Abstract
Background: Poor child growth increases risks of mortality and morbidity. Micronutrient supplements have the potential to improve child growth.
Objective: We assessed the effect of daily zinc, multivitamin (vitamins C, E, and B-complex), and zinc and multivitamin (Zn+MV) supplementation on growth in infants in Tanzania.
Design: In this randomized, 2 × 2 factorial, double-blind trial, 2400 infants were randomly assigned to receive zinc, multivitamins, Zn+MVs, or a placebo at 6 wk of age and were followed up for 18 mo with monthly growth measurements. Mixed-effects models with restricted cubic splines for the mean change in anthropometric z scores were fit for each group. Likelihood ratio tests were used to compare the effect of supplements on growth trajectories. Cox proportional hazards models were used to compare incidences of stunting, wasting, and underweight.
Results: Children in all groups experienced growth faltering. At 19 mo of age, prevalences of stunting, wasting, and underweight were 19.8%, 6.0%, and 10.8%, respectively. Changes in weight-for-age z scores (WAZs) and weight-for-height z scores (WHZs) were significantly different across the 4 groups (P < 0.001 for both). The mean ± SE decline in the WAZ from baseline to the end of follow-up in the Zn+MV group was significantly less than in the placebo group (−0.36 ± 0.04 compared with −0.50 ± 0.04; P = 0.020), whereas the decline in the WHZ was significantly greater in the zinc-only group than in the placebo group (−0.57 ± 0.07 compared with −0.35 ± 0.07; P = 0.021). Supplements did not have a significant effect on mean change in the height-for-age z score or on rates of stunting, wasting, or underweight.
Conclusions: Although there were small but significant improvements in the WAZ in the Zn+MV group, daily zinc supplementation alone, multivitamin supplementation alone, and the combined Zn+MV did not reduce the incidences of underweight, stunting, or wasting in Tanzanian infants. Alternative approaches to prevent growth faltering should be pursued. This trial was registered at clinicaltrials.gov as NCT00421668.
Keywords: infancy, growth, multiple micronutrients, supplementation, zinc
INTRODUCTION
Chronic undernutrition, which is manifested as stunting (height-for-age <−2 SDs), currently affects 165 million children <5 y old worldwide (1). Children who suffer from undernutrition in early life experience detrimental short- and long-term consequences. Undernutrition decreases immune function and increases risk of infection. In particular, severe wasting (weight-for-height <−3SDs of the 2006 WHO standard) has been shown to increase risk of mortality 12-fold (2). In children who survive early childhood, growth faltering can also lead to impaired cognitive development and increased risks of chronic disease as well pregnancy complications in women (3).
Micronutrient deficiencies are particularly prevalent in resource-constrained settings even when caloric intake may be sufficient (1, 4). An estimated 17% of the world’s population is at risk of inadequate intake of zinc, which is an essential mineral that plays an important role in growth, immune function, reproduction, and neurobehavioral development (5). The WHO currently recommends zinc supplementation as part of the treatment of diarrheal disease because it can help shorten the duration and severity of disease (6, 7). However, whether routine, preventative zinc supplementation can improve long-term child growth is less clear. Numerous studies have documented a small but significant effect of zinc supplementation on growth in children and adolescents (8–10); however, there has been substantial heterogeneity in the impact of zinc supplements on growth on the basis of children’s age, study setting, supplement dosage and duration, and underlying zinc status (10). In particular, there have been few studies that have initiated high-dose zinc supplementation [multiple times the Recommended Dietary Allowance (RDA)]11 during early infancy and longitudinally assessed whether there is an added benefit of zinc supplementation beyond what infants receive through breast milk (10).
Public health practitioners have also begun to emphasize the role of multiple-micronutrient interventions as opposed to single-nutrient interventions to improve child health (4). Because multiple deficiencies often coexist in the same individuals and communities, the use of multiple-micronutrient supplements are a particularly attractive and relatively inexpensive intervention (1, 11, 12). Research on which micronutrients, at which doses, and in which settings is essential to formulate policies on multiple-micronutrient interventions. The WHO currently supports the home fortification of food with micronutrient powders that contain at least iron, vitamin A, and zinc to improve iron status and reduce anemia in infants and children 6–23 mo of age (13); however, the evidence base to support the routine provision of other micronutrients remains incomplete. Our group has shown that the daily provision of vitamins B-complex, C, and E to pregnant women can improve birth weight (14) and postnatal infant weight gain (15). In the current study, we assess whether the daily provision of these micronutrients directly to infants born to HIV-negative mothers can have a long-term impact on child growth and, furthermore, whether multivitamins have a synergistic effect when combined with zinc supplements.
METHODS
Subjects in this study were part of a 2 × 2 factorial, randomized, double-blind, placebo-controlled trial that was designed to assess whether the daily administration of zinc or multivitamins to infants born to HIV-negative mothers from 6 wk of age for a period of 18 mo reduced risk of diarrheal disease and respiratory infection morbidity compared with the effects of a placebo (16). A secondary endpoint of this study was to assess whether these supplements improved child growth. Pregnant women at <34 wk of gestation who planned to stay in Dar es Salaam for ≥2 y were informed about the study and consented prenatally. In addition, mothers were also recruited from the labor ward from district hospitals in Dar es Salaam. Inclusion criteria included singleton, live births of HIV-negative mothers. All mothers in the study were confirmed to be HIV-negative 1–2 wk after delivery. HIV status was assessed with the use of 2 sequential ELISAs that used the Murex HIV antigen/antibody (Abbot Murex) followed by the Enzygnost anti–HIV-1/2 Plus (Dade Behring). Discordant results between the 2 ELISAs were resolved with the use of a Western blot assay. Low-birth-weight and premature infants were included in the study because they might be particularly likely to benefit from supplements; however, infants of multiple births and infants with congenital abnormalities or other medical conditions that would have interfered with the study proceedings were excluded.
