Abstract
Background: Flavonoids have been implicated in the prevention of cardiovascular disease; however, their mechanisms of action have yet to be elucidated, possibly because most previous in vitro studies have used supraphysiological concentrations of unmetabolized flavonoids, overlooking their more bioavailable phenolic metabolites.
Objective: We aimed to explore the effects of phenolic metabolites and their precursor flavonoids at physiologically achievable concentrations, in isolation and combination, on soluble vascular cellular adhesion molecule-1 (sVCAM-1).
Method: Fourteen phenolic acid metabolites and 6 flavonoids were screened at 1 μM for their relative effects on sVCAM-1 secretion by human umbilical vein endothelial cells stimulated with tumor necrosis factor alpha (TNF-α). The active metabolites were further studied for their response at different concentrations (0.01 μM–100 μM), structure-activity relationships, and effect on vascular cellular adhesion molecule (VCAM)-1 mRNA expression. In addition, the additive activity of the metabolites and flavonoids was investigated by screening 25 unique mixtures at cumulative equimolar concentrations of 1 μM.
Results: Of the 20 compounds screened at 1 μM, inhibition of sVCAM-1 secretion was elicited by 4 phenolic metabolites, of which protocatechuic acid (PCA) was the most active (−17.2%, P = 0.05). Investigations into their responses at different concentrations showed that PCA significantly reduced sVCAM-1 15.2–36.5% between 1 and 100 μM, protocatechuic acid-3-sulfate and isovanillic acid reduced sVCAM-1 levels 12.2–54.7% between 10 and 100 μM, and protocatechuic acid-4-sulfate and isovanillic acid-3-glucuronide reduced sVCAM-1 secretion 27.6% and 42.8%, respectively, only at 100 μM. PCA demonstrated the strongest protein response and was therefore explored for its effect on VCAM-1 mRNA, where 78.4% inhibition was observed only after treatment with 100 μM PCA. Mixtures of the metabolites showed no activity toward sVCAM-1, suggesting no additive activity at 1 μM.
Conclusions: The present findings suggest that metabolism of flavonoids increases their vascular efficacy, resulting in a diversity of structures of varying bioactivity in human endothelial cells.
Keywords: polyphenol, VCAM-1, metabolism, endothelial, inflammation, phase II conjugate
Introduction
Epidemiological studies have demonstrated associations between diets high in flavonoid-rich foods and the reduced risk of cardiovascular disease (1–3). Furthermore, the protective effects of dietary flavonoids have been observed in numerous randomized control trials (4–8) and animal feeding studies (9–14). Unfortunately, the direct mechanisms of action of flavonoids have been elusive. Much of the focus of previous studies has been on direct vascular reactivity, affecting blood pressure, blood flow, heart rate variability, and flow-mediated vasodilation (15, 16); however, low-level chronic inflammation, attributed to the expression of vascular adhesion molecules on the surface of the endothelium, has long been implicated as a driving factor in the early stages of atherosclerosis (17, 18). TNF-α is a cytokine that serves as a mediator in a number of diseases, such as atherosclerosis, and stimulates the production of a number of pro-inflammatory biomarkers (19), such as circulating levels of soluble vascular adhesion molecule-1 (sVCAM-1)8, an important predictor of risk of death from coronary heart disease (20). TNF-α–stimulated sVCAM-1 expression therefore provided a logical target for exploring the potential mechanisms of action of flavonoid metabolites in the present investigation. The present study used human umbilical vein endothelial cells (HUVECs), which are an established model for the study of endothelial dysfunction, with similar expression profiles to arterial endothelial cells in response to inflammatory stimuli (21).
Previous studies have demonstrated potentially beneficial effects of flavonoids on some inflammatory mechanisms in vitro, including inhibition of the adhesion of leukocytes to endothelial cells (22–24). Unfortunately again, the mechanisms underlining these effects are unknown, potentially because previous in vitro investigations have focused on the activity of unmetabolized flavonoids, which are found in relatively low abundance in the circulation compared with their metabolites, and have considerably shorter half-lives (25–28). It has therefore been suggested that the biological activity observed in human studies results from the activity of products of bacterial catabolism, absorption, and further phase II metabolism (29, 30), which were the focus of the present study. In addition, many past in vitro studies have used supraphysiological concentrations of precursor/unmetabolized flavonoids, while only a limited few have reported the activity of free-phenolic acids (31). Until recently (32, 33) few have explored the activity of phase II conjugates of phenolic acid derivatives (34–37), primarily as a result of the lack of availability of synthetic standards (29, 38). We hypothesized that phenolic metabolites of flavonoids will have differential biological activities to their precursor structures and that metabolites in combination may have additive or synergistic effects. We therefore screened 6 flavonoids found commonly in the Western diet (Supplemental Figure 1); 14 human metabolites, as previously reported (25–27, 39); and 25 combinations of the flavonoids and their metabolites (at equimolar concentrations) (Figure 1), for their ability to reduce sVCAM-1 protein secretion by TNF-α–stimulated HUVECs. Investigations into the response to different concentrations of active treatments were also explored, including 4 physiological (between 0.01 μM and 10 μM) and 1 supraphysiological (100 μM) concentration. The most active treatment was further assessed for its activity on transcription regulation via mRNA expression of vascular cellular adhesion molecule (VCAM)-1.
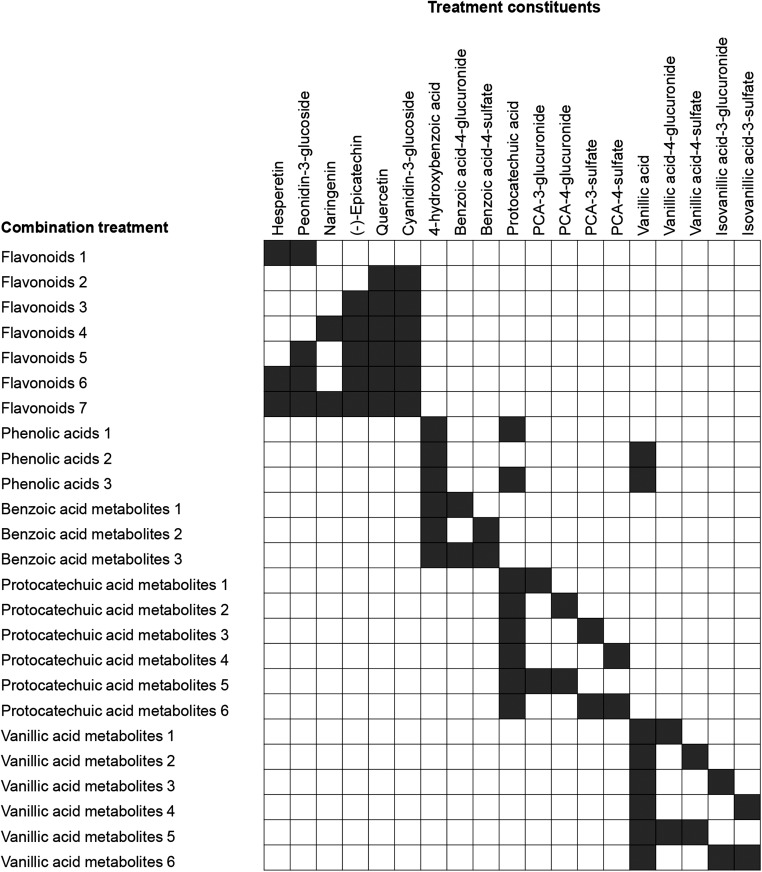
FIGURE 1.
