Abstract
Background: Studies that examined dietary energy requirements (DERs) of patients undergoing maintenance hemodialysis (MHD) have shown mixed results. Many studies reported normal DERs, but some described increased energy needs. DERs in MHD patients have been estimated primarily from indirect calorimetry and from nitrogen balance studies. The present study measured DERs in MHD patients on the basis of their dietary energy intake and changes in body composition.
Objective: This study assessed DERs in MHD patients who received a constant energy intake while changes in their body composition were measured.
Design: Seven male and 6 female sedentary, clinically stable MHD patients received a constant mean (±SD) energy intake for 92.2 ± 7.9 d while residing in a metabolic research ward. Changes in fat and fat-free mass, measured by dual-energy X-ray absorptiometry, were converted to calorie equivalents and added to energy intake to calculate energy requirements.
Results: The average DER was 31 ± 3 kcal · kg−1 · d−1 calculated from energy intake and change in fat and fat-free calories, which was 28 ± 197 kcal/d over the 92 d of the study. DERs of MHD patients correlated strongly with their body weight (r = 0.81, P = 0.002) and less closely with their measured resting energy expenditure expressed as kcal/d (r = 0.69, P = 0.01). Although the average observed DER in MHD patients was similar to published estimated values for normal sedentary individuals of similar age and sex, there was wide variability in DER among individual patients (range: 26–36 kcal · kg−1 · d−1).
Conclusions: Average DERs of sedentary, clinically stable patients receiving MHD are similar to those of sedentary normal individuals. Our data do not support the theory that MHD patients have increased DERs. Due to the high variability in DERs, careful monitoring of the nutritional status of individual MHD patients is essential. This trial was registered at clinicaltrials.gov as NCT02194114.
Keywords: dietary energy needs, chronic kidney disease, chronic kidney failure, renal nutrition, body composition
INTRODUCTION
A number of studies have examined the dietary energy requirements (DERs)7 of adult patients who receive maintenance hemodialysis (MHD). Energy requirements have generally been assessed in 2 ways: measuring energy expenditure by indirect calorimetry during different daily physical activities (1) and with the use of nitrogen balance studies during which clinically stable MHD patients ingested a constant protein diet while their energy intake was varied (2). The results are somewhat discordant. Some studies reported that dietary energy needs determined by indirect calorimetry are not different from those of normal adults of similar age, sex, and level of daily physical activity (3), whereas other studies reported increased energy requirements (1). Very few studies in MHD patients have measured energy expenditure during both resting conditions [resting energy expenditure (REE)] and during dialysis and various activities of typical daily living (4). This is a question of considerable importance because of the following reasons: protein-energy wasting (PEW) occurs commonly in MHD patients (5), protein-energy malnutrition is a common cause of PEW in MHD patients (5, 6), reduced dietary energy intake is common in MHD patients (7), and PEW is associated with increased mortality in these individuals (8, 9).
We recently examined DERs in 13 clinically stable patients receiving MHD by a novel method for advanced kidney disease. Patients ingested a constant energy diet for an average of 92 d in a metabolic research ward. Their body composition was measured at the beginning and end of this period. We calculated their DERs by adjusting each patient’s constant energy intake by the energy equivalent of the changes, if any, in their measured body fat and estimated protein mass.
METHODS
Many details of this study were described elsewhere (10). This project was primarily designed to assess DERs in MHD patients. Participants were 13 clinically stable patients receiving MHD who lived in the General Clinical Research Center (GCRC) at Harbor-UCLA Medical Center for an average of 92 d. For each patient, diets were designed to be constant in energy throughout the study and provided, in random order, 0.60, 0.80, 1.00, 1.15, and 1.30 g protein · kg body weight−1 · d−1. Except for one protein diet that was consumed by one patient for 11 d, each diet was consumed for 16–22 d. Patients underwent hemodialysis 3 times weekly with dialyzers manufactured by Fresenius Medical Care. Blood flows were 400 mL/min, and dialysate flows were 800 mL/min.
Inclusion criteria were as follows: 1) age of 25–65 y, 2) relatively healthy and receiving MHD 3 times weekly for ≥5 mo, 3) serum albumin ≥3.6 g/dL, 4) serum hemoglobin ≥11 g/dL, and 5) relative body weight of 90–115% of the NHANES II median body weight for normal individuals of the same height, frame size, age range, and sex (11). Exclusion criteria were as follows: 1) moderate or severe PEW; 2) regular intensive physical activity; 3) existing cancer other than basal cell carcinoma; 4) severe heart, lung, or liver disease; 5) poorly controlled hypertension, active asthma, chronic systemic infection, any systemic inflammatory process, symptomatic musculoskeletal disease or neuropathy, and amputations of extremities; 6) type 1 or 2 diabetes; 7) pregnancy; 8) alcohol or drug abuse; 9) treatment with l-carnitine or anabolic hormones within previous 6 mo; and 10) psychosis or inability to give informed consent or follow the protocol. This study was approved by the Institutional Review Board of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center (clinicaltrials.gov; NCT02194114).
Dietary intake
The controlled diets for each patient were designed by using ProNutra software (version 3.3.0.10, 2009; Viocare, Inc.). Diets were calculated so that each patient received a constant energy intake throughout the study. The prescribed energy intake was determined as described previously (10). The patient’s REE was measured by indirect calorimetry before the metabolic study commenced. The patient’s daily dietary energy needs were then calculated with the use of standard methods for estimating the patient’s total daily energy expenditure by multiplying his or her REE by a factor of 1.3 or 1.4 to adjust for the specific thermic effect of foods and estimated physical activity level (PAL) (12). These factors were selected because MHD patients appear to be very sedentary, even more so than sedentary normal individuals (13). Daily energy intake was then modified further according to the patient’s age, clinical status, and physical activity in the metabolic ward. The patient’s actual energy intake differed, at most, only modestly from the estimated total daily energy expenditure calculated from the product of REE and a PAL of 1.3 or 1.4.
