Abstract
Background: Given the long-term adverse sequelae of childhood obesity, identification of early life factors related to fetal growth and childhood obesity is warranted. Investigation on growth and obesity in early life in association with intrauterine exposure to maternal hyperglycemia, a common metabolic pregnancy complication, is of public health significance and clinical implications.
Objective: We investigated the association of fasting plasma glucose (FPG) concentrations during pregnancy with offspring growth and risk of overweight/obesity through age 7 y, after adjustment for confounders, including maternal prepregnancy obesity status.
Design: FPG concentrations at 28 gestational weeks (IQR: 22–32 wk) were extracted from medical records for 661 pregnancies complicated by gestational diabetes mellitus in the Danish National Birth Cohort (1996–2002). Offspring’s ponderal index was derived from birth weight and length; age- and sex-specific body mass index (BMI) z scores at 5 mo, 12 mo, and 7 y were calculated based on WHO reference data. Relations between FPG and offspring growth and obesity were assessed by linear and Poisson regression with robust standard errors, adjusting for maternal prepregnancy BMI and sociodemographic and perinatal factors.
Results: At birth, maternal FPG during pregnancy was significantly associated with offspring ponderal index (β = 0.46; 95% CI: 0.14, 0.78 per 1-mmol/L increase) and risk of macrosomia (birth weight >4000 g) (RR = 1.21; 95% CI: 1.07, 1.38 per 1-mmol/L increase). At 7 y, higher maternal FPG concentrations were significantly associated with increased BMI z scores (β = 0.20; 95% CI: 0.04, 0.36) and elevated risk of overweight/obesity (RR = 1.21; 95% CI: 1.01, 1.50). Additional adjustment for birth weight and childhood lifestyle factors did not appreciably alter results. No associations were observed at 5 or 12 mo.
Conclusion: Among women with gestational diabetes mellitus, maternal FPG concentrations during pregnancy were significantly and positively associated with offspring birth size and overweight/obesity risk at 7 y, adjusting for maternal prepregnancy BMI.
Keywords: childhood obesity, fasting plasma glucose, gestational diabetes, intrauterine exposure, macrosomia, prepregnancy obesity
INTRODUCTION
The childhood obesity epidemic remains an urgent public health priority because of its short-term and long-term health consequences, including an increased risk of type 2 diabetes and cardiovascular diseases (1, 2). Therefore, identifying risk factors related to childhood growth and obesity is of public health significance and clinical implications. Accumulating evidence suggests that maternal hyperglycemia during pregnancy may induce intrauterine over nutrition and fetal hyperinsulinemia, resulting in excessive fetal growth (3) and long-term unfavorable metabolic dysregulation, including obesity in the offspring (4–8). Therefore, children born to a hyperglycemia intrauterine environment, such as gestational diabetes mellitus (GDM),9 could serve as a unique model of a high-risk population to study fetal programming or early origins of obesity. However, epidemiologic data on GDM in association with offspring risk of obesity are inconsistent, at least partly due to heterogeneity in the different diagnosis criteria of GDM and severity of hyperglycemia status, as reflected by variations in glucose concentrations (9). Therefore, studies examining the spectrum of glucose concentrations on a continuum, among the high-risk population of women with GDM, in relation to offspring growth and obesity are warranted.
Emerging yet limited epidemiologic evidence did reveal that maternal glycemia during pregnancy may be associated with offspring adiposity at birth or later in life in a dose-response fashion. Such studies in high-risk children born from pregnancies complicated by GDM are scant (10, 11). Furthermore, findings from studies in the general population are inconsistent (12–16). For instance, increasing levels of pregnancy glycemia (per 1 SD, 0.4 mmol/L) have been related to greater offspring adiposity at birth in the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study in women with a subdiabetic glucose range (14). However, in the Belfast site of the HAPO study, pregnancy glycemia (in categories or per 1 mmol/L) was not associated with offspring obesity risk at age 2 or 5–7 y (15, 16). The limited and inconsistent findings may be at least partially attributable to differences in study design, length of follow-up of the offspring, and residual confounding. Furthermore, few studies have examined the association of glucose concentrations during pregnancy with longitudinal trajectories of offspring growth and obesity adjusting for women’s obesity status, which is an important confounder but not adequately adjusted for in all previous studies (9, 17). Therefore, prospective studies with longitudinal follow-up and adequate adjustment for important confounders, including maternal obesity status and other shared family lifestyle factors, are warranted. To address the data gaps, in the current study, we aimed to investigate the continuous association between fasting plasma glucose (FPG) concentrations during pregnancies and longitudinal trajectories of offspring growth and obesity through the first 7 y of life among the high-risk population of women with GDM, accounting for potential confounders such as prepregnancy BMI (in kg/m2).
METHODS
Study population and design
The present study was based on data from the Danish National Birth Cohort (DNBC), a longitudinal nationwide study including 101,042 pregnancies (91,827 women) (18). Briefly, pregnant women who spoke Danish, intended to carry their pregnancy to term, and had a permanent address in Denmark were invited to participate in the study at their first pregnancy visit (6–12 wk of gestation) between 1996 and 2002. Information on sociodemographic, perinatal, and medical factors was collected from 4 computer-assisted telephone interviews at gestational weeks 12 and 30 and postpartum months 6 and 18. When the child was 7 y old, a follow-up was conducted by sending an online or mailed questionnaire to the parents about child’s health and development, including weight and height. The DNBC was approved by the Danish National Committee on Biomedical Research Ethics. Informed consent was obtained from all participants.