Combination treatments used in this study. Shaded boxes represent inclusion of respective compounds in equimolar concentrations to a cumulative concentration of 1 μM; for example, a combination comprising 4 constituents would require 0.25 μM of each to yield a final concentration of 1 μM. PCA, protocatechuic acid.
Methods
Materials.
Early passage HUVECs (cryopreserved, pooled donors), large vessel endothelial growth medium (containing 2% fetal calf serum, human epidermal growth factor, human fibroblast growth factor, 25 μg/mL gentamycin, 50 μg/L amphotericin, hydrocortisone, and heparin), and trypsin passage pack were purchased from Caltag Medsystems (Buckingham, United Kingdom). Human-derived fibronectin, TNF-α, and BAY 11–7085 were purchased from Sigma Aldrich (Dorset, United Kingdom). The conjugated metabolites, as listed in Supplemental Figure 1, were synthesized at the University of St. Andrews (United Kingdom) (40). All flavonoids and unconjugated phenolic acids were obtained from Sigma Aldrich (Dorset, United Kingdom), with the exception of peonidin-3-glucoside (Extrasynthase, France).
Preparation of treatment solutions.
Stock solutions of all compounds screened were prepared in 100% DMSO at 200 mM and stored at −80°C with the exception of cyanidin-3-glucoside and peonidin-3-glucoside, which were prepared at 40 mM, and the sulfate-conjugated phenolic acids, which were prepared at 25 mM in 50% DMSO (50% PBS) to maintain stability while reducing final DMSO concentrations in working solutions. Working solutions of all treatments were made in supplemented media. Treatments containing mixtures of compounds (Figure 1) consisted of equimolar concentrations of the constituent treatment compounds to a cumulative concentration of 1 μM.
Cell culture.
HUVECs were maintained in supplemented large vessel endothelial medium on fibronectin-coated cell culture plates (0.25 μg/cm2) at 37°C, 5% CO2, in a humidified atmosphere, as previously described (40). Cells were reseeded at 90–95% confluence using a trypsin passage pack. HUVECs were used at passage 4 in all experiments.
Cell treatment and stimulation.
HUVECs were seeded into fibronectin-coated 24-well cell culture plates (0.25 μg/cm2) and incubated in supplemented media for 24 h before treatment. Media was then removed and replaced by 1 μM treatment solutions for the screen of individual compounds and treatment mixtures (1 μM cumulatively) and 0.01–100 μM for protein and mRNA concentration response experiments. IκBα inhibitor BAY 11–7085 (41) was included as a negative control for all mRNA experiments. Cells were incubated with treatment solutions for 30 min before the addition of TNF-α (10 μg/L) for 18 h, as used in previous studies (42, 43). A vehicle control of equivalent DMSO concentration was used in each experiment, where the concentration did not exceed 0.1%, as was the control for 100 μM solutions, and did not exceed 0.02% for all other treatments. Cell culture supernatants were collected and used immediately or stored at −80°C until required.
sVCAM-1 ELISA.
Human sVCAM-1 protein levels in recovered cell culture supernatants were assayed using a Human VCAM-1/CD106 DuoSet ELISA kit (R&D Systems; Abingdon, United Kingdom), according to the manufacturer’s instructions. sVCAM-1 was quantified via colorimetric assay at 450 nm, corrected for 570 nm, using a BMG (LABTECH) plate reader. The interassay coefficient of variation was 8.8%.
RNA extraction, reverse transcription, and real-time qPCR.
Total RNA was extracted from HUVECs and reverse transcribed to cDNA using conditions previously described by this group (32). Real-time qPCR was carried out using 25 ng of cDNA of each sample, with the addition of VCAM-1 primers (forward primer, 5′-CAGGCTAAGTTACATATTGATGACAT-3′; reverse primer, 5′-GAGGAAGGGCTGACCAAGAC-3′) and real time PCR Precision master mix with SYBR green (Primer Design). Real-time qPCR was carried out using the ABI7500 system, using cycle methods previously described (32). Relative changes in gene expression from the TNF-α control were quantified using the comparative Ct method (44). The difference between recorded Ct values for treatment and positive control was calculated in the first instance for all genes. VCAM-1 values were normalized to 2 geNORM housekeeping genes, UBE2D2 and PRDM4 (Primer Design), selected based on their stability, as established using qPCR data analysis software qbasePLUS2 (Biogazelle, Belgium), where the geometric mean of the 2 housekeeping genes was used as the normalization factor (45).
Data analysis.
sVCAM-1 protein (in pg/mL) or mRNA (fold change) were recorded as the mean of 2 technical duplicates and reported relative to the TNF-α positive control (containing TNF-α without DMSO), where data represents the mean ± SD of 3 independent measures (n = 3). Unequal variances were tested by use of Levene’s test, where the null hypothesis was rejected at the level of 0.05. Treatments containing combinations of metabolites were identified as nonparametric, whereas all other treatments satisfied Levene’s criteria. Where unequal variances were identified (i.e., treatments containing combinations of metabolites), between-group differences were established via Kruskal-Wallis ANOVA. Treatment effects for parametric variables were established by 1-factor ANOVA with post hoc least square difference. Analysis was conducted using SPSS for Windows (version 22.0; IBM). Data were considered significant where P ≤ 0.05. Untreated and negative controls were not included in the ANOVA for treatment effect but presented graphically, where a Student’s t test established difference relative to the vehicle control (DMSO). For screening purposes, treatments displaying nonsignificant values ≤0.15 were taken forward, for validation in subsequent concentration analysis.
Results
Effects of flavonoid and flavonoid metabolites on sVCAM-1 secretion.