Diets provided ∼0.6–1.3 g protein · kg body weight−1 · d−1 depending on the specific dietary study period and the randomly assigned order of dietary protein administration. A total of 30–35% of energy intake was from fat, with a polyunsaturated to saturated fat ratio of ∼1:1. Carbohydrate intake varied depending on the amount of protein given; fiber intake was ∼20 g/d. The study diets daily provided no more than 3000 mg sodium, 3120 mg potassium, 1000 mg phosphorus, and 1400 mg calcium. Patients were also given the multivitamin Nephro-Vite (Watson Pharmaceuticals). The glucose content of the hemodialysate was 200 mg/dL (∼182 mg anhydrous glucose/dL); therefore, glucose uptake during hemodialysis treatments should be very low (14). The patients were often in negative protein balance with lower-protein diets and in positive protein balance with higher-protein diets. We estimated that, overall during the course of the study, the various protein intakes would not substantially influence net protein balance. All of the foods for each patient were prepared in the metabolic kitchen in the GCRC and purchased at the same time (except for perishables) to minimize the risk of changes in nutrient content during the 92 d of study. Foods were weighed to the nearest 0.01 g, prepackaged for individual meals, and stored frozen until used (except for foods that could be damaged by freezing). Meal plans were homogenous so that the 3 meals during the day and throughout the study were approximately equal in nutrient composition for a given patient, except for the protocol-designed variations in protein intake. The snack provided one-half of the nutrients present in each of the meals. Each patient was strongly encouraged to consume all of the diet foods in their entirety. A spatula, squirt bottle, and the subject’s tongue were used to ensure 100% consumption of foods and beverages, including residual food or drink left on plates and in drinking glasses. Patients consumed meals in their hospital room under the supervision of the GCRC nursing and research nutrition staff, and actual intake was recorded after each meal.
Physical activities that are usually uncontrolled, such as standing, sitting, and walking, were gauged by the dietitian through interviews before and at the beginning of the metabolic study to accurately prescribe the energy intake for each patient. Patients were prescribed exercise on a stationary ergometer several times daily and by walking in the GCRC corridor. Exercise was tightly controlled to approximate the patient’s typical free-living daily activity level, determined by a careful history, to attempt to maintain the patient’s typical outpatient daily energy expenditure.
REE
REE was measured by open-circuit indirect calorimetry with the use of a ventilated hood system (Vmax Spectra Series model 29n; Sensor Medics Corporation/Viasys Health Care). The carbon dioxide elimination and oxygen uptake readouts from the Vmax was adjusted to standard temperature and pressure (dry conditions at a temperature of 273°K and pressure at 1013 hPa). REE was calculated by using the manufacturer’s software. Calibration of the equipment with 2 different standard gases and 1 standard volume was performed on a daily basis before starting the measurements. In addition, the system was automatically recalibrated every 5 min. REE was measured for each patient by the same operator at the same time of day (0800–0900 h) (15) in accordance with recommended best-practice guidelines (16). Measurements were performed after 6–8 h sleep and after a 10–12 h fast in a semidarkened room in the GCRC, with only the subject and tester present. Subjects were allowed to void before the test, but all other activity was prohibited. Subjects rested for an additional 20 min after voiding. Room temperature was 20–24°C, and blankets were available when requested. The metabolic cart canopy was positioned over the participant's head while he or she rested quietly and awake in a supine position. An initial 5- to 10-min period was allowed to accustom the participant to the device and for equilibration. Subsequently, oxygen consumption and carbon dioxide expiration were recorded for 20 min while the subject remained under strict resting conditions. Only steady state periods of measurement were selected for calculation according to the protocol for the ventilated hood system (<10% CV). Energy expenditure was calculated by using the Weir formula (17). Subjects underwent the REE measurements 5–6 wk before the start of the study to allow time for the diets to be planned and prepared.
DERs
The prescribed energy intake during this 92-d study may have underestimated or overestimated the patients’ true DERs for stability in body energy sources. Hence, the DER was calculated by adjusting the prescribed energy intake the patient’s estimated energy excesses or deficits as indicated by any changes during the study in body fat or protein mass, as measured by dual-energy X-ray absorptiometry (DXA). Protein mass was estimated from lean mass. The energy equivalents of changes in fat and lean body mass were considered to be 9.297 kcal/g body fat and 1.027 kcal/g lean body mass, respectively (18). These changes were subtracted from or added to the patient’s constant dietary energy intake during the study to indicate the patient’s actual DER.
DXA
DXA was measured at baseline (beginning of the metabolic study) and at the end of each of the 5 protein diets (periods 1–5, respectively) given to each patient. Fat mass, soft lean body mass (fat-free, edema-free mass), and bone mass were estimated by DXA 1 h postdialysis by using a Hologic Series model QDR 4500A-XP scanner (Hologic Inc.). The methods for DXA assessment of body composition have been described elsewhere (10, 19, 20). The precision of body-composition analysis was determined by daily spine phantom quality-control assessments in addition to weekly quality-control assessments by using the Hologic Anthropomorphic Spine Phantom. The bone densitometer is serviced biannually for preventative maintenance. The whole-body phantom was used to assess instrument stability and total body bone mineral density, total lean mass, total fat mass, total tissue mass, and total percentage fat. It was also used for weekly calibration of skeletal and whole-body composition measurements. The acceptable quality-control limits are ±1.5% of the mean value at calibration for bone density. Quality-control studies indicate that the QDR 4500A DXA model gives very precise and accurate measurements with excellent reproducibility (21).
Statistical methods
All of the reported data in this article concerning body weight refer to body weight obtained 1 d postdialysis. Data for the 13 subjects were analyzed by using linear regression or quadratic analyses, with significance set at P < 0.05. Presented data are means ± SDs, SEs, 95% CIs of the mean, and 95% prediction intervals unless otherwise indicated. Statistical analyses were conducted by using Microsoft Excel 2007 and Stata Statistical Software, release 12 (2011; StataCorp LP).
RESULTS
Patients were 7 men and 6 women, 8 of whom were Hispanic, 3 African American, 1 white, and 1 Asian. Patients’ mean age was 47.7 y, BMI (in kg/m2) was 25.5, and dialysis vintage was 51.9 ± 33.1 mo. Body fat mass was 19.9 ± 5.6 kg, and fat-free mass was 44.8 ± 9.3 kg (Table 1). The dietary energy provided to the patients during the study averaged 2134 ± 354 kcal/d (Table 2). Measured REE was 24 ± 4 kcal · kg−1 · d−1 and varied substantially, from 20 to 33 kcal · kg−1 · d−1 (Table 3).
