Abstract
Background: The high-fat and high-sugar Westernized diet that is popular worldwide is associated with increased body fat accumulation, which has been related to the development of nonalcoholic fatty liver disease (NAFLD). Without treatment, NAFLD may progress to hepatocellular carcinoma (HCC), a cancer with a high mortality rate. The consumption of broccoli in the United States has greatly increased in the last 2 decades. Epidemiologic studies show that incorporating brassica vegetables into the daily diet lowers the risk of several cancers, although, to our knowledge, this is the first study to evaluate HCC prevention through dietary broccoli.
Objective: We aimed to determine the impact of dietary broccoli on hepatic lipid metabolism and the progression of NAFLD to HCC. Our hypothesis was that broccoli decreases both hepatic lipidosis and the development of HCC in a mouse model of Western diet–enhanced liver cancer.
Methods: Adult 5-wk-old male B6C3F1 mice received a control diet (AIN-93M) or a Western diet (high in lard and sucrose, 19% and 31%, wt:wt, respectively), with or without freeze-dried broccoli (10%, wt:wt). Starting the following week, mice were treated once per week with diethylnitrosamine (DEN; 45 mg/kg body weight intraperitoneally at ages 6, 7, 8, 10, 11, and 12 wk). Hepatic gene expression, lipidosis, and tumor outcomes were analyzed 6 mo later, when mice were 9 mo old.
Results: Mice receiving broccoli exhibited lower hepatic triglycerides (P < 0.001) and NAFLD scores (P < 0.0001), decreased plasma alanine aminotransferase (P < 0.0001), suppressed activation of hepatic CD68+ macrophages (P < 0.0001), and slowed initiation and progression of hepatic neoplasm. Hepatic Cd36 was downregulated by broccoli feeding (P = 0.006), whereas microsomal triglyceride transfer protein was upregulated (P = 0.045), supporting the finding that dietary broccoli decreased hepatic triglycerides.
Conclusion: Long-term consumption of whole broccoli countered both NAFLD development enhanced by a Western diet and hepatic tumorigenesis induced by DEN in male B6C3F1 mice.
Keywords: broccoli, Western diet, NAFLD, liver cancer, diethylnitrosamine
Introduction
Nonalcoholic fatty liver disease (NAFLD)6, the term to describe a cluster of related liver diseases that range from uncomplicated steatosis, to nonalcoholic steatohepatitis (NASH), to cirrhosis, and potentially to hepatocellular carcinoma (HCC), is prevalent in the United States today, but there are few early symptoms (1). Almost 1 in every 2 adult Americans has some degree of NAFLD (2). Liver cancer is lethal and is the fifth most common cancer in men worldwide, with a 5-y survival rate of 17% (3, 4). This cancer is relatively preventable, because many of the risk factors for liver cancer are related to diet, lifestyle, and infection (5). Although >50% of HCC cases are related to hepatitis B viral infection, hepatitis B can be prevented by vaccination (6, 7). It has been reported that there is not only a greater prevalence of NAFLD in the obese population than in people with normal body weight (BW), but also a greater risk of mortality from liver cancer (8, 9). According to the newest report from the World Cancer Research Fund International, “body fatness” has been officially added to the list of factors that increase the risk of liver cancer (10). Fortunately, adiposity can be reduced by changing lifestyle, such as increasing physical activity and altering dietary patterns (11, 12).
The so-called Westernized diet, which is high in saturated fats and sugars, is well rooted in the lifestyle of the majority of Americans. Over 35% of daily energy consumed by Americans is from solid fat, including trans fats and saturated fats, and added sugars (13). Excess consumption of saturated fats and sugars, including sucrose, not only contributes to adiposity but also to the progression of NAFLD. SFAs are a poor source of energy, because they are not the primary substrate for β oxidation, and tend to be directed toward storage (14). In the liver, SFAs can increase endoplasmic reticulum stress and free radical production in the mitochondria, promoting the development of NASH (15). Moreover, fructose, which can be ingested directly or in the form of sucrose, is a highly lipogenic nutrient, enhancing hepatic lipid accumulation through de novo lipogenesis (16). Therefore, a Westernized diet disrupts lipid homeostasis and oxidative balance in the liver and worsens the development of NAFLD.
Broccoli, a brassica vegetable containing multiple bioactive compounds, including vitamin C, flavonoids, and glucosinolates, has shown substantial market growth in the last 20 y (17, 18). Epidemiologic studies show that incorporating brassicas into the daily diet can decrease cancer risk at several tissue sites, including the bladder, breasts, prostate, and colon (19–22). A clinical trial in China found that consuming tea from a boiling-water infusion of broccoli sprouts may decrease urinary concentrations of aflatoxin-N7-guanine, an aflatoxin–DNA adduct metabolite and biomarker (23). This finding suggests that brassicas may protect against liver cancer by decreasing aflatoxin bioactivation, but there are no direct reports of protection against liver cancer. However, preliminary studies suggested that broccoli impedes the development of NAFLD by decreasing hepatic triglyceride (HTG) accumulation in mice fed a Western diet (WD) (Y-J Chen, unpublished results, 2013). Therefore, we hypothesized that the long-term consumption of broccoli could regulate hepatic lipid metabolism, and thus decrease the amount of hepatic lipidosis that is typically increased by a WD. We further hypothesized that broccoli could hinder the initiation of liver tumorigenesis by the hepatic carcinogen diethylnitrosamine (DEN). To approach our goal, adult B6C3F1 mice, a carcinogen-sensitive strain, were used to observe the impact of broccoli on both the initiation and progression of liver tumorigenesis. The expression of hepatic lipid-metabolizing enzymes was evaluated to determine how dietary treatments (including a Westernized diet high in saturated fats and sucrose with a broccoli intervention) might interact to affect hepatic lipidosis.
Methods
Mice and diets.
Male 4-wk-old B6C3F1 mice (C57BL/6J × C3H/HeJ hybrid) were purchased from the Jackson Laboratory. Mice were housed individually under a 12-h light/dark cycle at 22°C and 60% humidity. Water and feed were consumed ad libitum. Mouse care was in compliance with the Institutional Animal Care and Use Committee at the University of Illinois, according to NIH guidelines.
A powdered control (CON) diet was prepared by following the AIN-93M formula with adjustments (24). The WD was formulated by modifying the AIN-93M diet, increasing the sucrose and saturated fat content (Supplemental Table 1). Freeze-dried broccoli powder (10% by weight; Brassica oleracea L. var. Green Magic), kindly provided by John A Juvik, University of Illinois, was incorporated into the broccoli diets, and macronutrients were replaced to balance the diet for the composition of the broccoli. Glucoraphanin (4.0 mmol/kg freeze-dried broccoli) was estimated by adding water to allow myrosinase-dependent hydrolysis to sulforaphane as previously reported (25). Diet ingredients were bought from Harlan.
Experimental design.