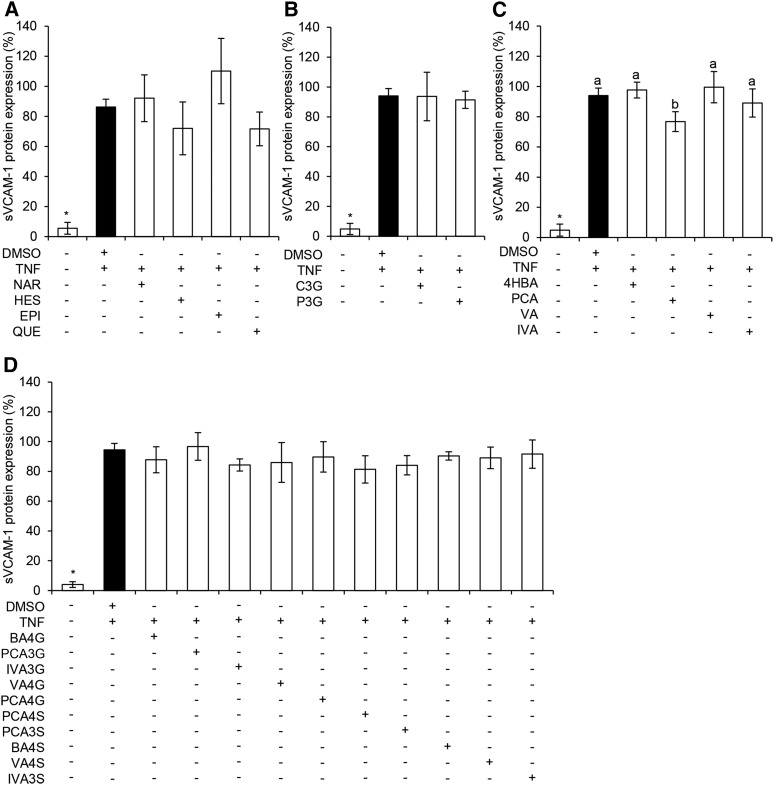
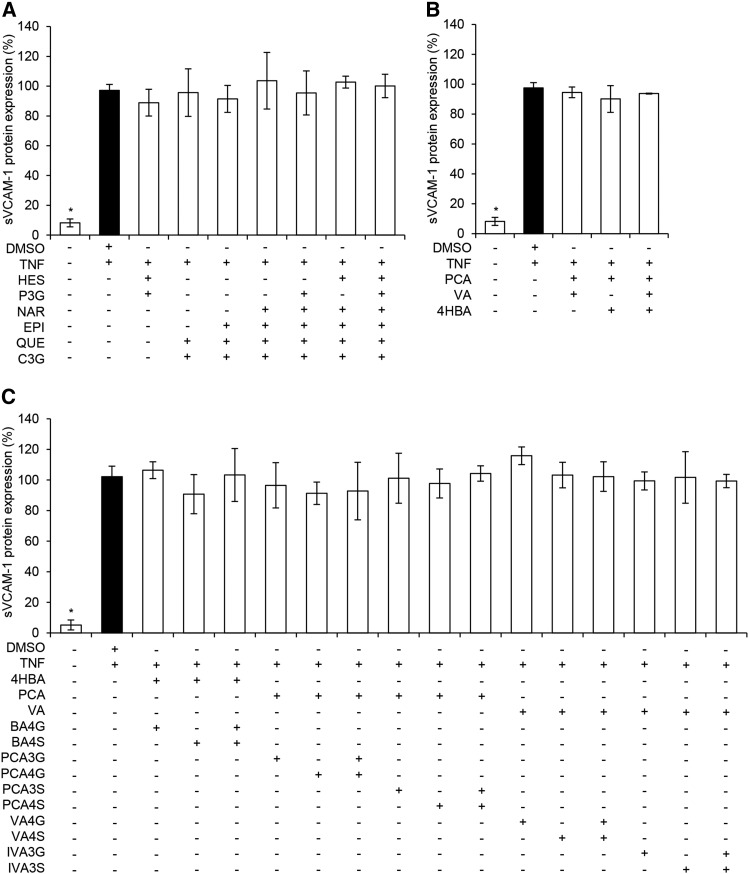
Six flavonoids and 14 phenolic metabolites were screened at a concentration of 1 μM for their ability to reduce TNF-α–stimulated sVCAM-1 secretion by HUVECs. Precursor flavonoids had no significant effect on sVCAM-1 secretion, although there was a moderate, nonsignificant increase in the secretion of sVCAM-1 (P = 0.14) following treatment with (-)-epicatechin (Figure 2). The metabolite protocatechuic acid (PCA) significantly decreased sVCAM-1 secretion (P = 0.05), whereas nonsignificant effects were observed for treatments with sulfate [protocatechuic acid-4-sulfate (PCA4S), P = 0.07; protocatechuic acid-3-sulfate (PCA3S), P = 0.14] and glucuronide [isovanillic acid-3-glucuronide (IVA3G), P = 0.15] conjugates of PCA. Treatments showing the greatest activity (P ≤ 0.15) were taken forward to explore response at different concentrations. Seven treatments containing mixtures of flavonoids and 18 treatments containing mixtures of phenolic metabolites were also investigated for their effect on sVCAM-1 secretion (Figure 3); however, no activity was observed at a cumulative concentration of 1 μM of the compounds (P ≥ 0.27), and therefore no combination treatments were taken forward for analysis of response to different concentrations.
FIGURE 2.
Effect of 1 μM flavonoids and phenolic acid metabolites on TNF-α–stimulated sVCAM-1 protein secretion by HUVECs. Flavonoids (A), anthocyanin glucosides (B), unconjugated phenolic acids (C), and conjugated phenolic acids (D) are shown. Data were normalized to a TNF-α control, and columns represent the mean ± SD (n = 3 independent measures). Labeled means without a common letter differ, P ≤ 0.05 (ANOVA with post hoc LSD). *Different from DMSO, P ≤ 0.05 (t test). BA4G, benzoic acid-4-glucuronide; BA4S, benzoic acid-4-sulfate; C3G, cyanidin-3-glucoside; EPI, (-) epicatechin; HES, hesperetin; HUVEC, human umbilical vein endothelial cell; IVA, isovanillic acid; IVA3G, IVA-3-glucuronide; IVA3S, IVA-3-sulfate; LSD, least square difference; NAR, naringenin; PCA, protocatechuic acid; PCA3G, PCA-3-glucuronide; PCA3S, PCA-3-sulfate; PCA4G, PCA-4-glucuronide; PCA4S, PCA-4-sulfate; P3G, peonidin-3-glucoside; QUE, quercetin; sVCAM-1, soluble vascular cellular adhesion molecule 1; VA, vanillic acid; VA4G, VA-4-glucuronide; VA4S, VA-4-sulfate; 4HBA, 4-hydroxybenzoic acid.
FIGURE 3.
Effect of 1 μM mixtures of flavonoids and phenolic acid metabolites on TNF-α stimulated sVCAM-1 protein secretion by HUVECs. Flavonoid mixtures (A), phenolic acid mixtures (B), and conjugated and unconjugated phenolic metabolite mixtures (C) are shown. Data were normalized to a TNF-α control, and columns represent the mean ± SD (n = 3 independent measures). *Different from DMSO, P ≤ 0.05 (t test). Where unequal variance was identified (B and C), group differences were established via Kruskal-Wallis nonparametric ANOVA. BA4G, benzoic acid-4-glucuronide; BA4S, benzoic acid-4-sulfate; C3G, cyanidin-3-glucoside; EPI, (-) epicatechin; HES, hesperetin; HUVEC, human umbilical vein endothelial cell; IVA3G, isovanillic acid-3-glucuronide; IVA3S, isovanillic acid-3-sulfate; NAR, naringenin; PCA, protocatechuic acid; PCA3G, PCA-3-glucuronide; PCA3S, PCA-3-sulfate; PCA4G, PCA-4-glucuronide; PCA4S, PCA-4-sulfate; P3G, peonidin-3-glucoside; QUE, quercetin; sVCAM-1, soluble vascular cellular adhesion molecule 1; VA, vanillic acid; VA4G, VA-4-glucuronide; VA4S, VA-4-sulfate; 4HBA, 4-hydroxybenzoic acid.
Response to different concentrations of active metabolites on sVCAM-1 secretion.