At 5–7 wk of age, 2400 infants were randomly assigned to receive one of the following 4 treatment regimens: zinc only, multivitamins only, zinc and multivitamins (Zn+MVs), or a placebo. A biostatistician in Boston prepared a random-assignment list from 1 to 2400 that used blocks of 20 and was stratified by study clinic. Capsules were packaged in a blister pack of 15 capsules each, and numbered boxes that contained 6 blister packs were prepared with the corresponding treatments. Each eligible infant was assigned the next numbered box of capsules at his or her respective site. All study physicians, nurses, and participants were blinded to treatment groups. The supplements were provided as opaque capsules containing an orange-flavored powder that was manufactured by Nutriset. Mothers were instructed to push the capsule through the back of the blister pack, open the capsule, and empty the contents into a clean, plastic cup, mix the contents with 5 mL sterile H2O, and administer the solution to the child orally. All 4 regimens were tested to ensure that they were indistinguishable in appearance, smell, and taste. For infants who received zinc, each capsule contained 5 mg Zn. For infants who received multivitamin supplements, each capsule contained 60 mg vitamin C, 8 mg vitamin E, 0.5 mg thiamin, 0.6 mg riboflavin, 4 mg niacin, 0.6 mg vitamin B-6, 130 μg folate, and 1 μg vitamin B-12. From the time of random assignment to 6 mo of age, infants received 1 capsule/d, that represented between 150% and 600% of the RDA or Adequate Intake for the different micronutrients for infants 0–6 mo of age. From 7 mo of age until the end of follow-up, infants received 2 capsules/d that represented between 150% and 400% of the RDA or Adequate Intake for their age group.
Mothers were instructed to return to the clinic monthly for follow-up visits. All mothers and children received the standard of perinatal and child health care on the basis of the guidelines of the Ministry of Health and Social Welfare in Tanzania. Mothers received prenatal care including an anthropometric assessment, iron–folic acid supplementation, and the intermittent preventive treatment of malaria. The standard of care for children included monthly growth monitoring, immunizations, routine treatment of illnesses, and periodic vitamin A supplementation (100,000 IU at 9 mo of age and 200,000 IU at 15 mo of age). Children who were diagnosed with anemia were treated with iron supplementation.
At each monthly follow-up visit, trained study nurses assessed the regimen compliance by counting the number of unconsumed capsules and obtained child anthropometric measurements with the use of standard techniques (17). Weight was measured on a digital infant balance scale with a 10-g precision (Tanita) and length with a 1-mm precision with the use of a rigid length board with an adjustable foot piece.
Ethics
Ethical approval was granted by the Harvard T.H. Chan School of Public Health Human Subjects Committee, the Muhimbili University of Health and Allied Science Committee of Research and Publications, the Tanzanian Institute of Medical Research, and the Tanzanian Food and Drug Authority. A data safety monitoring board also met twice annually over the course of the study. All mothers provided written informed consent to enroll themselves and their infants in the study. This trial was registered at clinicaltrials.gov as NCT00421668.
Statistics
Power calculations to determine the study sample size were based on the primary outcomes of the parent trial (i.e., clinical symptoms of diarrhea and lower respiratory infection) (16). Post hoc power calculations for our current growth analysis were conducted for the mean change in height-for-age z score (HAZ) without taking splines into account. On the basis of the power calculation approach described by Basagaña and Spiegelman (18), with the assumption that the coefficient of the change in HAZ and time interaction was 0.01, we had >99% power to detect a linearly divergent difference in the growth trajectory in the change in HAZ between treatment groups.
Descriptive statistics were used to summarize baseline maternal, child, and household characteristics. For categorical variables, frequencies in absolute numbers and percentages were assessed and compared with the use of chi-square tests. For continuous variables, means ± SDs were derived and compared with the use of an ANOVA. Regimen compliance was defined as the mean percentage of capsules a child consumed over the number of capsules he or she should have consumed between visits. For our analysis, we calculated age- and sex-specific z scores for the following 3 anthropometric indexes: weight-for-height z score (WHZ), HAZ, and weight-for-age z score (WAZ) with the use of the 2006 WHO growth standards (19). In accordance with WHO recommendations, we set all extreme HAZ (<−6 or >6), WHZ (<−5 or >5), and WAZ (<−6 or >5) values to missing as recommended (20). The measurement error in individual children’s growth trajectories was also identified by regressing height, weight, HAZ, and WAZ independently against age for each child. The greatest and lowest deciles of residual values were individually assessed, and extreme outliers were set to missing. The WHZ was set to missing for any visit where the HAZ or WAZ were set to missing. Dichotomous variables of wasting, stunting, and underweight were created for children who fell below −2 of the relevant z score.
For the primary analysis, mixed-effects models with restricted cubic splines for the change in mean anthropometric z score over time were created for each group with the use of the intent-to-treat principle (21). Changes in z scores were calculated by subtracting a child’s baseline z score from the appropriate anthropometric z score at each subsequent follow-up visit. For the mixed-effects models with restricted cubic splines, knots were placed at 3, 6, 9, 12, 15, and 18 mo of age with 2 additional knots at 6 and 10 wk of age to improve model fit by accounting for rapid growth in early infancy. To assess whether there was a significant interaction effect of combining supplements, we conducted likelihood ratio tests to compare mixed effects models created with the use of the maximum likelihood method. Full models contained all treatment terms (zinc, multivitamins, and Zn+MVs), age variables (age as a continuous variable as well as age spline variables), and interaction terms for treatment variables with age variables. Reduced models did not contain interaction terms for Zn+MVs with age variables.
