Abstract
Background:
Green tea is one of the most popular beverages in the world. It is believed to have beneficial effects in the prevention and treatment of many diseases, one of which is nonalcoholic fatty liver disease (NAFLD). The present study investigated the effects of consumption of green tea in NAFLD patients.
Methods:
This study was a double-blind, placebo-controlled, randomized clinical trial. Ultrasonography was used to diagnose fatty liver in patients with alanine aminotransferase (ALT) >31 mg/dl and 41 mg/dl and aspartate aminotransferase (AST) >31 mg/dl and 47 g/dl in women and men, respectively and without other hepatic diseases. A total of 80 participants (20–50 years) with NAFLD were randomly allocated into two groups to receive either green tea extract (GTE) supplement (500 mg GTE tablet per day) or placebo for 90 days. At baseline and at the end of the intervention weight, serum ALT, AST, and alkaline phosphatase (ALP) were measured in fasting state, and dietary data were collected at baseline and end of the study.
Results:
Green tea group showed significant reductions in ALT and AST levels after 12 weeks period (P < 0.001). The placebo group showed a reduction in ALT and AST levels at the end of the study, but it was no significant. ALP levels showed significant reductions in both groups after 12 weeks period (P < 0.001).
Conclusions:
According to the findings of this study, GTE supplementation decrease liver enzymes in patients with NAFLD. It can be claimed that GTE prescribed can be considered as a treatment to improve serum levels of liver enzymes in NAFLD patients.
Keywords: Alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase, green tea extract, nonalcoholic fatty liver disease
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a constellation of progressive liver disorders that are closely related to obesity, diabetes, and insulin resistance. NAFLD prevalence has increased with the change in eating habits, thus identifying an effective treatment for NAFLD is a significant public health objective. Lifestyle-related factors such as poor diet, obesity, excessive alcohol intake, diabetes, and hyperlipidemia have all been proposed to contribute to NAFLD. In addition to the development of a fatty liver, NAFLD patients may also exhibit inflammation, necrosis and fibrosis of the liver, which are known as nonalcoholic steatohepatitis (NASH).[1] This disease may progress to cirrhosis of the liver and hepatocellular carcinoma. Lifestyle interventions such as improvement of eating habits or physical activity are commonly recommended for NAFLD and NASH, but no effective medical therapy for these diseases has been established, although many medications for the treatment of NAFLD is undergoing clinical trials in the Western countries.
Green tea (Camellia sinensis) is one of the most popular beverages in the World. It contains high levels of flavonoids, which have antioxidant properties. Catechin, one of the main flavonoids in green tea has recently attracted attention for its anti-tumor[2] and anti-arteriosclerotic[3] effects. Catechins account for ~20% of the flavonoids in green tea leaves.[4] The major tea catechins include epicatechin, epicatechin gallate, epigallocatechin (EGC), and EGC gallate (EGCG).[5] They have been found to decrease oxidative stress[6] and to exert anti-virus,[7] anti-allergenic,[8] anti-hypertensive,[9] and anti-hyperglycemic effects.[10,11] In addition, results of animal experiments have indicated that catechins affect the lipid metabolism by decreasing triglyceride and total cholesterol levels[12] and enhancing energy utilization.[13] Experimental evidence supports a role of green tea extract (GTE) or its catechins in protecting against NAFLD by regulating energy homeostasis and decreasing oxidative stress and inflammatory responses.[14,15] However, the effect of the GTE on humans and its detailed mechanism have yet to be clarified. To the best of our knowledge, we conducted this randomized clinical trial to examine the effect of GTE on NAFLD in humans.