Seventy-two 4-wk-old male B6C3F1 mice were acclimated for 1 wk to the AIN-93M diet before starting diet treatments. At the age of 5 wk, mice were divided into 4 groups and provided a CON diet, a CON plus 10% broccoli diet, a WD, or a WD plus 10% broccoli (Supplemental Table 1). One week later, DEN (Sigma-Aldrich) in saline was given to mice (n = 12/group) at a dose of 45 mg/kg intraperitoneally 1 time/wk for 6 wk, with saline given as a vehicle control (n = 6/group), forming 8 treatment groups: Saline (S)–no broccoli (NB)–CON, S–broccoli (B)–CON, S-NB-WD, S-B-WD, DEN-NB-CON, DEN-B-CON, DEN-NB-WD, and DEN-B-WD. There was a 1-wk interval after 3 injections, for better recovery from DEN treatment (Supplemental Figure 1). BW and feed intake were monitored weekly. Two DEN-treated mice died during the injection period; 6 mice (1 in the DEN-NB-CON group, 2 in the DEN-B-CON group, 1 in the DEN-NB-WD group, and 2 in the DEN-B-WD group) were euthanized before the end of the study because of serious illness. Mice were anesthetized using ketamine:xylazine mixture (87 mg/mL:13 mg/mL) at 6 mo after starting the DEN treatments for collection of blood samples, and then killed by cervical dislocation, without recovering consciousness, for tissue collection and liver nodule count (diameter ≥1 mm). Liver tissue was divided for biochemical analysis (stored at –80°C) and histology (fixed in 10% neutral buffered formalin).
HTG content.
Liver lipid was extracted by the Folch method (26), with some modifications. Liver tissue was homogenized and lipid was extracted into chloroform:methanol (2:1, vol:vol) containing 0.01% butylated hydroxytoluene. The homogenate was washed with ultrapure water and centrifuged at room temperature (1080 × g for 20 min) to collect the organic phase. Chloroform was evaporated from the organic phase of the lipid extract with mild heating (50°C) and a stream of nitrogen gas. The remaining lipid extract was reconstituted with absolute ethanol for quantification. The HTG concentration was determined by the glyceride phosphate oxidase method with the use of a reagent kit (Pointe Scientific).
Liver histology.
Liver samples from the left, median, and right lobes were fixed in 10% neutral buffered formalin and embedded in paraffin. Sections of each lobe from hilar to lobe edge (3 μm thick) were stained with hematoxylin and eosin (H&E) for histologic examination. All histology was carried out at the Veterinary Diagnostic Laboratory at the University of Illinois. Murine NAFLD scores and microscopic hepatic neoplasm–related lesions were determined by a trained pathologist, based on the H&E-stained sections. The scoring criteria are shown in Supplemental Table 2.
Plasma alanine aminotransferase concentrations.
Plasma alanine aminotransferase (ALT) concentrations were determined with the use of a reagent kit (Pointe Scientific) in a 96-well format according to the manufacturer’s instructions.
Immunoblotting.
Liver tissue was homogenized 1:10 (wt:vol) with lysis buffer (0.15 mol/L NaCl, 5 mM EDTA, 10 mM Tris-HCl, 5 mM dithiothreitol, 1% Triton X-100, and a protease inhibitor cocktail). The protein extract was separated by centrifugation (13,400 × g; 30 min; 4°C). Protein concentration was determined by the Bio-Rad protein assay (Bio-Rad Laboratories). Liver protein samples were separated by SDS-PAGE and transferred to a nitrocellulose membrane (GE Health Care). After blocking in 5% nonfat dried milk, membranes were incubated with primary antimouse antibodies (1:500) with the use of goat polyclonal anti–microsomal triglyceride transfer protein (MTTP) (Santa Cruz Biotechnology), rabbit polyclonal anti–acetyl-CoA carboxylase (ACC; α form) (Cell Signaling Technology), or rabbit polyclonal anti–β-actin antibodies (Santa Cruz Biotechnology) overnight at 4°C, and then incubated with goat antirabbit or donkey antigoat IgG HRP secondary antibodies (Santa Cruz Biotechnology) at room temperature for 1 h. Enhanced chemiluminescence of the immunocomplex was detected with the use of a detection kit (GE Health Care). Chemiluminescence was visualized and quantified with the use of ImageQuant LAS 4000 and IQTL software (GE Health Care).
Real-time qPCR.
Liver RNA was extracted with Trizol Reagent (Life Technologies), following the manufacturer’s instructions. RNA integrity was monitored by 1% bleach agarose gel electrophoresis (27). Synthesis of cDNA from RNA was conducted with the use of a reverse transcription reagent kit (Life Technologies). PrimeTime qPCR 5′Nuclease primer and probe sets (Integrated DNA Technologies) and TaqMan Universal PCR Master Mix (Life Technologies; 4324020) were used for real-time qPCR. Hepatic gene expression of Cd36, Cd68, tumor necrosis factor (Tnf), interferon γ (Ifng), fatty acid synthase (Fasn), carnitine palmitoyltransferase 1α (Cpt1a), cytochrome P450 2E1 (Cyp2e1), and Gapdh (a housekeeping gene), were quantified. Sequences for primer sets and probes are shown in Supplemental Table 3. Thermal cycler procedures, including 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min, were conducted with the use of the 7900HT Fast Real-Time PCR System (Life Technologies). Data were analyzed with the use of the comparative threshold cycle method, and expressed as fold change (28).
Statistical analysis.
All results, except liver tumor– and neoplasm-related lesion incidence, are expressed as mean ± SD and were analyzed by a 2- or 3-factor ANOVA for the effect of DEN, broccoli, WD, and interactions, followed by Scheffé’s test when the differences were indicated. Differences in liver tumor– and neoplasm-related lesion incidence were evaluated by a Fisher’s exact test. Correlation between liver and epididymal adipose tissue (EAT) weights among the high-BW (final BW >40 g) mice (n = 53; 6 from the S-NB-CON group, 6 from the DEN-NB-CON group, 6 from the S-B-CON group, 6 from the DEN-B-CON group, 6 from the S-NB-WD group, 8 from the DEN-NB-WD group, 6 from the S-B-WD group, and 9 from the DEN-B-WD group) was analyzed by Pearson’s correlation coefficient. A P value of < 0.05 was considered to be significant in this study. All statistical analyses were performed with the use of SAS 9.4 software.
Results
Dietary broccoli decreased relative liver mass but not BW.
Feed intake was decreased in the DEN-treated mice through the age of 12 wk (the last DEN injection). Although the difference was resolved when DEN administration concluded from 13 wk of age, the effect of DEN was evident again by the end of the study (week 36; Supplemental Table 4). Moreover, broccoli had no impact on feed intake independent of DEN treatment and the WD (Supplemental Table 4). Final BW was increased by the WD but not influenced by broccoli (Table 1). However, DEN treatment exerted a negative impact on BW, adversely affecting BW increase during the period of DEN treatments, resulting in a decreased final BW (Table 1 and Supplemental Figure 2). Although broccoli showed no impact on final BW, it did decrease relative liver mass (Table 1). The WD not only increased body mass but also relative liver mass, whereas DEN treatments had no influence on liver as a percentage of BW (Table 1). Unexpectedly, relative EAT mass was decreased by the WD but increased with DEN treatments (Table 1). Upon further examination, it became evident that the absolute liver and EAT masses were negatively correlated (r = −0.71, P < 0.0001, data not shown), but only in the high-BW mice (final BW >40 g).