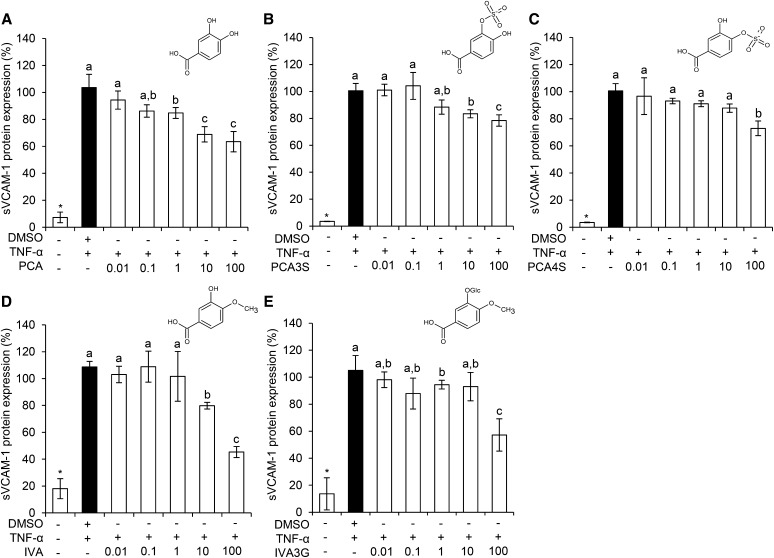
sVCAM-1 secretion was investigated following treatment with 0.01 μM–100 μM of the most active treatments PCA, PCA3S, PCA4S, and IVA3G (Figure 4). Isovanillic acid (IVA), although not active in the sVCAM-1 protein screen, was also included in order to establish structure-activity relationships (SARs) with PCA and IVA conjugates. Here, PCA significantly reduced sVCAM-1 levels in a concentration-dependent manner at concentrations between 1 μM and 100 μM, whereas PCA3S and IVA were active at levels between 10 μM and 100 μM, and PCA4S and IVA3G were only active at 100 μM.
FIGURE 4.
Effect of concentration of phenolic acid metabolites on TNF-α–stimulated sVCAM-1 protein secretion by HUVECs. PCA (A), PCA3S (B), PCA4S (C), IVA (D), and IVA3G (E) are shown. Data were normalized to a TNF-α control, and columns represent the mean ± SD (n = 3 independent measures). Labeled means without a common letter differ, P ≤ 0.05 (ANOVA with post hoc LSD). *Different from DMSO, P ≤ 0.05 (t test). HUVEC, human umbilical vein endothelial cell; IVA, isovanillic acid; IVA3G, IVA-3-glucuronide; LSD, least square difference; PCA, protocatechuic acid; PCA3S, PCA-3-sulfate; PCA4S, PCA-4-sulfate; sVCAM-1, soluble vascular cellular adhesion molecule 1.
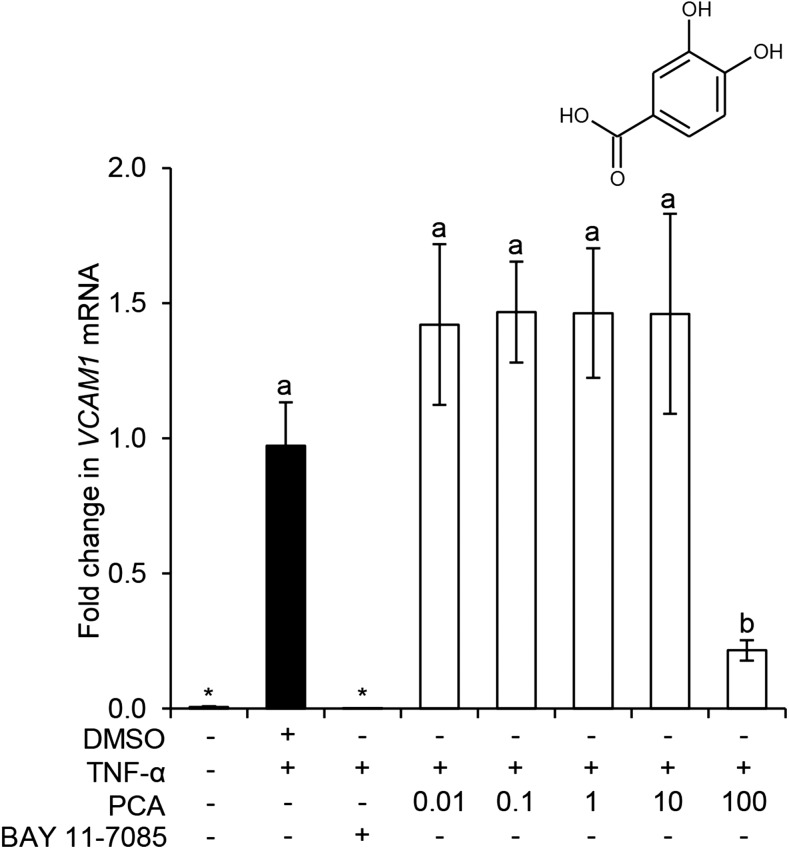
Response to different concentrations of PCA on VCAM-1 mRNA expression.
Because PCA showed the highest activity on protein secretion, and this effect was amplified with increased concentration, we further investigated whether this response was reciprocated in VCAM-1 mRNA expression (Figure 5). Here, TNF-α significantly induced VCAM-1 mRNA expression after 4 h (P ≤ 0.01), and this effect was fully inhibited by treatment with the negative control (IκBα-inhibitor BAY 11–7085). Treatment with 100 μM PCA was the only concentration to significantly inhibit VCAM-1 mRNA expression (78% inhibition; P = 0.05).
FIGURE 5.
Effect of concentration of PCA on TNF-α–stimulated VCAM-1 mRNA expression in HUVECs. Data were normalized to a TNF-α control, and columns represent the mean ± SD (n = 3 independent measures). Labeled means without a common letter differ, P ≤ 0.05 (ANOVA with post hoc LSD). *Different from DMSO, P ≤ 0.05 (t test). HUVEC, human umbilical vein endothelial cell; LSD, least square difference; PCA, protocatechuic acid; VCAM, vascular cellular adhesion molecule.
Discussion
The consumption of flavonoid-rich foods has been associated with reduced cardiovascular disease risk (1–3), yet their mechanisms of action have yet to be elucidated. It has been suggested that the beneficial effects of flavonoids are the result of degradation products (chemical degradation or bacterial catabolites) and phase II metabolites (29, 30). We investigated phenolic metabolites commonly reported for berry anthocyanins (39, 46), cocoa and tea (47–50), and citrus fruits (51), focusing on metabolites that differed primarily on their 3′ and 4′ positions (relative to the precursor B-ring structure) to draw conclusions regarding SARs. The focus on physiologically achievable concentrations of metabolites in cell culture studies is reasonably contemporary (29, 52) , whereas investigation of the additive activities of conjugated and unconjugated metabolites in combination, at concentrations achievable through diet, is novel. We investigated the differential effects of 20 flavonoids and common metabolites and 25 combinations thereof. We found that 4 phenolic metabolites modulated sVCAM-1 secretion, whereas no effects were observed for their precursor structures. The response to PCA appeared amplified with increasing concentration, which is suggestive of a dose-dependent response. Inhibition of VCAM-1 mRNA was observed in response to PCA; however, this was only at a supraphysiological concentration of 100 μM. Furthermore, mixtures of metabolites and flavonoids showed no activity toward sVCAM-1, suggesting no additive activity.
sVCAM-1 was a logical target to investigate the vasoprotective activity of flavonoids, because it is a clinical predictor of risk of death from cardiovascular disease (20) and previous studies have demonstrated beneficial effects of flavonoids on adhesion of leukocytes to endothelial cells (22–24). These findings are supported by additional recent studies by our group, where metabolites were active on IL-6 and sVCAM-1 production following stimulation with CD40 and oxidized LDL in vascular endothelial cells and TNF-α following LPS stimulation in human monocytes (32, 33).
