Abstract
Background:
The prevalence of colorectal cancer (CRC) is rapidly increasing in Iran. It holds the most prevalent cancer after skin, breast, and gastric cancers among the Iranian population. The current study was designed to investigate the effects of leisure time, occupational and household physical activity as well as exercise on the risk of CRC in the Iranian population.
Methods:
In this population-based case–control study, 100 individuals with a recent diagnosis of CRC who were eligible for the study were recruited between 2006 and 2008. The control groups were selected from patients’ companions (excluding first- and second-degree relatives) without past history of cancer or any physical disability. Physical activity of the participants was evaluated using a Kriska retrospective physical activity questionnaire. The relation between CRC and physical activity was assessed via logistic regression model and calculating the odds ratio (OR) as well as a confidence interval (CI) of 95%.
Results:
According to the findings, the adjusted OR of occupational (OR = 0.98, 95%, CI: 0.95–1.02) and house holding physical activities (OR = 1.03, 95% CI: 0.99–1.08) were not significantly different between the case and control groups for women (P > 0.05). The risk of CRC shows a significant reduction in individuals with moderate leisure physical activities compared to those with minimal activities (OR = 0.82, CI 95%: 0.73–0.98).
Conclusions:
The study suggests that the risk of CRC will decrease in individuals with higher leisure physical activities (especially with an increase in hours of brisk walking during the day).
Keywords: Case–control study, colorectal cancer, physical activity
INTRODUCTION
Colorectal cancers (CRCs) are among the most common cancers in the world. In developed countries, 6% of general population are at risk for CRC and it accounts for 10% of newly diagnosed cases in male as well as 11% in female.[1] Surveys by a federation of European gastroenterology announced CRC as the most common cancer in 2000. The incident rate of CRC varies in different parts of the world with a higher incidence in developed countries, such as US, Australia, Western Europe, and New Zealand compared with Southern America, Africa, and Asia.[2,3]
In Iran, the incident rate of CRC was 51.7%, 87.7%, and 12.8% in 2007, 2008, and 2009, respectively. CRC is the most common cancer after skin, breast, and gastric cancer in the Iranian population.[4] Despite preventive interventions, CRC exhibit increasing prevalence in recent years.[5] The most common prevalence is observed in sporadic group and individuals without any familial or hereditary past history. Lifestyle, nutrition, and physical activities play a key role in increased susceptibility to CRC.[6,7] CRC is considered as a multifactorial disease with environmental and genetic contributors. Studies in animal models have shown the importance protective effect of physical activities on chemical tumorigenic insults in the intestine.[8] The role of physical activities in susceptibility to CRC has been investigated extensively in epidemiological studies despite the controversial results of these studies. Different groups have reported converse correlation,[9,10] no effect[11,12] or increased risk[13,14,15,16] of physical activities on CRC. Considering a very few studies that were taking the effect of leisure time, occupational, and household physical activity on CRC, we designed this study to assess these factors on CRC in the Iranian population.
METHODS
Study design and participants
In this case–control population-based study, 100 individuals with confirmed diagnosis of CRC were recruited to the study in two referral hospitals in Isfahan city, Iran. Another inclusion criterion was the age between 40 and 65. We excluded patients with the history of metastatic colon cancer as well as patients with the history of any other chronic disease that could cause limitations in physical and mental activities and potentially interfere with comfortable interview or correct filling of the consent form. Among 400 patients that were considered for this study 100 new cases met the inclusion criteria and were recruited to the study between 2006 and 2008. Histopathology confirmed the disease in all participants. In this group, data collection was performed after the diagnosis of each patient.
The control group was chosen from companions of other patients (in other clinical sectors) without any history of cancer and limited physical activity. We matched the two groups for socioeconomic variables. The study was approved by research council of Isfahan University of Medical Sciences (study no: 385342) and written consent form was obtained from all the participants.
Data collection
All the participants in the study were interviewed by an expert. Physical activities of the participants were evaluated using a modified retrospective Kriska physical activity questionnaire.[17] This questionnaire is a well-established questionnaire to evaluate past physical history and allows robust analysis of data in retrospective studies.[18] In this questionnaire, 14 leisure physical activities were assessed, including slow easy walking, brisk walking, Yoga, swimming, and cycling. We also evaluated the household activities, such as cleaning, changing decoration, and cooking. Occupational physical activities were studied considering all physical activities that happen during the work time. To have a reliable assessment of physical activity level we categorized data into four different groups; number of years, number of months during the past year, number of days in a week, and number of hours in a day. We evaluated the number of each activity in a week by calculating the frequency of that activity per unit of time. Validity and reliability of the questionnaire in Iranian population have been considered and approved.[19,20]
Different studies have shown that it is difficult to reliably report brisk walking during the day[21] so we evaluated brisk walking separately from other activities.