TABLE 1.
Effect of DEN, a WD, and broccoli on BW, body composition, and hepatic lipidosis in male B6C3F1 mice1
| S |
DEN |
||||||||||||||
| NB |
B |
NB |
B |
P (3-factor ANOVA) |
|||||||||||
| Objective outcomes | CON (n = 6) | WD (n = 6) | CON (n = 6) | WD (n = 6) | CON (n = 10) | WD (n = 11) | CON (n = 10) | WD (n = 9) | DEN | B | WD | DEN × B | DEN × WD | B × WD | DEN × B × WD |
| BW and body composition | |||||||||||||||
| BW, g | 46.7 ± 2.8a,b | 52.6 ± 3.4a | 45.6 ± 1.8a,b | 53.9 ± 3.5a | 40.7 ± 4.0b | 42.6 ± 6.3b | 39.6 ± 5.6b | 47.1 ± 1.9a,b | <0.0001 | NS | <0.0001 | NS | NS | NS | NS |
| Liver mass,2 % | 6.0 ± 1.0a,b | 7.2 ± 0.9a | 5.2 ± 0.5a,b | 7.1 ± 0.6a,b | 5.6 ± 0.7a,b | 7.3 ± 1.7a | 5.0 ± 1.0b | 6.2 ± 1.4a,b | NS | 0.022 | <0.0001 | NS | NS | NS | NS |
| EAT mass,2 % | 3.3 ± 1.0a,b | 1.6 ± 0.4c,d | 3.0 ± 0.6a,b,c | 1.5 ± 0.2d | 3.9 ± 0.5a | 2.8 ± 0.6a,b,c,d | 4.0 ± 1.1a | 2.3 ± 0.5b,c,d | <0.0001 | NS | <0.0001 | NS | NS | NS | NS |
| Hepatic lipidosis endpoints | |||||||||||||||
| HTGs, mg/mg protein | 0.9 ± 0.2a | 0.8 ± 0.1a,b | 0.5 ± 0.2b | 0.7 ± 0.2a,b | 0.8 ± 0.2a,b | 0.8 ± 0.1a,b | 0.6 ± 0.2a,b | 0.7 ± 0.2a,b | NS | <0.0001 | NS | NS | NS | NS | NS |
| HTGs, mg | 238 ± 78a,b | 298 ± 44a | 132 ± 45b | 270 ± 97a | 175 ± 40a,b | 269 ± 82a | 125 ± 43b | 195 ± 55a,b | 0.011 | <0.001 | <0.0001 | NS | NS | NS | NS |
| NAFLD score | 3.2 ± 1.0a,b | 4.0 ± 0.6a | 1.5 ± 1.0b | 2.8 ± 1.0a,b | 3.1 ± 0.7a,b | 2.6 ± 0.9a,b | 1.7 ± 1.1b | 2.3 ± 0.5a,b | NS | <0.0001 | 0.012 | NS | 0.031 | NS | NS |
Data are means ± SDs. Means with different letters are statistically significant, P < 0.05. NS is defined as P > 0.05. B, broccoli; BW, body weight; CON, control; DEN, diethylnitrosamine; EAT, epididymal adipose tissue; HTG, hepatic triglyceride; NAFLD, nonalcoholic fatty liver disease; NB, no broccoli; S, saline; WD, Western diet.
Percentage BW.
The WD resulted in liver damage and increased HTG accumulation, whereas dietary broccoli blocked these effects.
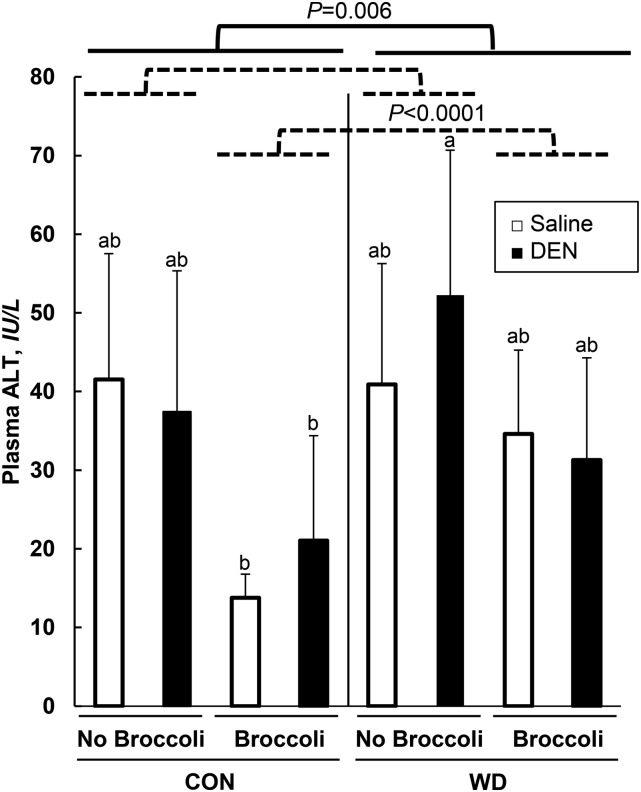
Feeding a WD increased plasma ALT concentrations, suggesting liver damage, but DEN treatments had no effect on plasma ALT (Figure 1). Dietary broccoli exerted a substantial protective effect, with decreased plasma ALT, independent of any effect from WD or DEN treatments (Figure 1). Moreover, HTG density was decreased by broccoli, but there was no impact of the WD or DEN treatment on HTG density (Table 1). HTGs were decreased by broccoli, whereas the WD increased HTGs (Table 1). However, DEN-treated mice had lower HTGs than did S-treated mice (Table 1). Representative hepatic histologic images are shown in Figure 2. The NAFLD scores, which were graded based on H&E-stained liver sections, showed a pattern similar to that of HTGs, being increased by the WD and decreased by broccoli, but these scores were not influenced by DEN treatment (Table 1). Furthermore, in reviewing the NAFLD scores, there was an interaction between the WD and DEN treatments in that DEN treatment decreased the NAFLD severity score in the WD group, but not in the CON group (Table 1).
FIGURE 1.
Effect of DEN, broccoli, and a WD on plasma ALT in male B6C3F1 mice. Data are means ± SDs; n = 6–11. Means with different letters are statistically significant, P < 0.05. ALT, alanine aminotransferase; CON, control; DEN, diethylnitrosamine; WD, Western diet.