Incidence rates of stunting, wasting, and underweight were compared with the use of Cox proportional hazards models with the exact method for ties. The assumption of proportional hazards was assessed by comparing Kaplan-Meier plots across treatment groups and also by testing an additional model that included treatment and age interactions in the Cox model. Chi-square tests were used to compare the number of children who were lost to follow-up and the number of children who completed follow-up across the 4 treatment groups, and t tests were used to compare the mean age of the last visit across each treatment group. In addition, baseline sociodemographic characteristics of infants who completed the study and infants who dropped out were compared with the use of t tests for continuous variables and chi-square tests for categorical outcomes. Because adjustment for baseline covariates has the potential to improve precision (22), and baseline z scores were somewhat different across the 4 groups, we repeated the primary analyses to assess whether adjustment for baseline anthropometric status or other baseline covariates changed the findings from the mixed-effects models with cubic splines. We also assessed effect modifications with the use of mixed-effects models with restricted cubic splines stratified first by infant sex and separately stratified by birth weight (infants in the lowest quartile of birth weight <3000 g compared with birth weight ≥3000 g). All analyses were conducted with SAS software (version 9.3; SAS Institute).
RESULTS
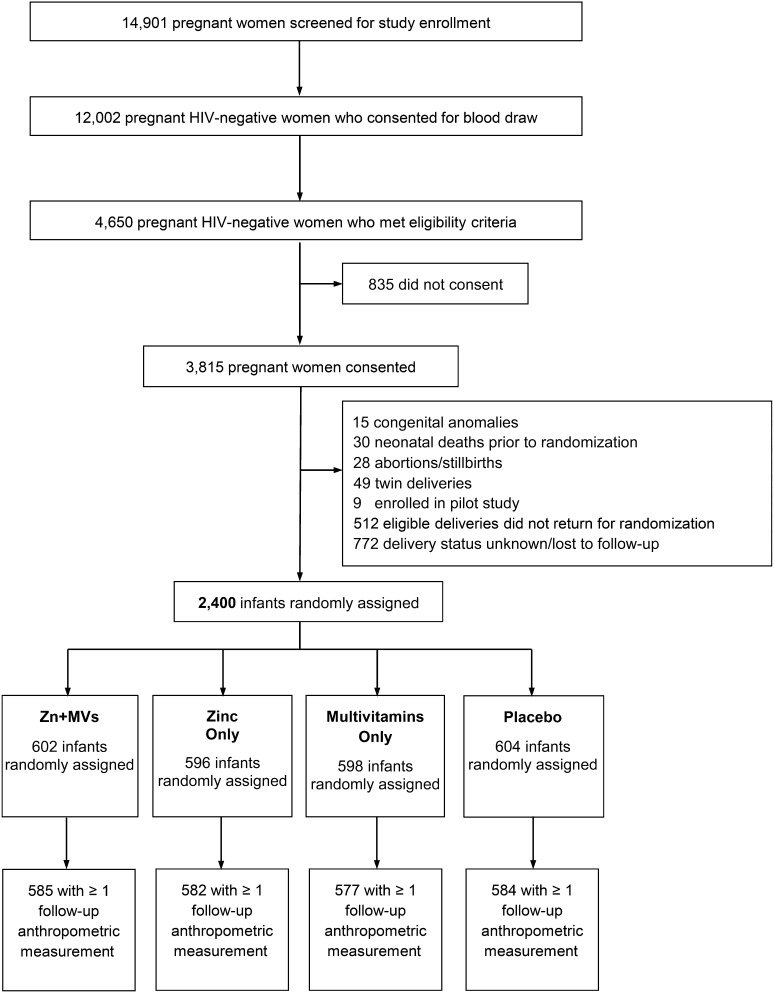
A total of 14,901 pregnant women were screened for study enrollment of whom 3815 women were HIV-uninfected women who met the eligibility criteria and consented to participate in the study. After the exclusion of infants with medical conditions at birth, infants with an unknown delivery status, or infants who did not return for random assignment, 2400 infants were randomly assigned to a zinc, multivitamin, Zn+MV, or placebo group (Figure 1). Maternal, child, and household characteristics at baseline were comparable across the 4 groups (Table 1). More than one-half of all mothers were housewives with no income, and three-quarters of mothers had ≤7 y of education. For almost one-third of mothers, their current pregnancy was their first pregnancy. Mean maternal age was significantly different across the 4 groups (P = 0.049) with the youngest mothers in the Zn+MV group (26.1 ± 5.0 y) and the oldest in the zinc-only group (26.8 ± 5.1 y). The mean age of children at random assignment was 5.9 wk in all 4 groups, and approximately one-half of the children were boys. The prevalence of low birth weight was approximately 3%, and 13% of children were born before 37 wk of gestation. The mean HAZ score at random assignment was significantly different across the 4 groups (P = 0.028). Infants in the Zn+MV group had the lowest mean HAZ at baseline (−0.37 ± 1.23), whereas infants in the placebo group had the highest mean HAZ at baseline (−0.17 ± 1.31).
FIGURE 1.
Study profile of the randomized trial of multivitamin and zinc supplementation to infants in Dar es Salaam, Tanzania. Zn+MV, zinc and multivitamin.
TABLE 1.
