Abstract
Background: Limited and inconsistent research findings exist about the effect of dietary protein intake on indexes of sleep.
Objective: We assessed the effect of protein intake during dietary energy restriction on indexes of sleep in overweight and obese adults in 2 randomized, controlled feeding studies.
Design: For study 1, 14 participants [3 men and 11 women; mean ± SE age: 56 ± 3 y; body mass index (BMI; in kg/m2): 30.9 ± 0.6] consumed energy-restricted diets (a 750-kcal/d deficit) with either beef and pork (BP; n = 5) or soy and legume (SL; n = 9) as the main protein sources for 3 consecutive 4-wk periods with 10% (control), 20%, or 30% of total energy from protein (random order). At baseline and the end of each period, the global sleep score (GSS) was assessed with the use of the Pittsburgh Sleep Quality Index (PSQI) questionnaire. For study 2, 44 participants (12 men and 32 women; age: 52 ± 1 y; BMI: 31.4 ± 0.5) consumed a 3-wk baseline energy-balance diet with 0.8 g protein · kg baseline body mass−1 · d−1. Then, study 2 subjects consumed either a normal-protein [NP (control); n = 23] or a high-protein (HP; n = 21) (0.8 compared with 1.5 g · kg−1 · d−1, respectively) energy-restricted diet (a 750-kcal/d deficit) for 16 wk. The PSQI was administered during baseline week 3 and intervention weeks 4, 8, 12, and 16. GSSs ranged from 0 to 21 arbitrary units (au), with a higher value representing a worse GSS during the preceding month.
Results: In study 1, we showed that a higher protein quantity improved GSSs independent of the protein source. The GSS was higher (P < 0.05) when 10% (6.0 ± 0.4 au) compared with 20% (5.0 ± 0.4 au) protein was consumed, with 30% protein (5.4 ± 0.6 au) intermediate. In study 2, at baseline, the GSS was not different between NP (5.2 ± 0.5 au) and HP (5.4 ± 0.5 au) groups. Over time, the GSS was unchanged for the NP group and improved for the HP group (P-group-by-time interaction < 0.05). After intervention (week 16), GSSs for NP and HP groups were 5.9 ± 0.5 and 4.0 ± 0.6 au, respectively (P < 0.01).
Conclusion: The consumption of a greater proportion of energy from protein while dieting may improve sleep in overweight and obese adults. This trial was registered at clinicaltrials.gov as NCT01005563 (study 1) and NCT01692860 (study 2).
Keywords: higher-protein diet, indexes of sleep, Pittsburgh Sleep Quality Index, weight loss, global sleep score
INTRODUCTION
Sleep is essential to health. Indexes of sleep including duration, quality, and patterning are related to obesity, type 2 diabetes, cardiovascular disease, hypertension, worsened lipid metabolism, and premature death (1–6). All of these morbidities and mortality are also affected by diet (7–11). The 2015 Dietary Guidelines Advisory Committee recognized “an insufficient body of evidence” in the “emerging area” of “associations between sleep patterns, dietary intakes, and obesity risk” and that “a paucity of research exists on the potential impact of diet on sleep-related outcomes” (12). Currently, the majority of research has assessed the influence of sleep on energy balance (13–15) or dietary choices (15–18). Limited research exists on the effects of dietary energy and macronutrients, especially protein intake, on indexes of sleep (19).
Cross-sectional studies have shown that dietary protein intake was associated with sleep duration, quality, and patterns. Habitual protein intake was positively correlated with sleep duration (except for adults who slept >9 h) (20), and normal sleepers consumed more protein than did people with insomnia (21). Also, higher protein intake was associated with an earlier midpoint of sleep (22), indicating that protein intake may influence the timing of sleep. More generally, middle-aged adults who consumed a moderate protein intake showed a lower OR for poor sleep-wake regularity than that of adults with low protein intake (23). In contrast, other research has shown that protein intake was negatively associated with sleep duration (24). Collectively, these epidemiologic studies provide support that dietary protein intake may be an important moderator of indexes of sleep, but evidence of how that moderation works has been inconsistent or not understood.