TABLE 1.
Patient demographic characteristics
| Sex | Age, y | Height, cm | Weight, kg | BMI, kg/m2 | Albumin, g/dL | Intact parathyroid hormone, pg/mL | Fat mass, kg | Fat-free mass, kg | |
| Patient number | |||||||||
| 1 | F | 53 | 155.2 | 58.2 | 24.2 | 3.9 | 373 | 21.6 | 33.8 |
| 2 | F | 49 | 153.9 | 49.1 | 20.7 | 3.8 | 75 | 14.7 | 33.0 |
| 3 | F | 54 | 153.1 | 62.3 | 26.3 | 3.8 | 492 | 23.6 | 33.8 |
| 4 | F | 49 | 151.9 | 53 | 22.9 | 3.5 | 296 | 17.9 | 32.8 |
| 5 | F | 39 | 170.9 | 74.2 | 25.4 | 3.6 | 138 | 17.0 | 54.7 |
| 6 | M | 49 | 156.2 | 70.7 | 29 | 3.6 | 74 | 20.9 | 47.1 |
| 7 | M | 26 | 173.3 | 67.6 | 22.6 | 4.2 | 355 | 9.5 | 51.2 |
| 8 | F | 44 | 171.5 | 72.1 | 24.5 | 3.5 | 189 | 25.1 | 44.2 |
| 9 | M | 46 | 173.5 | 69.9 | 23.2 | 3.5 | 494 | 17.1 | 50.1 |
| 10 | M | 65 | 157.7 | 65.4 | 26 | 3.7 | 59 | 16.4 | 45.5 |
| 11 | M | 45 | 175.9 | 94.2 | 30.5 | 4.5 | 291 | 30.2 | 57.9 |
| 12 | M | 60 | 170.9 | 81.7 | 28 | 3.6 | 108 | 25.2 | 53.8 |
| 13 | M | 41 | 166.1 | 75.3 | 27.3 | 4.4 | 51 | 19.9 | 44.8 |
| Mean ± SD | 48 ± 10 | 164 ± 9 | 69 ± 12 | 25 ± 3 | 3.8 ± 0.3 | 230 ± 163 | 21.6 ± 5.6 | 44.8 ± 9.3 |
TABLE 2.
Estimated and calculated DERs1
| Dietary energy intake2 |
Calculated DER3 |
||||
| kcal/d | kcal · kg−1 · d−1 | Requirement, kcal/d | kcal · kg−1 · d−1 | Fat-free mass, kcal · kg−1 · d−1 | |
| Patient number | |||||
| 1 | 1600 | 27.7 | 1585 | 27.2 | 46.9 |
| 2 | 1800 | 36.9 | 1634 | 33.3 | 49.5 |
| 3 | 1750 | 28.3 | 1675 | 26.9 | 49.6 |
| 4 | 1565 | 30.0 | 1913 | 36.1 | 58.3 |
| 5 | 2200 | 29.2 | 2028 | 27.3 | 37.1 |
| 6 | 2350 | 33.0 | 2151 | 30.4 | 45.7 |
| 7 | 2400 | 35.8 | 2090 | 30.9 | 40.8 |
| 8 | 2150 | 30.2 | 2266 | 31.4 | 51.3 |
| 9 | 2400 | 34.7 | 2289 | 32.8 | 45.7 |
| 10 | 2000 | 31.0 | 2349 | 35.9 | 51.6 |
| 11 | 2530 | 27.0 | 2494 | 26.5 | 43.1 |
| 12 | 2500 | 30.9 | 2482 | 30.4 | 46.1 |
| 13 | 2500 | 32.8 | 2427 | 32.2 | 54.2 |
| Mean ± SD | 2134 ± 353.9 | 31.3 ± 3.1 | 2106 ± 321.3 | 30.9 ± 3.3 | 47.7 ± 5.7 |
| SE | 98.2 | 0.9 | 89.1 | 0.9 | 1.6 |
| 95% CI4 | 1920, 2347 | 29.4, 33.2 | 1912, 2300 | 28.9, 32.9 | 44.3, 51.1 |
DER, dietary energy requirement.
The constant energy intake provided to a given patient throughout the study, estimated on the basis of measured resting energy expenditure.
Calculated on the basis of the sum of the constant energy intake of the maintenance hemodialysis patients and the changes in body calorie stores as determined by body fat and lean body mass (12).
95% CI of the mean.
TABLE 3.
Estimates and measurements of REE and estimates of DERs1
| REE2 |
Estimated REE from Mifflin–St. Jeor equation3 |
Estimated DER based on Dietary Reference Intakes4 |
Estimated DER from Redman et al.5 |
|||||
| kcal/d | kcal · kg−1 · d−1 | kcal/d | kcal · kg−1 · d−1 | kcal/d | kcal · kg−1 · d−1 | kcal/d | kcal · kg−1 · d−1 | |
| Patient number | ||||||||
| 1 | 1201 | 20.6 | 1130 | 19.4 | 1659 | 28.5 | 1998 | 34.3 |
| 2 | 1427 | 29.1 | 1050 | 21.4 | 1592 | 32.4 | 1985 | 40.4 |
| 3 | 1237 | 19.9 | 1152 | 18.5 | 1676 | 27.0 | 1991 | 32.0 |
| 4 | 1204 | 22.7 | 1076 | 20.3 | 1614 | 30.5 | 1960 | 37.0 |
| 5 | 1737 | 23.4 | 1456 | 19.6 | 2020 | 27.2 | 2765 | 37.3 |
| 6 | 1754 | 24.8 | 1446 | 19.6 | 2163 | 30.6 | 2394 | 33.9 |
| 7 | 2220 | 32.8 | 1635 | 24.2 | 2425 | 35.9 | 2506 | 37.1 |
| 8 | 1807 | 25.1 | 1414 | 19.6 | 1970 | 28 | 2339 | 32.4 |
| 9 | 2131 | 30.5 | 1561 | 22.3 | 2272 | 32.5 | 2517 | 36.0 |
| 10 | 1640 | 25.1 | 1403 | 21.5 | 1934 | 29.5 | 2432 | 37.2 |
| 11 | 1874 | 19.9 | 1824 | 19.3 | 2681 | 28.5 | 2743 | 29.1 |
| 12 | 1680 | 20.6 | 1594 | 19.5 | 2312 | 28.3 | 2682 | 32.8 |
| 13 | 1876 | 24.9 | 1593 | 21.1 | 2366 | 31.4 | 2273 | 30.2 |
| Mean ± SD | 1676 ± 330.9 | 24.6 ± 4.1 | 1410 ± 241.8 | 20.6 ± 1.5 | 2053 ± 325.3 | 30.0 ± 2.6 | 2353 ± 294.7 | 34.6 ± 3.2 |
| SE | 91.8 | 1.1 | 67.1 | 0.4 | 97.4 | 0.7 | 81.7 | 0.9 |
| 95% CI6 | 1476, 1876 | 22.2, 27.0 | 1264, 1556 | 19.7, 21.5 | 1841, 2265.4 | 28.4, 31.6 | 2175, 2353 | 32.6, 36.6 |
DER, dietary energy requirement; REE, resting energy expenditure.
