Abstract
Introduction: Hypovitaminosis D is reported to be associated with several medical complications. Recent studies have reported a high worldwide prevalence of Vitamin D deficiency in the general population (up to 80 %). This is even higher in patients with chronic kidney disease (CKD) and increases with advancing stages of CKD.
Objectives: To determine the difference in serum Vitamin D [25-hydroxyvitamin D, 25(OH) D] levels between CKD patients and normal healthy population.
Materials and Methods: A prospective cross-sectional study involving 50 normal volunteers (control) and 50 patients with CKD stages 2-4. Their demographic profiles were recorded and blood samples taken for serum 25(OH) D, intact parathyroid hormone (iPTH) and other routine blood tests.
Results: All subjects regardless of renal status had hypovitaminosis D (< 30ng/mL). The mean serum 25(OH) D were comparable in the control and CKD groups (15.3 ± 4.2 ng/mL vs 16.1 ± 6.2 ng/mL, p = NS). However, within the Vitamin D deficient group, the CKD group had lower levels of serum 25(OH) D [12.6(3.7) ng/mL vs 11.2(6.5) ng/mL, p = 0.039]. Female gender [OR 22.553; CI 95 % (2.16-235.48); p = 0.009] and diabetic status [OR 6.456; CI 95 % (1.144-36.433); p = 0.035] were independent predictors for 25(OH) D deficiency.
Conclusions: Vitamin D insufficiency and vitamin D deficiency are indeed prevalent and under-recognized. Although the vitamin D levels among the study subjects and their control are equally low, the CKD group had severe degree of vitamin D deficiency. Diabetic status and female gender were independent predictors of low serum 25(OH)D.
Keywords: 25-hydroxyvitamin D, vitamin D insufficiency and deficiency, normal population, CKD patients
Introduction
Vitamin D has been proven to modulate the immune system through the stimulation of expression of anti-microbial peptides (AMPs) are integral in defense mechanism (Schwalfenberg, 2011[38]). Several studies have shown that hypovitaminosis D to be associated with respiratory tract infections including TB (Laaksi et al., 2007[22]; Nnoaham and Clarke, 2008[32]). Vitamin D has been shown to be important in maintaining B cell homeostasis therefore may be useful in the treatment of B cell-mediated autoimmune disorders (Chen et al., 2007[5]). Hypovitaminosis D is associated with an increased risk of cardiovascular disease as shown by the Framingham study (Wang et al., 2008[42]). It increases the risk of myocardial infarction independent of diabetes, hypertension and dyslipidaemia and other known cardiovascular risk factors (Giovannucci et al., 2008[11]). Vitamin D is proven to prevent nephrosclerosis and retard CKD progression through its anti inflammatory and anti proliferative properties and inhibition of renin production (Danescu et al., 2009[6]). In patients with established CKD, treatment with calcitriol was associated with a trend towards a lower incidence of dialysis initiation and a decrease in overall mortality rates (Autier and Gandini, 2007[4]; Doorenbos et al., 2009[8]). Interestingly, vitamin D also has anti cancer properties by promoting cell differentiation and inhibiting cell proliferation and angiogenesis (Giovannucci et al., 2005[10]). Studies have shown patients with low levels of vitamin D to be associated with increased cancer incidence (Giovannucci, 2006[12]).
Recent studies worldwide have reported a high prevalence of vitamin D deficiency in the general population and is even more common in patients with chronic kidney disease (CKD) (Zadshir et al., 2005[44]; Adams and Hewison, 2010[2]). Apart from a reduction in renal 1α-hydroxylase activity, diabetes and proteinuria have also been implicated with vitamin D deficiency in CKD patients (Schiavi and Kumar, 2004[37]; Holden et al., 2010[15]). Interestingly, urinary protein losses were reported to be a more important predictor of vitamin D deficiency than the degree of CKD (Mehrotra et al., 2008[31]).
