chain interactions of C molecules account for 13 % of ET,Σ. Contacts between L-3 layers (molecules A + B) and C-2 stacks (molecule C) contribute 19 % to ET,Σ (@1), and the contacts @2 and @3 between neighbouring C-2 chains contribute 5 and 4 %, respectively (Figs. 8, 9). Due to their fundamentally different environments and different involvement in N–H···O=C bonds, the three independent molecules also differ substantially in their PIXEL energy sums: 143.1 kJ mol−1 (A), −103.8 kJ mol−1 (B) and −122.9 kJ mol−1 (C).
Fig. 9.

Packing diagram of polymorph I. One selected molecule of each type of A, B and C is drawn in ball-and-sticks-style. Together the internal L-3 (blue) and C-3 (orange) interactions account for 67 % of E T,Σ. Interactions between L-3 and C-3 chains (@1) account for 19 % and interactions between neighbouring C-3 chains (@2, @3) for 9 % of E T,Σ
HBS types L-3 + C-2: polymorph II
Polymorph II (space group P) is a Z′ = 3 structure whose molecules A and B are linked into an L-3 layer, whilst C-type molecules form a C-2 chain, and it exhibits a very close 2D packing similarity with polymorph I [26]. In fact, the only fundamental difference between these two modifications is the symmetry of the C-2 chain formed by the respective A-type molecules (I: glide symmetry, II: inversion; see Additional file 1: Fig. S4).
The comparison of interaction energy diagrams (Additional file 1: Fig. S7; see also Tables S1–S6) shows that this packing similarity results in a striking similarity of corresponding pairwise interaction energies. Therefore, the general assessment of relative energy contributions attributable to L-3 and C-2 units and to their packing in polymorph I (previous section) is also valid for polymorph II.
HBS type C-3: polymorph V
Williams [36] reported space group and unit cell data for polymorph V which indicated a crystal structure with two independent molecules, and these data are consistent, after unit cell transformation, with those of the full crystal structure analysis carried out by us (see footnote a of Table 3). Form V has the space group symmetry P21/c and contains two independent molecules, labelled A and B. It contains N–H···O=C bonded C-3 tapes (Fig. 10) which are arranged parallel to [010].
Fig. 10.
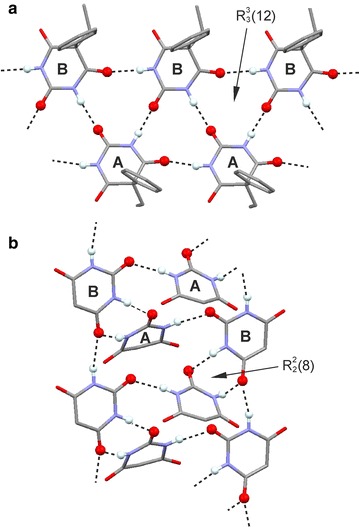
N–H···O=C bonded tapes C-3 in polymorph V (a) and C-5 in polymorph VI (b). Ethyl and phenyl groups are omitted for clarity. Hydrogen bonds are drawn as dashed lines; O and H atoms engaged in H-bond interactions are drawn as balls
Each molecule forms o-connections to four neighbouring molecules. A and B molecules are linked into separate H-bonded strands with translation symmetry, which are offset against one another by one half of a translation period. The linkage between the two parallel strands via N–H···O=C bonds results in fused rings. Although A and B molecules are crystallographically distinct, they are topologically equivalent in the context of the (uninodal) C-3 structure.
Interaction energies of −32.9 kJ mol−1 were obtained both for the o-interactions between A-type molecules (#1/1′) and the analogous interactions between B-molecules (#2/2′). Considerably lower stabilisation effects of −23.8 and −23.2 kJ mol−1 result from the o-interactions (#5/5′ and #10/10′) between A and B strands, which is the result of higher (by 9.9–6.4 kJ mol−1) dispersion terms. Two H-bonded molecules belonging to different strands have fewer van der Waals interactions with one another than two H-bonded molecules within the same strand (Fig. 11b, c). Moreover, the PIXEL energies of the o-connections #5/5′ and #10/10′ are very similar to those of seven non-H-bond interactions (#7, #8/8′, #12/12′, #14/14′; −23.5 to −20.9 kJ mol−1). Each of the latter involves extensive van der Waals contacts (ED = −21.9 to −30.7 kJ mol−1) which compensate for the lower EC contribution in the absence of any N–H···O=C bonding (Additional file 1: Tables S8 and S9). The interactions #12/12′ contain a single contact (mol. B)(CH2)C–H···O(mol. A) in which the C2 carbonyl group of molecule A is engaged (H···O 2.58 Å, CHO = 143°), but the associated Coulombic contribution (−11.7 kJ mol−1) is less stabilising than ED (−28.4 kJ mol−1).
Fig. 11.

Results of SCDS-PIXEL calculations for polymorph V. a Interaction energies, represented by balls, are separated into internal C-3 interactions (blue) and interactions between neighbouring C-3 tapes (highlighted @1, red; @2, orange; @3, green). The horizontal bars indicate cumulative PIXEL energies (summation from left to right) relative to the E T,Cry (scale on the right-hand side). A central molecule A (b) or B (c) (coloured orange) and neighbouring molecules involved in eight (b) or nine (c) pairwise interactions (see Additional file 1: Tables S8 and S9). The mean plane of the pyrimidine ring of the central molecule is drawn, H atoms are omitted for clarity and H-bonds are indicated by blue lines
The sum of all pairwise interaction energies involving molecule A is 6.5 kJ mol−1 higher than the corresponding sum for molecule B. This reflects somewhat different packing environments which are associated with different molecular conformations (see below). Internal C-3 interactions account for 46 % of ET,Cry. The C-3 tapes are arranged in centrosymmetric pairs (@2, see Fig. 12) in such a way that the pyrimidine rings of the two tapes are somewhat offset against one another, the ethyl groups are oriented towards the centre of the centrosymmetric unit and the phenyl rings are oriented in the opposite direction. Other centrosymmetric pairs of C-3 chains result in the mutual antiparallel interdigitation of sets of phenyl groups (@1, @3). The chain–chain interactions involve either three (@1) or two (@2 and @3) of the most stabilising non-H-bond interactions mentioned above (see Fig. 11a). The chain–chain interactions @1, @2 and @3 account for 21, 16 and 9 %, respectively, of ET,Cry. This means that 84 % of the stabilisation of the lattice is derived from columnar stacks of C-3 tapes parallel to [001] which involve the interactions @1 and @2 (Fig. 12).
Fig. 12.

Crystal packing of polymorph V. Interactions of selected A and B molecules (drawn in ball-and-sticks-style) within the same C-3 chain (blue) and with molecules belonging to four neighbouring chains (@1–@4; see Fig. 11). Together, C-3 hydrogen bonding and the @1 and @2 chain stacking interactions account for 84 % of E T,Cry
HBS type C-5: polymorph VI
Polymorph VI has the space group symmetry P21/n and contains two independent molecules, labelled A and B. It contains the novel N–H···O=C bonded tape structure C-5 (see Fig. 2) which possesses 21 symmetry. The two molecule types differ in their H-bond connectivity. Each A molecule forms three o-connections (to two A molecules and one B molecule) and one t-connection (to a second B molecule). Each B molecule forms one o- and one t-connection to A-type molecules (Fig. 10b).