Of all 101,042 pregnancies, we identified 1379 (1.4%) pregnancies complicated by GDM documented from study interviews at 30 wk of gestation or 6 mo postpartum and/or the Danish National Patient Registry. During the conduct of the DNBC (1996–2002), the WHO criteria (19) or local practices (20) were applied for diagnosis of GDM. We included both verified and self-reported cases to maximize the possibility of identifying pregnancies complicated by hyperglycemia. Among 682 (49.4%) pregnancies of which women’s FPG concentrations were measured at the first oral-glucose-tolerance test (OGTT) and data were available from medical charts, we sequentially excluded pregnancies with pregestational diabetes as verified by medical records (n = 1) and multiple- or still-birth deliveries (n = 20). The remaining pregnancies all had at least one offspring anthropometric measure at birth or follow-up, which rendered a total of 661 mother-offspring pairs as our analytic sample (see Supplemental Figure 1 for a flowchart). Due to loss to follow-up, we had 661, 405, 397, and 351 mother-offspring pairs with available data on offspring anthropometric measures at birth and the 5-mo, 12-mo, and 7-y follow-up, respectively.
Exposure assessment
Women’s FPG concentrations (mmol/L) measured during the first OGTT during pregnancy (median: 28 wk of gestation; IQR: 22–32 wk of gestation) were extracted from medical records. Most women underwent 75-g OGTT, whereas 0.3% (n = 2) had 1 g/kg body weight OGTT.
Outcome measures
Children’s birth weight and length were extracted from the Danish Medical Birth Registry (21). Ponderal index at birth was calculated as birth weight (kg)/[birth length (m)]3. Macrosomia was defined as birth weight >4000 g. Large-for-gestational age was defined as a birth weight greater than the sex- and gestational age–specific 90th percentile based on the entire DNBC population. During the postpartum 18-mo interview, mothers referred to the Child’s Book, which recorded children’s weight and recumbent length at 5 and 12 mo of age measured by the general practitioner (physician or nurse) at the 5- and 12-mo visits, respectively. Children’s weight and height on average at a mean ± SD age of 7.1 ± 0.26 y were reported by the parent(s) from the 7-y follow-up questionnaire based on measurements obtained by general practitioners, school nurses, or parents. Age- and sex-specific BMI z scores (BMIZs) were calculated by using the WHO Child Growth Standards for infants and children aged <5 y (22) and WHO Growth Reference for those ≥5 y (23). We further classified children as overweight/obese by using the corresponding age- and sex-specific WHO cutoffs [i.e., ≥85th percentile for children aged <5 y (22) and ≥2 SD for those aged ≥5 y (23)].
Covariates
Data on parity (nulliparous or not), socioeconomic status [high (high- or medium-level professionals), middle (skilled workers), or low (unskilled workers and others), determined by the highest level within the couple], prepregnancy BMI [<25, 25–29.9, or ≥30, calculated as self-reported prepregnancy weight (kg)/height (m)2], and smoking during pregnancy (yes or no) were obtained from interviews at gestational weeks 12 and 30. Information on age at index child’s delivery (years), gestational age at OGTT (weeks), and gestational age at delivery (weeks) was extracted from medical records. Gestational weight gain (kg) was extracted from medical records or self-reported during interviews at 6 mo postpartum. Information on breastfeeding duration (≥6 mo or not) was collected from interviews at postpartum months 6 and 18, whereas offspring physical activity (≥2 h/weekday or not) and food frequency of sugar-sweetened beverages (≥ once/wk or not) were obtained from the 7-y follow-up questionnaire.
Statistical analysis
Descriptive statistics for subject characteristics are presented as means ± SDs for parametric continuous variables, median (IQR) for nonparametric continuous variables, and percentages for categorical variables. Differences in subject characteristics by tertiles of maternal FPG concentrations were assessed by ANOVA (parametric) or Kruskal-Wallis test (nonparametric) for continuous variables and by χ2 test for categorical variables. Covariates were missing for <6.4% of the study population, and subject characteristics did not differ between complete cases and those with missing values. Thus, model-specific complete case analysis was conducted with respective sample size for each multivariate model specified in table footnotes. Examination of the association between maternal FPG quartiles and offspring growth/obesity suggested a linear relation. Therefore, maternal FPG concentrations were modeled in its original scale. Specifically, associations of maternal FPG concentrations (per 1 mmol/L) with continuous measures of childhood obesity (i.e., ponderal index at birth and BMIZ at follow-up) were assessed by using linear regression models, and associations with binary outcomes (i.e., macrosomia and overweight/obesity at follow-up) were assessed by using Poisson regression with robust standard errors (24) at each follow-up, after adjustment for the above-listed covariates. Generalized estimating equations were used to account for clustering due to multiple pregnancies of the same woman (1.4% women contributed more than one pregnancy). To evaluate the potential mediating effect of birth weight on the association between maternal FPG concentrations and offspring obesity independent of other covariates, we calculated the mediation proportion and its 95% CI in birth weight–adjusted models by using the method described by Hertzmark et al. (25).
To test for the longitudinal trajectory of offspring BMIZ from birth through 7 y (i.e., the velocity of BMIZ increase), we used linear mixed-effects models to account for within-subject correlations between repeated measures and different numbers of observations at follow-up (26). In addition, P values for the longitudinal changes of BMIZ were obtained by using the permutation test with 10,000 times resampling to correct for multiple testing (27). We also added a cross-product of maternal FPG (mmol/L) and prepregnancy BMI to examine the potential effect modification by maternal prepregnancy obesity. Although the cross-product terms at each age in the linear/Poisson regression models were not statistically significant, the term across ages in the linear mixed-effects model was significant, and the results were therefore stratified by maternal prepregnancy obesity at each age and across ages, respectively.