There is poor consensus as to what constitutes the optimal level of vitamin D or what defines vitamin D insufficiency or deficiency. At present, most experts define vitamin D insufficiency as a 25(OH) D level of less than 30 ng/mL and levels less than 15 ng/ml as vitamin D deficiency (Zadshir et al., 2005[44]; Holick, 2006[16], 2007[18]; Prentice, 2008[33]). With these definitions, it has been estimated that one billion people worldwide have either vitamin D deficiency or insufficiency (Lips et al., 2006[25]). It was reported that among 15,390 adults in the United State, half had vitamin D deficiency (Zadshir et al., 2005[44]). The prevalence of this condition increases with advancing age and is more predominant in Hispanics and Blacks compared to their white counterparts (Zadshir et al., 2005[44]; Tareen et al., 2005[40]; Yetley, 2008[43]). A study in post-menopausal Asian women reported, approximately 50 % of Siamese and Malaysian women had serum 25(OH)D of less than 30ng/mL and increased to 90 % in Japanese and Korean women (Lim et al., 2008[24]). Among the affected Malaysian, it was more prevalent in the Malay race compared to other races (Rahman et al., 2004[34]).
Although failure of 1,25(OH)2D3 synthesis is the cause of vitamin D deficiency in patients with CKD, studies on vitamin D levels are still based on measurements of serum 25(OH)D (Zittermann, 2003[45]; Al-Badr and Martin, 2008[3]). This is because the former is difficult to measure given that it's half-life is only 4-6 hours. Compared to 25(OH)D in which is the most stable and plentiful metabolite of vitamin D in humans and has a half-life of about 3 weeks, making it the most suitable indicator of vitamin D status (Thacher and Clarke, 2011[41]). Furthermore, 1,25 (OH)2D3 circulates at picogram concentrations that are 1000 times less than those of the precursor 25(OH)D. Hence this metabolite must be extensively purified before being measured by radioimmunoassay (Thacher and Clarke, 2011[41]). Therefore, earlier stages of vitamin D deficiency can be missed by measuring 1, 25(OH)2D3. Studies have found that circulating concentrations of 25(OH)D levels is a sensitive measurement of vitamin D status that reflects both intake and endogenous production in response to solar ultraviolet B exposure (Jones et al., 2007[19]; Holick, 2007[18]).
In view of the association of hypovitaminosis D with diseases and its implications, we decided to embark on this study to see the prevalence of hypovitaminosis D in our population.
Materials and Methods
A cross-sectional study comparing patients with CKD (attending follow-up at the Nephrology Clinic at our institution) with the normal healthy population. The study was approved by the Research and Ethics Committee with Research Grant FF-227-2010. We included CKD stages 2-4 and aged 18 to 65 years old. Our exclusion criteria included: acute renal failure, end stage renal disease, chronic liver disease, malabsorption syndromes, granulomatous disease and patients who were on medications known to affect vitamin D absorption or metabolism such as anticonvulsants, isoniazid, rifampicin, theophylline, glucocorticoids, calcium and vitamin D supplements. The control group were volunteers from our institution and with no medical illness.
Estimated Glomerular Filtration Rate (eGFR) was calculated with the equation of the Modification of Diet in Renal Disease (MDRD) Study Group and categorized based on the NKF Kidney Disease Outcomes Quality Initiative guidelines (K/DOQI guidelines, 2003[20]).
Clinical data of the control were obtained by patient interview. Weight and height were measured to calculate BMI and categorized based on the Malaysian Clinical Practice Guideline on Management of Obesity to: underweight (< 18.5 kg/m2), normal (18.5 to 22.9 kg/m2), overweight (23.0 to 27.4 kg/m2) and obese (27.4 kg/m2) (Academy of Medicine of Malaysia Ministry of Health, 2004[1]). Current smokers were defined as patients smoking at least one cigarette per day during the previous 6 months.
10 ml of fasting venous blood was collected for measurement of 25-hydroxyvitamin D (25(OH) D, intact parathyroid hormone (iPTH) and other routine blood tests. Urine was collected for urine dipstick, microscopy and urine protein creatinine index (uPCI).