The presence of two parallel strands of H-bonded molecules is reminiscent of the C-3 tape. The C-5 type displays an unusual asymmetric ring due to N–H···O=C bonds involving the C2 carbonyl function of molecule B and the C4 carbonyl function of molecule A. The energy contribution of −46.5 kJ mol−1 associated with this asymmetric t-connection (#1/1′) is very similar to the corresponding values obtained for the symmetric t-connections in forms I, II, III and X. The PIXEL energy calculated for the o-connections between A molecules which are related by a translation along [010] (#3/3′; −34.4 kJ mol−1) is similar to energies obtained for the analogous interactions in polymorph V (#1/1′, #3/3′). The interaction energy for the second set of o-connections (#5/5′) in the C-5 tape is somewhat higher, −28.4 kJ mol−1. In addition to the two o- and four t-connections, the C-5 tape contains six non-H-bond interactions with PIXEL energies between −13.9 and −8.3 kJ mol−1. Altogether, the internal interactions of the C-5 tape account for 63 % of ET,Cry.
The six strongest external interactions (#7, #8/8′, #12/12′, #18; −19.2 to −12.1 kJ mol−1) all involve molecules which belong to a single neighbouring C-5 tape (@1; see Figs. 13a, 14). Each of these molecule–molecule interactions is dominated by the ED term as a result of extensive van der Waals contacts, mainly between phenyl groups. In the structure of polymorph VI, each instance of C-5 is surrounded by six other C-5 tapes (three symmetrical interaction pairs, @1, @2, @3; Fig. 14). The chain–chain interaction @1 defines, together with the internal C-5 interactions, the packing within planes which accounts for 85 % of ET,Cry and @1 alone accounts for 21 %. Interactions @2 (six molecule–molecule contacts) and @3 (two molecule–molecule contacts) account for approximately 10 and 5 %, respectively, of the stabilisation energy.
Fig. 13.

Results of SCDS-PIXEL calculations for polymorph VI. a Interaction energies, represented by balls, are separated into internal C-5 interactions (blue) and interactions between neighbouring C-5 tapes (@1, red; @2, orange; @3, green). Internal C-5 interactions are labelled t (two-point H-bonded), o (one-point H-bonded) and n (non-H-bonded). The horizontal bars indicate cumulative PIXEL energies (summation from left to right) relative to the E T,Cry (scale on the right-hand side). A central molecule A (b) or B (c) (coloured orange) and neighbouring molecules involved in seven (b) or six (c) pairwise interactions (see Additional file 1: Tables S10 and S11). The mean plane of the pyrimidine ring of the central molecule is drawn, H atoms are omitted for clarity and H-bonds are indicated by blue lines
Fig. 14.

Crystal packing of polymorph VI. Interactions of selected A and B molecules (drawn in ball-and-sticks-style) within the same C-5 chain (blue) and with molecules belonging to three neighbouring chains (@1–@3; see Fig. 13). Together, C-5 hydrogen bonding and @1 chain stacking account for 84 % of E T,Cry
Molecular geometry
The PIXEL energy (ET,Cry) is an intermolecular energy derived by integration over the isolated molecule charge densities placed in the crystal structure. The electrostatic contribution (EC,Cry) is rigorously derived by this procedure and various approximations are used to estimate the polarisation (induction; EP,Cry), dispersion (ED,Cry) and repulsion (ER,Cry) contributions to the intermolecular lattice energy. To make the PIXEL crystal energies of different Pbtl polymorphs comparable with one another, we have estimated the intramolecular energy penalties (∆Eintra) of their experimental conformations (Additional file 1: Table S13) with respect to the global conformational energy minimum. The obtained ∆Eintra values were then added to the PIXEL energy ET,Cry.
The geometry of a Pbtl molecule can be characterised by two parameters, the torsion angle ϕ describing the ethyl rotation and the twist angle ω between the phenyl and pyrimidine rings [42] (Fig. 15a). The ϕ values for all previously reported experimental conformations lie within the narrow range of 0° ± 5°, indicating that the ethyl orientation perpendicular to the pyrimidinetrione ring might be the preferred one in the solid state of Pbtl. At the same time there is a wide variation in the corresponding ω angles from 0° to 75°, which is in agreement with the free rotability of the phenyl group as derived from energy scans for an isolated molecule in the gas phase.
Fig. 15.

a Definition of the torsion angles ϕ and ω used to characterise the molecular geometry of Pbtl. b Conformational energy surface of the Pbtl molecule with respect to ϕ and ω, calculated at the MP2 level of theory with the 6-31G(d,p) basis set, with the rest of the molecule optimised in 30° intervals of ϕ and ω. The data points (ϕ, ω)/(−ϕ, −ω) represent the experimental torsion angles in crystal forms of Pbtl, all of which are centrosymmetric. A, B and C are examples of characteristic conformations
Like all the previously reported Pbtl forms, the conformations of molecule A of polymorph V, (ϕ, ω) = (−3°, 31°) and both independent Pbtl molecules of polymorph VI, A: (ϕ, ω) = (−1°, 77°) and B: (ϕ, ω) = (1°, 42°) are located in the global energy minimum ‘valley’ (Fig. 15b). The geometry of molecule B of V, (ϕ, ω) = (−129°, 31°), is unique in that it can be assigned to the second (local) energy minimum rather than the global energy minimum. A conformational change from the conformer of molecule B to that of molecule A would involve a rotation of the ethyl group (ϕ) by approximately 120° and require approximately 20 kJ mol−1. The fact that modification V was obtained only from the melt or by sublimation, but never from solution crystallisation experiments, may indicate that a conformation related to the global energy minimum ‘valley’ is preferred in solution.
Comparison of IV, V and X with previous crystal structure predictions
Pbtl was used by Day et al. [42] as a model flexible molecule in a structure prediction study. 72 structures within 5 kJ mol−1 of the global minimum were identified as possible candidates for new polymorphs (in addition to the previously published forms I–III). Six additional Z′ = 2 candidate structures for polymorph V were proposed because they matched the original space group symmetry P21/c and the reduced cell (a = 12.66, b = 6.75, c = 26.89 Å; β = 99.9°) of Williams’ [36] original cell (a = 12.66, b = 6.75, c = 27.69 Å; β = 106.9°). However, we note that the transformation involved in this unit cell reduction implies a simultaneous transformation of the space group symmetry from P21/c to P21/n. Using the program XPac [41, 50], we have compared the new structure models for polymorphs V, VI and X with the 78 theoretical Pbtl structures proposed by Day et al. [42].
There is no complete 3D match for the experimental structure of V, but one of the Z′ = 2 candidates for form V (#6) with an energy difference from the global minimum of 7.71 kJ mol−1 (see Table 2 of Ref. [42]) displays certain features which are reminiscent of the experimental structure of V (Additional file 1: Fig. S8). Both structures contain centrosymmetric pairs of C-3 chains (propagating along [010]) which are arranged into stacks along the a-axis in such a way that phenyl groups belonging to neighbouring chain pairs interdigitate (Fig. 16). However, they differ fundamentally in the packing mode between adjacent stacks of H-bonded chains. The molecular conformations (ϕ, ω) = (1°, −21°) and (5°, 23°) for this theoretical structure are both well within the “valley” of low-energy conformations close to ϕ = 0°, whereas in the experimental structure one molecule shows an atypical ethyl rotation with ϕ = −129° (see Fig. 15).
Fig. 16.

a Crystal structure of form V of Pbtl (space group P21/n) and b the closest predicted structure for form V (space group setting P21/c) from Ref. [42]. Each structure is viewed along the b-axis, the direction of translation of its C-3 chains. Ethyl and phenyl groups are coloured orange and blue, respectively, and O and H engaged in N–H···O interactions are shown as balls; other H atoms are omitted for clarity. Note the fundamental differences in the packing of neighbouring ab planes composed of C-3 chain pairs
No close match was found for form VI, and it seems that its unique C-5 chain does not occur in any of the theoretical structures. However, there is a very close 3D match between the derived structure model for polymorph X (Table 3) and a theoretical structure (#72; reported in I2/a; transformed C2/c unit cell: a = 12.91 Å, b = 20.26 Å, c = 10.34 Å; β = 115.3°). An XPac comparison based on geometrical parameters derived from complete sets of non-H atoms gives a low dissimilarity index, x = 5.2 (see Additional file 1: Fig. S9).