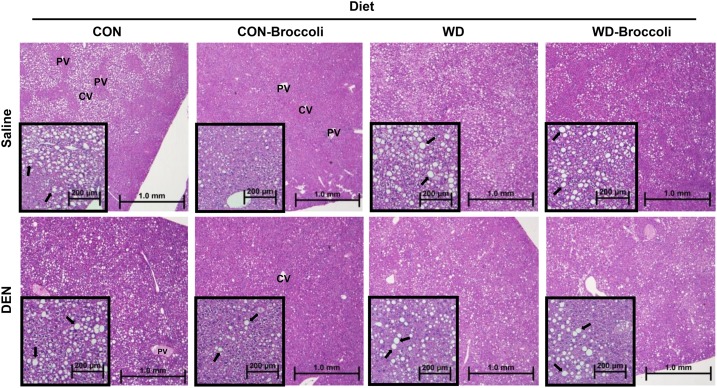
FIGURE 2.
Effect of DEN, broccoli, and a WD on liver histologic alteration in lipodosis in male B6C3F1 mice. Liver sections were stained with hematoxylin and eosin. Representative images from each group are shown as 40× (large image) and 100× (small image) magnifications. TG vacuoles are indicated by arrows. CON, control; CV, central vein; DEN, diethylnitrosamine; PV, portal vein; WD, Western diet.
Dietary broccoli decreased the expression of hepatic Cd36 and MTTP.
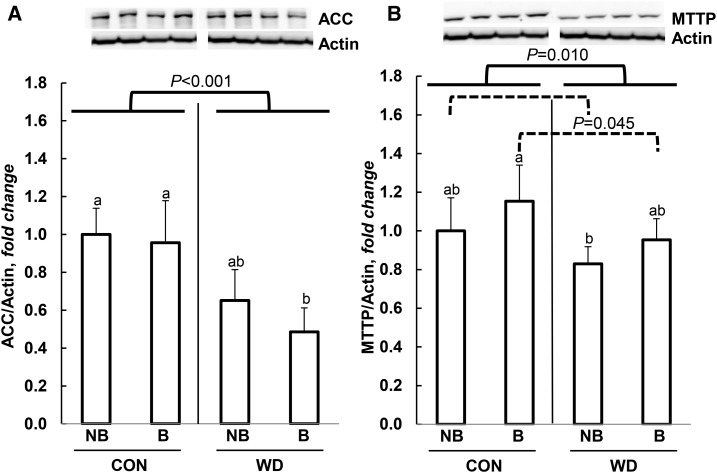
Hepatic lipid metabolism was influenced by DEN treatment, broccoli, and the WD in this study. The expression of Cd36, a major FA transporter for the uptake of lipids into the liver, was increased by the WD but decreased by long-term consumption of broccoli (Table 2). The DEN treatment also decreased hepatic Cd36 expression, but there was an overall interaction between all treatments (Table 2). This 3-way interaction indicates that, in mice not receiving the DEN treatment, there was a significant broccoli × WD interaction (P < 0.05), in which a broccoli-dependent decrease was seen in hepatic Cd36 expression in the CON-fed mice (P < 0.05), but this did not occur in the WD-fed mice. Furthermore, there was no broccoli × WD interaction in Cd36 concentrations in the DEN-treated mice. Fasn, a critical enzyme for de novo lipogenesis, was downregulated by both the WD and the DEN treatment but not by broccoli (Table 2). A similar effect was found for the protein concentration of ACC, in which the WD decreased ACC expression (Figure 3A).
TABLE 2.
Effect of DEN, a WD, and broccoli on hepatic gene expression (mRNA) in male B6C3F1 mice1
| S |
DEN |
||||||||||||||
| NB |
B |
NB |
B |
P (3-factor ANOVA) |
|||||||||||
| Hepatic gene expression | CON (n = 6) | WD (n = 6) | CON (n = 6) | WD (n = 6) | CON (n = 10) | WD (n = 11) | CON (n = 10) | WD (n = 9) | DEN | B | WD | DEN × B | DEN × WD | B × WD | DEN × B × WD |
| Lipid regulation, fold of CON | |||||||||||||||
| Cd36 | 1.0 ± 0.1a,b | 1.0 ± 0.2a,b | 0.7 ± 0.2a,b | 1.1 ± 0.2a | 0.7 ± 0.2a,b | 1.0 ± 0.2a,b | 0.6 ± 0.2b | 0.8 ± 0.2a,b | 0.008 | 0.006 | <0.001 | NS | NS | NS | 0.021 |
| Cpt1a | 1.0 ± 0.2a,b,c | 1.2 ± 0.1a,b | 0.9 ± 0.2b,c | 1.1 ± 0.2a,b,c | 0.9 ± 0.2b,c | 1.3 ± 0.2a | 0.9 ± 0.1c | 1.0 ± 0.1a,b,c | NS | 0.003 | <0.0001 | NS | NS | NS | NS |
| Cyp2e1 | 1.0 ± 0.1a,b | 1.0 ± 0.2a,b | 1.1 ± 0.3a,b | 0.9 ± 0.4a,b | 0.8 ± 0.2b | 1.5 ± 0.6a | 1.1 ± 0.2a,b | 1.2 ± 0.2a,b | NS | NS | NS | NS | 0.009 | 0.041 | NS |
| Fasn | 1.0 ± 0.2a,b | 0.6 ± 0.1b,c,d | 1.1 ± 0.2a | 0.8 ± 0.2a,b,c | 1.1 ± 0.2a | 0.4 ± 0.1d | 0.9 ± 0.2a,b | 0.5 ± 0.1c,d | <0.001 | NS | <0.0001 | NS | NS | NS | NS |
| Inflammation, fold of CON | |||||||||||||||
| Ifng | 1.0 ± 0.1 | 1.4 ± 0.9 | 0.8 ± 0.3 | 1.0 ± 0.5 | 1.8 ± 1.1 | 2.0 ± 0.6 | 0.9 ± 0.3 | 0.6 ± 0.2 | NS | <0.001 | NS | 0.027 | NS | NS | NS |
| Cd68 | 1.0 ± 0.2b | 1.2 ± 0.4a,b | 0.7 ± 0.1b | 0.9 ± 0.2b | 1.3 ± 0.2a,b | 1.7 ± 0.5a | 1.0 ± 0.3b | 1.0 ± 0.3b | <0.001 | <0.0001 | 0.009 | NS | NS | NS | NS |
| Tnf | 1.0 ± 0.2a,b | 1.0 ± 0.4a,b | 0.6 ± 0.2b | 0.5 ± 0.1b | 1.0 ± 0.3a,b | 1.2 ± 0.3a | 0.6 ± 0.2b | 0.6 ± 0.2b | NS | <0.0001 | NS | NS | NS | NS | NS |
Values are means ± SDs. Means with different letters are statistically significant, P < 0.05. NS is defined as P > 0.05. B, broccoli; CON, control; Cpt1a, carnitine palmitoyltransferase 1α; Cyp2e1, cytochrome P450 2E1; DEN, diethylnitrosamine; Fasn, fatty acid synthase; Ifng, interferon γ; NB, no broccoli; S, saline; Tnf, tumor necrosis factor; WD, Western diet.