25-Hydroxyvitamin D Assay 125 I RIA Kit
Serum for 25 (OH) D assays was centrifuged at 3000 rpm for 10 minutes at room temperature and stored at -80 °C until assayed. The 25(OH) D was tested in duplicate, in a single laboratory and was assayed in a single batch to avoid inter assay variations. We used the 25(OH)D assay Radioimmunoassay Kit (DiaSorin Stillwater, Minnesota, USA) which has been used in several other studies ((Del Valle et al., 2007[7]; Levin et al., 2007[23]; Mehrotra et al., 2008[31]; Holden et al., 2010[15]). For the purposes of analysis, 25(OH)D concentration was categorized based on current Kidney Disease Outcomes Quality Initiative guidelines (K/DOQI guidelines, 2003[20]) to:
Optimal level (> 30 ng/mL),
insufficient (15 - 30 ng/mL) and
deficient (< 15 ng/mL).
Statistical Analysis
Data were analysed using the Statistical Package for Social Science (SPSS) software version 19. Normally distributed data were expressed as mean ± standard deviation (SD) and non-normally distributed data were expressed as median (IQR). For normally distributed data Student's t-test or one-way ANOVA were used and for non-normally distributed data, Mann-Whitney U or Kruskal Wallis tests were used. Categorical variables were analysed using χ2 or Fisher's Exact test as appropriate. Correlations were tested using Pearson or Spearman correlation coefficients. Multivariate analyses were performed using binary logistic regression. All statistical tests were two sided and an unadjusted p value of < 0.05 was considered significant.
Results
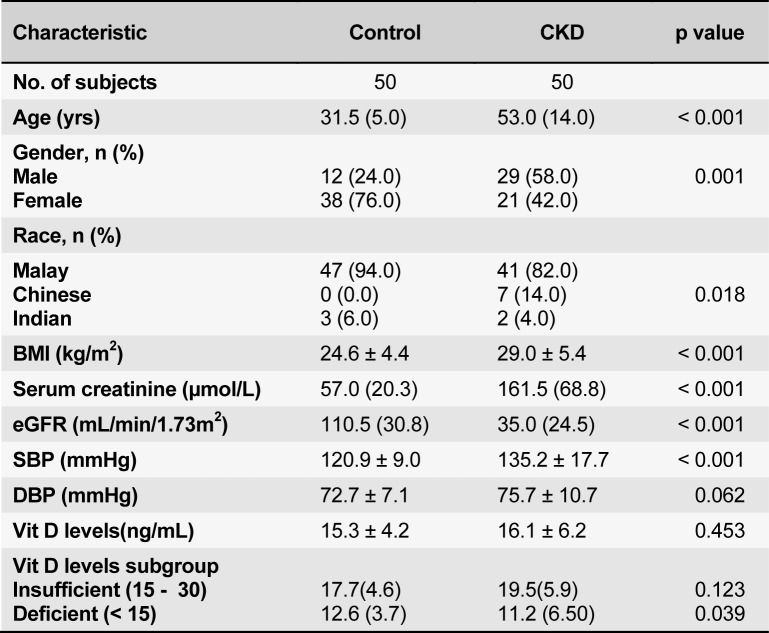
One hundred subjects were enrolled - 50 healthy controls and 50 CKD patients. Their baseline demographics are summarized in Table 1(Tab. 1). Subjects in the control group were significantly younger and predominantly female. CKD patients were overweight or obese compared to the controls. As expected, the control group had a normal serum creatinine, thus higher eGFR and a normal blood pressure. Majority of the patients (58.0 %) had CKD stage 3, followed by fifteen (30.0 %) with CKD stage 4 and six (12.0 %) subjects with CKD stage 2 (Table 2(Tab. 2)). Among the CKD group, there was a trend towards an increasing age with advancing CKD stage (p = 0.068). The leading cause of CKD in our patient cohort was diabetic nephropathy in 31(62.0 %) of the patients followed by chronic glomerulonephritis in 13 (26.0 %) patients.
Table 1. Demographic and clinical characteristics in the control and CKD subgroup.