Discussion
The PIXEL energies for all symmetrical (C-1, C-2, L-3) and asymmetrical (C-5) t-connections in Pbtl polymorphs lie between −45.4 and −49.2 kJ mol−1 (Table 4). The reason for this relatively narrow range is that the rigid ring geometry permits only small variations in van der Waals interactions and therefore dispersion contributions. The geometry of an o-connection is much less constrained than that of a t-connection, and the corresponding PIXEL energies (−23.1 to −40.5 kJ mol−1) can therefore vary by a wide margin. For example, the stabilisation contribution from the strongest o-connection encountered in this study (#5/5′ in the L-3 layer of I) is 5 kJ mol−1 lower than that from the weakest t-connection (#1/1′ in the C-2 chain of III), whereas the four weakest o-interactions in the C-3 chain of V (#5/5′, #10/10′) are only just as stabilising as the three strongest non-H-bond interactions in the same crystal structure (#7, #8/8′) (see Fig. 11a). The implied compensation effect arises from a large variation in the dispersion term (e.g. #10/10′: ED = −9.5 kJ mol−1 vs. #7: ED = −30.7 kJ mol−1). The observation that enhanced dispersion contributions can fully compensate for the absence of classical H-bonding contradicts the conventional view that H-bonds always dominate the interaction hierarchy but is consistent with recent analyses of chiral carboxylic acids [8] and primary amines [51].
Table 4.
Sums of internal energies, E HBS,Σ (kJ mol−1), from N–H···O=C bonded structures in polymorphs of Pbtl and their origin from different types of interaction
| HBS | Form | N HBS [N o, N t, N n] | E HBS,Σ | E T range (o) | E T range (t) | E n,Σ |
|---|---|---|---|---|---|---|
| C-1 | X | 2 [0, 2, 0] | −47.5 | −47.2 to −47.7 | ||
| C-2 | III | 2 [0, 2, 0] | −45.4 | −45.4 | ||
| C-2 | I (C) | 2 [0, 2, 0] | −48.9 | −48.1 to −49.7 | ||
| C-2 | II (C) | 2 [0, 2, 0] | −46.9 | −46.8 to −47.0 | ||
| C-3 | V | 4 [4, 0, 0] | −56.4 | −23.1 to −32.9 | ||
| C-5 | VI | 6 [2, 1, 3] | −72.0 | −28.4 to −34.4 | −46.5 | −17.3 |
| L-3 | I (A + B) | 10 [2, 1, 7] | −100.2 | −34.0 to −40.5 | −49.2 | −38.4 |
| L-3 | II (A + B) | 10 [2, 1, 7] | −98.6 | −35.1 to −38.2 | −45.7 to −47.5 | −38.7 |
Contributions arise from N HBS pairwise contacts, of which there are N o one-point H-bond connections, N T two-point connections and N n non-H-bond interactions and ranges of interaction energies E T (kJ mol−1) for the o- and t-connections involved. E n,Σ (kJ mol−1) is the sum of all significant (internal) non-H-bonded interaction energies within an HBS (C-5 and L-3 only)
The (internal) molecule–molecule interactions within an HBS can be classified as being either H-bonded (via an o- or t-connection) or non-H-bonded. The latter type is relevant for the complex C-5 tape and L-3 layer structures where it accounts for a PIXEL energy sum of −17 kJ mol−1 (VI) and approximately −39 kJ mol−1 (I, II), respectively. The first coordination shell of a molecule is of limited size and usually comprises no more than 14 significant interactions with other molecules. Therefore, the total number NHBS of internal (H-bond or non-H-bond) of a central molecule is an important characteristic of an HBS.
The average internal energy contribution (EHBS,Σ) from a C-1 or C-2 loop chain (NHBS = 2) is −47 kJ mol−1. The analogous PIXEL energy sums for the competing C-3 (NHBS = 4), C-5 (NHBS = 6) and L-3 (NHBS = 9) structures are ≈9, ≈25 and ≈52 kJ mol−1, respectively, lower than this C-1/C-2 value. Hence, HBSs containing exclusively t-connections result in the lowest and complex tape or layer structures result in the highest internal stabilisation contributions (Table 4). However, its lower NHBS number means that the first coordination shell of a t-connected molecule offers more accessible molecule sites for external interactions than that of an o-connected molecule. Specifically, a molecule in a C-1 or C-2 chain can engage in two more significant external interactions with molecules belonging to neighbouring chains than a molecule within a C-3 chain structure. These additional interactions should easily enable a compensation for the internal advantage of C-3 over C1/C-2 (≈9 kJ mol−1). Therefore, the comparison of EHBS,Σ and NHBS values suggests that an HBS with t-connections (C-1/C-2) should be inherently more favourable than any alternative HBS which is based solely on o-connections (C-3). In order for the latter to be a viable competitor, it has to enable a set of significantly more favourable external (packing) interactions in comparison to the former.
To analyse the packing effects associated with different HBS types, sums of molecule–molecule interaction energies, corrected for ΔEintra, have been plotted in a diagram (Fig. 17). For each polymorph, a series of molecular clusters was generated by sequentially adding the 14 most important molecule–molecule interactions (first coordination shell) in descending order of their contributions to the lattice energy. For Z′ > 1 structures (I, V, VI), separate cluster series were generated for independent molecules, whose energy sums were averaged. Each data point in Fig. 17 corresponds to a specific cluster size and represents the difference in energy sums between the indicated polymorph and form III. As mentioned above, HBSs dominated by t-connections (I–III, X) are favoured if only the strongest interactions are taken into account.
Fig. 17.

Differences between sums of PIXEL energies, corrected for ΔE intra, for molecule clusters in polymorphs I, II, V, VI and X in comparison to the corresponding energy sums calculated for polymorph III of Pbtl. For each polymorph, clusters were generated by sequentially adding the 14 most important pairwise energies, ranked in the order of their contribution to the lattice energy from highest to lowest. For each Pbtl polymorph, a broken horizontal line indicates the difference to the corrected E T,Σ value of polymorph III, i.e. (E T,Σ + ΔE intra)Pbtl polymorph − (E T,Σ + ΔE intra)III
For all Pbtl polymorphs, the cluster of size 4 contains the complete set of H-bond interactions. Corrected PIXEL energy sums for these clusters in forms I, II (both Nt = 4/3) and III, X (both Nt = 2) lie within a 2.4 kJ mol−1 interval, whereas the corresponding value for polymorph V (Nt = 0) exceeds that of form III by more than 12 kJ mol−1. The effects of packing multiple C-5 tapes in form V and multiple C-2 chains in form III are such that for each of the next seven highest ranked interactions average PIXEL energies of −17 and −12 kJ mol−1, respectively, are obtained. This means that the initial “disadvantage” of V has disappeared completely at cluster size 9, and V even becomes slightly more favourable than III at cluster size 11. If all weak contributions are taken into account, III has an overall 5.5 kJ mol−1 advantage over V. The plot in Fig. 17 illustrates that HBSs based on multiple H-bond connections result in the highest initial stabilisation of small clusters and that HBSs based on o-connections may overcome their inherent “disadvantage” only if they possess superior crystal packing characteristics.