Baseline characteristics of mothers and their children enrolled in a trial of zinc and multivitamin supplementation1
| Zn+MVs (n = 602) | Zinc only (n = 596) | Multivitamins only (n = 598) | Placebo (n = 604) | P | |
| Maternal characteristic | |||||
| Age, y | 26.1 ± 5.02 | 26.8 ± 5.1 | 26.2 ± 5.0 | 26.5 ± 5.0 | 0.049 |
| Formal education, n (%) | 0.538 | ||||
| None | 9 (1.5) | 8 (1.4) | 10 (1.7) | 9 (1.5) | |
| 1–7 y | 441 (73.4) | 421 (71.1) | 416 (70.2) | 453 (75.3) | |
| ≥8 y | 151 (25.1) | 163 (27.5) | 167 (28.2) | 140 (23.3) | |
| Employment, n (%) | 0.125 | ||||
| Housewife without income | 357 (59.8) | 365 (62.3) | 337 (56.7) | 386 (64.4) | |
| Housewife with income | 201 (33.7) | 175 (29.9) | 212 (35.7) | 176 (29.4) | |
| Other | 39 (6.5) | 46 (7.9) | 45 (7.6) | 37 (6.2) | |
| Marital status (married or living with partner), n (%) | 542 (90.5) | 534 (90.5) | 542 (91.4) | 537 (90.0) | 0.860 |
| Previous pregnancy, n (%) | 0.378 | ||||
| None | 187 (31.2) | 169 (28.6) | 205 (34.5) | 184 (30.6) | |
| 1–4 | 396 (66.1) | 410 (69.3) | 375 (63.1) | 398 (66.2) | |
| ≥5 | 16 (2.7) | 13 (2.2) | 14 (2.4) | 19 (3.2) | |
| Midupper arm circumference, cm | 26.7 ± 3.2 | 27.1 ± 3.1 | 27.1 ± 3.1 | 27.0 ± 3.1 | 0.090 |
| Socioeconomic characteristic | 0.925 | ||||
| Daily food expenditure per person in household <1000 Tanzanian shillings,3 n (%) | 170 (29.4) | 162 (28.6) | 164 (28.8) | 158 (27.6) | |
| Household possessions,4 n (%) | 0.292 | ||||
| None | 180 (30.0) | 192 (32.7) | 173 (29.2) | 171 (28.4) | |
| 1–3 | 339 (56.5) | 308 (52.4) | 330 (55.7) | 358 (59.5) | |
| ≥4 | 81 (13.5) | 88 (15.0) | 90 (15.2) | 73 (12.1) | |
| Child characteristics | |||||
| Age at random assignment, wk | 5.9 ± 0.4 | 5.9 ± 0.4 | 5.9 ± 0.4 | 5.9 ± 0.4 | 0.543 |
| Sex, M, n (%) | 289 (48.0) | 300 (50.3) | 316 (52.8) | 311 (51.5) | 0.388 |
| Low birth weight (<2500 g), n (%) | 22 (3.7) | 21 (3.6) | 21 (3.6) | 18 (3.0) | 0.920 |
| Born preterm (<37 wk),5 n (%) | 67 (12.1) | 80 (14.6) | 66 (12.0) | 77 (14.0) | 0.501 |
| Small for gestational age5,6 | 53 (9.9) | 48 (8.9) | 35 (6.5) | 55 (10.1) | 0.143 |
| z score7 | |||||
| Length-for-age | −0.37 ± 1.23 | −0.33 ± 1.19 | −0.26 ± 1.20 | −0.17 ± 1.31 | 0.028 |
| Weight-for-length | 0.15 ± 1.31 | 0.16 ± 1.33 | 0.14 ± 1.29 | 0.05 ± 1.28 | 0.477 |
| Weight-for-age | −0.26 ± 1.02 | −0.23 ± 0.97 | −0.17 ± 0.99 | −0.16 ± 1.05 | 0.247 |
P values for continuous variables were derived from ANOVA tests. P values for categorical variables were derived from chi-squared tests. Zn+MV, zinc and multivitamin.
Mean ± SD (all such values)
At the time of the study, this amount was roughly equal to US$0.75/d.
From a list that included a sofa, television, radio, refrigerator, and fan.
n = 2157
Calculated on the basis of the standards developed by Oken et al. (23).
Anthropometric z scores from baseline (ages 5–7 wk).
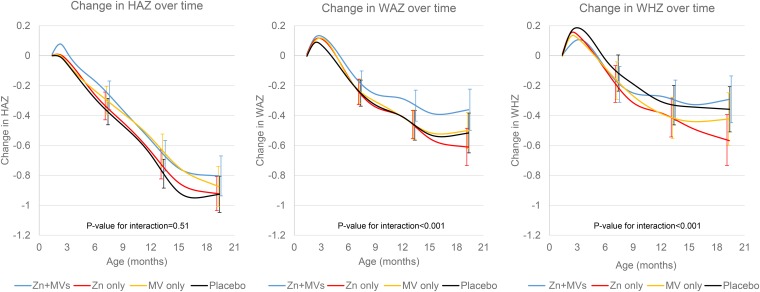
The median regimen compliance in children was 96% (25th and 75th percentiles: 91% and 99%, respectively) of the allocated regimen on the basis of pill counts at clinic visits. Children in all supplement groups experienced significant growth faltering during follow-up. At 19 mo of age, prevalences of stunting, wasting, and underweight were 19.8%, 6.0%, and 10.8% respectively. In the likelihood ratio tests that compared mixed-effects models with and without interaction terms for Zn+MVs and time spline variables, the models for the change in the WAZ (P < 0.001) and WHZ (P < 0.001) were significantly improved by including interaction terms for Zn+MVs, which indicated a significant interaction on growth outcomes by the combination of the 2 supplements (Figure 2). There was no significant improvement when Zn+MV interaction terms were added to the model for the change in HAZ (P = 0.505). Pairwise comparisons of the mean change in WAZ from baseline to the end of follow-up indicated that the Zn+MV group experienced a significantly smaller decline in WAZs than the placebo group did (mean ± SE change: −0.36 ± 0.04 compared with −0.50 ± 0.04, respectively; P-difference = 0.020) (Table 2). Pairwise comparisons for the mean change in WHZ revealed a significantly greater decline in WHZs in the zinc-only group than in the placebo group (−0.57 ± 0.07 compared with −0.35 ± 0.07, respectively; P = 0.021). Incidence rates of stunting, wasting, and underweight were not significantly different in the Zn+MV, zinc-only, or multivitamin-only groups compared with the placebo group (Table 3). In the analyses that were adjusted for baseline anthropometric measures, we found that the decline in the WAZ of the Zn+MV group remained significantly smaller than the decline in the placebo group (mean ± SE change: −0.42 ± 0.04 compared with −0.55 ± 0.04, respectively; P = 0.026). The decline in the mean WHZ in the zinc-only group was also steeper than the decline in the placebo group (mean ± SE change: −0.47 ± 0.06 compared with −0.33 ± 0.06, respectively; P = 0.089). The addition of other baseline covariates (maternal height, parity, infant sex, and household asset score) resulted in similar findings compared with models that contained baseline z scores only.