METHODS
This double-blind, randomized controlled clinical trial was carried out on 80 obese adult patients with NAFLD recruited from Metabolic Liver Disease Research Center in Isfahan University of Medical Sciences from September 2013 to February 2014. All subjects were randomly assigned to one of the two above-mentioned groups. A random number between 0.0 and 0.99 was generated by the computer for each subject. Subjects with a random number between 0.0 and 0.49 were assigned to the group with GTE, while those with a random number between 0.50 and 0.99 were assigned to the placebo group with cellulose. The same opaque capsules containing either dried powdered GTE or placebo (cellulose) were administered to the subjects by a research assistant blinded to the contents in the capsules. All subjects were treated in the same fashion. The study protocol was approved by the Ethics Committee of Isfahan University of Medical Sciences and was registered in the Iranian Registry of Clinical Trials website (IRCT2013092611763N12).
The sample size was computed 35 per group by considering α = 0.05 and a power of 90%. This number was increased to 40 per group to accommodate the anticipated dropout rate. All participants underwent ultrasonography for determining fatty liver by a single sonographist. Echogenisity grading was performed using SonoAce X4 Medison (South Korea, Seoul). NAFLD is defined by elevated liver enzymes (alanine aminotransferase [ALT] >31 mg/dl and 41 mg/dl and aspartate aminotransferase [AST] >31 mg/dl and 47 g/dl in women and men, respectively) and echogenicity grading of the liver was based on Saverymuttu et al.,[16] that is, “mild steatosis” as a slight increase in liver echogenicity and exaggeration of liver and kidney echo discrepancy, and relative preservation of echoes from the walls of the portal vein, “moderate steatosis” as a loss of echoes from the walls of the portal veins, particularly from the peripheral branches, resulting in a featureless appearance of the liver as well as a greater posterior beam attenuation and a greater discrepancy between hepatic and renal echoes, and “severe steatosis” as a greater reduction in beam penetration, loss of echoes from most of the portal vein wall. The main branches and a large discrepancy between hepatic and renal echoes.
Of totally 153 recruited patients, only 80 confirmed NAFLD patients met the inclusion criteria Inc. aged 20–50 years of gender and body mass index (BMI) equal or over 30 kg/m2. Patients with liver diseases such as Wilson's disease, autoimmune liver disease, hemochromatosis, virus infection, and alcoholic fatty liver as well as those with hepatotoxic, lipid lowering, metformin consumption and antihypertensive medication, contraceptive, and estrogen were excluded. The aim of the study was carefully explained to the patients, and their written informed consent was obtained. Personal characteristics Inc. demographic and disease history were obtained. Height and weight were measured by using standard protocols with participants in light clothes and without shoes. BMI was calculated as weight (kg) divided by height squared (m2), and body fat percentage was estimated with Omron body fat monitor (HBF-306) at baseline and at the end of the study. Dietary data were collected using a 3-day dietary record and averages of 3-day energy and macro-nutrients intakes were analyzed using Nutritionist Software (Version 4.1, First Databank Division, The Hearst Corporation, San Bruno, CA). A self-reported questionnaire was used to estimate physical activity level.[17] The subjects were randomly allocated into two groups “Green tea extract” and “placebo” groups.
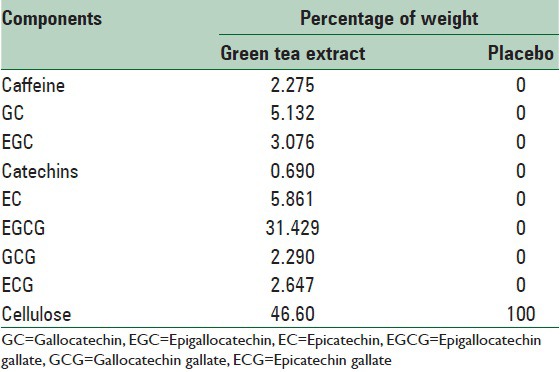
Our GTE samples were prepared by Giah Essence Phytopharm Corporation (Gorgan, Iran). GTE samples were extracted from dried leaves of green tea according to the present standard procedures with a certificate of analysis given. The placebo given to the control group comprised pure microcrystalline cellulose. The placebo tablets supplied by School of Pharmacy, Isfahan University of Medical Sciences, Iran were similar to green tea tablets in color and size. The subjects were asked to take one capsule containing 500 mg of either GTE or cellulose each day for 12 weeks. The above capsule was taken 30 min after lunch. Table 1 shows the components of caffeine and polyphenols in the capsules.