Five treatments (Figure 4) were further explored for the effect of increased concentration on sVCAM-1 protein; here, 1 metabolite (PCA) significantly inhibited sVCAM-1; 3 (PCA3S, PCA4S, IVA3G) had moderate, nonsignificant activity; and 1 (IVA) was selected to draw conclusions regarding SARs between PCA and IVA conjugates. Of the compounds screened, PCA was most active across the concentrations tested, which is in line with previous studies, where it was observed to inhibit the expression of inflammatory mediators, including adhesion molecules (53, 54). The activity of PCA was comparable to its aldehyde equivalent (protocatechuic aldehyde), in a study identifying a concentration-dependent reduction in TNF-α–stimulated sVCAM-1 (42); this supports the premise that the catechol moiety of flavonoid metabolites holds significant activity (55). However, given PCAs reactive catechol moiety is rapidly methylated by catechol-O-methyltransferase (56), it does not persist in the systemic circulation at any appreciable concentration for significant periods of time (25, 39, 46), whereas its metabolite, vanillic acid, exists at much higher concentrations and has a considerably longer half-life (25, 39, 46, 51). Vanillic acid may therefore make an appropriate target for future investigation, given that it was recently shown to have a significant effect on CD40-stimulated VCAM-1 mRNA (32).
The lack of dietary relevance of contemporary cell culture studies in the field of nutrition is apparent, given the use of precursor structures at supraphysiological concentrations, which may explain why the underlying mechanisms of action are still unknown (29). It is interesting that we observed significant inhibition of sVCAM-1 in response to PCA at 1 μM, because previous studies have identified serum concentrations of PCA ranging between 0.15 μM (25) and 1.5 μM (46, 57), suggesting this effect is achievable through diet.
Given the apparent strength and linearity of the concentration responsiveness of PCA, we sought to explore if this was reflected in the expression of VCAM-1 mRNA (Figure 5), as advocated by others (32, 58). Here, PCA was only active at 100 μM, suggesting PCA is not directly active on mRNA transcription at physiologically achievable concentrations, but likely acting post-translationally. It is conceivable metabolites could interact with the cleavage of the protein from the surface of endothelial cells (59), such as by interaction with TNF-α converting enzyme ADAM17 (60), an indicated mediator of VCAM-1 shedding from the surface of endothelial cells. Future studies exploring the mechanisms of action of PCA should therefore focus on post-translational or receptor-binding activities.
Investigations of SARs are important for understanding how metabolism alters phytochemical activity. Because previous studies have reported the SAR of flavonoids (61, 63), we aimed to draw conclusions based on relationships between conjugated and unconjugated phenolic metabolites (Supplemental Figure 2). Of the 5 metabolites studied, PCA had the greatest effect on sVCAM-1, with PCA3S, PCA4S, and IVA having equally lesser activity and IVA3G having no effect, suggesting conjugation of both the hydroxyl moieties reduces potency on sVCAM-1 secretion. Conjugation has also been shown to reduce the inhibitory activity of certain flavonoids on monocyte adhesion (52, 55); however, the opposite has recently been reported in oxidized-LDL–stimulated HUVECs, where conjugation of PCA increased the inhibition of sVCAM-1 (32), suggesting the effects of conjugation are dependent on the inflammatory stimulus, and thus the upstream signal transduction pathway involved, as suggested for other flavonoids (63).
After ingestion, flavonoids circulate as complex mixtures of metabolites at various concentrations (26, 39, 64), thus it is important that this is reflected in cell culture experiments. Few studies have explored the effects of flavonoids in combination, despite indication of differential activities when in combination relative to isolation (65, 66). The present study examined 25 mixtures of equimolar concentrations of structurally similar compounds. Treatments were designed in this manner because plasma concentrations of flavonoid/metabolites vary greatly between, and indeed within, subjects. Therefore, it was deemed unrealistic to model treatments mimicking human plasma compositions identified in any single feeding study. Here we did not observe any inhibitory effects on sVCAM-1 secretion from mixtures totaling 1 μM in concentration (cumulative concentration of analytes present in an equimolar ratio). This represented concentrations of each analyte between 0.5 and 0.17 μM, and although these concentrations represent physiologically relevant levels, it is possible that they were too low to elicit a quantifiable response. A recent human study identified total phenolic metabolites as high as 13.3 μM following consumption of orange juice flavanones (51), suggesting greater cumulative concentrations are indicated in future cell culture studies. Given that mixtures of phenolic metabolites have shown differential effects to their constituents in isolation in LPS stimulated THP-1 cells (33), cumulative effects of these metabolites may be cell-type specific.
Here we provide insight into the differential activity of flavonoid metabolites and have explored their potential for additive effects; however, there are certain limitations of this work. The use of soluble VCAM-1 over membrane-bound VCAM-1 could be a limitation because it is the membrane-bound VCAM-1 that binds directly to leukocytes in the progression of atherosclerosis (18). However, it has been reported that sVCAM-1 protein levels directly correlate with levels of surface-bound VCAM-1 (67) and may be a more appropriate biomarker of endothelial cell activation (59); that said, further investigation of the relative activity of these metabolites on surface-bound VCAM-1 would verify such correlations (67). The concentration of TNF-α used to stimulate sVCAM-1 could also be viewed as a limitation. The concentration 10 μg/L was selected because it is commonly reported in the literature (41, 42, 68, 69); however, physiologically, plasma concentrations are reported at 0.001–0.04 μg/L in patients with coronary artery disease (70) and can reach 2 μg/L in patients who have suffered myocardial infarction (71). Future studies could therefore consider using models that more closely reflect in vivo conditions. The stimulation time used in our pro-inflammatory model could also be considered a limitation. As previously discussed, the design of the treatment mixtures used in this investigation was quite artificial, because equimolar ratios would not mimic serum concentrations observed in human studies; future studies could therefore use metabolites at a range of concentrations, based on levels reported in human feeding studies (25–27). Finally, HUVECs do not originate from the arterial walls, and therefore their use may be considered a limitation. Verification was carried out using single donor, human coronary artery endothelial cells, where induction of VCAM-1 in response to TNF-α was greater than in HUVECs; however, the response to increasing concentrations of PCA was similar in both cell types (data not shown).