Table 2. Demographic and clinical characteristics at different stages of CKD.
Serum 25(OH) D levels
All study subjects regardless of renal status were found to have hypovitaminosis D. Their mean levels of 25(OH) D were comparable in the control and CKD groups (15.3 ± 4.2 ng/mL and 16.1 ± 6.2 ng/mL (p = 0.453) respectively. The proportion of subjects with 25(OH)D insufficiency and 25(OH)D deficiency were also comparable in both groups. However in 25(OH)D deficiency group, the mean levels of 25(OH)D was significantly lower in the CKD groups [11.2 (6.5) ng/mL vs 12.6 (3.7), p = 0.039]. The serum 25(OH) D levels were also not different across the different CKD stages (p = 0.87). A further sub-analysis was done to compare the serum 25(OH) D levels amongst CKD patients with and without diabetes mellitus. The mean serum 25(OH) D tended to be lower in diabetic patients compared with those in non-diabetics. Additionally, more of the diabetic CKD patients were 25(OH)D deficient (16/31 vs 4/19, p = 0.032). Female gender (p < 0.001), diabetic status (p = 0.032) and lower serum haemoglobin levels (p = 0.002) were significantly associated with hypovitaminosis D status. Multivariate analysis showed female gender and diabetes were independent predictors for Vitamin D deficiency in CKD patients (Table 3(Tab. 3)).
Table 3. Independent predictive factor for hypovitaminosis D in CKD patients.
Discussion
All patients in both groups of our study cohort have hypovitaminosis D. The control subjects despite being younger, predominantly reproductive aged females and almost exclusively Malay doctors and nurses of our hospital, their prevalence of hypovitaminosis D was no far better than the CKD group. Their prevalence of vitamin D insufficiency (15 to 30 ng/mL) was 46 % and vitamin D deficiency (< 15 ng/mL) was 54 % as opposed to 60 % with vitamin D insufficiency and 40 % with vitamin D deficiency in the CKD group. The reported prevalence of hypovitaminosis D has been consistently and unexpectedly high in almost all populations studied worldwide and across the age spectrum. A previous local study in primary school children aged 7-12 years old (n = 402) showed the prevalence of hypovitaminosis D(< 30 ng/ml) to be 73 % (Khor et al., 2011[21]). Whereas, Rahman et al. found in post-menopausal women aged 50 to 65 years the level of 25(OH) D to be significantly lower in the postmenopausal Malay women compared to Chinese women(p < 0.05) (Rahman et al., 2004[34]).
However the true serum vitamin D level that defines hypovitaminosis D remains debatable. Most studies used a serum 25(OH) D level of less than 30 ng/mL to define hypovitaminosis D. Nonetheless a recent United States guideline on dietary requirements for calcium and vitamin D suggested a serum 25(OH) D levels of at least 20 ng/mL should be considered as an adequate vitamin D level which meets the requirements of at least 97.5 % of the population (Ross et al., 2011[35]). Using the same definition, the prevalence of hypovitaminosis D in our control and CKD group would be reduced to 86 % and 76 % respectively (p = 0.202).
Hypovitaminosis D (< 30mg/mL) has been shown to be associated with increased risk of cardiovascular disease and its risk factors i.e obesity, hypertension and diabetes (Martins et al., 2007[28]). However at present the exact cut off between increased risk is not clear with some studies saying less than 20 mg/mL and also varies with different races (Holick, 2004[17]). As to what the optimum level we need to target to confer benefits is not clear although levels > 37 mg/mL has been suggested by one study (Martins et al., 2007[28]).
Since the prevalence of hypovitaminosis D in both of our groups was high, it raised the question of what were the common similar factors in both groups. What could be the trigger factors? Could it be genetic? Could it be dietary? The two main determinants of vitamin D levels are sunlight exposure and dietary intake. In general, Malaysian diet lacks good sources of vitamin D as they rarely take cereal (fortified), margarine, milk or cheese regularly. However in assessing diet we need a validated food intake recall. Previous studies have used a single 24 hour dietary recall (Holden et al., 2010[15]; Mehrotra et al., 2008[31]). However this method is prone to recall bias and may not represent an individual's general dietary intake and habits.