FIGURE 2.
Changes (95% CIs) in HAZ, WAZ, and WHZ from 6 to 84 wk of age in 4 treatment arms. Curves were created with the use of mixed-effects models with restricted cubic splines with knots at 6 and 10 wk and 3, 6, 9, 12, 15, and 18 mo of age. n = 2336 for the HAZ analysis, n = 2347 for the WAZ analysis, and n = 2332 for the WHZ analysis. Bars represent 95% CIs for each of the 4 treatment groups at 6, 12, and 18 mo of follow-up. P-interaction values were derived from likelihood ratio tests that compared mixed-effects models with restricted cubic splines. The full model contained a Zn+MV interaction term as well as interaction terms between Zn+MVs and time and all time spline variables. The reduced model did not contain interaction terms for Zn+MVs and time or time spline variables. HAZ, height-for-age z score; MV, multivitamins; WAZ, weight-for-age z score; WHZ, weight-for-height z score; Zn+MV, zinc and multivitamin.
TABLE 2.
Changes in HAZs, WAZs, and WHZs over 18 mo of follow-up across the 4 treatment groups1
| Zn+MVs (n = 602) | Zinc only (n = 596) | Multivitamins only (n = 598) | Placebo (n = 604) | |
| Change in HAZ | −0.79 ± 0.05 | −0.93 ± 0.05 | −0.88 ± 0.05 | −0.93 ± 0.05 |
| P | 0.058 | 0.956 | 0.478 | Referent |
| Change in WAZ | −0.36 ± 0.04 | −0.61 ± 0.04 | −0.52 ± 0.04 | −0.50 ± 0.04 |
| P | 0.020 | 0.063 | 0.792 | Referent |
| Change in WHZ | −0.31 ± 0.07 | −0.57 ± 0.07 | −0.42 ± 0.07 | −0.35 ± 0.07 |
| P | 0.655 | 0.021 | 0.473 | Referent |
All values are mean ± SEs. The analysis was derived from a mixed-effects model with restricted cubic splines. P values are for comparisons of the mean change in z score compared with the mean change in z score in the placebo group. HAZ, height-for-age z score; WAZ, weight-for-age z score; WHZ, weight-for-height z score; Zn+MV, zinc and multivitamin.
TABLE 3.
Incidences of stunting, wasting, and underweight from 6 to 84 wk of age in 4 treatment groups
| Zn+MVs1 | Zinc | Multivitamins | Placebo | |
| Stunting | ||||
| Events/child-years, n | 173/460 | 176/478 | 169/470 | 164/459 |
| HR2 (95% CI) | 0.84 (0.67, 1.04) | 0.96 (0.77, 1.18) | 0.93 (0.75, 1.15) | — |
| P | 0.102 | 0.687 | 0.486 | Referent |
| Wasting | ||||
| Events/child-years, n | 89/514 | 118/523 | 110/505 | 99/513 |
| HR3 (95% CI) | 0.92 (0.69, 1.22) | 1.19 (0.91, 1.55) | 1.11 (0.85, 1.46) | — |
| P | 0.546 | 0.203 | 0.458 | Referent |
| Underweight | ||||
| Events/child-years, n | 107/530 | 131/542 | 116/516 | 99/541 |
| HR4 (95% CI) | 0.97 (0.74, 1.27) | 1.23 (0.95, 1.60) | 1.29 (0.99, 1.69) | — |
| P | 0.818 | 0.116 | 0.060 | Referent |
Zn+MV, zinc and multivitamin.
Adjusted for baseline height-for-age z score.
Adjusted for baseline weight-for-height z score
Adjusted for baseline weight-for-age z score.
Because we did not see a significant interaction between the zinc and multivitamin supplements on the change in HAZs, we collapsed the 4 treatment groups so that we could conduct 2 separate comparisons of infants who received zinc with infants who did not receive zinc, and infants who received multivitamins with infants who did not receive multivitamins. In the comparison of infants who received zinc with infants who did not receive zinc, we did not see a significant difference in the mean change in HAZ, WAZ, or WHZ (Supplemental Figure 1). Children who received multivitamins experienced a small but significant reduction in decline in HAZ and WAZ compared with infants who did not receive multivitamins (Supplemental Figure 2). The mean ± SE decline in HAZ over the 18 mo of follow-up in infants who received multivitamins was −0.82 ± 0.03 compared with −0.93 ± 0.03 in infants who did not receive multivitamins (P-difference = 0.012). In WAZ, the infants who received multivitamins experienced a decline of −0.46 ± 0.03 compared with −0.56 ± 0.03 in infants who did not receive multivitamins (P-difference = 0.004). In Cox proportional hazards models, however, there was no significant difference in rates of stunting, wasting or underweight when comparing infants who received zinc with infants who did not receive zinc, nor in the comparison of infants who received multivitamins and those who did not receive multivitamins (Supplemental Tables 1 and 2).
In analyses that assessed the loss to follow-up, rates of completion were similar across all 4 treatment groups. No baseline sociodemographic characteristics in early dropouts were significantly different from the characteristics of those who remained in the study until completion with the exception of gestational age. Children with shorter gestational ages were less likely to complete the study; however, this was not significantly different across treatment groups.