To test the robustness of our findings against the potential effect modification by the use of insulin or other medications for GDM, we conducted a sensitivity analysis by further excluding pregnancies during which women received medications for GDM (n = 42). Effect modification by child’s sex was examined as well. Selection bias was assessed by comparing maternal/offspring characteristics between the full analytic sample (n = 661) and those excluded due to FPG concentrations not available from medical charts (n = 697). Similarly, nonresponse analyses were conducted to assess whether participant characteristics differed between mother-offspring pairs with missing offspring anthropometric measurements and those retained at each follow-up (n for mother-offspring pairs with missing/nonmissing anthropometric data: 256/405, 264/397, and 310/351 at the 5-mo, 12-mo, and 7-y follow-up, respectively). All analyses were conducted with SAS version 9.4 (SAS Institute). Two-tailed P values <0.05 were considered statistically significant.
RESULTS
Among the 661 pregnancies complicated by GDM, the age at the index childbirth was 31.5 ± 4.5 y (Table 1). Overall, women tended to be overweight/obese before the index pregnancy and have middle/high socioeconomic status. Maternal FPG concentrations were measured on average at 28.1 (IQR: 22.1–32.7) wk of gestation, with a median concentration of 4.7 (IQR: 4.2–5.3) mmol/L. Among the offspring, 33.6% were born macrosomic and 25.9% were overweight/obese at 7 y of age. Across increasing tertiles of FPG concentrations, women tended to be heavier (P < 0.001); their offspring were more likely to be macrosomic at birth and overweight/obese at 7 y but not at 5 or 12 mo of age.
TABLE 1.
Maternal and child characteristics of the study population according to tertiles of maternal FPG concentrations during pregnancy (n = 661)1
| Overall (n = 661) | Tertile 1 (n = 202) | Tertile 2 (n = 215) | Tertile 3 (n = 244) | P value | |
| FPG concentrations, mmol/L | 4.7 (4.2–5.3)2 | 4.0 (3.8–4.2) | 4.6 (4.5–4.8) | 5.5 (5.2–5.9) | <0.001 |
| Maternal characteristics | |||||
| Age at index child's birth, y | 31.5 ± 4.53 | 30.9 ± 4.8 | 32.0 ± 4.3 | 31.7 ± 4.3 | 0.11 |
| Socioeconomic status, n (%) | 0.2 | ||||
| High | 282 (42.7) | 97 (48) | 92 (42.8) | 93 (38.1) | |
| Middle | 203 (30.7) | 53 (26.2) | 71 (33) | 79 (32.4) | |
| Low | 146 (22.1) | 45 (22.3) | 41 (19.1) | 60 (24.6) | |
| Unknown | 30 (4.5) | 7 (3.5) | 11 (5.1) | 12 (4.9) | |
| Prepregnancy BMI (kg/m2), n (%) | <0.001 | ||||
| <25.0 | 206 (31.2) | 92 (45.5) | 68 (31.6) | 46 (18.9) | |
| 25.0–29.9 | 176 (26.6) | 52 (25.7) | 65 (30.2) | 59 (24.2) | |
| ≥30.0 | 237 (35.9) | 48 (23.8) | 67 (31.2) | 122 (50.0) | |
| Unknown | 42 (6.4) | 10 (5.0) | 15 (7.0) | 17 (7.0) | |
| Nulliparity, n (%) | 245 (38.7) | 70 (35.7) | 73 (35.6) | 102 (44.0) | 0.12 |
| Gestational age at OGTT, wk | 28.1 (22.1–32.7) | 28.3 (22.1–33.0) | 27.6 (20.6–32.4) | 27.9 (22.1–32.1) | 0.31 |
| Gestational weight gain, kg | 13.0 ± 7.2 | 13.4 ± 6.7 | 13.1 ± 7.2 | 12.5 ± 7.5 | 0.29 |
| Smoking during pregnancy, n (%) | 110 (16.8) | 37 (18.5) | 34 (15.8) | 39 (16.2) | 0.73 |
| Offspring characteristics | |||||
| Male, n (%) | 323 (51.0) | 106 (52.5) | 102 (47.4) | 129 (52.9) | 0.45 |
| Gestational age at delivery, wk | 39.5 ± 1.6 | 39.7 ± 1.6 | 39.6 ± 1.6 | 39.4 ± 1.6 | 0.1 |
| Birth weight, kg | 3.7 ± 0.6 | 3.6 ± 0.6 | 3.8 ± 0.6 | 3.8 ± 0.6 | 0.006 |
| Ponderal index, kg/m3 | 25.7 ± 2.9 | 25.5 ± 2.9 | 25.4 ± 2.3 | 26.1 ± 3.2 | 0.027 |
| Macrosomia (>4000 g), n (%) | 222 (33.6) | 51 (25.2) | 84 (39.1) | 87 (35.8) | 0.008 |
| Large-for-gestational age,4 n (%) | 197 (29.8) | 43 (21.3) | 73 (34.0) | 81 (33.3) | 0.006 |
| BMI-for-age z score | |||||
| Birth | 0.01 ± 1.2 | −0.2 ± 1.2 | −0.0 ± 1.1 | 0.2 ± 1.2 | 0.015 |
| 5 mo | −0.39 ± 1.1 | −0.4 ± 1.1 | −0.4 ± 1.0 | −0.4 ± 1.1 | 0.78 |
| 12 mo | 0.28 ± 1.1 | 0.1 ± 1.1 | 0.4 ± 1.1 | 0.3 ± 1.1 | 0.13 |
| 7 y | 0.32 ± 1.1 | −0.0 ± 1.1 | 0.3 ± 1.0 | 0.6 ± 1.1 | <0.001 |
| Overweight/obese,5 n (%) | |||||
| Birth | 109 (16.6) | 31 (15.3) | 28 (13.2) | 50 (20.7) | 0.083 |
| 5 mo | 41 (10.1) | 13 (10.9) | 11 (7.6) | 17 (12.0) | 0.45 |
| 12 mo | 92 (23.2) | 23 (19.7) | 32 (23.4) | 37 (25.9) | 0.5 |
| 7 y | 91 (25.9) | 19 (17.3) | 28 (23.7) | 44 (35.8) | 0.005 |
P values denote differences across tertiles of FPG concentrations determined by ANOVA (parametric) or Kruskal-Wallis test (nonparametric) for continuous variables and by χ2 test for categorical variables. FPG, fasting plasma glucose; OGTT, oral-glucose-tolerance test.