Table 1.
Components of caffeine and polyphenols (500 mg each capsule)
All participants were asked to maintain their usual lifestyle. Blood samples were taken after 12–14 h of overnight fasting at baseline and after the intervention period. Serum samples were transferred into microtubes and were stored at −70°C until analysis. ALT and AST were determined by the method developed by International Federation of Clinical Chemistry.[18,19] Alkaline phosphatase (ALP) was determined by Deutschen Gesellschaft für Klinische Chemie.[20] All statistical procedures were performed using SPSS Software (Version 16, Spss Inc. Chicago, IL). Normality of continuous variable was tested by Kolmogorov–Smirnov test. Data are expressed as mean ± standard deviation (SD) for normally distributed variables. Differences in the mean of the continuous variables between the two groups were tested using analysis of covariance for adjusting for baseline measurements and covariates. Changes in biochemical parameters over the study period were estimated by after intervention minus baseline amount was done using paired t-test. P < 0.05 was considered statistically significant.
RESULTS
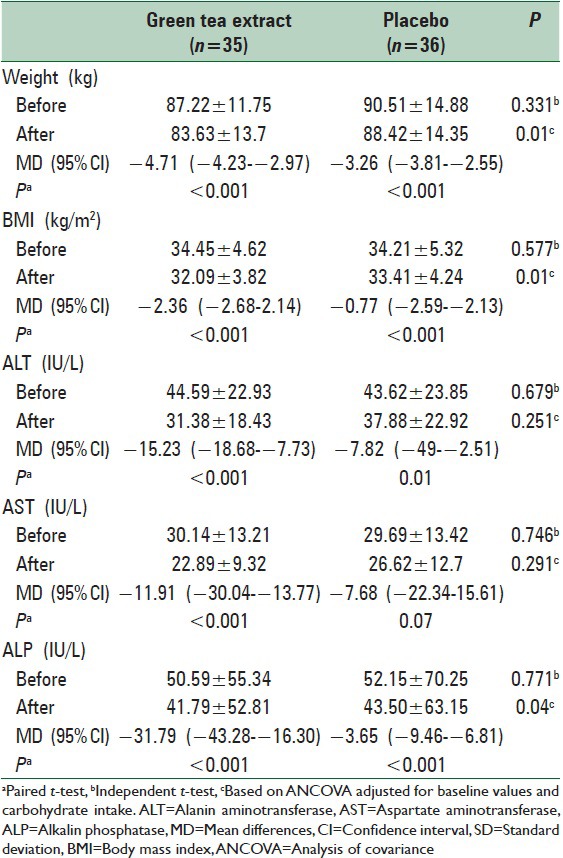
Of totally 80 patients, 71 patients completed the study (n = 35 and 36 for green tea and placebo groups, respectively) [Figure 1]. General characteristics of the study subjects are shown in Table 2. There were no significant differences at baseline between the two groups in terms of age, gender, marital status, education level, physical activity, and the severity of fatty liver. The mean daily macronutrient and dietary fiber intakes were shown in Table 3. Dietary data assessment over the study showed no significant changes in total energy intake, fat, protein, and dietary fiber over the study in both groups. There was a significant reduction in carbohydrate intake in green tea group (P = 0.02). There were no significant differences in the mean nutrient intakes between the two groups at baseline and also after 12 weeks. The mean ± SD of biochemical factors before and after intervention are shown in Table 4. At the beginning of the study, the groups were similar based upon weight (87.22 ± 11.75 kg and 90.51 ± 14.88 kg in intervention and placebo group, respectively) and liver enzymes. Weight decreased significantly in both groups (P < 0.001). The mean weight change in the green tea group was significantly greater than the placebo group (P = 0.001). Green tea group showed significant reductions in ALT and AST levels after 12 weeks period (P < 0.001). The placebo group showed a reduction in ALT and AST levels at the end of the study, but it was no significant. ALP levels showed significant reductions in both group after 12 weeks period (P < 0.001).