We registered the following data in the questionnaire: Demographic data including, age gender, date of birth, current address, marital status, and level of education. We also evaluated other relevant data, such as hormone replacement therapy (starting age, dosage, duration, method of administration), smoking (package/year), alcohol (amount and duration), aspirin usage (regularly at least for 1 year), cholecystectomy, history of cancer, family history of cancer in the first- and second-degree relatives. Anthropometric evaluation was conducted in self-report format and it included height (cm), average of weight during the past 5 years (kg) and body mass index (BMI) according to the formula of (weight [kg]/height2 [m]).
Statistical analysis
SPSS statistical software version 15 (2006 by SPSS Inc, Chicago, IL, Inc. USA) was used. Frequency distributions of socioeconomic and confounding variables were analyzed using Chi-square test. The risk of CRC was evaluated using multiple binary logistic regression and odds ratio (OR) with confidence interval (CI) = 95%. We considered the group with lowest physical activity as the reference group. The model was also adjusted for confounding variables including smoking and hormone use. Significant level was considered P < 0.05.
RESULTS
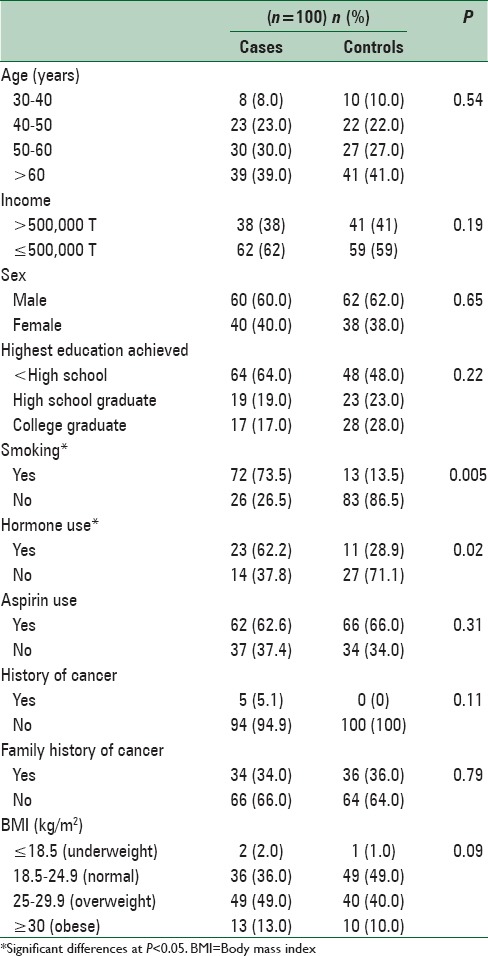
The average of the age was 59.40 ± 11.82 and 55.81 ± 11.96 in case and control group, respectively. We did not observe any significant difference in two groups regarding the age (P = 0.92), gender (P = 0.77), educational level (P = 0.07) and BMI (P = 0.09). The case group showed higher scores for smoking, hormone therapy and in the past 5 years compared to the control [Table 1].
Table 1.