FIGURE 3.
Effect of broccoli and a WD on ACC (A) and MTTP (B) in male B6C3F1 mice. Data are shown as fold change from the S-NB-CON group and are means ± SDs; n = 6. Means with different letters are statistically significant, P < 0.05. ACC, acetyl-CoA carboxylase; B, broccoli; CON, control; MTTP, microsomal triglyceride transfer protein; NB, no broccoli; S, saline; WD, Western diet.
Mitochondrial β oxidation is tightly controlled by carnitine palmitoyltransferase, which was downregulated by broccoli and upregulated by the WD, but there was no significant treatment effect of DEN treatment, broccoli, or the WD on hepatic Cyp2e1, which is responsible for microsomal ω oxidation (Table 2). However, there was an interaction between the DEN treatment and the WD, indicating that DEN treatments enhanced Cyp2e1 expression in the WD-fed mice (Table 2). Moreover, broccoli may downregulate Cyp2e1 in mice receiving a WD because of a broccoli × WD interaction (Table 2). Our data show that MTTP, which is essential for VLDL excretion, was increased by broccoli but decreased by the WD (Figure 3B).
Hepatic Cd68, a marker of macrophage activation, was decreased by dietary broccoli.
Activated hepatic macrophages (monitored as Cd68 expression), including Kupffer cells (KCs) and infiltrating macrophages, were increased by the WD and the DEN treatment (Table 2). However, the broccoli-fed mice showed a decreased expression of hepatic Cd68 (Table 2). Moreover, broccoli also downregulated Tnf expression in the liver (Table 2). Hepatic Ifng, a cytokine that triggers the activation of macrophages, was downregulated by broccoli as well (Table 2). The interaction between broccoli and the DEN treatment indicates that the suppression of Ifng expression by broccoli was greater when there was a DEN carcinogen challenge (Table 2).
Long-term consumption of broccoli decreased liver nodule numbers.
The formation of liver nodules was induced by DEN, and both liver nodule number and size, as well as hepatic adenoma incidence, were increased by the WD (Table 3). However, broccoli showed a substantial protective effect, decreasing liver nodule numbers (Table 3). As expected, there were no microscopic hepatic neoplasm–related lesions seen in liver sections from the DEN-B-CON group, and a lower total hepatic neoplasm–related lesion incidence in the DEN-B-WD group, compared with the DEN-NB-WD mice (Table 3).
TABLE 3.
Effect of DEN, a WD, and broccoli on hepatic neoplasm–related lesions in male B6C3F1 mice1
| S |
DEN |
||||||||||||||
| NB |
B |
NB |
B |
P (2- or 3-factor ANOVA) |
|||||||||||
| CON (n = 6) | WD (n = 6) | CON (n = 6) | WD (n = 6) | CON (n = 10) | WD (n = 11) | CON (n = 10) | WD (n = 9) | DEN | B | WD | DEN × B | DEN × WD | B × WD | DEN × B × WD | |
| Macroscopic lesions | |||||||||||||||
| Nodule incidence2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (70) | 11 (100) | 5 (50) | 8 (89) | — | — | — | — | — | — | — |
| Nodules,2 n | NDb | NDb | NDb | NDb | 2.3 ± 1.9b | 8.4 ± 5.7a | 0.5 ± 0.5b | 3.0 ± 2.4a,b | <0.0001 | 0.001 | 0.003 | NS | NS | NS | NS |
| Max nodule diameter, mm | — | — | — | — | 4.1 ± 2.4a,b | 9.3 ± 4.6a | 3.2 ± 3.5b | 5.4 ± 3.0a,b | — | NS | 0.011 | — | — | NS | — |
| Microscopic lesions | |||||||||||||||
| AHF incidence | 0 (0) | 0 (0) | 0 (0) | 2 (33) | 2 (20) | 4 (36) | 0 (0) | 4 (44) | |||||||
| HA incidence | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (46)* | 0 (0) | 1 (11) | |||||||
| HCC incidence | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (10) | 1 (9) | 0 (0) | 1 (11) | |||||||
| Total incidence | 0 (0) | 0 (0) | 0 (0) | 2 (33) | 3 (30) | 9 (82)* | 0 (0) | 5 (56) | |||||||
Data are means ± SDs or n (%). Means with different letters are statistically significant, P < 0.05. NS is defined as P > 0.05. *P < 0.05 compared with DEN-NB-CON group by Fisher’s exact test. AHF, altered hepatic foci; B, broccoli; CON, control; DEN, diethylnitrosamine; HA, hepatic adenoma; HCC, hepatocellular carcinoma; max, maximum; NB, no broccoli; ND, not detectable; S, saline; WD, Western diet.
Visible liver nodules, diameter ≥1 mm; ND, diameter <1 mm.
Discussion
In this study, DEN administration retarded the growth of mice, possibly because of decreased feed intake during administration. DEN may have caused acute toxicity, leading to the temporary reduction in feed intake that we observed. A similar result has been reported for a DEN rat model, in which BW and feed intake were both adversely affected immediately after DEN administration (100 mg/kg BW) (29). In adult mice, a single high dose of DEN (80 mg/kg BW) also has been reported to cause a loss of BW (30). Moreover, we observed a negative correlation between lipodystrophy as measured by EAT and hepatomegaly for mice with a high BW, which also has been reported in a murine high-fat diet model in which EAT mass increased with growing BW during the first 12 wk of the diet; however, when BW was >40 g, a negative correlation (r = −0.85) was observed (31). This hepatomegaly-related lipodystrophy could be tissue-specific, because the subcutaneous inguinal adipose mass has been reported to increase with the feeding of a long-term high-fat diet, showing no tendency toward weight loss, regardless of the weight of the mice (31, 32).
This study revealed that broccoli had an impact on the HTG pool by decreasing HTGs and lipidosis in both the CON and WD groups, which is consistent with reports of the impact of isolated bioactives from broccoli. Sulforaphane, the hydrolyzed glucosinolate product that is rich in broccoli, has been reported to show the potential to decrease HTG concentrations in mice receiving a high-fat diet, but the effect was not significant (33). Our results suggest that the whole broccoli intervention exerted a substantial beneficial health effect. Furthermore, the broccoli diets in our study, which contained only 0.4 mmol glucoraphanin/kg diet, showed a greater influence on hepatic lipid regulation than pure sulforaphane, which was given at a substantially greater dose (5.6 mmol/kg) (33). This phenomenon has been noted for other endpoints in our previous study (34). A recent clinical study has identified that 400 g broccoli/wk for 12 wk significantly decreases plasma LDL cholesterol (35). Although that study did not include obese subjects, it suggests that broccoli may affect hepatic lipid metabolism in humans. A clinical study of obesity, NAFLD, and broccoli ingestion should be carried out to translate our findings to human health.