Figure 1.
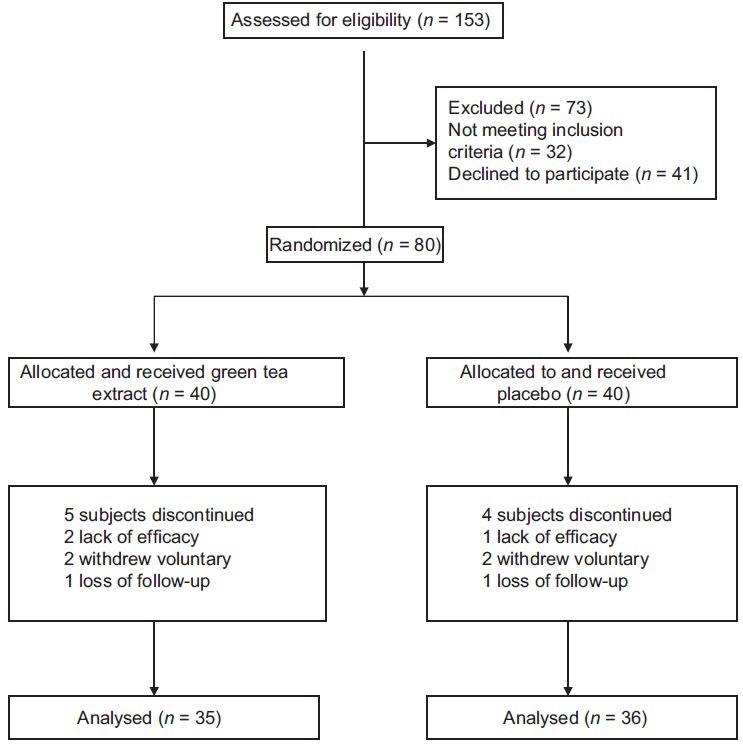
Study flow diagram
Table 2.
Baseline characteristics of the NAFLD patients in each group
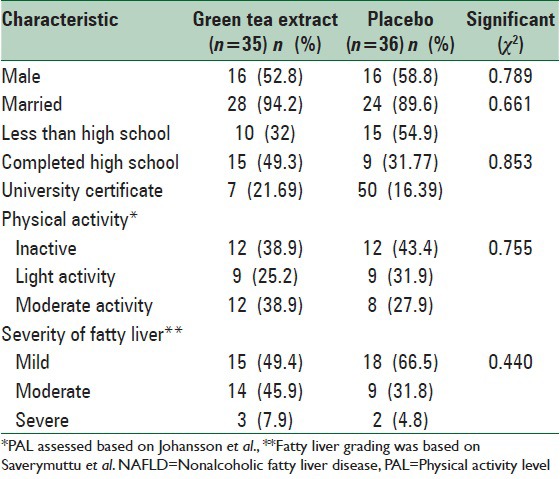
Table 3.