In analyses that assessed the effect modification of the supplements by birth weight, we found that, among infants in the lowest quartile of birth weight (<3000 g), infants who received multivitamins had improved growth outcomes across all 3 anthropometric measures compared with infants who did not receive multivitamins. Specifically, infants with birth weight <3000 g who received multivitamins experienced a mean ± SE decline in HAZ of −0.30 ± 0.10 compared with a decline of −0.68 ± 0.10 in infants who did not receive multivitamins (P-difference = 0.001). This trend was replicated in the WHZ (−0.35 ± 0.11 compared with −0.60 ± 0.12, respectively; P-difference = 0.070). Of particular note, infants in the lowest quartile of birth weight who received multivitamins experienced improvements in WAZ, with a mean ± SE increase of 0.16 ± 0.10 compared with a decline of −0.19 ± 0.11 in infants in the lowest quartile of birth weight who did not receive multivitamins (P-difference < 0.001). There was no significant effect of multivitamins in infants born with weight ≥3000 g. By contrast, infants who received zinc supplements did not have significantly different declines in WHZ or WAZ in either birth-weight category, whereas infants in the lowest quartile of birth weight who received zinc experienced greater declines in HAZ than did infants in this birth-weight range who did not receive zinc (mean ± SE change: −0.61 ± 0.10 compared with −0.34 ± 0.11, respectively; P-difference = 0.020). There was no significant effect modification of treatment by infant sex.
DISCUSSION
In this randomized, 2 × 2 factorial, clinical trial in infants born to HIV-negative mothers in Dar es Salaam, Tanzania, neither daily zinc nor multivitamin supplements, alone or in combination, had a significant effect on the incidence of stunting, wasting, or underweight. There was a small difference in the mean change in WAZ and WHZ across treatment groups. Children in the Zn+MV group experienced a significantly smaller decline in WAZs than did children in the placebo group, which was a trend that was replicated in the change in HAZs. By contrast, children in the zinc-only group experienced a significantly greater decline in WHZs, which was a trend that was also replicated in the decline in WAZs compared with in the placebo group.
Our findings on the effect of zinc supplementation were somewhat surprising. Several meta-analyses have shown a small but significant positive effect of zinc supplementation on child growth. In 2 meta-analyses in 2002 and again in 2009, Brown et al. (8, 24) showed that zinc supplementation had a positive effect on linear growth in prepubertal children (including infants) in both developed and resource-constrained countries. In 2 separate meta-analyses published in 2011, Ramakrishnan et al. (25) and Imdad and Bhutta (9) narrowed inclusion criteria to focus on the effect of zinc supplements on growth specifically in children <5 y of age in low- and middle-income countries. Although Imdad and Bhutta (9) concluded that a dose of 10 mg Zn/d for 24 wk led to a net gain in height of 0.37 ± 0.25 cm, Ramakrishnan et al. (25) showed no effect of zinc supplementation on height gain and only modest increases in the WHZ. More recently, a Cochrane review by Mayo-Wilson et al. (10) showed a significant positive effect of zinc supplementation on both height and weight in children from 6 mo through 12 y of age. However, note that the effect on height and weight in the review by Mayo-Wilson et al. (10) was driven by studies in children >5 y old. In children <5 y of age, zinc supplementation appeared to reduce both height and weight gain; there was no effect of zinc supplementation on the prevalence of stunting in any age range.
Our findings support the argument that, if there is an effect of zinc supplementation on growth in infancy and early childhood, it is quite small and may not be beneficial, particularly if it is not balanced with the addition of other micronutrients. Note that infants in our study initiated zinc supplementation at 6 wk of age. In early infancy, the zinc concentration in breast milk and infant absorption of zinc are both relatively high, which often result in sufficient zinc intake for estimated requirements (26). In our study, it is likely that zinc supplements provided no additional benefit and, by contrast, may have led to a nutrient imbalance by inhibiting the absorption of nutrients such as iron and copper (27–29). The significant difference in decline in the WAZ in the zinc-only group highlights the importance of establishing the appropriate micronutrient dosage and combination to minimize potential harm from supplements.
Our finding that zinc supplements, when given in combination with multivitamin supplements, had a small but significant beneficial effect on growth outcomes is in line with the literature on multiple-micronutrient supplements. Multiple-micronutrient supplements have gained significant attention as an inexpensive strategy to combat deficiencies and potentially improve child growth because several micronutrient deficiencies often coexist within the same individual and communities in resource-constrained settings (4, 30). Numerous studies have assessed the effect of multiple-micronutrient supplements on anemia and hemoglobin concentrations (11, 31–36), but fewer studies have assessed long-term outcomes including child growth (34, 36). Some studies have shown significant benefits of multiple-micronutrient supplements on growth (11, 37, 38); however, micronutrient combinations and supplement dosages vary significantly across studies, which has complicated efforts to compare the evidence.
In 2 different studies, one study in HIV-positive mothers (15) and the other study in HIV-negative mothers (14), our group showed that the provision of this particular combination of multiple micronutrients (vitamins B-complex, C, and E) to pregnant women could improve growth outcomes in their offspring. However, our group recently reported the results of a trial that was similar in design to the current study whereby we provided direct daily supplementation to infants of HIV-infected women in Dar es Salaam with vitamins B-complex, C, and E from 6 wk to 24 mo of age (39). In the study of infants born to HIV-infected mothers, mothers in both the placebo and intervention groups received daily multivitamin supplements as well, thereby potentially reducing the benefit of multivitamin supplementation in their infants. We did not find a significant difference in child growth across treatment arms. In the current study, although we showed a significant improvement in the WHZ in children in the Zn+MV group, the difference in the change in mean z scores across treatment groups was quite small, and there was no difference in any of the treatment groups in incidence rates of stunting, wasting, or underweight. These findings do not support the use of this particular combination of micronutrient supplements to infants as a strategy to promote growth in the general population.