Sunlight exposure between studies is also non-consistent and often there is no objective measurement of sun exposure (Levin et al., 2007[23]; Del Valle et al., 2007[7]). The validated measures of sun exposure has only been published very recently (Saliba et al., 2012[36]). Hypovitaminosis D of both normal and CKD subjects due to the lack of sun exposure is undeniably important as Malaysia is a tropical country with a hot and humid climate. Surprisingly, many other countries with a lot of sun exposure such as in the Middle East also showed a similar prevalence of hypovitaminosis D (Sedrani, 1984[39]; McGrath et al., 2001[30]; Marwaha et al., 2005[29]). This may be explained by the fact that these Muslim countries require women to be fully covered except for the hands and face thus reducing their exposure to sunlight. This finding has been supported by studies in European countries where the prevalence hypovitaminosis D is high in immigrants who are covered and veiled (Lowe et al., 2010[26]). As our study also consists of predominantly Muslim women (76 % in control group and 42 % in CKD group) who shared similar attire code as their counterparts worldwide, this is likely to play a major part in contributing to the reduced serum vitamin D levels from sun exposure.
Race is a major contributor to hypovitaminosis D worldwide. The Blacks and Hispanics were reported to have higher prevalence of hypovitaminosis D compared to the Caucasians (Gutiérrez et al., 2011[14]). South Asian women had significantly higher serum PTH and lower serum 25(OH)D concentrations than Caucasian women in the UK (Lowe et al., 2010[26]). However, this was not associated with higher levels of markers of bone resorption or reduced bone quality in the South Asian women (Lowe et al., 2010[26]).
In the present study, female gender and diabetes mellitus were found to be independently associated with lower levels of 25(OH)D. Female gender was found to have an inverse relationship with serum 25(OH)D levels in previous study (Mehrotra et al., 2008[31]). This could explain the high prevalence of hypovitaminosis D in our population whereas in diabetes, there is emerging data showing Vitamin D deficiency to be a contributing factor for the development of both type 1 and 2 diabetes. The β cells of pancreas not only secrets insulin but also contains vitamin D receptor (VDRs) as well as 1-α hydroxylase enzymes. It has been proven that treatment with vitamin D improves glucose tolerance and insulin resistance (Martin and Campbell, 2011[27]).
BMI and obesity have also been implicated with hypovitaminosis D. Obesity reduces the availability of vitamin D due to sequestration of vitamin D in body fat (Holick, 2007[18]). These findings emphasize the need for appropriate interventions to address the problem of obesity in our younger population to reduce the risk of the metabolic syndrome later in life. As shown in our study, the control group was found to be overweight whereas in the CKD group were noted to be obese.
In CKD, the loss of the renal1α-hydroxylase is largely responsible for the hypovitaminosis D. Several epidemiologic studies have reported patients with CKD stages 3 to 5 to have lower 25(OH) D concentrations than the general population (González et al., 2004[13]; Levin et al., 2007[23]). These in keeping with our findings. The identification of extrarenal 1α-hydroxylase suggests that 25(OH)D status is an important consideration in patients with CKD (Gal-Moscovici and Sprague, 2007[9]). Ideally, the serum levels of 1,25 (OH)2D3 which is the biologically active form of Vitamin D should be measured. However, low levels of the 25(OH)D itself may contribute to decreased levels of 1,25(OH)2D3 production, particularly in CKD patients with nephrotic range proteinuria (Levin et al., 2007[23]).