Notably, the WD had opposing impacts on HTG concentrations and NAFLD scores in the DEN-treated mice, showing that the DEN-NB-WD group had lower NAFLD scores than did the DEN-NB-CON group, but exhibited elevated HTGs. These divergent results are possibly a distortion because of interference by the presence of hepatic preneoplastic and neoplastic lesions. It has been reported that it is rare for liver tumors to contain a high quantity of fat in humans (36). Moreover, in the end stage of NAFLD, the initiation of HCC is considered to be related to cirrhosis (37). It has also been observed that, in NAFLD patients, once cirrhosis develops, hepatic lipid accumulation decreases without BW loss (38). These reports suggested that cirrhotic tissue or liver tumor tissue, which could be responsible for a substantial amount of the liver weight in the DEN-NB-WD group, may have relatively little TG compared with simple (uncomplicated) steatosis, as found in the S-NB-WD mice. Therefore, although the DEN-NB-WD mice had a greater liver mass than the DEN-NB-CON mice, the lack of homogeneity in liver tissue actually may have resulted in less total lipid. Also, the decrease in NAFLD score that we observed in the DEN-NB-WD group, as well as the loss in BW during the final month before the end of the study (33–36 wk of age; Supplemental Figure 2), may reflect the severity of liver tumorigenesis.
HTGs mainly derive from circulating nonesterified fatty acids (NEFAs; 60%), whereas minor sources are de novo lipogenesis (25%) and dietary fat (15%) (39). The liver can obtain NEFAs from the circulation by both passive diffusion and facilitated uptake by membrane FA transporters (40). The FA translocator CD36 facilitates the hepatic uptake of long-chain FAs, and is highly upregulated during the progression of NAFLD, compared with other FA transporters, such as FA binding protein and caveolin (41, 42). The present results show that broccoli decreased hepatic Cd36 expression by as much as 35% (Table 2), which could have greatly reduced the influx of NEFAs, the major source of HTGs. Moreover, an increased expression of MTTP in broccoli-fed mice may imply enhanced excretion of VLDLs, which also may contribute to the reduction of HTG deposition. In contrast, the WD had a negative effect on MTTP. Although plasma lipoproteins were not estimated in this study, MTTP is critical for VLDL formation (43). Future studies could determine whether plasma VLDLs are decreased in mice receiving a WD, but elevated by broccoli feeding.
Several clinical studies suggest that de novo lipogenesis is elevated in NAFLD. Liver biopsy shows that the expression of the lipogenic enzymes Fasn and Acc are both upregulated in patients diagnosed with NAFLD (44). Furthermore, people diagnosed with NAFLD excrete more TGs generated from de novo hepatic lipogenesis (45). However, in our study, long-term broccoli consumption had no impact on Fasn or ACC, which were significantly decreased, not increased, by the WD. Although high-fat diets (with or without high carbohydrate) are able to increase de novo lipogenesis in rodents (46), diet-induced hepatic steatosis models have not always provided consistent results (47–50). The cause of this discrepancy in reports is still not understood, but it might relate to feedback mechanisms from the high-fat diet that develop over time (51).
Our data indicate that the WD raised hepatic Cpt1a expression, suggesting an increased influx of FAs into the mitochondria for hepatic β oxidation, whereas broccoli exerted the converse effect. Mitochondria produce reactive oxygen species (ROS) through incomplete reduction of oxygen in the electron transport chain, releasing superoxide anions (52). High dietary fat may cause the mitochondrial lipid overload and increased uptake of oxygen needed for β oxidation, potentially leading to enhanced superoxide production (53, 54). Furthermore, the metabolic enzyme Cyp2E1 not only oxidized FAs through the ω-oxidation pathway, but also generated ROS (55). Therefore, although upregulated FA oxidation can promote the utilization of lipid, it can also cause oxidative stress and increase the production of ROS, a second hit known to cause liver inflammation and damage in the 2-hit model for the development of NASH (56, 57).
The bioactivation of DEN is highly controlled by Cyp2E1 (58), and a high-fat diet can increase hepatic Cyp2e1 expression (59), suggesting that the WD might upregulate Cyp2E1- and DEN-induced carcinogenesis. Separately, sulforaphane and phenethyl isothiocyanate, isothiocyanates from broccoli, have been reported to decrease Cyp2E1 activity (60–62). Furthermore, phenethyl isothiocyanate is also reported to inhibit cytochrome P450 1A and cytochrome P450 3A activity (62). Our results are consistent with broccoli protection from increased DEN bioactivation by the WD. The nuclear factor (erythroid-derived 2)–like 2 pathway, activated by broccoli components and promoting phase-2 and antioxidant enzymes (25), may be downregulated by DEN (63). Thus, a WD may increase the bioactivation of DEN and support the promotion of tumorigenesis, as was evident in this study.
Our results also show that the WD and DEN enhanced the activation of hepatic macrophages, including KCs, whereas broccoli exhibited a counter-effect, downregulating Tnf and suppressing the expression of the activated macrophage biomarker Cd68. The activation of KCs is considered to contribute greatly to the progression of NAFLD (64, 65). Activated KCs are also critical in triggering the development of NASH from hepatic steatosis (66). C–C motif chemokine receptor 2 (CCL2) promotes TNFα production in KCs and the recruitment of bone marrow-derived macrophages (66, 67). Furthermore, elevated concentrations of circulating monocyte chemoattractant protein 1, the CCL2 ligand, have been reported in NASH patients (68). Therefore, broccoli, by inhibiting KC activation, may impede not only the promotion of hepatic steatosis, but also of NASH. Future studies could determine whether CCL2 or its ligands are altered by broccoli feeding.
As expected, the WD increased liver nodule size and number, which was also reported in a similar model that used a high-fat diet (30). It should be noted that this study uncovered an effect of broccoli on both the initiation and progression of liver tumorigenesis; there were no microscopic hepatic neoplasm–related lesions in the DEN-B-CON group, indicating that broccoli can suppress the initiation of hepatic neoplasms by DEN. However, although the DEN-NB-WD and DEN-B-WD groups showed a similar incidence of altered hepatic foci, which are preneoplastic lesions that have the potential to progress to hepatic adenomas and possibly HCC (69), there was a lower incidence of hepatic adenoma in DEN-B-WD mice, suggesting a delay or inhibition of the progression of preneoplasia to neoplasia, rather than a complete blockage of tumorigenesis. To our knowledge, our study is the first to show protection against murine liver cancer by the consumption of broccoli, and adds support to the accumulating knowledge showing that broccoli effects in whole-animal cancer studies reflect the positive implications in human studies in which broccoli-sprout tea possibly may slow or inhibit aflatoxin-induced liver cancer (23). It would certainly be interesting to carry out a study on the prevention of aflatoxin-induced liver cancer with the use of broccoli in mice.