Baseline characteristics of study participants
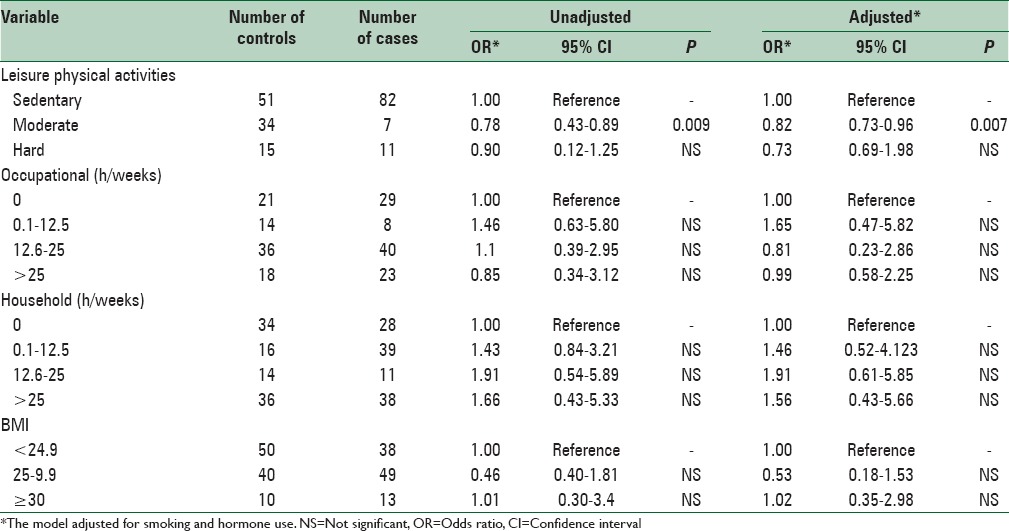
After assessing the age, BMI, smoking, and hormone therapy, next, we evaluated the risk of CRC in groups with high leisure physical activity level compared to those with low physical activity and we found 27% reduction in the risk of CRC. This is of great importance because we could not detect any significant difference in the risk of CRC when considering occupational and house holding physical activities (P = 0.67 and P = 0.73, respectively). The OR of the moderate leisure physical activity level in the adjusted model was 0.82 (P = 0.007) and in the nonadjusted model was determined 0.78 (P = 0.009). Investigating the BMI in both case and controls did not show a significant difference with the risk of CRC [Table 2]. According to the findings of our study, the protective effect of leisure physical activities in the age groups of 40–50 years (OR = 0.69, 95% CI: 0.56–1.9), 50–60 (OR = 0.74, 95% CI: 0.48–1.05) and above 60 (OR = 0.64, 95% CI: 0.45–0.52) shows a significant difference although these data in the group of 30–40 did not reach significance. Our data suggest that the correlation between brisk walking and CRC is significant in female versus male (OR = 0.76, 95% CI: 0.42–3.56 and OR = 0.79, 95 percent CI: 0.51–1.56, respectively).
Table 2.
Odds ratios and 95% confidence intervals of colorectal cancer in relation to physical activity
DISCUSSION
Our results suggest that there is a significant reverse correlation between CRC and physical activity. We observed 27% decrease in the risk of CRC in individuals with high level of leisure physical activities. Our results are in agreement with the study of Slattery et al., that reported a substantial decrease in the risk of CRC and rectal cancer in individuals with high physical activity.[9] There are other studies that suggest an even stronger correlation, for example, in a study conducted in Yale University, a 40–50% decrease in CRC was reported in female and male with high physical activity.[22] Cohort studies also show a strong correlation between the risk of CRC and leisure physical activities.[23]
There are still many aspects of physical activities and their impact on our daily life that needs to be elucidated. We still do not know the mechanism by which physical activity will decrease the risk of CRC but resting lifestyle and hyperinsulinemia are among the major causes.
Like other members of growth factor family, insulin is correlated with tumor growth and anti-apoptotic effects and that may partially explain the higher risk of CRC in individuals with increased blood levels of insulin, C-peptide and insulin-like growth factor 1.[24,25,26] Studies suggest that the increased levels of Insulin and having diabetes mellitus are correlated with the risk of proximal, and not distal, CRC.[27]
Reduced exposure of mucosa to carcinogens in diet after exercise, decreased body fat and insulin resistance, improved immune function of T-cells, B-cells and NK-cells, altered interleukin-1 levels, as well as changes in prostaglandin synthesis, modifications in gallbladder acids and cholesterol level as well as gastrointestinal hormones, are among different mechanisms that were suggested to explain the correlation between risk of CRC and physical activities.[8]
We could not detect any significant correlation between occupational as well as household activities and the risk of CRC. Although a meta-analysis of 16 cohort studies conducted in 2006 reported a significant decrease in CRC risk in individuals with high physical and work activities,[28] but another review in 2007 report no correlation between CRC and work activities.[22] It seems that type of study and method of classifying work activities are determining factors that need to be of more attention when analyzing the data. In most of the other studies, no preventive effect of work activities has been reported.[9,29,30] Other than physical and work activities we also assessed housekeeping activities in our study. Housekeeping activities are more reliably measured compared to leisure physical activities because of their routine nature. One of the reasons that may explain why we did not observe any correlation between house holding physical activities and risk of CRC is the fact that we studies both colon and rectum cancers together. In the study conducted by Larsson et al., a reverse correlation between housekeeping activities and colon cancer has been reported,[31] although in another study conducted by Hou et al., there was no detectable correlation between rectum cancer and physical activity.[32]
Obesity is one of the determining factors in insulin resistance and hyperinsulinemia. Some studies have mentioned a strong correlation between obesity and CRC in male and to a lesser extent in female.[33] Our results do not show any significant correlation between BMI and CRC in both genders. There are controversial reports on this correlation in the literature.[23,33,34] We think that recall bias in reporting BMI should be considered in more depth when studying BMI and CRC risk in case–control studies. In Dai et al., meta-analysis, obesity was reported to have a significant positive effect on CRC.[28] Although another meta-analysis in 2007 considered this correlation less significant.[16] The most common physical activity among our participants was walking. According to our finding, brisk walking can reduce the risk of CRC 21%. Other studies have also suggested brisk walking as the most common activity with up to 25% preventive effect on CRC when happens more than 40 min/day.[30]
One of the limitations of the study was the limited sample size, so the generalizability of the results to the broader community should be done with caution. In addition, numbers of case and controls were equal. Another important limitation of this study is a lack of Food Intake Assessment. Although due to similarities in the socioeconomic status of both groups, we expected similar dietary habits between the two groups.