Intervention studies that have investigated dietary protein effects on sleep have been mostly relatively short-term (4–21-d) studies. In 1978, Lacey et al. (25) reported that, after a 7-d dietary intervention, consumption of higher (>100 g/d) and lower (<15 g/d) protein diets increased restlessness in sleep more than a normal protein diet did, whereas slow-wave sleep was not different in the interventions. More recently, a 4-d intervention study showed that the ingestion of a very high–protein diet (56% of energy from protein) reduced wake episodes more than did a normal-protein diet (15% of energy from protein) (26). A 21-d study showed that no difference in sleep outcomes existed in 3 protein-intake trials (2.4 compared with 1.6 compared with 0.8 g · kg body weight−1 · d−1) during weight loss (a 40% energy deficit) in normal and overweight healthy military individuals (27). Collectively, these short-term studies that included extreme protein intakes and energy deficits have provided inconsistent findings on whether protein intake affects indexes of sleep.
The purpose of the current research was to assess the effect of sustained protein intake on indexes of sleep during weight loss in middle-aged overweight and obese adults in 2 randomized, controlled intervention studies. Study 1 was designed to assess the effects of the protein source and quantity on appetite responses, and study 2 aimed to investigate the effects of higher protein intake on body composition and metabolic health. Indexes of sleep were measured as part of these studies as secondary outcomes. We hypothesized that indexes of sleep would improve with higher protein intake.
METHODS
Studies 1 and 2: ethics and protocol registration
Both study protocols received approval from the Institutional Review Board at Purdue University. The studies complied with the Declaration of Helsinki as revised in 2013. Each participant signed an informed consent document and received a monetary stipend for participation. Studies 1 and 2 were registered at clinicaltrials.gov as NCT01005563 and NCT01692860, respectively.
Study 1: participants
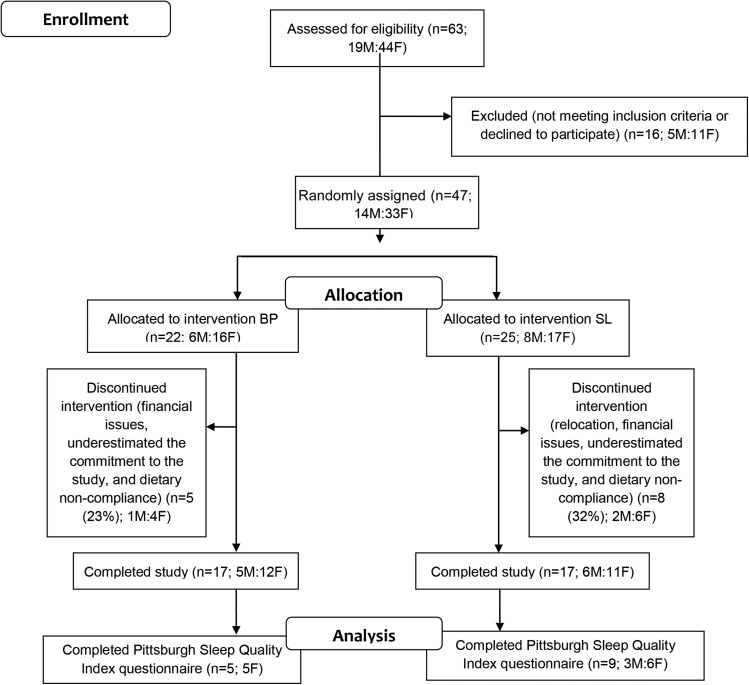
Thirty-four healthy, overweight and obese adults from the greater Lafayette, Indiana, region completed the intervention study (Figure 1). Inclusion criteria were as follows: ≥21 y of age; BMI (in kg/m2) range from 27.0 to 37.9; nonsmoking; weight stable (±3 kg) and habitual or stable activity patterns 3 mo before the study; and the absence of acute or chronic diseases or use of medications known to influence protein or energy metabolism. A subset of 14 participants [n = 5 in the beef and pork (BP)6 group, and n = 9 in the soy and legume (SL) group] were assessed for indexes of sleep with the use of the Pittsburgh Sleep Quality Index (PSQI) (28).
FIGURE 1.
Consolidated Standards of Reporting Trials flow diagrams for study 1. BP, beef and pork; SL, soy and legume.
To document responses to the designed protein intakes, postprandial plasma amino acid profiles were assessed in a separate subset of 10 participants (n = 5 for BP, and n = 5 for SL; of these 10 participants, 7 subjects also completed the PSQI (n = 4 for BP, and n = 3 for SL).
Study 1: experimental design
The randomized crossover study included an initial 2-wk baseline period and 3 consecutive 4-wk periods of energy restriction (ER) (750-kcal/d deficits from each individual’s energy need). Randomizer.org [Research Randomizer (Version 4.0)] was used to create a balanced block design. First, participants were randomly assigned to BP and SL groups (separate for men and women). Then, for each group, participants were randomly assigned to the 6 combinations of 10%, 20%, and 30% protein intakes. During baseline and day 28 of each intervention period, body composition measurements and the PSQI were completed. In addition, postprandial plasma concentrations of amino acids were measured on day 28 of each intervention period.