Measured by using indirect calorimetry (22).
The predicted resting DER of adults based on the Mifflin–St. Jeor equations (23): REE (men) = 10 × weight (kg) + 6.25 × height (cm) − 5 × age (y) + 5; REE (women) = 10 × weight (kg) + 6.25 × height (cm) − 5 × age (y) − 161.
Predicted DER of normal sedentary adults according to the Dietary Reference Intakes (24).
Predicted DER of adults based Redman et al. (25). Model B: Total daily energy expenditure (kcal/d) = 454 + 38.7 × fat-free mass (kg) − 5.4 × fat mass (kg) + 4.7 × age (y) + 103 × sex (1 = female, 0 = male).
95% CI of the mean.
Table 4 shows the changes in body fat and lean body mass and the energy equivalents of these changes that were calculated from the beginning of the study until its end, an average of 92.2 d later. It is possible that some of the changes in body composition at the beginning of the study were due to alterations in body water caused by more careful control of ultrafiltration during hemodialysis and of fluid balance in the GCRC. However, weight loss during the first diet period, which lasted 17.9 ± 5.6 d, was −0.18 ± 0.79 kg, and weight loss from the beginning of period 2 until the end of study, which comprised 4 periods lasting 74.3 ± 7.0 d, was −0.45 ± 1.92 kg (Table 4). Thus, there was no major difference in daily body weight loss during the first period compared with the subsequent 4 diet periods. Weight, fat mass, lean body mass, and body calorie balance also did not vary significantly according to the chronological order of administration of the 5 protein intakes (data not shown). Moreover, there was no trend in change in body weight, fat mass, lean body mass, or calorie balance according to the amount of dietary protein intake (data not shown). There was no visible edema in any of the 13 patients when they entered the metabolic research ward. Therefore, for estimating DERs, we calculated body-composition changes from the beginning of diet period 1 until the end of diet period 5.
TABLE 4.
Change in body mass and composition from baseline to end of study
| Calorie equivalent of change in fat mass |
Calorie equivalent of change in fat-free mass |
|||||||
| Days from baseline to end of study, n | Change in body weight throughout study, kg | Change in fat mass throughout study, kg | Throughout study,1 kcal | Per day, kcal/d | Change in fat-free body mass throughout study,2 kg | Throughout study,3 kcal | Per day, kcal/d | |
| Patient number | ||||||||
| 1 | 99 | −0.32 | 0.27 | 2500 | 25 | −1.0142 | −1042 | −11 |
| 2 | 93 | −0.08 | 1.87 | 17,388 | 187 | −1.933 | −1985 | −21 |
| 3 | 91 | 1.89 | 0.59 | 5521 | 61 | 1.2822 | 1317 | 14 |
| 4 | 96 | −4.59 | −3.48 | −32,324 | −337 | −1.017 | −1044 | −11 |
| 5 | 90 | −2.54 | 2.18 | 20,237 | 225 | −4.6576 | −4784 | −53 |
| 6 | 100 | −0.74 | 2.49 | 23,177 | 232 | −3.222 | −3309 | −33 |
| 7 | 78 | −0.21 | 2.93 | 27,194 | 349 | −2.9543 | −3034 | −39 |
| 8 | 91 | 0.02 | −1.27 | −11,817 | −130 | 1.2641 | 1298 | 14 |
| 9 | 101 | 1.85 | 1.13 | 10,455 | 104 | 0.7115 | 31 | 7 |
| 10 | 75 | −3.95 | −2.69 | −24,986 | −333 | −1.1789 | −1211 | −16 |
| 11 | 98 | 0.39 | 0.4 | 3687 | 38 | −0.1125 | −116 | −1 |
| 12 | 93 | −0.08 | 0.21 | 1912 | 21 | −0.201 | −206 | −2 |
| 13 | 94 | 0.16 | 0.81 | 7490 | 80 | −0.6208 | −638 | −7 |
| Mean ± SD | 92.2 ± 7.9 | −0.6 ± 2.0 | 0.4 ± 1.9 | 3880 ± 17,844 | 40.2 ± 205 | −1.1 ± 1.8 | −1079 ± 1824 | −12 ± 20 |
| SE | 2.18 | 0.54 | 0.53 | 4949.15 | 56.90 | 0.49 | 505.82 | 5.61 |
| 95% CI4 | 87.5, 97.0 | −1.78, 0.58 | −0.76, 1.56 | −6904, 14,664 | −88.8, 164.2 | −2.17, −0.03 | −2181, 23 | −24.22, 0.22 |
Calculated as change in fat mass (kg) multiplied by 9291.11 kcal/kg fat.
Fat-free mass is considered to be edema-free.
Calculated as change in fat-free mass (kg) multiplied by 1027.04 kcal/kg fat-free mass.
95% CI of the mean.