An HBS based on multiple-point connections is more compact and often also of lower dimensionality than an alternative which contains exclusively o-connections (e.g. dimer vs. catemer or C-1/C-2 vs. C-3). Therefore, a higher number of theoretical 3D packing options exist for a multiple-point HBS than for a one-point competitor so that it seems likely that more viable crystal packing arrangements would emerge for the former than for the latter. Moreover, compact entities with multiple-point connections may be more likely to exist prior to nucleation and could therefore be kinetically favoured. The domination of the barbiturate set of crystal structures by C-1 and C-2 chains (Table 1) could be interpreted in terms of a general preference for HBSs which are based on multiple-point connections.2
As discussed above, an interaction between two non-H bonded molecules which involves strong dispersion effects can be as stabilising as an o-interaction with a smaller dispersion contribution (polymorph V). The importance of dispersion interactions [51] is not usually recognised in crystal structure discussions, which tend to focus on the interpretation of intermolecular atom–atom distances (with reference to van der Waals radii and standard geometries), for example in terms of conventional or weak hydrogen bonds [52, 53]. The formation of conventional N–H···O=C bonds in barbiturates is largely predictable (but not the exact characteristics of the resulting HBS). By contrast, short intermolecular C–H···O contacts [1], which usually involve a small but significant Coulombic contribution, occur in a rather irregular fashion (see footnotes for Additional file 1: Tables S1–S12). However, in each such case, the crystal contains at least one other molecule–molecule interaction with a lower or only slightly higher PIXEL energy which involves neither an N–H···O=C bond nor a short C–H···O contact. The size of associated EC terms (relative to differences in ED between individual molecule–molecule interactions) as well as the irregularity of their occurrence suggest an opportunistic rather than systematic formation of short C–H···O contacts in Pbtl polymorphs as part of an effort to optimise the stability of the crystal.
The SCDS-PIXEL method allows the comparison of energy sums ET,Σ(A, B,…) of interactions originating from the crystallographically distinct molecule types (A, B,…) of a Z′ > 1 structure [54]. In the case of forms I and II, ET,Σ(A) is approximately 20 and 40 kJ mol−1 lower than ET,Σ(C) and ET,Σ(B), respectively (Table 3), which reflects the different involvement of the three independent molecules in o- and t-connections, e.g. [No, Nt] = [2, 2] (A) or [2, 0] (B) or [0, 2] (C). This means for example that the interactions of molecule B contribute 27.5 % less to the PIXEL energy of the crystal than those of molecule A. A comparison with an overview compiled by Gavezzotti for Z′ = 2 structures (Fig. 7 in Ref. [54]) suggests that the differences in ET,Σ(A, B,…) found in Pbtl forms I and II are unusually large.
In order to demonstrate that the results of the PIXEL calculations presented above are both realistic and consistent, we have attempted to rank the Pbtl polymorphs according to their PIXEL energies and have compared the result with available experimental data. This ranking was based on PIXEL energy sums, ET,Σ (Table 3), rather than total PIXEL energies, ET,Cry, which are not possible to calculate for the Z′ = 3 polymorphs I and II. Due to the non-additive character of the polarisation contribution, the ET,Σ value obtained for each of III, V, VI and X is between 1.7 and 3.0 kJ mol−1 lower than the corresponding ET,Cry value. In order to make the PIXEL crystal energies of all Pbtl forms comparable to one another, experimental molecular conformations (Additional file 1: Table S13) were estimated with respect to the global conformational energy minimum, individual ΔEintra values were calculated (Table 3) and added to ET,Cry. The stability order implied by this procedure is III > I > II > VI > X > V, where the first three forms differ by just 1.7 kJ mol−1. This result is in good overall agreement with the findings of a previous experimental study (see Table 3) [26]. Low-temperature (173 K; II, V, VI, X) as well as room-temperature (I, III) structure models were used for our PIXEL calculations. On the basis of a previous report [55] describing two separate PIXEL calculations performed with a room-temperature and a low-temperature structure model of olanzapine, we estimate that the ET,Σ values quoted for I and III in Table 3 should be corrected by approximately −2 % to adjust for different temperatures. Moreover, an optimisation of the model for X (derived from the disordered co-crystal structure) would probably have resulted in a slightly lower ET,Σ.
The ΔEintra contributions of the experimental conformations located in the global energy minimum ‘valley’ were estimated to lie within a range of 0.3–8.9 kJ mol−1 from the global minimum, with only molecule B of modification V adopting a distinct high-energy conformation (17.6 kJ mol−1). This higher ΔEintra penalty is compensated for by more stable intermolecular interactions.
Conclusions
There cannot be a straightforward answer to the question whether, for a given group of compounds, an HBS based on multiple-point connections should generally be more favourable than an alternative HBS containing one-point connections (“dimer or catemer?”). Beside geometry restraints and factors such as accessibility and relative strength of H-bond donor and acceptor functions, the competition between alternative HBSs is governed by an interplay between internal energy contributions (from H-bond and non-H-bond molecule–molecule interactions) and stabilisation effects arising from the packing of multiple HBS instances. An HBS based on multiple-point H-bond connections (i.e. a dimer or a C-1 chain) possesses a more compact architecture than a one-point alternative (i.e. a catemer or a C-3 tape) and offers a higher number of packing alternatives, which may ultimately result in a higher number of potentially viable low-energy structures. The observation that 60 % of the experimental crystal structures of barbiturates listed in Table 1 contain HBSs which are based exclusively on t-connections may be interpreted in this regard. However, the importance of (external) HBS packing characteristics implies that the competition situation between alternative HBSs can be critically affected by relatively small differences in molecular geometry, for example by the size of the C5 ring substituents in the case of the aforementioned barbiturates.
Experimental
Materials
The Pbtl sample used in this study was purchased from Mallinckrodt Chemical Works (U.S.P. XIII Powder, USA) and consisted of a mixture of forms I and II.
Preparation of forms V and VI
Fine needles of V were obtained, together with crystals of II and III from sublimation experiments carried out on a Kofler hot bench, using a setup of two glass slides separated by a 1 cm spacer ring and a sublimation temperature of 135 °C (Additional file 1: Fig. S1). Single crystals of V, stored at 5 °C, were stable for at least 2 months, whereas a melt film of form V was previously reported to have transformed into either II or III within hours [26].
Polymorph VI was produced, on a hot bench, by the melting and partial dissolution of Pbtl powder immersed in paraffin oil and subsequent crystallisation at 100° C. Prismatic single crystals and spherical polycrystalline aggregates of VI were obtained (Additional file 1: Fig. S1).
The identity of the obtained crystals with the Pbtl polymorphs V and VI was established by comparison of their IR spectra with reference data recorded in a previous study [26] (Additional file 1: Fig. S6).
Single-crystal X-ray structure analysis3
Intensity data were collected, using Cu radiation (V) or Mo radiation (VI), on an Oxford Diffraction Gemini-R Ultra diffractometer operated by the CrysAlis software [56]. The data were corrected for absorption effects by means of comparison of equivalent reflections using the program SADABS [57]. The structures were solved using the direct methods procedure in SHELXS97 and refined by full-matrix least squares on F2 using SHELXL97 [58]. Non-hydrogen atoms were refined anisotropically. Hydrogen atoms were located in difference maps and those bonded to carbon atoms were fixed in idealised positions. NH hydrogen atoms were refined with a distance restraint of N–H = 0.88(2) Å. In the case of V, the displacement parameters of H atoms were set to 1.2Ueq (for NH, CH and CH2) or 1.5Ueq (for the CH3 group) of the parent N or C atom. In the case of VI, these parameters were refined freely. The molecular structures are shown in Additional file 1: Figs. S2 and S3 and the geometric parameters of hydrogen bonds are listed in Table 5. The crystal structure data of polymorphs V (CCDC 1035977) and VI (CCDC 103598) have been deposited with Cambridge Crystallographic Data Centre.
Table 5.