In some of the previous studies, selection bias and recall bias had affected the data. To avoid this type of biases we conducted this study in a double-blind format. All the interviewers were trained according to protocols and these trainings were repeated during the study to ensure the validity and reliability of the data. We also ensured recruiting the recently diagnosed individuals to participate in this study.
CONCLUSIONS
In summary, our study shows a reverse correlation between risk of CRC and leisure physical activities (especially brisk walking during the day). Our study highlights the importance of leisure physical activities and not occupational and housekeeping activities in preventing CRC, especially at the age of 40 and above.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Martínez ME. Primary prevention of colorectal cancer: Lifestyle, nutrition, exercise. Recent Results Cancer Res. 2005;166:177–211. doi: 10.1007/3-540-26980-0_13. [DOI] [PubMed] [Google Scholar]
- 2.James AS, Campbell MK, Hudson MA. Perceived barriers and benefits to colon cancer screening among African Americans in North Carolina: How does perception relate to screening behavior? Cancer Epidemiol Biomarkers Prev. 2002;11:529–34. [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Mohler MJ, Coons SJ, Hornbrook MC, Herrinton LJ, Wendel CS, Grant M, et al. The health-related quality of life in long-term colorectal cancer survivors study: Objectives, methods and patient sample. Curr Med Res Opin. 2008;24:2059–70. doi: 10.1185/03007990802118360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–71. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 6.Harriss DJ, Atkinson G, Batterham A, George K, Cable NT, Reilly T, et al. Lifestyle factors and colorectal cancer risk (2): A systematic review and meta-analysis of associations with leisure-time physical activity. Colorectal Dis. 2009;11:689–701. doi: 10.1111/j.1463-1318.2009.01767.x. [DOI] [PubMed] [Google Scholar]
- 7.Kuriki K, Tajima K. The increasing incidence of colorectal cancer and the preventive strategy in Japan. Asian Pac J Cancer Prev. 2006;7:495–501. [PubMed] [Google Scholar]
- 8.Quadrilatero J, Hoffman-Goetz L. Physical activity and colon cancer. A systematic review of potential mechanisms. J Sports Med Phys Fitness. 2003;43:121–38. [PubMed] [Google Scholar]
- 9.Slattery ML, Edwards S, Curtin K, Ma K, Edwards R, Holubkov R, et al. Physical activity and colorectal cancer. Am J Epidemiol. 2003;158:214–24. doi: 10.1093/aje/kwg134. [DOI] [PubMed] [Google Scholar]
- 10.Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31:876–85. doi: 10.1200/JCO.2012.45.9735. [DOI] [PubMed] [Google Scholar]
- 11.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: A systematic review and meta-analysis. Ann Oncol. 2014;25:1293–311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 12.Colbert LH, Hartman TJ, Malila N, Limburg PJ, Pietinen P, Virtamo J, et al. Physical activity in relation to cancer of the colon and rectum in a cohort of male smokers. Cancer Epidemiol Biomarkers Prev. 2001;10:265–8. [PubMed] [Google Scholar]
- 13.Thune I, Lund E. Physical activity and risk of colorectal cancer in men and women. Br J Cancer. 1996;73:1134–40. doi: 10.1038/bjc.1996.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee IM, Paffenbarger RS, Jr, Hsieh C. Physical activity and risk of developing colorectal cancer among college alumni. J Natl Cancer Inst. 1991;83:1324–9. doi: 10.1093/jnci/83.18.1324. [DOI] [PubMed] [Google Scholar]