Most of the change in energy sources due to changes in body composition was caused by a net change in body fat mass that accounted for a mean accrual of 3880 ± 17,844 kcal/patient (+40.2 ± 205 kcal · d−1 · patient−1) (Table 4). The changes in fat-free mass accounted for a net loss of 1079 ± 1824 kcal/patient (−12 ± 20 kcal · d−1 · patient−1). Although the average total change in fuel sources due to changes in fat and fat-free mass was not great, the change in fuel sources in some individual patients was often large. For example, patients 4 and 10 exhibited total fat and fat-free mass fuel source losses of 337 and 333 kcal/d (Table 4).
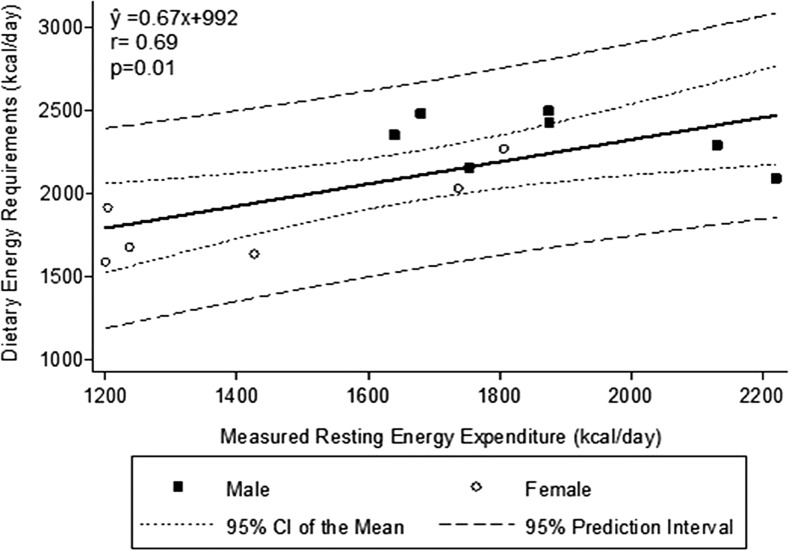
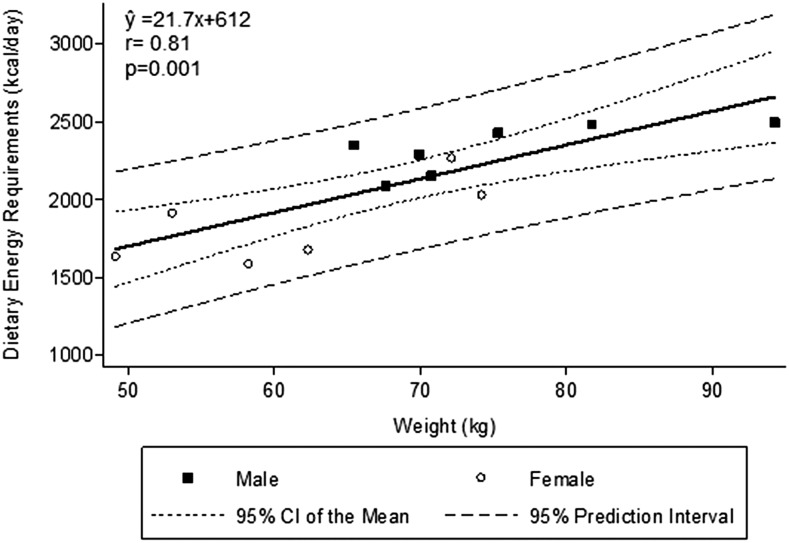
Each patient’s DER, calculated from his or her dietary energy intake during the metabolic study adjusted for any changes in his or her fat and fat-free mass, is shown in Table 2. The DER averaged 30.9 ± 3.3 kcal · kg−1 · d−1 (range: 26–36 kcal · kg−1 · d−1) (Table 2). DERs were 2326 ± 159 kcal/d (31.1± 3.0 kcal · kg−1 · d−1) and 1850 ± 267 kcal/d (30.8 ± 3.9 kcal · kg−1 · d−1) in male and female MHD patients, respectively. As expected, DERs correlated strongly and positively with both REE and body weight (Figures 1 and 2). The correlation between DER and REE was significant when both factors were expressed as kcal/d (r = 0.69, P < 0.01) (Figure 1). When evaluating REE compared with REE for sexes separately, the correlation was slightly negative in men, but this reflects an even smaller sample size of 7 and is not significantly different from zero (r = 0.50, P = 0.25). DERs could not be precisely predicted from REE. The DER was not significantly correlated with REE when both variables were expressed as kcal · kg−1 · d−1 (r = 0.47, P = 0.1).
FIGURE 1.
Dietary energy requirements compared with resting energy expenditure. There is a positive correlation between resting energy expenditure and dietary energy requirements.
FIGURE 2.
Dietary energy requirements compared with weight. Dietary energy requirement is positively correlated with weight.
The CIs of the mean and the prediction intervals for the relation between DERs and REE and between DERs and body weight are shown in Figures 1 and 2. As would be expected with a small sample size of 13 MHD patients, the prediction intervals are rather large. Analysis of the data showed a slightly higher r value for a quadratic relation between REE and DERs (r = 0.81). However, the biological plausibility of a condition in which REE substantially increases but the DER does not is dubious. The significance of this relation likely reflects the chance variability in the measurements in the setting of a small sample size. Analyzing DERs compared with body weight by a quadratic equation gave essentially the same r value (r = 0.82) as with linear regression analysis (r = 0.81; Figure 2).
DISCUSSION
The DERs of MHD patients have been examined in a number of studies (1, 2, 12, 26–28). DERs in MHD patients have generally been determined by indirect calorimetry during the resting state; in one study during such daily activities as resting, sitting, and postprandially after a defined meal (4); and in another study before, during, and after hemodialysis treatment (1). We also examined dietary energy needs by measuring nitrogen balances in MHD patients while they ingested a constant protein diet and their dietary energy intake was varied every 21–23 d (2). Most of these studies described normal energy requirements in clinically stable MHD patients, although one study reported increased energy needs in MHD patients (1). Inflammation is associated with increased energy requirements in these individuals (29).