Geometric parameters for N–H···O=C bonds
| D-H···A | d(D-H)/Å | d(H···A)/Å | d(D···A)/Å | ∠(DHA)/° |
|---|---|---|---|---|
| Pbtl-V (C-3) | ||||
| (a) N1–H1···O4 | 0.88(2) | 1.92(2) | 2.772(5) | 164(5) |
| (b) N3–H3···O2′ii | 0.878(19) | 1.91(2) | 2.790(5) | 177(5) |
| (c) N1′–H1′···O4′iii | 0.87(2) | 2.00(2) | 2.832(5) | 160(4) |
| (d) N3′–H3′···O2ii | 0.903(19) | 1.99(2) | 2.874 (5) | 168(5) |
| Pbtl-VI (C-5) | ||||
| (a) N1–H1···O4i | 0.887(14) | 2.108(15) | 2.974(2) | 165.2(18) |
| (b) N3–H3···O2′ | 0.887(15) | 1.966(15) | 2.838(2) | 167.3(19) |
| (c) N1′–H1′···O4 | 0.898(15) | 2.052(16) | 2.936(2) | 168.0(17) |
| (d) N3′–H3′···O2v | 0.892(15) | 1.969(16) | 2.852(2) | 170.7(19) |
Symmetry transformations: (i) x, y + 1, z; (ii) 1 − x, 1 − y, 2 − z; (iii) x, y − 1, z; (iv) 1 − x, 2 − y, 2 −z; (v) −x + 3/2, y − ½, −z + ½
Calculation of specific energy contributions
Intermolecular interaction energies were calculated with the semi-classical density sums (SCDS-PIXEL) [37–40] method using the program OPiX [59]. Details of these calculations are available in section 5 of Additional file 1. The structure models listed in Table 3 were used, and C–H and N–H distances were re-calculated to standard lengths within OPiX. No optimisation of the molecular geometry was performed. An electron density map was calculated on a three-dimensional grid with a step size of 0.08 Å at the MP2/6-31G(d,p) level using Gaussian 09 [60]. A PIXEL condensation factor of 3 was applied, giving superpixels with dimensions 0.24 × 0.24 × 0.24 Å3. The calculations yielded interaction energies partitioned into Coulombic, polarisation, dispersion and repulsion terms with an expected accuracy of 1–2 kJ mol−1. No more than two independent molecules can be processed in a single OPiX procedure. Three separate calculations were therefore carried out for each of the Z′ = 3 forms I and II in order to obtain a full set of pairwise interaction energies.
Potential-energy surface scan
The deformation energy for the Pbtl molecule was computed on a 13 × 13 grid, equivalent to a 30° grid spacing for each dihedral angle in the range from 0° to 360° for ϕ and ω, using Gaussian 09 [60]. At each grid point the deformation energy was calculated with the flexible torsions fixed and the rest of the molecule (i.e. all other torsions, angles and bond lengths) optimised at the MP2/6-31G(d,p) level of theory. Additionally, the conformational energy penalties (ΔEintra) with respect to the global conformational energy minimum were calculated, keeping the experimental ϕ and ω torsions fixed, and the rest of the molecule was minimised using the same method as applied for the grid calculations.
Analysis and comparison of crystal structure data
The topologies of HBSs (Table 2) were determined and classified with the programs ADS and IsoTest of the TOPOS package [61] in the manner described by Baburin and Blatov [62].
Geometrical comparisons between crystal structures were carried with the program XPac [41, 50]. The underlying calculations were based on intermolecular geometrical parameters obtained from all 11 non-H atomic positions of the Pbtl molecule (for details, see Additional file 1: Section 7). In order to minimise effects arising from different molecular conformations, a second set of calculations was performed which was based only on the 1,3,5-pyrimidinetrione unit and the C atoms bonded to ring atom C5.
Authors’ contributions
TG carried out crystallisations and structure analyses, the analysis of HBSs, SCDS-PIXEL and XPac calculations. DEB carried out energy calculations of molecular conformations. All authors were involved in the drafting of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
DEB gratefully acknowledges funding by the Elise Richter programme of the Austrian Science Fund (FWF, project V436-N34). Calculations were supported by the Austrian Ministry of Science (BMWF) as part of the UniInfrastrukturprogramm of the Research Platform Scientific Computing at the University of Innsbruck.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- Pbtl
phenobarbital
- I, II, III…XI
polymorphic forms of phenobarbital
- HBS
hydrogen-bonded structure
- C-n, L-n, F-n, (n = 1, 2, 3…)
types of H-bonded structures of barbiturates (Ref. [23])
- o-connection
connection of two molecules by a single H-bond interaction
- t-connection
connection of two molecules by two H-bond interactions
- No
number of o-connections per molecule
- Nt
number of t-connections per molecule
- EC
PIXEL Coulombic energy term (pairwise interaction)
- EP
PIXEL polarisation energy term (pairwise interaction)
- ED
PIXEL dispersion energy term (pairwise interaction)
- ER
PIXEL repulsion energy term (pairwise interaction)
- ET
total PIXEL interaction energy (pairwise interaction)
- ET,Cry
total PIXEL energy of the crystal
- ET,Σ
sum of all ET energies of the crystal
- ET,Σ(A or B or C)
sum of all ET energies in a Z′ > 1 structure involving a specific independent molecule (A or B or C)
- ΔEintra
intramolecular energy penalty
- EHBS,Σ
sum of all internal molecule–molecule energy contributions of an HBS
- #1, #2, #3…
labels for pairwise interactions between molecules
- @1, @2, @3…
labels for pairwise interactions between different H-bonded entities
Additional file
10.1186/s13065-016-0152-5 Details of the preparation of forms V and VI, thermal ellipsoid plots for V and VI, structural information about forms I–III, IR spectra of V and VI, details of PIXEL calculations and XPac comparisons, derived structure model for X.
Footnotes
The carbonyl group at C2 will be referred to as “C2 carbonyl group” and any one of the two topologically equivalent carbonyl groups at C4 or C6 will be referred to as “C4/C6 carbonyl group”.
The fact that only 12 of the theoretical low energy structures reported by Day et al. [35] contain C-1 or C-2 chains may be due to modelling errors. We note also that 15 of the 72 predicted Pbtl structures contain one NH group which is not engaged in an intermolecular N−H∙∙∙O interaction, a characteristic not encountered in any relevant experimental crystal structures of Pbtl analogues (Table 1).
Crystal data for form V: C12H12N2O3, M = 232.24, monoclinic, a = 12.7606(12) Å, b = 6.7624(5) Å, c = 26.847(3) Å, β = 98.829(9)°, V = 2289.2(4) Å3, T = 173(2) K, space group P21/n, Z = 8, 7565 reflections measured, 3822 independent reflections (Rint = 0.1439); 3.3° ≤ θ ≤ 65.0° (λ = 1.5418 Å). The final R1 value was 0.0718 (I > 2σ(I)). The final wR(F2) values were 0.1310 (I > 2σ(I)) and 0.1775 (all data). The max. and min. residual densities were 0.23 and −0.22 e Å−3, respectively. Crystal data for form VI: C12H12N2O3, M = 232.24, monoclinic, a = 14.6701(11) Å, b = 6.9000(5) Å, c = 23.0308(19) Å, β = 94.072(7)°, V = 2325.4(3) Å3, T = 173(2) K, space group P21/n, Z = 8, 9105 reflections measured, 4104 independent reflections (Rint = 0.0499); 3.1° ≤ θ ≤ 25.1° (λ = 0.71073 Å). The final R1 value was 0.0458 (I > 2σ (I)) and the final wR(F2) values were 0.0763 (I > 2σ(I)) and 0.0866 (all data). The max. and min. residual densities were 0.20 and −0.21 e Å−3, respectively.
Contributor Information
Thomas Gelbrich, Email: thomas.gelbrich@uibk.ac.at.