Median; IQR in parentheses (all such values).
Mean ± SD (all such values).
Defined as a birth weight greater than the sex- and gestational age–specific 90th percentile based on the entire Danish National Birth Cohort study population.
Defined as ≥85th percentile (children aged <5 y) or ≥2 SD (≥5 y) according to the WHO Child Growth Standards or WHO growth reference, respectively.
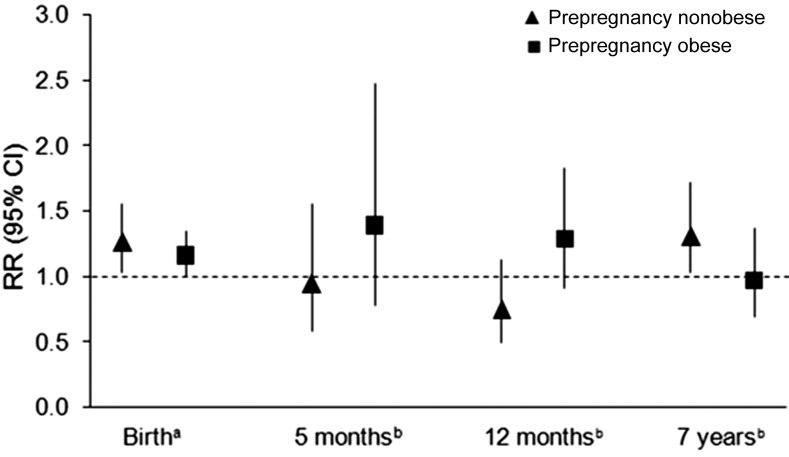
Every 1-mmol/L increment in women’s FPG concentrations was significantly associated with an increase of 0.46 (95% CI: 0.14, 0.78) units in offspring ponderal index at birth and an increase of 0.20 (95% CI: 0.04, 0.36) SD in BMIZ at 7 y of age, after adjustment for prepregnancy BMI and other sociodemographic and perinatal factors (model 1 in Table 2). Per a 1-mmol/L increase in maternal FPG, the risks of offspring macrosomia, large-for-gestational age, and overweight/obesity at 7 y were elevated by 21% (95% CI: 1.07, 1.38; P = 0.003), 19% (95% CI: 1.02, 1.39; P = 0.026), and 21% (95% CI: 1.01, 1.50; P = 0.041), respectively, after adjustment for confounders (model 1 in Table 3). Results at 7 y remained similar after further adjustment for child’s birth weight (model 2 in Tables 2 and 3). Mediation analysis indicated that the effect of maternal FPG on birth weight was responsible for a relatively small and declining proportion of offspring overweight/obesity at 5 mo, 12 mo, and 7 y (i.e., 10.9%, 4.5%, and 0.9%, respectively), and none of these estimates reached statistical significance (P = 0.06, 0.08, and 0.80, respectively). Furthermore, additional adjustment for breastfeeding duration and child’s physical activity and intake of sugar-sweetened beverages at 7 y did not appreciably alter the results for overweight/obesity at 7 y (RR = 1.26; 95% CI: 1.02, 1.57; data not shown). The association appeared more pronounced in children who had sugar-sweetened beverages more than once/wk (n = 131, 37.3%; RR = 1.59; 95% CI: 1.05, 2.40) compared with their counterparts with a lower frequency (RR = 1.10; 95% CI: 0.81, 1.48). In addition, the trend of increased risk of macrosomia associated with maternal FPG was consistent regardless of maternal prepregnancy obesity status, whereas the association with overweight/obesity at 7 y was statistically significant only among children born to women who were nonobese before pregnancy (Figure 1).
TABLE 2.
Linear regression β coefficients (95% CIs) for the association between each 1-mmol/L increase in maternal fasting plasma glucose concentrations and offspring growth parameters at birth and follow-up
| Crude |
Model 11 |
Model 1 + birth weight2 |
|||||
| n | β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | |
| Ponderal index at birth, kg/m3 | 655 | 0.44 (0.18, 0.71) | 0.001 | 0.46 (0.14, 0.78) | 0.005 | NA3 | NA |
| BMI z scores at 5 mo, SD | 405 | 0.002 (−0.18, 0.13) | 0.98 | 0.034 (−0.13, 0.20) | 0.68 | −0.016 (−0.18, 0.15) | 0.84 |
| BMI z scores at 12 mo, SD | 397 | 0.064 (−0.07, 0.20) | 0.36 | 0.020 (−0.15, 0.19) | 0.81 | −0.029 (−0.20, 0.14) | 0.73 |
| BMI z scores at 7 y, SD | 351 | 0.27 (0.13, 0.41) | <0.001 | 0.20 (0.04, 0.36) | 0.014 | 0.182 (0.023, 0.34) | 0.025 |
Adjusted for maternal age at index child's birth, parity, socioeconomic status, prepregnancy BMI, smoking during pregnancy, gestational age at oral-glucose-tolerance test, gestational weight gain, and gestational age at delivery. For multivariate analyses, the final sample sizes were 611, 381, 374, and 325 for ponderal index and BMI z scores at 5 mo, 12 mo, and 7 y, respectively, due to missing data on prepregnancy BMI, parity, and socioeconomic status.