To our knowledge, the present study is the first to measure DERs in clinically stable MHD patients by providing a constant diet for an extended period of time (mean: 92 d) in a research ward; the DER was calculated from the sum of their dietary energy intake and the energy gain or loss as determined from any changes in body fat mass and fat-free mass. This method enabled us to estimate energy expenditure in an integrated fashion, throughout this entire 92-d period of time, integrating changes in energy balance that may occur with the hemodialysis treatment, which itself may be a catabolic stress (30), during physical exercise, and during other activities of daily living. The laborious nature of this type of investigation would not make it suitable for use in routine measurement of DERs, but it does allow us to examine DERs of MHD patients by another, independent method.
The current study enabled us to compare DERs, estimated from REE measured by indirect calorimetry, with the above technique on the basis of changes in body mass and composition while the patients ingested a constant energy diet over a period of 3 mo. Our data indicated that the association between DER and REE in our MHD patients was not very precise (Figure 1). Moreover, this relation was significant only when both variables were expressed as kcal/d and when given as kcal · kg body weight−1 · d−1.
Our patient interviews indicated that they led sedentary lives outside the hospital, like most MHD patients (13), and this exercise pattern was continued in the research ward, notwithstanding the exercise that they performed several times daily on a stationary ergometer and walking back and forth in the GCRC corridor. We therefore designed their dietary energy intake for the metabolic study by multiplying their REE by 1.3 or 1.4, an adjustment factor for the thermic effect of foods and low PAL of very sedentary people, as recommended by the US National Academies of Science, Institute of Medicine (24). This would seem to be a distinctly unusual PAL for healthy adults but might not be uncommon for sedentary MHD patients (13). The less-active inpatient lifestyle imposed by the constraints of the study might have also contributed to their low PAL in this GCRC study.
As indicated in Methods, we made further minor adjustments to the patients’ prescribed energy intake according to their age, clinical status, and physical activity in the research ward. Notwithstanding these adjustments, the DER to REE ratio was only 1.28 ± 0.17 (Table 5). This ratio is lower than that observed for normal adults by Redman et al. (25) or in the FAO/WHO/United Nations University report on Human Energy Requirements (32). The REE in the MHD patients in our study appears to be greater than published REE values observed in 2 large-scale studies in normal adults (23, 25), but the DER of our MHD patients was not elevated, possibly because daily physical activity was less in our patients, as we previously reported for other MHD patients (13). The low PAL of our sedentary MHD patients may also lower their DER to REE ratio (13). This might also be due, in some part, to the common presence of inflammation related to uremia, which may not be detected by standard laboratory assessments, or other morbidities in these patients that may increase REE (22, 28, 29). In this regard, the serum concentration of albumin, a negative acute-phase protein, was 3.8 g/dL in these patients, which was only slightly lower than normal.
TABLE 5.
Ratios of measured DER to different estimates of energy requirements1
| Ratio of DER (from the present study) to |
||||
| Ratio of measured DER to REE2 (data from present study) | KDOQI estimate3 | DER from DRIs4 | DER from Redman et al. model B5 | |
| Patient number | ||||
| 1 | 1.32 | 0.78 | 0.96 | 0.79 |
| 2 | 1.15 | 0.95 | 1.03 | 0.82 |
| 3 | 1.35 | 0.77 | 1.00 | 0.84 |
| 4 | 1.59 | 1.03 | 1.18 | 0.98 |
| 5 | 1.17 | 0.78 | 1.00 | 0.73 |
| 6 | 1.23 | 0.87 | 0.99 | 0.90 |
| 7 | 0.94 | 0.88 | 0.86 | 0.83 |
| 8 | 1.25 | 0.90 | 1.15 | 0.97 |
| 9 | 1.07 | 0.94 | 1.01 | 0.91 |
| 10 | 1.43 | 1.20 | 1.21 | 0.97 |
| 11 | 1.33 | 0.76 | 0.93 | 0.91 |
| 12 | 1.48 | 1.01 | 1.07 | 0.93 |
| 13 | 1.29 | 0.92 | 1.03 | 1.07 |
| Mean ± SD | 1.28 ± 0.17 | 0.91 ± 0.13 | 1.0 ± 0.10 | 0.90 ± 0.09 |
| SE | 0.05 | 0.03 | 0.03 | 0.03 |
| 95% CI6 | 1.18, 1.38 | 0.83, 0.99 | 0.94, 1.06 | 0.84, 0.96 |
DER, dietary energy requirement; DRI, Dietary Reference Intake; KDOQI, Kidney Disease Outcomes Quality Initiative; REE, resting energy expenditure.
Measured by indirect calorimetry.
Estimated from KDOQI guidelines (31).
DERs of normal sedentary adults aged ≥19 y according to the DRIs (24).
Estimated by using the model B equation, derived from the data in Redman et al. (25); the equation includes body-composition measurements to predict DER and was used because of its higher coefficient than model A. Model B: Total daily energy expenditure (kcal/d) = 454+38.7 × fat-free mass (kg) − 5.4 × fat mass (kg) + 4.7 × age (y) + 103 × sex (1 = female, 0 = male).
95% CI of the mean.
The 1.0 ± 0.10 ratio of our MHD patients’ DER to the DER of healthy sedentary, nonpregnant adults of similar weight, age, and sex distribution, as estimated from the Institute of Medicine, suggests that the DERs of sedentary MHD patients are the same as those for normal sedentary adults. On the other hand, the ratio of our measured DER to the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI)–recommended energy intake guidelines (31) of 0.91 ± 0.10 suggests that the KDOQI DER recommendations may overestimate the energy needs of sedentary MHD patients by ∼9% (Table 5). The KDOQI guidelines of 35 kcal · kg−1 · d−1 for MHD patients <60 y of age and 30 kcal · kg−1 · d−1 for patients ≥60 y are based on expert opinion (31).