Mean±SD daily total energy and macronutrient intakes before and after intervention
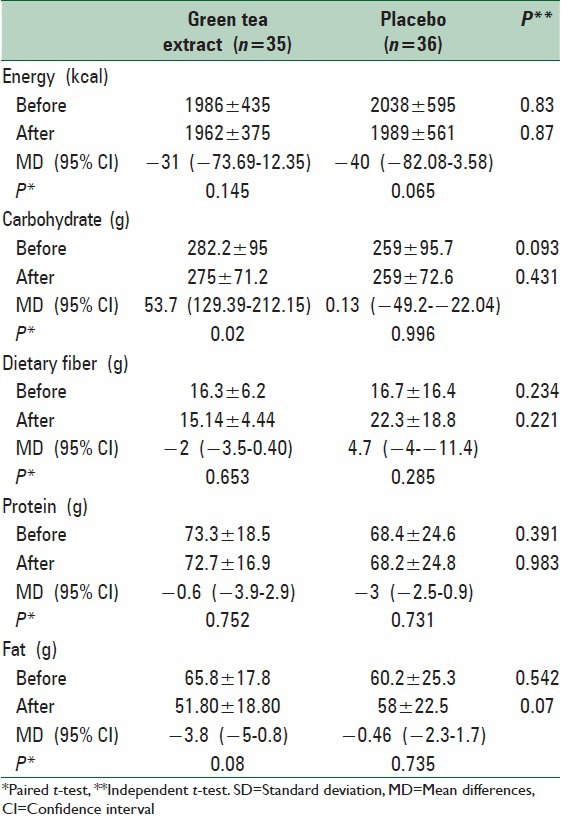
Table 4.
Mean±SD biochemical factors before and after intervention
DISCUSSION
NAFLD was identified for the first time in people without alcohol usage experience in 1980.[21] In fact, this disease includes a wide range of disorders from accumulated lipid in the big vesicular form to accumulated lipid with inflammation, cirrhosis, and hepatic damage.[22] In fact, when NAFLD appears that fat comprises 5–10% of liver's weight.[23,24,25] The prevalence of this is 34–46% in adults, although the prevalence of it is 21.5–31.5% in Iran.[26,27,28] There have been no certain treatment for NAFLD so far, but losing weight, controlling metabolic syndromes such as diabetes and hyperlipidemia, taking antioxidant drugs like Vitamin D and those which are sensitive to insulin are recommended.[29,30] In the present study, the effects of GTE prescription on liver function test were evaluated. The results indicated a decrease in weight, after and before the intervention, in both groups. In Jablonski et al., 2013 study which was done on 53 NAFLD patients, the average weight of patients was more than control group, so that patients had high BMI and 10% weight addition to their idea weight.[31] In our study before any intervention, both groups were homogenized in terms of BMI and weight so that there was no significant statistical difference. The role of BMI and weight in the fatty liver has been the topic of many studies.[32,33] In the past, BMI was the more important independent predictor agent of accumulated fat in the liver. Other researchers have accentuated that the peripheral accumulated lipid is a more important predictor agent in comparison with BMI.[34,35] So according to the significant reduction in BMI and weight effected by taking GTE daily in the intervention group, this drug can be considered as an effective drug and can be prescribed for NAFLD patients. In this present, green tea group showed significant reductions in ALT and AST levels after 12 weeks period (P < 0.001). The placebo group showed a reduction in ALT and AST levels at the end of the study, but it was no significant. ALP levels showed significant reductions in both group after 12 weeks period (P < 0.001). According to these results, it can be claimed that GTE 500 mg prescribed can be considered as an absolute treatment to improve serum levels of liver enzymes in NAFLD patients. While in the results of Takato et al., in 2013 study that evaluated laboratory parameters, BMI, weight, and histological observed liver after taking GTE 100 mg 3 times each day in 12 weeks, ALT declined in green tea group while AST and ALP levels remained unchanged.[36] The difference between the results of Sakata's study and ours study can be because of the GTE dosage during the study.