Adjusted for covariates in model 1 and child’s birth weight.
NA, not applicable.
TABLE 3.
RRs (95% CIs) for the association between each 1-mmol/L increase in maternal fasting plasma glucose concentrations and offspring adiposity measures at birth and follow-up
| Crude |
Model 11 |
Model 1 + birth weight2 |
|||||
| n | RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | |
| Macrosomia (birth weight >4000 g) | 661 | 1.18 (1.05, 1.32) | 0.006 | 1.21 (1.07, 1.38) | 0.003 | NA3 | NA |
| Large-for-gestational age | 661 | 1.18 (1.03, 1.34) | 0.016 | 1.19 (1.02, 1.39) | 0.026 | NA | NA |
| Overweight/obesity at 5 mo4 | 405 | 1.07 (0.77, 1.48) | 0.697 | 1.18 (0.79, 1.78) | 0.417 | 1.07 (0.70, 1.65) | 0.755 |
| Overweight/obesity at 12 mo4 | 397 | 1.04 (0.84, 1.28) | 0.724 | 1.09 (0.84, 1.40) | 0.514 | 1.03 (0.80, 1.33) | 0.824 |
| Overweight/obesity at 7 y5 | 351 | 1.27 (1.07, 1.51) | 0.007 | 1.21 (1.01, 1.50) | 0.041 | 1.20 (1.01, 1.49) | 0.042 |
Adjusted for maternal age at index child's birth, parity, socioeconomic status, prepregnancy BMI, smoking during pregnancy, gestational age at oral-glucose-tolerance test, gestational weight gain, and gestational age at delivery. For multivariate analyses, the final sample sizes were 616, 381, 374, and 325 for ponderal index and BMI z scores at 5 mo, 12 mo, and 7 y, respectively, because of missing data on prepregnancy BMI, parity, and socioeconomic status.
Adjusted for covariates in model 1 and child’s birth weight.
NA, not applicable.
Defined as age- and sex-specific BMI-for-age z scores >85th percentile according to the WHO Child Growth Standards.
Defined as age- and sex-specific BMI-for-age z scores >2 SD according to the WHO growth reference.
FIGURE 1.
RR and 95% CI for the association between each 1-mmol/L increase in fasting plasma glucose concentrations during pregnancy and offspring risk of macrosomia at birth and overweight/obesity at age 5 mo, 12 mo, and 7 y by prepregnancy obesity status [nonobese: BMI (in kg/m2) <30; obese: ≥30]. The numbers of children born to women who were nonobese/obese before pregnancy were 382/237, 241/142, 231/145, and 210/117 at birth and the 5-mo, 12-mo, and 7-y follow-up, respectively. aRR (95% CI) for macrosomia at birth was calculated by using Poisson regression with robust SEs, adjusted for maternal age at index child's birth, parity, socioeconomic status, prepregnancy BMI, smoking during pregnancy, gestational age at oral-glucose-tolerance test, gestational weight gain, and gestational age at delivery. bRR (95% CI) for overweight/obesity at each follow-up was calculated by using Poisson regression with robust SEs, also adjusted for birth weight besides covariates for macrosomia. P-interaction for fasting plasma glucose concentrations by prepregnancy obesity = 0.61, 0.50, 0.57, and 0.07 for macrosomia at birth and overweight/obesity at age 5 mo, 12 mo, and 7 y, respectively.
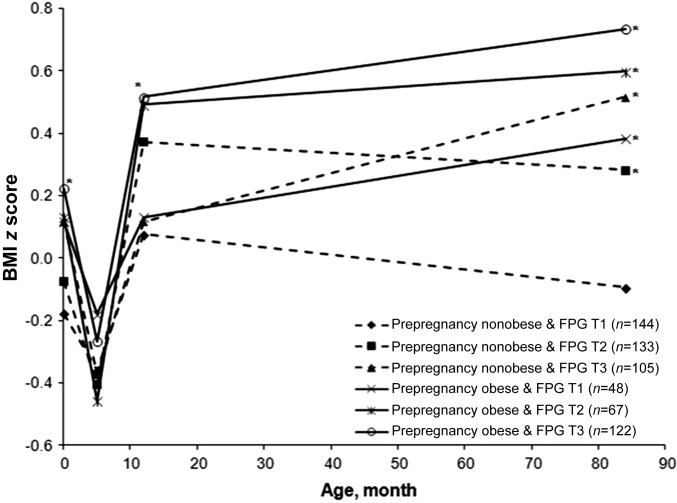
We further examined the association of growth trajectory from birth through 7 y with maternal FPG concentrations by prepregnancy obesity status and FPG tertiles (Figure 2). The BMIZ trajectory exhibited a continuously rising trend after 5 mo of age in offspring born to women who were obese before pregnancy, regardless of FPG categories (P < 0.05 after permutation test correcting for multiple testing). Similarly, the rising trend was also observed in women who were nonobese before pregnancy and whose FPG concentrations were in the highest tertile (P < 0.05 after permutation). In addition, offspring BMIZ at 7 y was highest in those born to women who were obese before pregnancy and whose FPG concentrations during pregnancy were in the highest tertile.
FIGURE 2.