However, note that the prevalence of low birth weight (<2500 g) in our current study was very low. In our analyses of an effect modification, we showed that multivitamins were particularly beneficial for infants with birth weights <3000 g, but there was no effect of multivitamins in infants born with weights ≥3000 g. These findings indicate that the minimal effect of supplementation in the current study may have been due to the low prevalence of low birth weight at baseline. Additional research on multivitamin supplementation that specifically targets infants with poor nutritional status at baseline may provide insight into the potential mechanisms and effectiveness of this intervention.
The limitations of our study were similar to those of other large-scale randomized controlled trials in Sub-Saharan Africa. Our study experienced a loss to follow-up over the 18 mo of follow-up; however, our analyses indicated that this loss was nondifferential across treatment groups and, thus, was unlikely to cause bias in our findings on the effect of treatment on growth. In addition, although our findings may not be generalizable to rural communities where the baseline micronutrient status and prevalence of other risk factors for poor growth may differ, our study is likely generalizable to periurban settings in Sub-Saharan Africa.
In conclusion, to our knowledge, this is the largest randomized controlled trial of zinc and multivitamin supplements in African infants to date. Our 2 × 2 factorial design and extended follow-up enabled a rigorous assessment of the effect of both nutrient interventions alone and in combination on early childhood growth. In particular, the initiation of supplements at a very young age (6 wk), the provision of dosages at multiple times the RDA, and the long duration of supplementation enhanced our ability to assess the impact of the provision of these micronutrients on child growth. Although our group previously reported improvements in morbidity in the children in the current study who received zinc supplements (16), our current findings do not support the use of daily zinc, multivitamins (vitamins B-complex, C and E), or a combination of zinc and multivitamins to improve early childhood growth. Alternative approaches, including interventions that improve the feeding practices of infants and young children, increase nutrient density, and prevent infection would likely have a greater impact on long-term growth outcomes (1, 40).
Acknowledgments
We thank the following field and study staff for their tireless efforts: Esther Kibona (deceased), Illuminata Ballonzi, Godwin Njiro, Frank Killa, Edgar Basheka, Susie Welty, Rachel Steinfeld, Anne Marie Darling, James Okuma, Angela Jardin, Elizabeth Long, Jenna Golan, and Emily Dantzer. We also thank Enju Liu and Ellen Hertzmark for their expert advice, and the members of the data safety monitoring board [Paul Jacques (chair), Davidson Hamer, Roger Mbise, and Zul Premji].
The authors’ responsibilities were as follows—LML: wrote the manuscript; LML, CMM, R Kupka, and MW: analyzed the data and performed the statistical analyses; LML and CPD: had primary responsibility for the final content of the manuscript; KPM, R Kupka, R Kisenge, and SA: conducted the research (hands-on conduct of the experiments and data collection); KPM, WWF, and CPD: designed the research (project conception, development of overall research plan, and study oversight); and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: HAZ, height-for-age z score; RDA, Recommended Dietary Allowance; WAZ, weight-for-age z score; WHZ, weight-for-height z score; Zn+MV, zinc and multivitamin.
REFERENCES
- 1.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382:427–51. [DOI] [PubMed] [Google Scholar]
- 2.Olofin I, McDonald CM, Ezzati M, Flaxman S, Black RE, Fawzi WW, Caulfield LE, Danaei G. Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: a pooled analysis of ten prospective studies. PLoS One 2013;88:e64636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 2008;371:340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christian P, Tielsch JM. Evidence for multiple micronutrient effects based on randomized controlled trials and meta-analyses in developing countries. J Nutr 2012;142:173S–7S. [DOI] [PubMed] [Google Scholar]
- 5.Gibson RS. Zinc: the missing link in combating micronutrient malnutrition in developing countries. Proc Nutr Soc 2006;65:51–60. [DOI] [PubMed] [Google Scholar]
- 6. Clinical management of acute diarrhoea. Geneva (Switzerland): World Health Organisation and United Nations Childrens Fund; 2004;WHO/FCH/CSH 04.7. [Google Scholar]
- 7.Lazzerini M, Luca R. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev 2013. [DOI] [PubMed] [Google Scholar]
- 8.Brown K, Peerson J, Rivera J, Allen L. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2002;75:1062–71. [DOI] [PubMed] [Google Scholar]
- 9.Imdad A, Bhutta Z. Effect of preventive zinc supplementation on linear growth in children under 5 years of age in developing countries: a meta-analysis of studies for input to the lives saved tool. BMC Public Health 2011;11(Suppl 3):S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayo-Wilson E, Junior JA, Imdad A, Dean S, Chan XHS, Chan ES, Jaswal A, Bhutta ZA. Zinc supplementation for preventing mortality, morbidity, and growth failure in children aged 6 months to 12 years of age. Cochrane Database Syst Rev 2014;5:CD009384. [DOI] [PubMed] [Google Scholar]
- 11.Allen LH, Peerson JM, Olney DK. Provision of multiple rather than two or fewer micronutrients more effectively improves growth and other outcomes in micronutrient-deficient children and adults. J Nutr 2009;139:1022–30. [DOI] [PubMed] [Google Scholar]
- 12.Harrison GG. Public health interventions to combat micronutrient deficiencies. Public Health Reviews 2010;32;256–66. [Google Scholar]
- 13.World Health Organisation Guideline. Use of multiple micronutrient powders for home fortification of foods consumed by infants and children 6–23 months of age. Geneva (Switzerland): World Health Organsation; 2011. [PubMed] [Google Scholar]
- 14.Fawzi WW, Msamanga GI, Urassa W, Hertzmark E, Petraro P, Willett WC, Spiegelman D. Vitamins and perinatal outcomes among HIV-negative women in Tanzania. N Engl J Med 2007;356:1423–31. [DOI] [PubMed] [Google Scholar]
- 15.Villamor E, Saathoff E, Bosch RJ, Hertzmark E, Baylin A, Manji K, Msamanga G, Hunter DJ, Fawzi WW. Vitamin supplementation of HIV-infected women improves postnatal child growth. Am J Clin Nutr 2005;81:880–8. [DOI] [PubMed] [Google Scholar]
- 16.McDonald CM, Manji KP, Kisenge R, Aboud S, Spiegelman D, Fawzi WW, Duggan CP. Daily Zinc but not multivitamin supplementation reduces diarrhea and upper respiratory infections in Tanzanian infants: a randomized, double-blind, placebo-controlled clinical Trial. J Nutr 2015;145:2153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organisation. Physical status: the use and interpretation of anthropometry: report of a WHO Expert Committee. Geneva (Switzerland): World Health Organization; 1995. [PubMed] [Google Scholar]
- 18.Basagaña X, Spiegelman D.. Power and sample size calculations for longitudinal studies comparing rates of change with a time-varying exposure. Statist Med 2010;29:181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Onis M. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. World Health Organisation; 2006. [Google Scholar]
- 20.World Health Organisation. WHO child growth standards SAS macro (version 3.2.2). Geneva (Switzerland): World Health Organization. [Internet]. Updated January 2011 [cited 2012 Sep]. Available from: http://www.who.int/childgrowth/software/readme_sas.pdf.