- 15.Kuchiba A, Morikawa T, Yamauchi M, Imamura Y, Liao X, Chan AT, et al. Body mass index and risk of colorectal cancer according to fatty acid synthase expression in the nurses’ health study. J Natl Cancer Inst. 2012;104:415–20. doi: 10.1093/jnci/djr542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: A meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev. 2007;16:2533–47. doi: 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- 17.Kriska AM. Historical leisure activity questionnaire. Med Sci Sports Exerc. 1997;29:S43–5. [Google Scholar]
- 18.Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13:401–11. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 19.Momenan AA, Delshad M, Sarbazi N, Rezaei Ghaleh N, Ghanbarian A, Azizi F. Reliability and validity of the modifiable activity questionnaire (MAQ) in an Iranian urban adult population. Arch Iran Med. 2012;15:279–82. [PubMed] [Google Scholar]
- 20.Delshad M, Ghanbarian A, Ghaleh NR, Amirshekari G, Askari S, Azizi F. Reliability and validity of the modifiable activity questionnaire for an Iranian urban adolescent population. Int J Prev Med. 2015;6:3. doi: 10.4103/2008-7802.151433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cottreau CM, Ness RB, Kriska AM. Physical activity and reduced risk of ovarian cancer. Obstet Gynecol. 2000;96:609–14. doi: 10.1016/s0029-7844(00)00972-8. [DOI] [PubMed] [Google Scholar]
- 22.Miles L. Physical activity and the prevention of cancer: A review of recent findings. Nutr Bull. 2007;32:250–82. [Google Scholar]
- 23.Nilsen TI, Vatten LJ. Prospective study of colorectal cancer risk and physical activity, diabetes, blood glucose and BMI: Exploring the hyperinsulinaemia hypothesis. Br J Cancer. 2001;84:417–22. doi: 10.1054/bjoc.2000.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138:2029–43.e10. doi: 10.1053/j.gastro.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gualdrini UA, Sambuelli A, Barugel M, Gutiérrez A, Avila KC. Prevention of colorectal cancer. Acta Gastroenterol Latinoam. 2005;35:104–40. [PubMed] [Google Scholar]
- 26.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: A population-based, case-control study. Ann Intern Med. 2011;154:22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 27.Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127:1044–50. doi: 10.1053/j.gastro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Dai Z, Xu YC, Niu L. Obesity and colorectal cancer risk: A meta-analysis of cohort studies. World J Gastroenterol. 2007;13:4199–206. doi: 10.3748/wjg.v13.i31.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isomura K, Kono S, Moore MA, Toyomura K, Nagano J, Mizoue T, et al. Physical activity and colorectal cancer: The Fukuoka colorectal cancer study. Cancer Sci. 2006;97:1099–104. doi: 10.1111/j.1349-7006.2006.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mao Y, Pan S, Wen SW, Johnson KC. Canadian Cancer Registries Epidemiology Research Group. Physical inactivity, energy intake, obesity and the risk of rectal cancer in Canada. Int J Cancer. 2003;105:831–7. doi: 10.1002/ijc.11159. [DOI] [PubMed] [Google Scholar]
- 31.Larsson SC, Rutegård J, Bergkvist L, Wolk A. Physical activity, obesity, and risk of colon and rectal cancer in a cohort of Swedish men. Eur J Cancer. 2006;42:2590–7. doi: 10.1016/j.ejca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Hou L, Ji BT, Blair A, Dai Q, Gao YT, Chow WH. Commuting physical activity and risk of colon cancer in Shanghai, China. Am J Epidemiol. 2004;160:860–7. doi: 10.1093/aje/kwh301. [DOI] [PubMed] [Google Scholar]
- 33.Kune GA, Kune S, Watson LF. Body weight and physical activity as predictors of colorectal cancer risk. Nutr Cancer. 1990;13:9–17. doi: 10.1080/01635589009514041. [DOI] [PubMed] [Google Scholar]
- 34.Terry PD, Miller AB, Rohan TE. Obesity and colorectal cancer risk in women. Gut. 2002;51:191–4. doi: 10.1136/gut.51.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]