Offspring’s sex- and age-specific BMI z scores by maternal prepregnancy obesity status [nonobese: BMI (in kg/m2) <30; obese: ≥30] and tertiles of fasting plasma glucose concentrations (T1: 2–4.3 mmol/L; T2: 4.4–4.9 mmol/L, and T3: 5–9 mmol/L) at birth and ages 5 mo, 12 mo, and 7 y. *P < 0.05, compared with the reference group “Prepregnancy nonobese & FPG T1,” obtained by Mann-Whitney test after permutation test correcting for multiple testing. FPG, fasting plasma glucose; T, tertile.
Sensitivity analysis restricted to pregnant women not taking medications for GDM (n = 619, 93%) showed similar results. Stratified analysis by child’s sex revealed no statistically significant sex-specific associations between maternal FPG and offspring birth size or risk of childhood overweight/obesity. In addition, pregnant women with FPG concentrations not available from medical records (n = 697) did not significantly differ from the full analytic sample (n = 661) with respect to maternal/child characteristics, except that gestational age at delivery was slightly earlier among the former than the latter (median: 39.3 wk compared with 39.6 wk). Nonresponse analyses showed that the major characteristics of mother-offspring pairs whose offspring anthropometric measures were missing did not appreciably differ from those with data available at each follow-up, except that those lost to 12-mo follow-up were more likely to smoke during pregnancy and those lost to 7-y follow-up had slightly lower socioeconomic status than did those with data available at respective follow-up (see Supplemental Table 1).
DISCUSSION
In this prospective DNBC study, women’s FPG concentrations during pregnancies complicated by GDM were significantly and positively associated with offspring birth size (i.e., ponderal index, macrosomia, and large-for-gestational age) after adjustment for prepregnancy BMI. The statistically significant association did not persist in infancy (i.e., 5 or 12 mo of age) but reappeared at 7 y.
Findings from previous studies on maternal pregnancy glycemia and offspring growth have been limited and findings were inconsistent. Our findings are, in general, in line with a previous longitudinal study of nondiabetic women, in whom statistically significant and positive linear associations of maternal glucose concentrations with offspring BMIZ at ages 5 and 7 y were observed but not at 2 or 3.5 y (13). In addition, Pima Indian children born to mothers with pre-existing diabetes or GDM exhibited age-specific patterns of growth trajectories with significantly higher weight-for-age z scores at birth and 7.7 y but not at 1.5 y, compared with their counterparts of nondiabetic women (28). However, among children of women in the Belfast center of the HAPO study, levels of maternal pregnancy glycemia were not associated with offspring obesity at 2 or 5–7 y after further adjustment for maternal BMI at the time of OGTT (15, 16). The discordant findings could be partially attributable to the variation in underlying characteristics of the study population and the potential threshold effect of maternal glycemia (15). Of note, compared with women in our study, women in the Belfast center of the HAPO study were less overweight/obese and had less severe hyperglycemia because of the exclusion of women with an FPG concentration >5.8 mmol/L or 75-g OGTT 2-h glucose concentration >11.1 mmol/L. Taken together, these findings suggest that the long-term impact of maternal glycemia during pregnancy on offspring growth and obesity might be age specific, particularly apparent at birth and later ages, and might vary by levels of glycemia. Furthermore, we observed that the BMIZ decreased between birth and early infancy around 5 mo, followed by growth acceleration through 7 y, especially among children of women who were obese before pregnancy and who had higher FPG concentrations. This pattern of restricted growth in early infancy preceding the subsequent catchup growth resembles the growth trajectory of children who later develop impaired glucose tolerance or type 2 diabetes in adulthood (29, 30).
Moreover, our findings suggest that the long-term impact of maternal hyperglycemia on offspring overweight/obesity is not entirely mediated by excessive fetal growth as estimated by birth weight in our models. Consistently, Deierlein et al. (12) and Kubo et al. (11) also reported that additional adjustment for birth weight did not substantially alter the effect estimates of the statistically significant and positive associations of maternal hyperglycemia with offspring BMIZ at 3 and 8–14 y, respectively. Collectively, these data suggest that maternal hyperglycemia during pregnancy may predispose offspring to increased risk of overweight/obesity in later life via mechanisms beyond excessive fetal growth.
The effect of maternal FPG in pregnancy on offspring obesity at 7 y appeared more pronounced in nonobese GDM women in this study and in nonobese nondiabetic women as reported previously (13). Given that both maternal obesity and hyperglycemia may induce a proinflammatory state, excessive fuel substrates, and dysregulation in energy balance (31, 32), these common pathways might attenuate the independent association of glycemia levels in the presence of maternal obesity as a statistically significant predictor of offspring obesity. In addition, it is worth noting that the sample size of prepregnancy nonobese women retained at the 7-y follow-up was larger than that of their obese counterparts. Therefore, it is possible that the statistically nonsignificant association between FPG and offspring risk of overweight/obesity at 7 y among prepregnancy obese women could be due to insufficient statistical power.
Biologic mechanisms underlying the putative independent effect of maternal glycemia during pregnancy on offspring obesity remain to be elucidated. However, animal studies show that intrauterine exposure to hyperglycemia may induce disruptions in hypothalamic neuropeptidergic neurons (33), impaired glucose homeostasis (34), and altered nephrogenesis and β cell dysfunction (35) in the offspring. Consistently, epidemiologic data corroborate that offspring of diabetic women are predisposed to fetal programming or metabolic imprinting via hyperinsulinemia (36), glucose intolerance (37), augmented inflammation response (38), or perturbations in epigenetic modifications (39), which in turn are positively related to childhood overweight/obesity (40).