EGCG, the main catechin in green tea is believed to reduce liver oxidation stress. The components of NAFLD have not yet been fully elucidated, but the following steps are considered to be the main mechanism. Free fatty acids are absorbed by the liver through the intestinal tract after a meal and are oxidized by mitochondria and peroxisomes. If fatty acid uptake by hepatocytes increases, fatty acid pools in the liver increase and accumulate in the hepatocytes as acylglycerol, increasing the load on hepatic mitochondria. Fatty acids that are not metabolized by mitochondria undergo ω or ß oxidation by microsomes or peroxisomes. If a large quantity of fatty acids continues to be deposited in the liver, accumulation of acylglycerol in the hepatocytes induces oxidative stress that may progress to NAFLD.[37] It has been shown that catechins promote lipid metabolism in the liver.[38] Body weight and adiposity were blunted by catechin administration in the obese mouse model C57BL/6J. Increased mRNA expression of acyl-CoA oxidase, one of the peroxisomal β-oxidizing enzymes and medium-chain acyl-CoA dehydrogenase, a mitochondrial β-oxidizing enzyme, was observed in the liver of the catechin administration group. Increased hepatocellular mitochondrial β-oxidation activity promotes the breakdown of fatty acids and it is thought that it acts as a protective mechanism against NAFLD. Catechins are a natural iron chelator and also serve to influence internal absorption of iron. A controlled study looking at the effects of EGCG on nonheme iron absorption showed that it was decreased by 27% in patients consuming 300 mg EGCG compared with controls consuming placebo.[39] Reports on NASH patients showed that elevated iron stores, iron absorption in the liver[40] and serum ALT levels were decreased by bloodletting treatment.[41] Restricting iron absorption through catechins may therefore be effective treatment for NAFLD.
CONCLUSIONS
Results of this randomized clinical trial shows that GTE supplementation decrease ALT and AST levels after 12 weeks period in patients with NAFLD. According to these results, it can be claimed that GTE prescribed can be considered as an absolute treatment to improve serum levels of Liver enzymes in NAFLD patients. Also Lifestyles, modifications, particularly weight reduction may more improve the condition of this disease.
ACKNOWLEDGEMENTS
This article was extracted from MSc. dissertation which was approved by School of Nutrition and Food Sciences, Isfahan University of Medical Sciences with code 393254. We thank all the participants and Colleague of Food Security Research Center and Department of Community Nutrition, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–19. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 2.Khan N, Mukhtar H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008;8(269):269–80. doi: 10.1016/j.canlet.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stangl V, Lorenz M, Stangl K. The role of tea and tea flavonoids in cardiovascular health. Mol Nutr Food Res. 2006;50:218–28. doi: 10.1002/mnfr.200500118. [DOI] [PubMed] [Google Scholar]
- 4.Balentine DA, Wiseman SA, Bouwens LC. The chemistry of tea flavonoids. Crit Rev Food Sci Nutr. 1997;37:693–704. doi: 10.1080/10408399709527797. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Ho CT. Polyphenolic chemistry of tea and coffee: A century of progress. J Agric Food Chem. 2009;57:8109–14. doi: 10.1021/jf804025c. [DOI] [PubMed] [Google Scholar]
- 6.Hakim IA, Harris RB, Brown S, Chow HH, Wiseman S, Agarwal S, et al. Effect of increased tea consumption on oxidative DNA damage among smokers: A randomized controlled study. J Nutr. 2003;133:3303S–9S. doi: 10.1093/jn/133.10.3303S. [DOI] [PubMed] [Google Scholar]
- 7.Hamza A, Zhan CG. How can (-)-epigallocatechin gallate from green tea prevent HIV-1 infection. Mechanistic insights from computational modeling and the implication for rational design of anti-HIV-1 entry inhibitors? J Phys Chem B. 2006;110:2910–7. doi: 10.1021/jp0550762. [DOI] [PubMed] [Google Scholar]