Doris E. Braun, Email: doris.braun@uibk.ac.at
Ulrich J. Griesser, Email: ulrich.griesser@uibk.ac.at
References
- 1.Desiraju GR, Steiner T (1999) Conventional and non-conventional bonds. In: The weak hydrogen bond in structural chemistry and biology. IUCr monographs on crystallography, vol 9. Oxford University Press, Oxford
- 2.Beyer T, Price SL. Dimer or catemer? Low-energy crystal packings for small carboxylic acids. J Phys Chem B. 2000;104:2647–2655. doi: 10.1021/jp9941413. [DOI] [Google Scholar]
- 3.Sanphui P, Bolla G, Das U, Mukherjee AK, Nangia A. Acemetacin polymorphs: a rare case of carboxylic acid catemer and dimer synthons. CrystEngComm. 2013;15:34–38. doi: 10.1039/C2CE26534F. [DOI] [Google Scholar]
- 4.Gavezzotti A, Filippini G. Polymorphic forms of organic crystals at room conditions: thermodynamic and structural implications. J Am Chem Soc. 1995;117:12299–12305. doi: 10.1021/ja00154a032. [DOI] [Google Scholar]
- 5.Neumann MA, Perrin M-A. Can crystal structure prediction guide experimentalists to a new polymorph of paracetamol? CrystEngComm. 2009;11:2475–2479. doi: 10.1039/b909819d. [DOI] [Google Scholar]
- 6.Chan HCS, Kendrick J, Leusen FJJ. Molecule VI, a benchmark crystal-structure-prediction sulfonimide: are its polymorphs predictable? Angew Chem Int Ed. 2011;50:2979–2981. doi: 10.1002/anie.201007488. [DOI] [PubMed] [Google Scholar]
- 7.Nyman J, Day GM. Static and lattice vibrational energy differences between polymorphs. CrystEngComm. 2015;17:5154–5165. doi: 10.1039/C5CE00045A. [DOI] [Google Scholar]
- 8.Gavezzotti A, Lo Presti L. Theoretical study of chiral carboxylic acids. Structural and energetic aspects of crystalline and liquid states. Cryst Growth Des. 2015;15:3792–3803. doi: 10.1021/acs.cgd.5b00442. [DOI] [Google Scholar]
- 9.Hisamatsu S, Masu H, Azumaya I, Takahashi M, Kishikawa K, Kohmoto S. U-Shaped aromatic ureadicarboxylic acids as versatile building blocks: construction of ladder and zigzag networks and channels. Cryst Growth Des. 2011;11:5387–5395. doi: 10.1021/cg200988w. [DOI] [Google Scholar]
- 10.Barnett SA, Hulme AT, Issa N, Lewis TC, Price LS, Tocher DA, Price SL. The observed and energetically feasible crystal structures of 5-substituted uracils. New J Chem. 2008;32:1761–1775. doi: 10.1039/b806763e. [DOI] [Google Scholar]
- 11.Florence AJ, Bedford CT, Fabbiani FPA, Shankland K, Gelbrich T, Hursthouse MB, Shankland N, Johnston A, Fernandes P. Two-dimensional similarity between forms I and II of cytenamide, a carbamazepine analogue. CrystEngComm. 2008;10:811–813. doi: 10.1039/b719717a. [DOI] [Google Scholar]
- 12.Florence AJ, Shankland K, Gelbrich T, Hursthouse MB, Shankland N, Johnston A, Fernandes P, Leech CK. A catemer-to-dimer structural transformation in cyheptamide. CrystEngComm. 2008;10:26–28. doi: 10.1039/B712547J. [DOI] [Google Scholar]
- 13.Arlin J-B, Price LS, Price SL, Florence AJ. A strategy for producing predicted polymorphs: catemeric carbamazepine form V. Chem Commun. 2011;47:7074–7076. doi: 10.1039/c1cc11634g. [DOI] [PubMed] [Google Scholar]
- 14.Arlin J-B, Johnston A, Miller GJ, Kennedy AR, Price SL, Florence AJ. A predicted dimer-based polymorph of 10,11-dihydrocarbamazepine (form IV) CrystEngComm. 2010;12:64–66. doi: 10.1039/B914365C. [DOI] [Google Scholar]
- 15.Braun DE, McMahon JA, Koztecki LH, Price SL, Reutzel-Edens SM. Contrasting polymorphism of related small molecule drugs correlated and guided by the computed crystal energy landscape. Cryst Growth Des. 2014;14:2056–2072. doi: 10.1021/cg500185h. [DOI] [Google Scholar]
- 16.Braun DE, Gelbrich T, Kahlenberg V, Tessadri R, Wieser J, Griesser UJ. Conformational polymorphism in aripiprazole: preparation, stability and structure of five modifications. J Pharm Sci. 2009;98:2010–2026. doi: 10.1002/jps.21574. [DOI] [PubMed] [Google Scholar]
- 17.Nanubolu JB, Sridhar B, Babu VSP, Jagadeesh B, Ravikumar K. Sixth polymorph of aripiprazole: an antipsychotic drug. CrystEngComm. 2012;14:4677–4685. doi: 10.1039/c2ce25306b. [DOI] [Google Scholar]
- 18.Delaney SP, Pan D, Yin SX, Smith TM, Korter TM. Evaluating the roles of conformational strain and cohesive binding in crystalline polymorphs of aripiprazole. Cryst Growth Des. 2013;13:2943–2952. doi: 10.1021/cg400358e. [DOI] [Google Scholar]
- 19.Hursthouse MB, Hughes DS, Gelbrich T, Threlfall TL. Describing hydrogen-bonded structures; topology graphs, nodal symbols and connectivity tables, exemplified by five polymorphs of each of sulfathiazole and sulfapyridine. Chem Cent J. 2015;9:1. doi: 10.1186/s13065-014-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelbrich T, Hursthouse MB. Threlfall TL (2007) Structural systematics of 4,4′-disubstituted benzenesulfonamidobenzenes. 1. Overview and dimer-based isostructures. Acta Crystallogr, Sect B: Struct Sci. 2007;63:621–632. doi: 10.1107/S010876810701395X. [DOI] [PubMed] [Google Scholar]
- 21.Sanphui P, Rajput L. Tuning solubility and stability of hydrochlorothiazide co-crystals. Acta Crystallogr Sect B: Struct Sci. 2014;70:81–90. doi: 10.1107/S2052520613026917. [DOI] [PubMed] [Google Scholar]
- 22.Roux MV, Temprado M, Notario R, Foces-Foces C, Emel’yanenko VN, Verevkin SP. Structure-energy relationship in barbituric acid: a calorimetric, computational, and crystallographic study. J Phys Chem A. 2008;112:7455–7465. doi: 10.1021/jp803370u. [DOI] [PubMed] [Google Scholar]
- 23.Gelbrich T, Rossi D, Häfele CA, Griesser UJ. Barbiturates with hydrogen-bonded layer and framework structures. CrystEngComm. 2011;13:5502–5509. doi: 10.1039/c1ce05430a. [DOI] [Google Scholar]
- 24.Gelbrich T, Griesser UJ. Crystal structure of the α-racemate of methohexital. Acta Crystallogr Sect E: Struct Rep Online. 2015;71:206–209. doi: 10.1107/S205698901500105X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zencirci N, Gelbrich T, Kahlenberg V, Griesser UJ. Crystallization of metastable polymorphs of phenobarbital by isomorphic seeding. Cryst Growth Des. 2009;9:3444–3456. doi: 10.1021/cg801416a. [DOI] [Google Scholar]
- 26.Zencirci N, Gelbrich T, Apperley DC, Harris RK, Kahlenberg V, Griesser UJ. Structural features, phase relationships and transformation behavior of the polymorphs I–VI of phenobarbital. Cryst Growth Des. 2010;10:302–313. doi: 10.1021/cg901062n. [DOI] [Google Scholar]