The current prospective design and repeated measures of childhood anthropometric measurements provide a unique opportunity and strength to investigate the long-term effect of maternal glycemia levels during pregnancy on offspring obesity with adjustment for important confounders. We also explored the effect modification by childhood factors as indicators of shared family lifestyle. Findings suggest that a combination of high maternal FPG during pregnancy and unhealthful childhood lifestyle (i.e., high intake of sugar-sweetened beverages) may exacerbate the risk of overweight/obesity at 7 y. However, our study has several potential limitations. Childhood weight and height at 7 y were obtained from parental report based on measurements by general practitioners, school nurses, or parents with inevitable measurement errors. Nonetheless, previous data showed high correlations between parentally reported and measured anthropometric measures in children aged 7–9 y (r = 0.942 for height, r = 0.925 for weight, r = 0.813 for BMI; P < 0.001) (41). Furthermore, the potential misclassification should be unrelated to maternal FPG concentrations during pregnancy and would likely attenuate the effect sizes of the true associations. The loss to follow-up could have reduced the statistical power and introduced selection bias. However, the selection bias seems unlikely or minimal based on the nonresponse analyses, which demonstrated that the major maternal/child characteristics and the exposure of interest (FPG concentrations) did not differ appreciably between mother-offspring pairs lost to and those retained at each follow-up. The study population was homogeneous and composed of Danish-speaking participants. Thus, generalizability to other racial-ethnic groups at high risk of GDM such as Hispanics and Asians may be limited. Further research may be warranted among other racial-ethnic groups.
In conclusion, our findings illustrate a statistically significant and positive association of birth size and overweight/obesity risk at age 7 y with maternal FPG concentrations during pregnancy, after adjustment for prepregnancy BMI. Our study adds to the growing, yet limited, literature on the long-term pathophysiologic consequences of high levels of maternal glycemia during pregnancy on the offspring and suggests that these consequences may differ by offspring age. Future prospective studies with longer follow-up through adolescence and adulthood of the offspring are warranted to evaluate whether our findings may persist in later life.
Acknowledgments
The authors’ responsibilities were as follows—YZ: analyzed data and drafted the manuscript; SFO, AL, and KM: contributed to data analyses or the acquisition of the data and critical revision of the manuscript; PM, EHY, AV, KB, WB, SL, CM, LGG, CG, SH, JEC, FBH, JL-R, and PD: contributed to the interpretation of data analyses and critical revision of the manuscript; CZ: conceptualized and designed the analysis, interpreted data analyses, critically revised the manuscript for important intellectual content, and had primary responsibility for the final content; and all authors: read and approved the final manuscript. The authors declared no conflicts of interest.
Footnotes
Abbreviations used: BMIZ, BMI z score; DNBC, Danish National Birth Cohort; FPG, fasting plasma glucose; GDM, gestational diabetes mellitus; HAPO, Hyperglycemia and Adverse Pregnancy Outcomes; OGTT, oral-glucose-tolerance test.
REFERENCES
- 1.Dabelea D, Harrod CS. Role of developmental overnutrition in pediatric obesity and type 2 diabetes. Nutr Rev 2013;71(Suppl 1):S62–7. [DOI] [PubMed] [Google Scholar]
- 2.Nadeau KJ, Maahs DM, Daniels SR, Eckel RH. Childhood obesity and cardiovascular disease: links and prevention strategies. Nat Rev Cardiol 2011;8:513–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen J. The pregnant diabetic and her newborn: problems and management. 2nd ed. Baltimore: Williams & Wilkins; 1977. [Google Scholar]
- 4.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, et al. . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002. [DOI] [PubMed] [Google Scholar]
- 5.Petitt DJ, Bennett PH, Knowler WC, Baird HR, Aleck KA. Gestational diabetes mellitus and impaired glucose tolerance during pregnancy: long-term effects on obesity and glucose tolerance in the offspring. Diabetes 1985;34(Suppl 2):119–22. [DOI] [PubMed] [Google Scholar]
- 6.Nehring I, Chmitorz A, Reulen H, von Kries R, Ensenauer R. Gestational diabetes predicts the risk of childhood overweight and abdominal circumference independent of maternal obesity. Diabet Med 2013;30:1449–56. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson C, Carlsson A, Landin-Olsson M. Increased risk for overweight among Swedish children born to mothers with gestational diabetes mellitus. Pediatr Diabetes 2014;15:57–66. [DOI] [PubMed] [Google Scholar]
- 8.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Schmidt L, Damm P. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab 2009;94:2464–70. [DOI] [PubMed] [Google Scholar]
- 9.Kim SY, England JL, Sharma JA, Njoroge T. Gestational diabetes mellitus and risk of childhood overweight and obesity in offspring: a systematic review. Exp Diabetes Res 2011;2011:541308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting—the ongoing effects of maternal hyperglycemia. Diabetes Care 2007;30:2287–92. [DOI] [PubMed] [Google Scholar]