There is an association between lower serum 25(OH)D levels and poorer kidney function, higher BMI and higher albuminuria, lower serum calcium and higher serum phosphorus, intact parathyroid hormone (iPTH) and alkaline phosphatase (ALP) . Nutritional factors associated with higher 25(OH)D levels were stable weight, higher albumin and HDL cholesterol (Holden et al., 2010[15]). In our CKD patients, the serum levels of albumin, haemoglobin, calcium and high density lipoprotein-cholesterol (HDL) decreased proportionately with advancing CKD stage. Whereas, the levels of serum phosphorus, ALP and iPTH rose significantly. Once eGFR drops to less than 30 ml/min/1.73m2 (CKD stage 4), the 1α-hydroxylase activity becomes impaired. This in turn will cause a significant decrease in intestinal calcium absorption leading to a reduction in circulating serum calcium. As a result, secondary hyperparathyroidism develops which results into osteopenia, osteoporosis and increasing fracture risk. Parathyroid hormone also causes increased phosphaturia, resulting in low-normal serum phosphorus. Without an adequate calcium-phosphorus product, mineralization of the collagen matrix is diminished hence aggravating the renal bone disease in CKD.
In view of such a high prevalence of hypovitaminosis D in CKD and its possible complications, we have proceeded with a further study to see the effects of vitamin D replacement in this group of patient. However this is beyond the scope of this paper.
Conclusions and Clinical Implications
There is enough evidence to treat patients with hypovitaminosis D and CKD. However the role of vitamin D replacement in the general healthy population remains debatable. Thus a larger study with longer follow up is required.
References
- 1.Academy of Medicine of Malaysia Ministry of Health. Clinical practice guidelines on management of obesity. Malaysia: 2004. [Google Scholar]
- 2.Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Badr W, Martin KJ. Vitamin D and kidney disease. Clin J Am Soc Nephrol. 2008;3:1555–1560. doi: 10.2215/CJN.01150308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 6.Danescu LG, Levy S, Levy J. Vitamin D and diabetes mellitus. Endocrine. 2009;35:11–17. doi: 10.1007/s12020-008-9115-5. [DOI] [PubMed] [Google Scholar]
- 7.Del Valle E, Negri AL, Aguirre C, Fradinger E, Zanchetta JR. Prevalence of 25(OH) vitamin D insufficiency and deficiency in chronic kidney disease stage 5 patients on hemodialysis. Hemodial Int. 2007;11:315–321. doi: 10.1111/j.1542-4758.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 8.Doorenbos CRC, van den Born J, Navis G, de Borst MH. Possible renoprotection by vitamin D in chronic renal disease: beyond mineral metabolism. Nature reviews. Nephrology. 2009;5:691–700. doi: 10.1038/nrneph.2009.185. [DOI] [PubMed] [Google Scholar]
- 9.Gal-Moscovici A, Sprague SM. Role of vitamin D deficiency in chronic kidney disease. J Bone Miner Res. 2007;22(Suppl 2):V91–V94. doi: 10.1359/jbmr.07s203. [DOI] [PubMed] [Google Scholar]
- 10.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States) Cancer Causes Control. 2005;16:83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 11.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 13.González EA, Sachdeva A, Oliver DA, Martin KJ. Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am J Nephrol. 2004;24:503–510. doi: 10.1159/000081023. [DOI] [PubMed] [Google Scholar]
- 14.Gutiérrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22:1745–1753. doi: 10.1007/s00198-010-1383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holden RM, Morton AR, Garland JS, Pavlov A, Day AG, Booth SL. Vitamins K and D status in stages 3-5 chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:590–597. doi: 10.2215/CJN.06420909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 Suppl):1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 19.Jones G, Horst R, Carter G, Makin HL. Contemporary diagnosis and treatment of vitamin D-related disorders. J Bone Miner Res. 2007;22(Suppl 2):V11–V15. doi: 10.1359/jbmr.07s219. [DOI] [PubMed] [Google Scholar]