- 27.Abraham A, Apperley DC, Gelbrich T, Harris RK, Griesser UJ. NMR crystallography: three polymorphs of phenobarbital. Can J Chem. 2011;89:770–778. doi: 10.1139/v11-011. [DOI] [Google Scholar]
- 28.Brandstätter-Kuhnert M, Aepkers M. Molecular compounds, crystalline solid solutions, and new cases of polymorphism in barbiturates. II. Microchim Acta. 1962;50:1055–1074. doi: 10.1007/BF01217548. [DOI] [Google Scholar]
- 29.Brandstätter-Kuhnert M, Aepkers M. Molecular compounds, crystalline solid solutions, and new cases of polymorphism in barbiturates. III. Microchim Acta. 1963;51:360–375. doi: 10.1007/BF01234703. [DOI] [Google Scholar]
- 30.Brandstätter-Kuhnert M, Aepkers M. Polymorphism of barbiturates by microscopical thermal analysis of two-component systems. Mikroskopie. 1961;16:181–197. [Google Scholar]
- 31.Kuhnert-Brandstätter M, Vlachopoulos A. Molecular compounds, crystalline solid solutions, and new polymorphism of barbiturates. IV. Mikrochim Acta. 1967;55:201–217. doi: 10.1007/BF01216332. [DOI] [Google Scholar]
- 32.Zencirci N, Griesser UJ, Gelbrich T, Apperley DC, Harris RK. Crystal polymorphs of barbital: news about a classic polymorphic system. Mol Pharm. 2014;11:338–350. doi: 10.1021/mp400515f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams P. Polymorphism of phenobarbitone. II. The crystal structure of modification III. Acta Crystallogr, Sect B: Struct Sci. 1974;30:12–17. [Google Scholar]
- 34.Platteau C, Lefebvre J, Hemon S, Baehtz C, Danede F, Prevost D. Structure determination of forms I and II of phenobarbital from X-ray powder diffraction. Acta Crystallogr Sect B: Struct Sci. 2005;61:80–88. doi: 10.1107/S0108768104031143. [DOI] [PubMed] [Google Scholar]
- 35.Zencirci N, Griesser UJ, Gelbrich T, Kahlenberg V, Jetti RKR, Apperley DC, Harris RK. New solvates of an old drug compound (phenobarbital): structure and stability. J Phys Chem B. 2014;118:3267–3280. doi: 10.1021/jp409201v. [DOI] [PubMed] [Google Scholar]
- 36.Williams PP. Polymorphism of phenobarbitone: the crystal structure of 5-ethyl-5-phenylbarbituric acid monohydrate. Acta Crystallogr Sect B: Struct Sci. 1973;29:1572–1579. doi: 10.1107/S0567740873005078. [DOI] [Google Scholar]
- 37.Dunitz JD, Gavezzotti A. Molecular recognition in organic crystals: directed intermolecular bonds or nonlocalized bonding? Angew Chem Int Ed. 2005;44:1766–1787. doi: 10.1002/anie.200460157. [DOI] [PubMed] [Google Scholar]
- 38.Gavezzotti A. Molecular aggregation: Structure analysis and molecular simulation of crystals and liquids. Oxford: Oxford University Press; 2007. [Google Scholar]
- 39.Gavezzotti A. Calculation of lattice energies of organic crystals: the PIXEL integration method in comparison with more traditional methods. Z Kristallogr. 2005;220:499–510. [Google Scholar]
- 40.Gavezzotti A. Quantitative ranking of crystal packing modes by systematic calculations on potential energies and vibrational amplitudes of molecular dimers. J Chem Theory Comput. 2005;1:834–840. doi: 10.1021/ct050091w. [DOI] [PubMed] [Google Scholar]
- 41.Gelbrich T, Hursthouse MB. A versatile procedure for the identification, description and quantification of structural similarity in molecular crystals. CrystEngComm. 2005;7:324–336. doi: 10.1039/b502484f. [DOI] [Google Scholar]
- 42.Day GM, Motherwell WDS, Jones W. A strategy for predicting the crystal structures of flexible molecules: the polymorphism of phenobarbital. Phys Chem Chem Phys. 2007;9:1693–1704. doi: 10.1039/b612190j. [DOI] [PubMed] [Google Scholar]
- 43.Groom CR, Allen FH. The Cambridge Structural Database in retrospect and prospect. Angew Chem Int Ed. 2014;53:662–671. doi: 10.1002/anie.201306438. [DOI] [PubMed] [Google Scholar]
- 44.Etter MC, MacDonald JC, Bernstein J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr Sect B: Struct Sci. 1990;46:256–262. doi: 10.1107/S0108768189012929. [DOI] [PubMed] [Google Scholar]
- 45.Bernstein J, Davis RE, Shimoni L, Chang N-L. Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew Chem Int Ed. 1995;34:1555–1573. doi: 10.1002/anie.199515551. [DOI] [Google Scholar]
- 46.Lewis TC, Tocher DA, Price SL. An experimental and theoretical search for polymorphs of barbituric acid: the challenges of even limited conformational flexibility. Cryst Growth Des. 2004;4:979–987. doi: 10.1021/cg034209a. [DOI] [Google Scholar]
- 47.DesMarteau DD, Pennington WT, Resnati G. Fluorinated barbituric acid derivatives. Acta Crystallogr Sect C: Cryst Struct Commun. 1994;50:1305–1308. doi: 10.1107/S0108270193012582. [DOI] [Google Scholar]
- 48.Rossi D, Gelbrich T, Kahlenberg V, Griesser UJ. Supramolecular constructs and thermodynamic stability of four polymorphs and a co-crystal of pentobarbital (nembutal) CrystEngComm. 2012;14:2494–2506. doi: 10.1039/c2ce06659a. [DOI] [Google Scholar]
- 49.Gelbrich T, Meischberger I, Griesser UJ. Two polymorphs of 5-cyclohexyl-5-ethylbarbituric acid and their packing relationships with other barbiturates. Acta Crystallogr Sect C: Cryst Struct Commun. 2015;71:204–210. doi: 10.1107/S2053229615002880. [DOI] [PubMed] [Google Scholar]
- 50.Gelbrich T, Threlfall TL, Hursthouse MB. XPac dissimilarity parameters as quantitative descriptors of isostructurality: the case of fourteen 4,5′-substituted benzenesulfonamido-2-pyridines obtained by substituent interchange involving Cf3/I/Br/Cl/F/Me/H. CrystEngComm. 2012;14:5454–5464. doi: 10.1039/c2ce25508a. [DOI] [Google Scholar]
- 51.Maloney AGP, Wood PA, Parsons S. Competition between hydrogen bonding and dispersion interactions in the crystal structures of the primary amines. CrystEngComm. 2014;16:3867–3882. doi: 10.1039/C3CE42639D. [DOI] [Google Scholar]
- 52.Dunitz J. Intermolecular atom–atom bonds in crystals? IUCrJ. 2015;2:157–158. doi: 10.1107/S2052252515002006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thakur TS, Dubey R, Desiraju GR. Intermolecular atom–atom bonds in crystals: a chemical perspective. IUCrJ. 2015;2:159–160. doi: 10.1107/S205225251500189X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gavezzotti A. Structure and energy in organic crystals with two molecules in the asymmetric unit: causality or chance? CrystEngComm. 2008;10:389–398. [Google Scholar]