Study 1: diet
Each participant’s total energy requirement was estimated with the use of the sex-specific equations for overweight and obese adults published by the US Institute of Medicine (29). The 10%, 20%, and 30% of energy intakes from dietary protein were chosen because they approximately spanned the Acceptable Macronutrient Distribution Range for protein (10–35% of energy intake). Participants consumed their habitual diets during the 2-wk baseline period. Macronutrient compositions of intervention diets were 25% fat; 10%, 20%, or 30% protein; and 65%, 55%, or 45% carbohydrate; respectively. The protein sources were as follows: 30% SL (SL group) or 30% BP (BP group); 20% dairy; 5% egg; 20% grains, breads, and flours; and 25% other (i.e., vegetables, fruit, nuts, and beverages). Participants were given shopping lists to self-purchase most of the foods and beverages with the use of a 3-d rotating menu with BP and SL foods provided. Each participant met with the registered dietitians weekly for dietary counseling and was given instructions on the amount (e.g., cups, teaspoons, tablespoons, and grams) of each food and beverage to consume. A sample menu is shown in Supplemental Table 1A. Fasting blood (serum) urea nitrogen (BUN), which was measured at baseline and on day 28 of each 4-wk period, was used as a crude index of dietary protein intakes during the study (30, 31) and as a measure of dietary compliance.
Study 2: participants
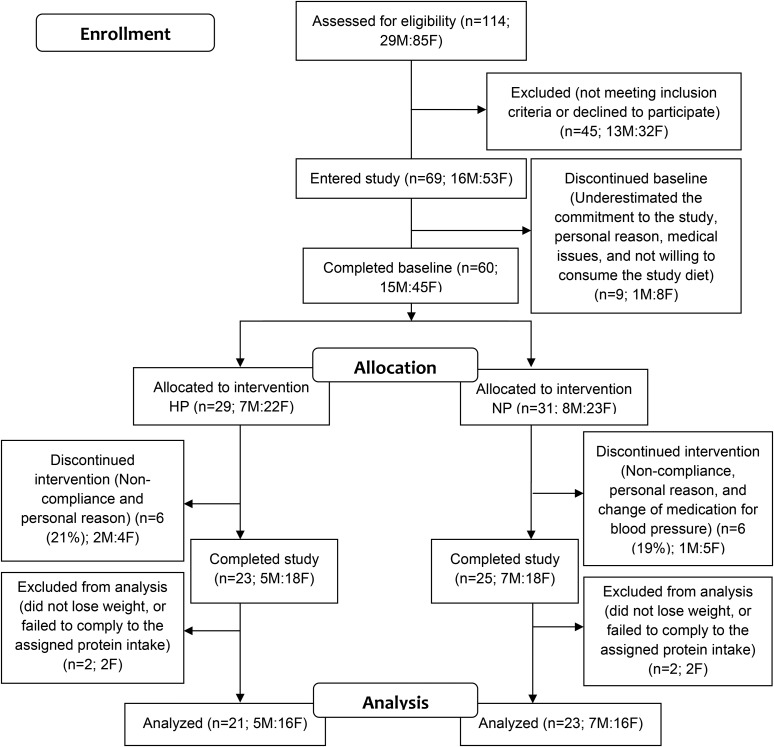
We recruited 69 overweight or obese adults (BMI range: 25–38) from the greater Lafayette, Indiana, region, of whom 48 adults completed the study, and 44 adults were included in the data analyses (Figure 2). Inclusion criteria were as follows: either male or female; age: 35–65 y; weight stable (±3 kg) 3 mo before the study; no acute illness; not diabetic, pregnant, or lactating; not currently (or ≤3 mo before the study) following an exercise or weight-loss program; nonsmoking; lactose tolerant; natural waist circumference ≥102 cm for men and ≥88 cm for women; fasting serum glucose concentration <110 mg/dL; systolic and diastolic blood pressures <140/90 mm Hg; serum total cholesterol concentration <260 mg/dL; LDL-cholesterol concentration <160 mg/dL; triacylglycerol concentration <400 mg/dL; and clinically normal serum albumin and prealbumin concentrations.
FIGURE 2.
Consolidated Standards of Reporting Trials flow diagrams for study 2. HP, high protein; NP, normal protein.