The present study supports previous reports that metabolism of flavonoids to phenolic acids alters their anti-inflammatory effects (61, 72–74). Our data indicate that the degradation of flavonoids to phenolic acids, which is believed to be largely facilitated by microbiota in the colon (25, 64), increases their overall bioactivity, whereas further conjugation by phase II enzymes may have differential effects on activity (32, 33). Therefore, certain flavonoids consumed in our habitual diet may require prior metabolism before they can exert their maximal effects and metabolites may possess differential bioactivities as they are systematically metabolized and eliminated from the circulation.
In conclusion, the present study provides insight into the activity of conjugated and unconjugated phenolic metabolites of flavonoids, thus contributing to our understanding of how these dietary phytochemicals influence cardiovascular health.
Acknowledgments
We thank the late Nigel Botting (St. Andrews University), who helped establish this research collaboration, including the design of the synthesis project objectives. EFW conducted the cell culture work and collected and graphed the data; EFW, MAOC, and CDK designed the culture experiments and methodology and contributed to the analysis and interpretation of the data; EFW and CDK drafted the initial manuscript; QZ and KSR synthesized the phenolic metabolites; QZ, KSR, and DOH jointly developed the synthetic strategy; MAOC and CDK conceived and managed the project. All authors reviewed and approved the final manuscript.
Footnotes
Abbreviations used: HUVEC, human umbilical vein endothelial cell; IVA, isovanillic acid; IVA3G, isovanillic acid-3-glucuronide; PCA, protocatechuic acid; PCA3S, protocatechuic acid-3-sulfate; PCA4S, protocatechuic acid-4-sulfate; SAR, structure-activity relationship; sVCAM-1, soluble vascular cellular adhesion molecule 1; VCAM, vascular cellular adhesion molecule.
References
- 1.Andújar I, Recio MC, Giner RM, Rios JL. Cocoa polyphenols and their potential benefits for human health. Oxid Med Cell Longev 2012;2012:906252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geleijnse JM, Launer LJ, Van der Kuip DA, Hofman A, Witteman JC. Inverse association of tea and flavonoid intakes with incident myocardial infarction: the Rotterdam Study. Am J Clin Nutr 2002;75:880–6. [DOI] [PubMed] [Google Scholar]
- 3.Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DR Jr. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr 2007;85:895–909. [DOI] [PubMed] [Google Scholar]
- 4.Barona J, Aristizabal JC, Blesso CN, Volek JS, Fernandez ML. Grape polyphenols reduce blood pressure and increase flow-mediated vasodilation in men with metabolic syndrome. J Nutr 2012;142:1626–32. [DOI] [PubMed] [Google Scholar]
- 5.Weseler AR, Ruijters EJ, Drittij-Reijnders MJ, Reesink KD, Haenen GR, Bast A. Pleiotropic benefit of monomeric and oligomeric flavanols on vascular health–a randomized controlled clinical pilot study. PLoS One 2011;6:e28460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bondonno CP, Yang X, Croft KD, Considine MJ, Ward NC, Rich L, Puddey IB, Swinny E, Mubarak A, Hodgson JM. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: a randomized controlled trial. Free Radic Biol Med 2012;52:95–102. [DOI] [PubMed] [Google Scholar]
- 7.Curtis PJ, Kroon PA, Hollands WJ, Walls R, Jenkins G, Kay CD, Cassidy A. Cardiovascular disease risk biomarkers and liver and kidney function are not altered in postmenopausal women after ingesting an elderberry extract rich in anthocyanins for 12 weeks. J Nutr 2009;139:2266–71. [DOI] [PubMed] [Google Scholar]
- 8.Faridi Z, Njike VY, Dutta S, Ali A, Katz DL. Acute dark chocolate and cocoa ingestion and endothelial function: a randomized controlled crossover trial. Am J Clin Nutr 2008;88:58–63. [DOI] [PubMed] [Google Scholar]
- 9.Bornhoeft J, Castaneda D, Nemoseck T, Wang PW, Henning SM, Hong MY. The protective effects of green tea polyphenols: lipid profile, inflammation, and antioxidant capacity in rats fed an atherogenic diet and dextran sodium sulfate. J Med Food 2012;15:726–32. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi C, Upaganalawar A, Balaraman R. Protection against in vivo focal myocardial ischemia/reperfusion injury-induced arrhythmias and apoptosis by Hesperidin. Free Radic Res 2009;43:817–27. [DOI] [PubMed] [Google Scholar]
- 11.Heeba GH, Mahmoud ME, El Hanafy AA. Anti-inflammatory potential of curcumin and quercetin in rats: Role of oxidative stress, heme oxygenase-1 and TNF-alpha. Toxicol Ind Health 2014; 30:551–60. [DOI] [PubMed] [Google Scholar]
- 12.Loke WM, Proudfoot JM, Hodgson JM, McKinley AJ, Hime N, Magat M, Stocker R, Croft KD. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler Thromb Vasc Biol 2010;30:749–57. [DOI] [PubMed] [Google Scholar]
- 13.Nabavi SF, Nabavi SM, Mirzaei M, Moghaddam AH. Protective effect of quercetin against sodium fluoride induced oxidative stress in rat’s heart. Food Funct 2012;3:437–41.22314573 [Google Scholar]
- 14.Sheng R, Gu ZL, Xie ML, Zhou WX, Guo CY. EGCG inhibits proliferation of cardiac fibroblasts in rats with cardiac hypertrophy. Planta Med 2009;75:113–20. [DOI] [PubMed] [Google Scholar]
- 15.Kay CD, Hooper L, Kroon PA, Rimm EB, Cassidy A. Relative impact of flavonoid composition, dose and structure on vascular function: a systematic review of randomised controlled trials of flavonoid-rich food products. Mol Nutr Food Res 2012;56:1605–16. [DOI] [PubMed] [Google Scholar]
- 16.Ried K, Sullivan TR, Fakler P, Frank OR, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev 2012;8:CD008893. [DOI] [PubMed] [Google Scholar]
- 17.Ley K, Huo Y. VCAM-1 is critical in atherosclerosis. J Clin Invest 2001;107:1209–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lusis AJ. Atherosclerosis. Nature 2000;407:233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakao S, Kuwano T, Ishibashi T, Kuwano M, Ono M. Synergistic effect of TNF-alpha in soluble VCAM-1-induced angiogenesis through alpha 4 integrins. J Immunol 2003;170:5704–11. [DOI] [PubMed] [Google Scholar]
- 20.Blankenberg S, Rupprecht HJ, Bickel C, Peetz D, Hafner G, Tiret L, Meyer J. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation 2001;104:1336–42. [DOI] [PubMed] [Google Scholar]
- 21.Luu NT, Rahman M, Stone PC, Rainger GE, Nash GB. Responses of endothelial cells from different vessels to inflammatory cytokines and shear stress: evidence for the pliability of endothelial phenotype. J Vasc Res 2010;47:451–61. [DOI] [PubMed] [Google Scholar]