However, the DER of our patients was ∼10% lower than the total daily energy expenditure and, hence, the DER of normal adults reported by Redman et al. (Table 5) (25). These investigators determined energy expenditure by the doubly labeled water method. Their model B equation, derived from their data, predicted that total daily energy expenditure should be 2507 ± 163 kcal/d in our male MHD patients and 2173 ± 323 kcal/d in our female MHD patients (25) (compare with Table 2). Their model B equation was used because of its somewhat tighter correlation with their doubly labeled water measurements compared with their model A (model B compared with model A: R2 0.65 compared with R2 0.57). Another advantage to the model B equation is that, unlike the model A equation, it includes body-composition measurements to predict DER, and body composition, including the proportion of fat mass, may be altered in MHD patients. It is possible that our DER was somewhat lower than that of the normal adults studied by Redman et al., because these latter subjects were younger, free-living, and not constrained by the inactivity associated with the commute to and from hemodialysis, the hemodialysis procedure itself conducted 3 times weekly, and the frequent occurrence of malaise lasting for up to several hours postdialysis.
A Bland-Altman analysis was conducted to analyze the relations between our measured REE data and the Mifflin–St. Jeor equation and between our calculated DER and the DER estimated for normal adults from the Dietary Reference Intake equations of the Institute of Medicine and from the Redman model B equation (data not shown).This analysis confirms that our REE data are greater than predicted from the Mifflin–St. Jeor data, that our observed DER data are similar to the Dietary Reference Intake data but possibly slightly lower than those predicted from the Redman data, and that there appear to be no trends with regard to change in the discrepancies in either REE or DER with increasing REE or DER.
These considerations, taken together, indicate that when estimating the dietary energy needs of clinically stable MHD patients, one might use the previously published DER values for sedentary normal people of the same weight, age range, and sex (21) and add appropriate adjustment factors for the usual PAL of MHD patients. If the recent data of Redman et al. (25) are closer to the true DERs of sedentary normal people, the results from our study support the theory that MHD patients do not have an elevated DER when compared with normal individuals.
A major challenge in the examination of energy expenditure or DER is how to adjust for differences in body mass. Kushner and Schoeller (33) pointed out that, in underweight patients, REE and daily total energy expenditure, when adjusted by dividing by body weight or fat-free mass, may overestimate the energy intake needed to maintain body mass. Conversely, in an obese patient, adjustment for body weight or fat-free mass may underestimate body energy needs. Clearly, some factor must be added to adjust for body size. The daily energy expenditure or DER of a 1.3-m, 45-kg man is different from a man who is 2.1 m and weighs 110 kg. Possibly energy needs and DER could be better expressed as a function of body height, of height and weight, or of height and fat-free mass rather than simply body weight or fat-free mass. On the other hand, the energy intake necessary to replete the lost body weight or fat-free mass in these underweight patients may require more energy than indicated by daily energy expenditure when it is not adjusted for their weight. As with all individuals, the energy intake, body weight, and body composition of MHD patients should be monitored, and their dietary energy intake should be adjusted as appropriate.
Limitations of this study include the rather small sample size, although this sample is not small for this type of labor-intensive metabolic study. Only one person of pure European descent was studied. The fat-free, edema-free body mass was estimated by DXA, which could be affected by hydration status. This is of particular importance for interpreting the DXA measurements, although no patient was observed to have either edema or hypertension or to develop these disorders during this study. Only 13 MHD patients were studied, and they were selected to be relatively healthy; not very obese, underweight, or aged; and without type 1 or type 2 diabetes, vasculitis, or other chronic catabolic or inflammatory illnesses. Thus, the data from this study may not be applicable to some of these other conditions. Indeed, some acutely or chronically ill MHD patients may have increased energy needs. Variations in protein intake in this study might have affected body mass or composition by causing positive or negative protein balance. We doubt the presence of such an effect, because protein balance over the course of study in each patient was approximately zero. Some data indicate that higher-protein diets may maintain greater REE and affect fat gain or loss differently than lower-protein diets in persons who consume deficient or excess-calorie diets (34, 35). However, our patients did not receive excess-calorie or restricted diets, and their protein intakes did not vary as much as in some of these studies. The strengths of this study include the rather long and intensive study under a constant dietary energy intake in a substantial cohort of MHD patients, the fact that all patients were clinically stable without overt evidence of inflammation during the study, and the rather novel use of this experimental design in dialysis patients.
Acknowledgments
We acknowledge the expert nutritional and nursing support of MacKenzie Kerr and Rose Umali of the GCRC at Harbor-UCLA for their assistance in carrying out this study.
The authors’ responsibilities were as follows—AS and JDK: prepared the manuscript and discussion; BBS: aided in data analysis; and RB, GM, and JDK: designed the metabolic study from which the data were gleaned. There were no conflicts of interest to report.
Footnotes
Abbreviations used: DER, dietary energy requirement; DXA, dual-energy X-ray absorptiometry; GCRC, General Clinical Research Center; KDOQI, Kidney Disease Outcomes Quality Initiative; MHD, maintenance hemodialysis; PAL, physical activity level; PEW, protein-energy wasting; REE, resting energy expenditure.