In conclusion, broccoli consumption decreased hepatic lipidosis in mice receiving both the CON diet and the WD, without changing body mass. The data support the possibility that the lowering of HTGs by broccoli may be due to a decreased influx of NEFAs and increased efflux or excretion of VLDLs. In this study, daily consumption of broccoli was able to suppress the activation of hepatic macrophages, decrease liver damage, and protect against the initiation and progression of liver tumorigenesis. Broccoli promoted liver health and countered NAFLD development. In this age during which obesity is such a problem, including broccoli in the diet may have substantial public health implications for maintenance of a healthy liver, particularly in those who are greatly overweight.
Acknowledgments
We thank Edward Dosz for his assistance with the quantification of the sulforaphane content of broccoli samples. Y-JC and EHJ designed the project; MAW performed the pathology; Y-JC carried out the assays and statistical analysis and wrote the manuscript; and EHJ edited the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ACC, acetyl-CoA carboxylase; ALT, alanine aminotransferase; B, broccoli; BW, body weight; CCL2, C–C motif chemokine receptor 2; CON, control; Cpt1a, carnitine palmitoyltransferase 1α; Cyp2e1, cytochrome P450 2E1; DEN, diethylnitrosamine; EAT, epididymal adipose tissue; Fasn, fatty acid synthase; HCC, hepatocellular carcinoma; H&E, hematoxylin and eosin; HTG, hepatic triglyceride; Ifng, interferon γ; KC, Kupffer cell; MTTP, microsomal triglyceride transfer protein; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NB, no broccoli; NEFA, nonesterified fatty acid; ROS, reactive oxygen species; S, saline; Tnf, tumor necrosis factor; WD, Western diet.
References
- 1.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science 2011;332:1519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124–31. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Liver cancer: estimated incidence, mortality and prevalence worldwide in 2012 [Internet]. [cited 2015 Jul 6]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 4.American Cancer Society. Cancer facts and figures 2015 [Internet]. Atlanta, GA: American Cancer Society, 2015 [cited 2015 Jun 3]. Available from: http://www.cancer.org/acs/groups/content/@ editorial/documents/document/acspc-044552.pdf.
- 5.Laursen L. A preventable cancer. Nature 2014;516:S2–3. [DOI] [PubMed] [Google Scholar]
- 6.Perz JF, Armstrong GL, Farrington LA, Hutin YJF, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006;45:529–38. [DOI] [PubMed] [Google Scholar]
- 7.Poland GA, Jacobson RM. Clinical practice: prevention of hepatitis B with the hepatitis B vaccine. N Engl J Med 2004;351:2832–8. [DOI] [PubMed] [Google Scholar]
- 8.Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in northern Italy. Ann Intern Med 2000;132:112–7. [DOI] [PubMed] [Google Scholar]
- 9.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625–38. [DOI] [PubMed] [Google Scholar]
- 10.World Cancer Research Fund International/American Institute for Cancer Research. Continuous update project report: diet, nutrition, physical activity and liver cancer [Internet]. [cited 2015 Jun 12]. Available from: http://wcrf.org/sites/default/files/Liver-Cancer-2015-Report.pdf.
- 11.Swinburn BA, Caterson I, Seidell JC, James WP. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr 2004;7 1A:123–46. [DOI] [PubMed] [Google Scholar]
- 12.Jeffery RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr 2003;78:684–9. [DOI] [PubMed] [Google Scholar]
- 13.United States Department of Agriculture and United States Department of Health and Human Services. Dietary guidelines for Americans, 2010, 7th Edition [Internet]. [cited 2015 Jun 10]. Washington (DC): U.S. Government Printing Office, 2010. Available from: http://health.gov/dietaryguidelines/dga2010/ DietaryGuidelines2010.pdf. [DOI] [PMC free article] [PubMed]
- 14.Storlien LH, Huang XF, Lin S, Xin X, Wang HQ, Else PL. Dietary fat subtypes and obesity. World Rev Nutr Diet 2001;88:148–54. [DOI] [PubMed] [Google Scholar]
- 15.Leamy AK, Egnatchik RA, Young JD. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog Lipid Res 2013;52:165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dekker MJ, Su QZ, Baker C, Rutledge AC, Adeli K. Fructose: a highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am J Physiol Endocrinol Metab 2010;299:E685–94. [DOI] [PubMed] [Google Scholar]
- 17.United States Department of Agriculture Economics Rersearch Service. U.S. per capita use of selected, commercially produced, fresh, and processing vegetables and dry pulse crops, 1970–2014 [Internet]. [cited 2015 Jul 10]. Available from: http://www.ers.usda.gov/datafiles/Vegetable_and_Pulses_Yearbook_Tables/ General /YRBK2015_Section%201_General.pdf.
- 18.Jeffery EH, Araya M. Physiological effects of broccoli consumption. Phytochem Rev 2009;8:283–98. [Google Scholar]
- 19.Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC, Giovannucci EL. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J Natl Cancer Inst 1999;91:605–13. [DOI] [PubMed] [Google Scholar]
- 20.Voorrips LE, Goldbohm RA, van Poppel G, Sturmans F, Hermus RJJ, van den Brandt PA. Vegetable and fruit consumption and risks of colon and rectal cancer in a prospective cohort study—The Netherlands Cohort Study on Diet and Cancer. Am J Epidemiol 2000;152:1081–92. [DOI] [PubMed] [Google Scholar]
- 21.Terry P, Wolk A, Persson I, Magnusson C. Brassica vegetables and breast cancer risk. JAMA 2001;285:2975–7. [DOI] [PubMed] [Google Scholar]
- 22.Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR, John EM, Howe GR, Dreon DM, West DW, et al. . Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev 2000;9:795–804. [PubMed] [Google Scholar]
- 23.Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, Ye LX, Coady JL, Wang JB, Wu Y, et al. . Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol Biomarkers Prev 2005;14:2605–13. [DOI] [PubMed] [Google Scholar]
- 24.Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 25.Dosz EB, Jeffery EH. Commercially produced frozen broccoli lacks the ability to form sulforaphane. J Funct Foods 2013;5:987–90. [Google Scholar]
- 26.Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 27.Aranda PS, LaJoie DM, Jorcyk CL. Bleach gel: a simple agarose gel for analyzing RNA quality. Electrophoresis 2012;33:366–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008;3:1101–8. [DOI] [PubMed] [Google Scholar]
- 29.Barbisan LF, Miyamoto M, Scolastici C, Salvadori DMF, Ribeiro LR, Eira AF, de Camargo JLV. Influence of aqueous extract of Agaricus blazei on rat liver toxicity induced by different doses of diethylnitrosamine. J Ethnopharmacol 2002;83:25–32. [DOI] [PubMed] [Google Scholar]
- 30.Park EJ, Lee JH, Yu GY, He GB, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 2010;140:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 2007;56:2910–8. [DOI] [PubMed] [Google Scholar]