- 20.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–201. [PubMed] [Google Scholar]
- 21.Khor GL, Winnie Chee SS, Zalilah MS, Poh BK, Mohan A, Jamalludin AR, et al. High prevalence of vitamin D insufficiency and its association with BMI-for-age among primary school children in Kuala Lumpur, Malaysia. BMC Publ Health. 2011;11:95. doi: 10.1186/1471-2458-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laaksi I, Ruohola JP, Tuohimaa P, Auvinen A, Haataja R, Pihlajamäki H, et al. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86:714–717. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- 23.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 24.Lim SK, Kung AWC, Sompongse S, Soontrapa S, Tsai KS. Vitamin D inadequacy in postmenopausal women in Eastern Asia. Curr Med Res Opin. 2008;24:99–106. doi: 10.1185/030079908x253429. [DOI] [PubMed] [Google Scholar]
- 25.Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006;260:245–254. doi: 10.1111/j.1365-2796.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 26.Lowe NM, Mitra SR, Foster PC, Bhojani I, McCann JF. Vitamin D status and markers of bone turnover in Caucasian and South Asian postmenopausal women living in the UK. Br J Nutr. 2010;103:1706–1710. doi: 10.1017/S0007114509993850. [DOI] [PubMed] [Google Scholar]
- 27.Martin T, Campbell RK. Vitamin D and diabetes. Diabetes Spectrum. 2011;24:113–118. [Google Scholar]
- 28.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 29.Marwaha RK, Tandon N, Reddy DRHK, Aggarwal R, Singh R, Sawhney RC, et al. Vitamin D and bone mineral density status of healthy schoolchildren in northern India. Am J Clin Nutr. 2005;82:477–482. doi: 10.1093/ajcn.82.2.477. [DOI] [PubMed] [Google Scholar]
- 30.McGrath JJ, Kimlin MG, Saha S, Eyles DW, Parisi AV. Vitamin D insufficiency in south-east Queensland. Med J Aust. 2001;174:150–151. doi: 10.5694/j.1326-5377.2001.tb143195.x. [DOI] [PubMed] [Google Scholar]
- 31.Mehrotra R, Kermah D, Budoff M, Salusky IB, Mao SS, Gao YL, et al. Hypovitaminosis D in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1144–1151. doi: 10.2215/CJN.05781207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37:113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 33.Prentice A. Vitamin D deficiency: a global perspective. Nutr Rev. 2008;66(10 Suppl 2):S153–S164. doi: 10.1111/j.1753-4887.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- 34.Rahman SA, Chee WSS, Yassin Z, Chan SP. Vitamin D status among postmenopausal Malaysian women. Asia Pac J Clin Nutr. 2004;13:255–260. [PubMed] [Google Scholar]
- 35.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The. 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saliba W, Rennert HS, Kershenbaum A, Rennert G. Serum 25(OH)D concentrations in sunny Israel. Osteoporos Int. 2012;23:687–694. doi: 10.1007/s00198-011-1597-y. [DOI] [PubMed] [Google Scholar]
- 37.Schiavi SC, Kumar R. The phosphatonin pathway: new insights in phosphate homeostasis. Kidney Int. 2004;65:1–4. doi: 10.1111/j.1523-1755.2004.00355.x. [DOI] [PubMed] [Google Scholar]
- 38.Schwalfenberg GK. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res. 2011;55:96–108. doi: 10.1002/mnfr.201000174. [DOI] [PubMed] [Google Scholar]
- 39.Sedrani SH. Low 25-hydroxyvitamin D and normal serum calcium concentrations in Saudi Arabia: Riyadh region. Ann Nutr Metab. 1984;28:181–185. doi: 10.1159/000176801. [DOI] [PubMed] [Google Scholar]
- 40.Tareen N, Martins D, Zadshir A, Pan D, Norris KC. The impact of routine vitamin supplementation on serum levels of 25 (OH) D3 among the general adult population and patients with chronic kidney disease. Ethn Dis. 2005;15(4 Suppl 5):S5–102. [PubMed] [Google Scholar]
- 41.Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86:50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S–564S. doi: 10.1093/ajcn/88.2.558S. [DOI] [PubMed] [Google Scholar]
- 44.Zadshir A, Tareen N, Pan D, Norris K, Martins D. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005;15(4 Suppl 5):S5–97. [PubMed] [Google Scholar]
- 45.Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr. 2003;89:552–572. doi: 10.1079/BJN2003837. [DOI] [PubMed] [Google Scholar]