- 8.Maeda-Yamamoto M, Inagaki N, Kitaura J, Chikumoto T, Kawahara H, Kawakami Y, et al. O-methylated catechins from tea leaves inhibit multiple protein kinases in mast cells. J Immunol. 2004;172:4486–92. doi: 10.4049/jimmunol.172.7.4486. [DOI] [PubMed] [Google Scholar]
- 9.Kim JA, Formoso G, Li Y, Potenza MA, Marasciulo FL, Montagnani M, et al. Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J Biol Chem. 2007;282:13736–45. doi: 10.1074/jbc.M609725200. [DOI] [PubMed] [Google Scholar]
- 10.Wolfram S. Effects of green tea and EGCG on cardiovascular and metabolic health. J Am Coll Nutr. 2007;26:373S–88S. doi: 10.1080/07315724.2007.10719626. [DOI] [PubMed] [Google Scholar]
- 11.Ueda M, Nishiumi S, Nagayasu H, Fukuda I, Yoshida K, Ashida H. Epigallocatechin gallate promotes GLUT4 translocation in skeletal muscle. Biochem Biophys Res Commun. 2008;377:286–90. doi: 10.1016/j.bbrc.2008.09.128. [DOI] [PubMed] [Google Scholar]
- 12.Koo SI, Noh SK. Green tea as inhibitor of the intestinal absorption of lipids: Potential mechanism for its lipid-lowering effect. J Nutr Biochem. 2007;18:179–83. doi: 10.1016/j.jnutbio.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murase T, Haramizu S, Shimotoyodome A, Tokimitsu I, Hase T. Green tea extract improves running endurance in mice by stimulating lipid utilization during exercise. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1550–6. doi: 10.1152/ajpregu.00752.2005. [DOI] [PubMed] [Google Scholar]
- 14.Bruno RS, Dugan CE, Smyth JA, DiNatale DA, Koo SI. Green tea extract protects leptin-deficient, spontaneously obese mice from hepatic steatosis and injury. J Nutr. 2008;138:323–31. doi: 10.1093/jn/138.2.323. [DOI] [PubMed] [Google Scholar]
- 15.Park HJ, DiNatale DA, Chung MY, Park YK, Lee JY, Koo SI, et al. Green tea extract attenuates hepatic steatosis by decreasing adipose lipogenesis and enhancing hepatic antioxidant defenses in ob/ob mice. J Nutr Biochem. 2011;22:393–400. doi: 10.1016/j.jnutbio.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed) 1986;292:13–5. doi: 10.1136/bmj.292.6512.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson G, Westerterp KR. Assessment of the physical activity level with two questions: Validation with doubly labeled water. Int J Obes (Lond) 2008;32:1031–3. doi: 10.1038/ijo.2008.42. [DOI] [PubMed] [Google Scholar]
- 18.Bergmeyer HU, Hørder M, Rej R. International Federation of Clinical Chemistry (IFCC) Scientific Committee, Analytical Section: Approved recommendation (1985) on IFCC methods for the measurement of catalytic concentration of enzymes. Part 3. IFCC method for alanine aminotransferase (L-alanine: 2-oxoglutarate aminotransferase, EC 2.6.1.2) J Clin Chem Clin Biochem. 1986;24:481–95. [PubMed] [Google Scholar]
- 19.Bergmeyer HU, Hørder M, Rej R. International Federation of Clinical Chemistry (IFCC) Scientific Committee, Analytical Section: Approved recommendation (1985) on IFCC methods for the measurement of catalytic concentration of enzymes. Part 2. IFCC method for aspartate aminotransferase (L-aspartate: 2-oxoglutarate aminotransferase, EC 2.6.1.1) J Clin Chem Clin Biochem. 1986;24:497–510. [PubMed] [Google Scholar]
- 20.Recommendations of the German Society for Clinical Chemistry. Standardization of methods for the determination of enzyme activities in biological fluids. Z Klin Chem Klin Biochem. 1970;8:658–60. [PubMed] [Google Scholar]
- 21.Angulo P. Obesity and nonalcoholic fatty liver disease. Nutr Rev. 2007;65(Suppl S1):S57–63. doi: 10.1111/j.1753-4887.2007.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 22.Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29:113–9. doi: 10.1111/j.1478-3231.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- 23.Nomura H, Kashiwagi S, Hayashi J, Kajiyama W, Tani S, Goto M. Prevalence of fatty liver in a general population of Okinawa, Japan. Jpn J Med. 1988;27:142–9. doi: 10.2169/internalmedicine1962.27.142. [DOI] [PubMed] [Google Scholar]