- 32.Altintas MM, Rossetti MA, Nayer B, Puig A, Zagallo P, Ortega LM, Johnson KB, McNamara G, Reiser J, Mendez AJ, et al. . Apoptosis, mastocytosis, and diminished adipocytokine gene expression accompany reduced epididymal fat mass in long-standing diet-induced obese mice. Lipids Health Dis 2011;10:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi KM, Lee YS, Kim W, Kim SJ, Shin KO, Yu JY, Lee MK, Lee YM, Hong JT, Yun YP, et al. . Sulforaphane attenuates obesity by inhibiting adipogenesis and activating the AMPK pathway in obese mice. J Nutr Biochem 2014;25:201–7. [DOI] [PubMed] [Google Scholar]
- 34.Zhu N, Soendergaard M, Jeffery EH, Lai RH. The impact of loss of myrosinase on the bioactivity of broccoli products in F344 rats. J Agric Food Chem 2010;58:1558–63. [DOI] [PubMed] [Google Scholar]
- 35.Armah CN, Derdemezis C, Traka MH, Dainty JR, Doleman JF, Saha S, Leung W, Potter JF, Lovegrove JA, Mithen RF. Diet rich in high glucoraphanin broccoli reduces plasma LDL cholesterol: evidence from randomised controlled trials. Mol Nutr Food Res 2015;59:918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valls C, Iannacconne R, Alba E, Murakami T, Hori M, Passariello R, Vilgrain V. Fat in the liver: diagnosis and characterization. Eur Radiol 2006;16:2292–308. [DOI] [PubMed] [Google Scholar]
- 37.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, et al. . Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002;123:134–40. [DOI] [PubMed] [Google Scholar]
- 38.Powell EE, Cooksley WGE, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of 42 patients for up to 21 years. Hepatology 1990;11:74–80. [DOI] [PubMed] [Google Scholar]
- 39.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab 2009;20:72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bechmann LP, Gieseler RK, Sowa JP, Kahraman A, Erhard J, Wedemeyer I, Emons B, Jochum C, Feldkamp T, Gerken G, et al. . Apoptosis is associated with CD36/fatty acid translocase upregulation in non-alcoholic steatohepatitis. Liver Int 2010;30:850–9. [DOI] [PubMed] [Google Scholar]
- 42.Miquilena-Colina ME, Lima-Cabello E, Sanchez-Campos S, Garcia-Mediavilla MV, Fernandez-Bermejo M, Lozano-Rodriguez T, Vargas-Castrillon J, Buque X, Ochoa B, Aspichueta P, et al. . Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut 2011;60:1394–402. [DOI] [PubMed] [Google Scholar]
- 43.Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res 2003;44:22–32. [DOI] [PubMed] [Google Scholar]
- 44.Kohjima M, Enjoji M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N, et al. . Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med 2007;20:351–8. [PubMed] [Google Scholar]
- 45.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab 2003;29:478–85. [DOI] [PubMed] [Google Scholar]
- 46.Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol 2010;45:199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Scholmerich J, Bollheimer LC. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol 2006;36:485–501. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, et al. . AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 2011;13:376–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim S, Sohn I, Ahn JI, Lee KH, Lee YS. Hepatic gene expression profiles in a long-term high-fat diet-induced obesity mouse model. Gene 2004;340:99–109. [DOI] [PubMed] [Google Scholar]
- 50.Inoue M, Ohtake T, Motomura W, Takahashi N, Hosoki Y, Miyoshi S, Suzuki Y, Saito H, Kohgo Y, Okumura T. Increased expression of PPAR gamma in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun 2005;336:215–22. [DOI] [PubMed] [Google Scholar]
- 51.Gregoire FM, Zhang Q, Smith SJ, Tong C, Ross D, Lopez H, West DB. Diet-induced obesity and hepatic gene expression alterations in C57BL/6J and ICAM-1-deficient mice. Am J Physiol Endocrinol Metab 2002;282:E703–13. [DOI] [PubMed] [Google Scholar]
- 52.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol 2003;552:335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JRB, Newgard CB, et al. . Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45–56. [DOI] [PubMed] [Google Scholar]
- 54.Lambertucci RH, Hirabara SM, Silveira LD, Levada-Pires AC, Curi R, Pithon-Curi TC. Palmitate increases superoxide production through mitochondrial electron transport chain and NADPH oxidase activity in skeletal muscle cells. J Cell Physiol 2008;216:796–804. [DOI] [PubMed] [Google Scholar]
- 55.Cytochrome DA. P450 2E1: its role in disease and drug metabolism, 2013. Netherlands: Springer; 2013. [Google Scholar]
- 56.Day CP. Pathogenesis of steatohepatitis. Best Pract Res Clin Gastroenterol 2002;16:663–78. [DOI] [PubMed] [Google Scholar]
- 57.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology 1998;114:842–5. [DOI] [PubMed] [Google Scholar]
- 58.Kang JS, Wanibuchi H, Morimura K, Gonzalez FJ, Fukushima S. Role of CYP2E1 in diethylnitrosamine-induced hepatocarcinogenesis in vivo. Cancer Res 2007;67:11141–6. [DOI] [PubMed] [Google Scholar]
- 59.Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, Ota T, Yokoyama M, Honda M, Miyamoto K, et al. . Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism 2008;57:1071–7. [DOI] [PubMed] [Google Scholar]
- 60.Zhou R, Lin JJ, Wu DF. Sulforaphane induces Nrf2 and protects against CYP2E1-dependent binge alcohol-induced liver steatosis. Biochim Biophys Acta 2014;1840:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barcelo S, Gardiner JM, Gescher A, Chipman JK. CYP2E1-mediated mechanism of anti-genotoxicity of the broccoli constituent sulforaphane. Carcinogenesis 1996;17:277–82. [DOI] [PubMed] [Google Scholar]
- 62.Guo Z, Smith TJ, Wang E, Sadrieh N, Ma Q, Thomas PE, Yang CS. Effects of phenethyl isothiocyanate, a carcinogenesis inhibitor, on xenobiotic-metabolizing enzymes and nitrosamine metabolism in rats. Carcinogenesis 1992;13:2205–10. [DOI] [PubMed] [Google Scholar]
- 63.Sahin K, Orhan C, Tuzcu M, Sahin N, Ali S, Bahcecioglu IH, Guler O, Ozercan I, Ilhan N, Kucuk O. Orally administered lycopene attenuates diethylnitrosamine-induced hepatocarcinogenesis in rats by modulating Nrf-2/HO-1 and Akt/mTOR pathways. Nutr Cancer 2014;66:590–8. [DOI] [PubMed] [Google Scholar]
- 64.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol 2009;51:212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, Scott DK, O’Doherty RM. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes 2010;59:347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production. J Biol Chem 2012;287:40161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol 2012;302:G1310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haukeland JW, Damas JK, Konopski Z, Loberg EM, Haaland T, Goverud I, Torjesen PA, Birkeland K, Bjoro K, Aukrust P. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol 2006;44:1167–74. [DOI] [PubMed] [Google Scholar]
- 69.Gad SC. Animal models in toxicology. 2nd ed. Boca Raton (FL): CRC/Taylor & Francis; 2007.