REFERENCES
- 1.Ikizler TA, Wingard RL, Sun M, Harvell J, Parker RA, Hakim RM. Increased energy expenditure in hemodialysis patients. J Am Soc Nephrol 1996;7:2646–53. [DOI] [PubMed] [Google Scholar]
- 2.Slomowitz LA, Monteon FJ, Grosvenor M, Laidlaw SA, Kopple JD. Effect of energy intake on nutritional status in maintenance hemodialysis patients. Kidney Int 1989;35:704–11. [DOI] [PubMed] [Google Scholar]
- 3.Cuppari L, Avesani CM. Energy requirements in patients with chronic kidney disease. J Ren Nutr 2004;14(3):121–6. [DOI] [PubMed] [Google Scholar]
- 4.Monteon FJ, Laidlaw SA, Shaib JK, Kopple JD. Energy expenditure in patients with chronic renal failure. Kidney Int 1986;30:741–7. [DOI] [PubMed] [Google Scholar]
- 5.Dukkipati R, Kopple JD. Causes and prevention of protein-energy wasting in chronic kidney failure. Semin Nephrol 2009;29:39–49. [DOI] [PubMed] [Google Scholar]
- 6.Kopple JD. Pathophysiology of protein-energy wasting in chronic renal failure. J Nutr 1999;129(1 Suppl):247S–51S. [DOI] [PubMed] [Google Scholar]
- 7.Duenhas MR, Draibe SA, Avesani CM, Sesso R, Cuppari L. Influence of renal function on spontaneous dietary intake and on nutritional status of chronic renal insufficiency patients. Eur J Clin Nutr 2003;57:1473–8. [DOI] [PubMed] [Google Scholar]
- 8.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 2001;38:1251–63. [DOI] [PubMed] [Google Scholar]
- 9.Kovesdy CP, Kalantar-Zadeh K. Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin Nephrol 2009;29:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro BB, Bross R, Morrison G, Kalantar-Zadeh K, Kopple JD. Self-reported interview-assisted diet records underreport energy intake in maintenance hemodialysis patients. J Ren Nutr 2015;25:357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frisancho AR. New standards of weight and body composition by frame size and height for assessment of nutritional status of adults and the elderly. Am J Clin Nutr 1984;40:808–19. [DOI] [PubMed] [Google Scholar]
- 12.Goran MI. Estimating energy requirements: regression based prediction equations or multiples of resting metabolic rate. Public Health Nutr 2005;8(7A):1184–6. [DOI] [PubMed] [Google Scholar]
- 13.Kim JC, Shapiro BB, Zhang M, Li Y, Porszasz J, Bross R, Feroze U, Upreti R, Kalantar-Zadeh K, Kopple JD. Daily physical activity and physical function in adult maintenance hemodialysis patients. J Cachexia Sarcopenia Muscle 2014;5:209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grodstein GP, Blumenkrantz MJ, Kopple JD, Moran JK, Coburn JW. Glucose absorption during continuous ambulatory peritoneal dialysis. Kidney Int 1981;19:564–7. [DOI] [PubMed] [Google Scholar]
- 15.Garrow JS. Energy balance and obesity in man. 2nd ed. Amsterdam/New York: Elsevier/North-Holland Biomedical Press; 1978. [Google Scholar]
- 16.Compher C, Frankenfield D, Keim N, Roth-Yousey L. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc 2006;106:881–903. [DOI] [PubMed] [Google Scholar]
- 17.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tremblay A, Despres JP, Theriault G, Fournier G, Bouchard C. Overfeeding and energy expenditure in humans. Am J Clin Nutr 1992;56:857–62. [DOI] [PubMed] [Google Scholar]
- 19.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy X-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr 1990;51:1106–12. [DOI] [PubMed] [Google Scholar]
- 20.Heymsfield SB, Wang J, Heshka S, Kehayias JJ, Pierson RN. Dual-photon absorptiometry: comparison of bone mineral and soft tissue mass measurements in vivo with established methods. Am J Clin Nutr 1989;49:1283–9. [DOI] [PubMed] [Google Scholar]
- 21.Scafoglieri A, Provyn S, Wallace J, Louis O, Tresignie J, Bautmans I, De Mey J, Clarys-Robion JP. Whole body composition by Hologic QDR 4500/A DXA: system reliability versus user accuracy and precision. in O Ivanov, editor. Applications and experiences of quality control. InTech; 2011. p. 45–60. [Google Scholar]
- 22.McClave SA, Snider HL. Use of indirect calorimetry in clinical nutrition. Nutr Clin Pract 1992;7(5):207–21. [DOI] [PubMed] [Google Scholar]
- 23.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241–7. [DOI] [PubMed] [Google Scholar]
- 24.Trumbo P, Schlicker S, Yates AA, Poos M. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc 2002;102:1621–30. [DOI] [PubMed] [Google Scholar]
- 25.Redman LM, Kraus WE, Bhapkar M, Das SK, Racette SB, Martin CK, Fontana L, Wong WW, Roberts SB, Ravussin E. Energy requirements in nonobese men and women: results from CALERIE. Am J Clin Nutr 2014;99:71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamimura MA, Draibe SA, Avesani CM, Canziani ME, Colugnati FA, Cuppari L. Resting energy expenditure and its determinants in hemodialysis patients. Eur J Clin Nutr 2007;61:362–7. [DOI] [PubMed] [Google Scholar]
- 27.Schneeweiss B, Graninger W, Stockenhuber F, Druml W, Ferenci P, Eichinger S, Grimm G, Laggner AN, Lenz K. Energy metabolism in acute and chronic renal failure. Am J Clin Nutr 1990;52:596–601. [DOI] [PubMed] [Google Scholar]
- 28.Cuppari L, de Carvalho AB, Avesani CM, Kamimura MA, Dos Santos Lobao RR, Draibe SA. Increased resting energy expenditure in hemodialysis patients with severe hyperparathyroidism. J Am Soc Nephrol 2004;15:2933–9. [DOI] [PubMed] [Google Scholar]
- 29.Utaka S, Avesani CM, Draibe SA, Kamimura MA, Andreoni S, Cuppari L. Inflammation is associated with increased energy expenditure in patients with chronic kidney disease. Am J Clin Nutr 2005;82:801–5. [DOI] [PubMed] [Google Scholar]
- 30.Borah MF, Schoenfeld PY, Gotch FA, Sargent JA, Wolfsen M, Humphreys MH. Nitrogen balance during intermittent dialysis therapy of uremia. Kidney Int 1978;14:491–500. [DOI] [PubMed] [Google Scholar]
- 31.National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 update. Am J Kidney Dis 2012;60:850–86. [DOI] [PubMed] [Google Scholar]
- 32.Human energy requirements: report of a joint FAO/ WHO/UNU Expert Consultation. Food Nutr Bull 2005;26:166. [PubMed] [Google Scholar]
- 33.Kushner RF, Schoeller DA. Resting and total energy expenditure in patients with inflammatory bowel disease. Am J Clin Nutr 1991;53:161–5. [DOI] [PubMed] [Google Scholar]
- 34.Soenen S, Martens EA, Hochstenbach-Waelen A, Lemmens SG, Westerterp-Plantenga MS. Normal protein intake is required for body weight loss and weight maintenance, and elevated protein intake for additional preservation of resting energy expenditure and fat free mass. J Nutr 2013;143:591–6. [DOI] [PubMed] [Google Scholar]
- 35.Bray GA, Smith SR, de Jonge L, Xie H, Rood J, Martin CK, Most M, Brock C, Mancuso S, Redman LM. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA 2012;307:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]