- 21.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 22.Streiner DL. Best (but oft-forgotten) practices: the multiple problems of multiplicity—whether and how to correct for many statistical tests. Am J Clin Nutr 2015;102:721–8. [DOI] [PubMed] [Google Scholar]
- 23.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown KH, Peerson JM, Baker SK, Hess SY. Preventive zinc supplementation among infants, preschoolers, and older prepubertal children. Food Nutr Bull 2009;30:S12S–40. [DOI] [PubMed] [Google Scholar]
- 25.Ramakrishnan U, Goldenberg T, Allen LH. Do multiple micronutrient interventions improve child health, growth, and development? J Nutr 2011;141:2066–75. [DOI] [PubMed] [Google Scholar]
- 26.Krebs NF, Miller LV, Hambidge KM. Zinc deficiency in infants and children: a review of its complex and synergistic interactions. Paediatr Int Child Health 2014;34:279–88. [DOI] [PubMed] [Google Scholar]
- 27.Herman S, Griffin IJ, Suwarti S, Ernawati F, Permaesih D, Pambudi D, Abrams SA. Cofortification of iron-fortified flour with zinc sulfate, but not zinc oxide, decreases iron absorption in Indonesian children. Am J Clin Nutr 2002;76:813–7. [DOI] [PubMed] [Google Scholar]
- 28.Wapnir RA, Balkman C. Inhibition of copper absorption by zinc. Biol Trace Elem Res 1991;29:193–202. [DOI] [PubMed] [Google Scholar]
- 29.Sandström B. Micronutrient interactions: effects on absorption and bioavailability. Br J Nutr 2001;85(Suppl 2):S181–5. [PubMed] [Google Scholar]
- 30.Ramakrishnan U. Prevalence of micronutrient malnutrition worldwide. Nutr Rev 2002;60:S46–52. [DOI] [PubMed] [Google Scholar]
- 31.Menon P, Ruel MT, Loechl CU, Arimond M, Habicht J-P, Pelto G, Michaud L. Micronutrient sprinkles reduce anemia among 9- to 24-mo-old children when delivered through an integrated health and nutrition program in Rural Haiti. J Nutr 2007;137:1023–30. [DOI] [PubMed] [Google Scholar]
- 32.Lombard CJ, Benadé AJ, Dhansay MA, Berger J, Hop le T, López de Romaña G, Untoro J, Karyadi E, Erhardt J, et al. Efficacy of a foodlet-based multiple micronutrient supplement for preventing growth faltering, anemia, and micronutrient deficiency of infants: the four country IRIS trial pooled data analysis. J Nutr 2005;135:631S–8S. [DOI] [PubMed] [Google Scholar]
- 33.Untoro J, Karyadi E, Wibowo L, Erhardt M, Gross R. Multiple micronutrient supplements improve micronutrient status and anemia but not growth and morbidity of Indonesian infants: a randomized, double-blind, placebo-controlled trial. J Nutr 2005;135:639S–45S. [DOI] [PubMed] [Google Scholar]
- 34.Chhagan MK, Van den Broeck J, Luabeya K-K, Mpontshane N, Tomkins A, Bennish M. Effect on longitudinal growth and anemia of zinc or multiple micronutrients added to vitamin A: a randomized controlled trial in children aged 6-24 months. BMC Public Health 2010;10:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.López de Romaña G, Cusirramos S, Lopez de Romana D, Gross R. Efficacy of multiple micronutrient supplementation for improving anemia, micronutrient status, growth, and morbidity of Peruvian infants. J Nutr 2005;135:646S–52S. [DOI] [PubMed] [Google Scholar]
- 36.Hop T, Berger J. Multiple micronutrient supplementation improves anemia, micronutrient nutrient status, and growth of Vietnamese infants: double-blind, randomized, placebo-controlled trial. J Nutr 2005;135:660S–5S. [DOI] [PubMed] [Google Scholar]
- 37.Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J, Ahmed I, Zaidi AKM, Bhutta ZA. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial. Lancet 2013;382:29–40. [DOI] [PubMed] [Google Scholar]
- 38.Ramakrishnan U, Nguyen P, Martorell R. Effects of micronutrients on growth of children under 5 y of age: meta-analyses of single and multiple nutrient interventions. Am J Clin Nutr 2009;89:191–203. [DOI] [PubMed] [Google Scholar]
- 39.Kupka R, Manji KP, Bosch RJ, Aboud S, Kisenge R, Okuma J, Fawzi WW, Duggan C. Multivitamin supplements have no effect on growth of Tanzanian children born to HIV-infected mothers. J Nutr 2013;143:722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, Haider BA, Kirkwood B, Morris SS, Sachdev H. What works? Interventions for maternal and child undernutrition and survival. Lancet 2008;371:417–40. [DOI] [PubMed] [Google Scholar]