- 24.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29:478–85. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 25.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 26.Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, et al. Disease progression of non-alcoholic fatty liver disease: A prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969–74. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 27.Foroughi M, Maghsoudi Z, Ghiasvand R, Iraj B, Askari G. Effect of Vitamin D Supplementation on C-reactive Protein in Patients with Nonalcoholic Fatty Liver. Int J Prev Med. 2014;5:969–75. [PMC free article] [PubMed] [Google Scholar]
- 28.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: A follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 29.Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, Goto T, Westerbacka J, Sovijärvi A, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–8. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 30.Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Pichiri I, et al. Prevalence of non-alcoholic fatty liver disease and its association with cardiovascular disease in patients with type 1 diabetes. J Hepatol. 2010;53:713–8. doi: 10.1016/j.jhep.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 31.Jablonski KL, Jovanovich A, Holmen J, Targher G, McFann K, Kendrick J, et al. Low 25-hydroxyvitamin D level is independently associated with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2013;23:792–8. doi: 10.1016/j.numecd.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sae-tan S, Grove KA, Lambert JD. Weight control and prevention of metabolic syndrome by green tea. Pharmacol Res. 2011;64:146–54. doi: 10.1016/j.phrs.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daskalopoulou SS, Mikhailidis DP, Elisaf M. Prevention and treatment of the metabolic syndrome. Angiology. 2004;55:589–612. doi: 10.1177/00033197040550i601. [DOI] [PubMed] [Google Scholar]
- 34.Shrestha S, Ehlers SJ, Lee JY, Fernandez ML, Koo SI. Dietary green tea extract lowers plasma and hepatic triglycerides and decreases the expression of sterol regulatory element-binding protein-1c mRNA and its responsive genes in fructose-fed, ovariectomized rats. J Nutr. 2009;139:640–5. doi: 10.3945/jn.108.103341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamoto K, Takayama F, Mankura M, Hidaka Y, Egashira T, Ogino T, et al. Beneficial effects of fermented green tea extract in a rat model of non-alcoholic steatohepatitis. J Clin Biochem Nutr. 2009;44:239–46. doi: 10.3164/jcbn.08-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takato U, Ryuichiro S, Toru N, Takuji T, Michio S. Green tea with high-density catechins improves liver function and fat infiltration in non-alcoholic fatty liver disease (NAFLD) patients. Phytomedicine. 2013;12:410–5. doi: 10.3892/ijmm.2013.1503. [DOI] [PubMed] [Google Scholar]
- 37.Barchetta I, Angelico F, Del Ben M, Baroni MG, Pozzilli P, Morini S, et al. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med. 2011;9:85. doi: 10.1186/1741-7015-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murase T, Nagasawa A, Suzuki J, Hase T, Tokimitsu I. Beneficial effects of tea catechins on diet-induced obesity: Stimulation of lipid catabolism in the liver. Int J Obes Relat Metab Disord. 2002;26:1459–64. doi: 10.1038/sj.ijo.0802141. [DOI] [PubMed] [Google Scholar]
- 39.Ullmann U, Haller J, Bakker GC, Brink EJ, Weber P. Epigallocatechin gallate (EGCG) (TEAVIGO) does not impair nonhaem-iron absorption in man. Phytomedicine. 2005;12:410–5. doi: 10.1016/j.phymed.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Kohgo Y, Ohtake T, Ikuta K, Suzuki Y, Torimoto Y, Kato J. Dysregulation of systemic iron metabolism in alcoholic liver diseases. J Gastroenterol Hepatol. 2008;23(Suppl 1):S78–81. doi: 10.1111/j.1440-1746.2007.05290.x. [DOI] [PubMed] [Google Scholar]
- 41.Sumida Y, Kanemasa K, Fukumoto K, Yoshida N, Sakai K, Nakashima T, et al. Effect of iron reduction by phlebotomy in Japanese patients with nonalcoholic steatohepatitis: A pilot study. Hepatol Res. 2006;36:315–21. doi: 10.1016/j.hepres.2006.08.003. [DOI] [PubMed] [Google Scholar]