- 11.Kubo A, Ferrara A, Windham GC, Greenspan L, Deardorff J, Hiatt RA, Quesenberry CP Jr, Laurent C, Mirabedi AS, Kushi LH. Maternal hyperglycemia during pregnancy predicts adiposity of the offspring. Diabetes Care 2014;37:2996–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deierlein AL, Siega-Riz AM, Chantala K, Herring AH. The association between maternal glucose concentration and child BMI at age 3 years. Diabetes Care 2011;34:480–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehrlich SF, Rosas LG, Ferrara A, King JC, Abrams B, Harley KG, Hedderson MM, Eskenazi B. Pregnancy glycemia in Mexican-American women without diabetes or gestational diabetes and programming for childhood obesity. Am J Epidemiol 2013;177:768–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.HAPO Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes 2009;58:453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thaware PK, McKenna S, Patterson CC, Hadden DR, Pettitt DJ, McCance DR. Untreated mild hyperglycemia during pregnancy and anthropometric measures of obesity in offspring at age 5-7 years. Diabetes Care 2015;38:1701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettitt DJ, McKenna S, McLaughlin C, Patterson CC, Hadden DR, McCance DR. Maternal glucose at 28 weeks of gestation is not associated with obesity in 2-year-old offspring the Belfast Hyperglycemia and Adverse Pregnancy Outcome (HAPO) family study. Diabetes Care 2010;33:1219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philipps LH, Santhakumaran S, Gale C, Prior E, Logan KM, Hyde MJ, Modi N. The diabetic pregnancy and offspring BMI in childhood: a systematic review and meta-analysis. Diabetologia 2011;54:1957–66. [DOI] [PubMed] [Google Scholar]
- 18.Olsen J, Melbye M, Olsen SF, Sorensen TI, Aaby P, Andersen AM, Taxbol D, Hansen KD, Juhl M, Schow TB, et al. . The Danish National Birth Cohort—its background, structure and aim. Scand J Public Health 2001;29:300–7. [DOI] [PubMed] [Google Scholar]
- 19.WHO. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Geneva (Switzerland): WHO; 1999. [Google Scholar]
- 20.Kühl C. Glucose metabolism during and after pregnancy in normal and gestational diabetic women. 1. Influence of normal pregnancy on serum glucose and insulin concentration during basal fasting conditions and after a challenge with glucose. Acta Endocrinol (Copenh) 1975;79:709–19. [PubMed] [Google Scholar]
- 21.Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull 1998;45:320–3. [PubMed] [Google Scholar]
- 22.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva (Switzerland): World Health Organization; 2006. [Google Scholar]
- 23.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 2007;85:660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- 25.Hertzmark E, Pazaris M, Spiegelman D. The SAS MEDIATE Macro [Internet]. [cited 2015 Dec 29]. Boston: Harvard T.H. Chan School of Public Health; 2012. Available from: http://www.hsph.harvard.edu/donna-spiegelman/software/mediate/.
- 26.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38:963–74. [PubMed] [Google Scholar]
- 27.Pesarin F. Multivariate permutation tests: with applications in biostatistics. New York: John Wiley; 2001. [Google Scholar]
- 28.Touger L, Looker HC, Krakoff J, Lindsay RS, Cook V, Knowler WC. Early growth in offspring of diabetic mothers. Diabetes Care 2005;28:585–9. [DOI] [PubMed] [Google Scholar]
- 29.Bhargava SK, Sachdev HS, Fall CH, Osmond C, Lakshmy R, Barker DJ, Biswas SK, Ramji S, Prabhakaran D, Reddy KS. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med 2004;350:865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Early adiposity rebound in childhood and risk of type 2 diabetes in adult life. Diabetologia 2003;46:190–4. [DOI] [PubMed] [Google Scholar]
- 31.King JC. Maternal obesity, metabolism, and pregnancy outcomes. Annu Rev Nutr 2006;26:271–91. [DOI] [PubMed] [Google Scholar]
- 32.Kitajima M, Oka S, Yasuhi I, Fukuda M, Rii Y, Ishimaru T. Maternal serum triglyceride at 24–32 weeks’ gestation and newborn weight in nondiabetic women with positive diabetic screens. Obstet Gynecol 2001;97:776–80. [DOI] [PubMed] [Google Scholar]
- 33.Franke K, Harder T, Aerts L, Melchior K, Fahrenkrog S, Rodekamp E, Ziska T, Van Assche FA, Dudenhausen JW, Plagemann A. ‘Programming’ of orexigenic and anorexigenic hypothalamic neurons in offspring of treated and untreated diabetic mother rats. Brain Res 2005;1031:276–83. [DOI] [PubMed] [Google Scholar]
- 34.Bihoreau MT, Ktorza A, Kinebanyan MF, Picon L. Impaired glucose homeostasis in adult rats from hyperglycemic mothers. Diabetes 1986;35:979–84. [DOI] [PubMed] [Google Scholar]
- 35.Amri K, Freund N, Van Huyen JPD, Merlet-Benichou C, Lelievre-Pegorier M. Altered nephrogenesis due to maternal diabetes is associated with increased expression of IGF-II/mannose-6-phosphate receptor in the fetal kidney. Diabetes 2001;50:1069–75. [DOI] [PubMed] [Google Scholar]
- 36.Silverman BL, Landsberg L, Metzger BE. Fetal hyperinsulinism in offspring of diabetic mothers: association with the subsequent development of childhood obesity. Ann N Y Acad Sci 1993;699:36–45. [DOI] [PubMed] [Google Scholar]
- 37.Krishnaveni GV, Hill JC, Leary SD, Veena SR, Saperia J, Saroja A, Karat SC, Fall CH. Anthropometry, glucose tolerance, and insulin concentrations in Indian children: relationships to maternal glucose and insulin concentrations during pregnancy. Diabetes Care 2005;28:2919–25. [DOI] [PubMed] [Google Scholar]
- 38.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 2002;106:2067–72. [DOI] [PubMed] [Google Scholar]
- 39.Young LE. Imprinting of genes and the Barker hypothesis. Twin Res 2001;4:307–17. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan EL, Grove KL. Metabolic imprinting in obesity. Forum Nutr 2010;63:186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Cauwenberghe J, Delvaux I, Michels N, Den Hond E, Schoeters G, Nelen V, Croes K, Van Larebeke N, Sioen I. Validity of parentally reported versus measured weight, length and waist in 7-to 9-year-old children for use in follow-up studies. Eur J Pediatr 2014;173:921–8. [DOI] [PubMed] [Google Scholar]