- 22.Chanet A, Milenkovic D, Claude S, Maier JA, Khan MK, Rakotomanomana N, Shinkaruk S, Berard AM, Bennetau-Pelissero C, Mazur A, et al. . Flavanone metabolites decrease monocyte adhesion to TNF-alpha-activated endothelial cells by modulating expression of atherosclerosis-related genes. Br J Nutr 2013;110:587–98. [DOI] [PubMed] [Google Scholar]
- 23.Chen JW, Lin FY, Chen YH, Wu TC, Chen YL, Lin SJ. Carvedilol inhibits tumor necrosis factor-alpha-induced endothelial transcription factor activation, adhesion molecule expression, and adhesiveness to human mononuclear cells. Arterioscler Thromb Vasc Biol 2004;24:2075–81. [DOI] [PubMed] [Google Scholar]
- 24.Claude S, Boby C, Rodriguez-Mateos A, Spencer JP, Gerard N, Morand C, Milenkovic D. Flavanol metabolites reduce monocyte adhesion to endothelial cells through modulation of expression of genes via p38-MAPK and p65-Nf-kB pathways. Mol Nutr Food Res 2014;58:1016–27. [DOI] [PubMed] [Google Scholar]
- 25.de Ferrars RM, Czank C, Zhang Q, Botting NP, Kroon PA, Cassidy A, Kay CD. The pharmacokinetics of anthocyanins and their metabolites in humans. Br J Pharmacol 2014;171:3268–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereira-Caro G, Borges G, van der Hooft J, Clifford MN, Del Rio D, Lean ME, Roberts SA, Kellerhals MB, Crozier A. Orange juice (poly)phenols are highly bioavailable in humans. Am J Clin Nutr 2014;100:1378–84. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Mateos A, Del Pino-Garcia R, George TW, Vidal-Diez A, Heiss C, Spencer JP. Impact of processing on the bioavailability and vascular effects of blueberry (poly)phenols. Mol Nutr Food Res 2014;58:1952–61. [DOI] [PubMed] [Google Scholar]
- 28.McKay DL, Chen CY, Zampariello CA, Blumberg JB. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chem 2015;168:233–40. [DOI] [PubMed] [Google Scholar]
- 29.Kay CD. The future of flavonoid research. Br J Nutr 2010;104 Suppl 3:S91–5. [DOI] [PubMed] [Google Scholar]
- 30.Murota K, Terao J. Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch Biochem Biophys 2003;417:12–7. [DOI] [PubMed] [Google Scholar]
- 31.Edwards M, Czank C, Woodward GM, Cassidy A, Kay CD. Phenolic metabolites of anthocyanins modulate mechanisms of endothelial function. J Agric Food Chem 2015;63:2423–31. [DOI] [PubMed] [Google Scholar]
- 32.Amin HP, Czank C, Raheem KS, Zhang Q, Botting NP, Cassidy A, Kay CD. Anthocyanin and its physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol Nutr Food Res 2015;59:1095–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.di Gesso JL, Kerr JS, Zhang Q, Raheem KS, Yalamanchili SK, O’Hagan D, Kay CD, O’Connell MA. Flavonoid metabolites reduce tumor necrosis factor-alpha secretion to a greater extent than their precursor compounds in human THP-1 monocytes. Mol Nutr Food Res 2015;59:1143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kling B, Bucherl D, Palatzky P, Matysik FM, Decker M, Wegener J, Heilmann J. Flavonoids, flavonoid metabolites, and phenolic acids inhibit oxidative stress in the neuronal cell line HT-22 monitored by ECIS and MTT assay: a comparative study. J Nat Prod 2014;77:446–54. [DOI] [PubMed] [Google Scholar]
- 35.Olejarz W, Bryk D, Zapolska-Downar D, Malecki M, Stachurska A, Sitkiewicz D. Mycophenolic acid attenuates the tumour necrosis factor-alpha-mediated proinflammatory response in endothelial cells by blocking the MAPK/NF-kappaB and ROS pathways. Eur J Clin Invest 2014;44:54–64. [DOI] [PubMed] [Google Scholar]
- 36.Sevgi K, Tepe B, Sarikurkcu C. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem Toxicol 2015;77:12–21. [DOI] [PubMed] [Google Scholar]
- 37.Juurlink BH, Azouz HJ, Aldalati AM, AlTinawi BM, Ganguly P. Hydroxybenzoic acid isomers and the cardiovascular system. Nutr J 2014;13:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Mateos A, Vauzour D, Krueger CG, Shanmuganayagam D, Reed J, Calani L, Mena P, Del Rio D, Crozier A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch Toxicol 2014;88:1803–53. [DOI] [PubMed] [Google Scholar]
- 39.Czank C, Cassidy A, Zhang Q, Morrison DJ, Preston T, Kroon PA, Botting NP, Kay CD. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a (13)C-tracer study. Am J Clin Nutr 2013;97:995–1003. [DOI] [PubMed] [Google Scholar]
- 40.Zhang QZ, Raheem KS, Botting NP, Slawin AMZ, Kay CD, O’Hagan D. Flavonoid metabolism: the synthesis of phenolic glucuronides and sulfates as candidate metabolites for bioactivity studies of dietary flavonoids. Tetrahedron 2012;68:4194–201. [Google Scholar]
- 41.Catalán U, Fernandez-Castillejo S, Pons L, Heras M, Aragones G, Angles N, Morello JR, Sola R. Alpha-tocopherol and BAY 11–7082 reduce vascular cell adhesion molecule in human aortic endothelial cells. J Vasc Res 2012;49:319–28. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Z, Liu Y, Miao AD, Wang SQ. Protocatechuic aldehyde suppresses TNF-alpha-induced ICAM-1 and VCAM-1 expression in human umbilical vein endothelial cells. Eur J Pharmacol 2005;513:1–8. [DOI] [PubMed] [Google Scholar]
- 43.Chen YH, Lin SJ, Ku HH, Shiao MS, Lin FY, Chen JW, Chen YL. Salvianolic acid B attenuates VCAM-1 and ICAM-1 expression in TNF-alpha-treated human aortic endothelial cells. J Cell Biochem 2001;82:512–21. [DOI] [PubMed] [Google Scholar]
- 44.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 45.Geomela PA, Kontos CK, Yiotakis I, Fragoulis EG, Scorilas A. L-DOPA decarboxylase mRNA expression is associated with tumor stage and size in head and neck squamous cell carcinoma: a retrospective cohort study. BMC Cancer 2012;12:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Ferrars RM, Cassidy A, Curtis P, Kay CD. Phenolic metabolites of anthocyanins following a dietary intervention study in post-menopausal women. Mol Nutr Food Res 2014;58:490–502. [DOI] [PubMed] [Google Scholar]
- 47.Urpi-Sarda M, Monagas M, Khan N, Llorach R, Lamuela-Raventos RM, Jauregui O, Estruch R, Izquierdo-Pulido M, Andres-Lacueva C. Targeted metabolic profiling of phenolics in urine and plasma after regular consumption of cocoa by liquid chromatography-tandem mass spectrometry. J Chromatogr A 2009;1216:7258–67. [DOI] [PubMed] [Google Scholar]
- 48.Rios LY, Gonthier MP, Remesy C, Mila I, Lapierre C, Lazarus SA, Williamson G, Scalbert A. Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. Am J Clin Nutr 2003;77:912–8. [DOI] [PubMed] [Google Scholar]