- 55.Bhardwaj RM, Price LS, Price SL, Reutzel-Edens SM, Miller GJ, Oswald IDH, Johnston BF, Florence AJ. Exploring the experimental and computed crystal energy landscape of olanzapine. Cryst Growth Des. 2013;13:1602–1617. doi: 10.1021/cg301826s. [DOI] [Google Scholar]
- 56.CrysAlis CCD, CrysAlis RED. Oxford Diffraction Ltd. Oxford: Abingdon; 2003. [Google Scholar]
- 57.Sheldrick GM. SADABS. Version 2007/7. Madison: Bruker AXS Inc.; 2007. [Google Scholar]
- 58.Sheldrick GM. A short history of SHELX. Acta Crystallogr, Sect A: Found Crystallogr. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 59.Gavezzotti A. OPiX: A computer program package for the calculation of intermolecular interactions and crystal energies. Italy: University of Milan; 2007. [Google Scholar]
- 60.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, et al. Gaussian 09. Wallingford: Gaussian Inc.; 2009. [Google Scholar]
- 61.Blatov VA. Multipurpose crystallochemical analysis with the program package topos. IUCr Compcomm Newsl. 2006;7:4–38. [Google Scholar]
- 62.Baburin IA, Blatov VA. Three-dimensional hydrogen-bonded frameworks in organic crystals: a topological study. Acta Crystallogr Sect B: Struct Sci. 2007;63:791–802. doi: 10.1107/S0108768107033137. [DOI] [PubMed] [Google Scholar]
- 63.Roux MV, Notario R, Foces-Foces C, Temprado M, Ros F, Emel’yanenko VN, Verevkin SP. Experimental and computational thermochemical study and solid-phase structure of 5,5-dimethylbarbituric acid. J Phys Chem A. 2010;114:3583–3590. doi: 10.1021/jp910778j. [DOI] [PubMed] [Google Scholar]
- 64.Bideau JP. Crystal structure of 5-ethyl-5-butylbarbituric acid. C R Acad Sci, Ser C: Sci Chim. 1971;272:757–760. [Google Scholar]
- 65.Nichol GS, Clegg W. 5-Butyl-5-ethylbarbituric acid: a phase transition at low temperature. Acta Crystallogr Sect C: Cryst Struct Commun. 2005;61:o297–o299. doi: 10.1107/S0108270105008486. [DOI] [PubMed] [Google Scholar]
- 66.Nichol GS, Clegg W. A second C2/c polymorph of butobarbitone. Acta Crystallogr Sect E: Struct Rep Online. 2007;63:o4147. doi: 10.1107/S1600536807045448. [DOI] [Google Scholar]
- 67.Bideau JP, Marsau P. 5-Ethyl-5-n-pentyl barbituric acid. Cryst Struct Commun. 1974;3:511–514. [Google Scholar]
- 68.Craven BM, Vizzini EA. The crystal structures of two polymorphs of 5-ethyl-5-isoamylbarbituric acid (amobarbital) Acta Crystallogr Sect B: Struct Sci. 1969;25:1993–2009. doi: 10.1107/S0567740869005061. [DOI] [PubMed] [Google Scholar]
- 69.Jones GP, Andrews PR. Conformations of barbiturates related to pentobarbitone. I. Crystal structure of trans-5-ethyl-5-but-1′-enyl barbituric acid. J Chem Cryst. 1981;11:125–133. [Google Scholar]
- 70.Jones GP, Andrews PR. Conformations of barbiturates related to pentobarbitone. III. Crystal structure of 5-ethyl-5-(3′-methylbut-2′-enyl) barbituric acid. J Chem Cryst. 1981;11:145–156. [Google Scholar]
- 71.Andrews PR, Jones GP. Conformations of barbiturates related to pentobarbitone. II. Crystal structure of trans-5-ethyl-5-(1′,3′-dimethylbut-1′-enyl) barbituric acid. J Chem Cryst. 1981;11:135–144. [Google Scholar]
- 72.Jones GP, Horn E. Conformations of barbiturates related to pentobarbitone. IV. Crystal structure of 5-ethyl,5-(1′,3′-dimethylbut-2′-enyl) barbituric acid. J Chem Cryst. 1986;16:629–637. [Google Scholar]
- 73.Smit PH, Kanters JA. The crystal and molecular structure of 5-ethyl-5-(l,3-dimethylbutyl)barbituric acid (α-methylamobarbital) Acta Crystallogr Sect B: Struct Sci. 1974;30:784–790. doi: 10.1107/S0567740874003700. [DOI] [Google Scholar]
- 74.Escobar C. Molecular and crystal structure of 5,5-diallylbarbituric acid. Acta Crystallogr Sect B: Struct Sci. 1975;31:1059–1064. doi: 10.1107/S0567740875004499. [DOI] [Google Scholar]
- 75.Rae AD. Polymorph of 5-allyl-5-isopropylbarbituric acid (aprobarbital I) Cryst Struct Commun. 1975;4:457–460. [Google Scholar]
- 76.Craven BM, Vizzini EA, Rodrigues MM. The crystal structure of two polymorphs of 5,5′-diethylbarbituric acid (barbital) Acta Crystallogr Sect B: Struct Sci. 1969;25:1978–1993. doi: 10.1107/S056774086900505X. [DOI] [PubMed] [Google Scholar]
- 77.Chentli-Benchikha F, Declercq JP, Germain G, Van Meerssche M, Bouche R, Draguet-Brughmans M. Structures des oxo-3 et oxo-6 cyclobarbitals. Acta Crystallogr Sect B: Struct Sci. 1977;33:2739–2743. doi: 10.1107/S056774087700939X. [DOI] [Google Scholar]
- 78.Gelbrich T, Rossi D, Griesser UJ. Tetragonal polymorph of 5,5-dichlorobarbituric acid. Acta Crystallogr Sect E: Struct Rep Online. 2012;68:o235–o236. doi: 10.1107/S1600536811054626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gartland GL, Craven BM. The crystal structure of 5-ethyl-5-(3,3-dimethylbutyl)barbituric acid (γ-methylamobarbital) Acta Crystallogr Sect B: Struct Sci. 1971;27:1909–1916. doi: 10.1107/S0567740871005089. [DOI] [Google Scholar]
- 80.Nichol GS, Clegg W. 5-(1-Methylbutyl)-5-propenylbarbituric acid (quinal barbitone) Acta Crystallogr Sect E: Struct Rep Online. 2007;63:o1632–o1634. doi: 10.1107/S1600536807009610. [DOI] [Google Scholar]
- 81.Gatehouse BM, Craven BM. The crystal structures of monoclinic 5-ethylbarbituric acid and 5-hydroxy-5-ethylbarbituric acid. Acta Crystallogr Sect B: Struct Sci. 1971;27:1337–1344. doi: 10.1107/S0567740871003960. [DOI] [Google Scholar]
- 82.Bravic G, Housty J, Bideau JP. Crystal structure of methylphenylbarbital (mephebarbital) C R Acad Sci, Ser C: Sci Chim. 1968;266:969–971. [Google Scholar]
- 83.Gelbrich T, Zencirci N, Griesser UJ. A polymorph of butobarbital with two distinct hydrogen-bonding motifs. Acta Crystallogr Sect C: Cryst Struct Commun. 2007;63:o751–o753. doi: 10.1107/S0108270107057174. [DOI] [PubMed] [Google Scholar]
- 84.Craven BM, Vizzini EA. The crystal structure of polymorph IV of 5,5-diethylbarbituric acid (barbital) Acta Crystallogr Sect B: Struct Sci. 1971;27:1917–1924. doi: 10.1107/S0567740871005090. [DOI] [PubMed] [Google Scholar]
- 85.Craven BM, Cusatis C. The crystal structure of 5-ethyl-5-(1-methylbutenyl)-barbituric acid. Acta Crystallogr Sect B: Struct Sci. 1969;25:2291–2298. doi: 10.1107/S0567740869005590. [DOI] [Google Scholar]