- 49.Dall’Asta M, Calani L, Tedeschi M, Jechiu L, Brighenti F, Del Rio D. Identification of microbial metabolites derived from in vitro fecal fermentation of different polyphenolic food sources. Nutrition 2012;28:197–203. [DOI] [PubMed] [Google Scholar]
- 50.Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep 2009;26:1001–43. [DOI] [PubMed] [Google Scholar]
- 51.Schär MY, Curtis PJ, Hazim S, Ostertag LM, Kay CD, Potter JF, Cassidy A. Orange juice-derived flavanone and phenolic metabolites do not acutely affect cardiovascular risk biomarkers: a randomized, placebo-controlled, crossover trial in men at moderate risk of cardiovascular disease. Am J Clin Nutr 2015;101:931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winterbone MS, Tribolo S, Needs PW, Kroon PA, Hughes DA. Physiologically relevant metabolites of quercetin have no effect on adhesion molecule or chemokine expression in human vascular smooth muscle cells. Atherosclerosis 2009;202:431–8. [DOI] [PubMed] [Google Scholar]
- 53.Kakkar S, Bais S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol 2014;2014:952943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Min SW, Ryu SN, Kim DH. Anti-inflammatory effects of black rice, cyanidin-3-O-beta-D-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int Immunopharmacol 2010;10:959–66. [DOI] [PubMed] [Google Scholar]
- 55.Rimbach G, Weinberg PD, de Pascual-Teresa S, Alonso MG, Ewins BA, Turner R, Minihane AM, Botting N, Fairley B, Matsugo S, et al. . Sulfation of genistein alters its antioxidant properties and its effect on platelet aggregation and monocyte and endothelial function. Biochim Biophys Acta 2004;1670:229–37. [DOI] [PubMed] [Google Scholar]
- 56.Zhu BT. Catechol-O-Methyltransferase (COMT)-mediated methylation metabolism of endogenous bioactive catechols and modulation by endobiotics and xenobiotics: importance in pathophysiology and pathogenesis. Curr Drug Metab 2002;3:321–49. [DOI] [PubMed] [Google Scholar]
- 57.Vitaglione P, Donnarumma G, Napolitano A, Galvano F, Gallo A, Scalfi L, Fogliano V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J Nutr 2007;137:2043–8. [DOI] [PubMed] [Google Scholar]
- 58.Wang D, Wei X, Yan X, Jin T, Ling W. Protocatechuic acid, a metabolite of anthocyanins, inhibits monocyte adhesion and reduces atherosclerosis in apolipoprotein E-deficient mice. J Agric Food Chem 2010;58:12722–8. [DOI] [PubMed] [Google Scholar]
- 59.Videm V, Albrigtsen M. Soluble ICAM-1 and VCAM-1 as markers of endothelial activation. Scand J Immunol 2008;67:523–31. [DOI] [PubMed] [Google Scholar]
- 60.Garton KJ, Gough PJ, Philalay J, Wille PT, Blobel CP, Whitehead RH, Dempsey PJ, Raines EW. Stimulated shedding of vascular cell adhesion molecule 1 (VCAM-1) is mediated by tumor necrosis factor-alpha-converting enzyme (ADAM 17). J Biol Chem 2003;278:37459–64. [DOI] [PubMed] [Google Scholar]
- 61.Lotito SB, Frei B. Dietary flavonoids attenuate tumor necrosis factor alpha-induced adhesion molecule expression in human aortic endothelial cells. Structure-function relationships and activity after first pass metabolism. J Biol Chem 2006;281:37102–10. [DOI] [PubMed] [Google Scholar]
- 62.Chen CC, Chow MP, Huang WC, Lin YC, Chang YJ. Flavonoids inhibit tumor necrosis factor-alpha-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-kappaB: structure-activity relationships. Mol Pharmacol 2004;66:683–93. [DOI] [PubMed] [Google Scholar]
- 63.Xu JW, Ikeda K, Yamori Y. Inhibitory effect of polyphenol cyanidin on TNF-alpha-induced apoptosis through multiple signaling pathways in endothelial cells. Atherosclerosis 2007;193:299–308. [DOI] [PubMed] [Google Scholar]
- 64.Serra A, Macia A, Romero MP, Reguant J, Ortega N, Motilva MJ. Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem 2012;130:383–93. [Google Scholar]
- 65.Koga T, Meydani M. Effect of plasma metabolites of (+)-catechin and quercetin on monocyte adhesion to human aortic endothelial cells. Am J Clin Nutr 2001;73:941–8. [DOI] [PubMed] [Google Scholar]
- 66.Harasstani OA, Moin S, Tham CL, Liew CY, Ismail N, Rajajendram R, Harith HH, Zakaria ZA, Mohamad AS, Sulaiman MR, et al. . Flavonoid combinations cause synergistic inhibition of proinflammatory mediator secretion from lipopolysaccharide-induced RAW 264.7 cells. Inflamm Res 2010;59:711–21. [DOI] [PubMed] [Google Scholar]
- 67.Kjaergaard AG, Dige A, Krog J, Tonnesen E, Wogensen L. Soluble adhesion molecules correlate with surface expression in an in vitro model of endothelial activation. Basic Clin Pharmacol Toxicol 2013;113:273–9. [DOI] [PubMed] [Google Scholar]
- 68.Chen YH, Lin SJ, Chen JW, Ku HH, Chen YL. Magnolol attenuates VCAM-1 expression in vitro in TNF-alpha-treated human aortic endothelial cells and in vivo in the aorta of cholesterol-fed rabbits. Br J Pharmacol 2002;135:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kwon HM, Choi YJ, Jeong YJ, Kang SW, Kang IJ, Lim SS, Kang YH. Anti-inflammatory inhibition of endothelial cell adhesion molecule expression by flavone derivatives. J Agric Food Chem 2005;53:5150–7. [DOI] [PubMed] [Google Scholar]
- 70.Aydin M, Tekin IO, Dogan SM, Yildirim N, Arasli M, Sayin MR, Aktop Z. The levels of tumor necrosis factor-alpha and interleukin-6 in patients with isolated coronary artery ectasia. Mediators Inflamm 2009;2009:106145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skoog T, Dichtl W, Boquist S, Skoglund-Andersson C, Karpe F, Tang R, Bond MG, de Faire U, Nilsson J, Eriksson P, et al. . Plasma tumour necrosis factor-alpha and early carotid atherosclerosis in healthy middle-aged men. Eur Heart J 2002;23:376–83. [DOI] [PubMed] [Google Scholar]
- 72.Lotito SB, Zhang WJ, Yang CS, Crozier A, Frei B. Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free Radic Biol Med 2011;51:454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Al-Shalmani S, Suri S, Hughes DA, Kroon PA, Needs PW, Taylor MA, Tribolo S, Wilson VG. Quercetin and its principal metabolites, but not myricetin, oppose lipopolysaccharide-induced hyporesponsiveness of the porcine isolated coronary artery. Br J Pharmacol 2011;162:1485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lodi F, Winterbone MS, Tribolo S, Needs PW, Hughes DA, Kroon PA. Human quercetin conjugated metabolites attenuate TNF-alpha-induced changes in vasomodulatory molecules in an HUASMCs/HUVECs co-culture model. Planta Med 2012;78:1571–3. [DOI] [PubMed] [Google Scholar]