Study 2: experimental design
Study 2 was a 20-wk randomized, parallel, placebo-controlled, double-blind study (with a 1-wk prestudy measurement, a 3-wk baseline, and a 16-wk ER intervention). The week before baseline, participant habitual diet intakes and general health status were accessed via 3-d dietary recalls and blood samples, respectively. During a 3-wk baseline period, subjects consumed an energy-balanced diet with 0.8 g protein · kg baseline body mass−1 · d−1. Then, subjects were randomly assigned with the use of the first generator (6 participants/block with separate random assignment; 10 blocks for women and 4 blocks for men) on Randomization.com and assigned to either a normal-protein (NP; n = 23) or a high-protein (HP; n = 21) diet (0.8 compared with 1.5 g · kg−1 · d−1) with ER (a 750-kcal/d deficit) during a 16-wk intervention period.
Study 2: diet
Each participant’s total energy requirement was estimated with the use of the sex-specific equations for overweight and obese adults published by the US Institute of Medicine (29). During the 3-wk baseline period, all participants consumed a weight-maintenance diet with 0.8 g protein · kg−1 · d−1 and selected food and beverage items (breakfast: biscuit, gravy, and pancake; lunch: ham and cheese casserole, chowders, and pizza crust; and snack: muffins and smoothies) with 0.7 g · kg−1 · d−1 of the carbohydrate powder maltodextrin (Muscle Feast LLC) mixed in. During the 16-wk intervention period, participants were randomly assigned to either the NP group (total protein intake: 0.8 g protein · kg−1 · d−1) or the HP group (total protein intake: 1.5 g protein · kg−1 · d−1). The NP group continued to consume the selected food and beverage items that contained maltodextrin, whereas the HP group switched to the consumption of these selected food and beverage items that contained 0.7 g protein · kg−1 · d−1 with the use of milk protein isolate-85 (MPI-85; Idaho Milk Products Inc.). The energy deficit was achieved by removing 750 kcal of nonprotein foods and beverages/d from each participant’s menus. Participants were counseled with 7-d rotating menus and shopping lists to self-purchase most foods and beverages and provided with the selected foods and beverages that contained the carbohydrate or MPI-85 powders. Each participant met with a registered dietitian weekly for dietary counseling and was given instructions on the amount (e.g., cups, teaspoons, tablespoons, and grams) of each food and beverage to consume. A sample menu is shown in Supplemental Table 1B. Fasting BUN was measured every 4 wk to indicate the compliance with protein intake.
Studies 1 and 2: body composition
BMI was calculated from the fasting-state body mass measured to the nearest 0.01 kg with the use of a digital platform scale (model ES200L; Ohaus Corp.), and standing height without shoes was measured to the nearest 0.1 cm with the use of a wall-mounted stadiometer. The fasting-state body mass was measured monthly (study 1) or weekly (study 2) during the intervention to document the effectiveness of and compliance with the energy-restricted diets.
Studies 1 and 2: PSQI
The PSQI questionnaire is a clinical sleep-behavior questionnaire that has been validated for use in patients with insomnia, cancer, Parkinson disease, and the general population (28). The questionnaire is designed to assess indexes of sleep during the preceding 1-mo interval and contains 19 questions that use Likert scales from 0 to 3. All questions are categorized into the following 7 subvariables: duration of sleep, sleep disturbance, sleep latency, day dysfunction because of sleepiness, sleep efficiency, subjective sleep quality, and use of a sleeping medication. Each of these 7 variables is scored between 0 and 3 arbitrary units (au), which generates a summed total score of 0–21 au. This total score is termed the global sleep score (GSS) with >5 au associated with a poor sleep condition and ≤5 au associated with a good sleep condition. The PSQI also has a question that indicates the routine sleep duration in hours (numerical).
In study 1, the PSQI questionnaire was completed by 14 participants at baseline and at the end of each 4-wk intervention period. In study 2, the PSQI questionnaire was completed by 48 participants at baseline week 3 and at intervention weeks 4, 8, 12, and 16.
Study 1: plasma free amino acid analyses
On day 28 of each of the 3 intervention periods, participants reported to the clinical laboratory after fasting overnight. Participants consumed a test meal that provided 25% of their individualized daily energy intakes, which corresponded to their assigned diets during the period. Fasting and postmeal (25-, 60-, 120-, 180-, and 240-min) blood samples were collected into plasma separator tubes, centrifuged for 10 min at 4000 × g and 4°C, transferred into 1-mL aliquot tubes, and stored at −80°C until they were thawed for analysis. Plasma free amino acids for a subsample of participants (5 women in the BP group; 1 man and 4 women in the SL group) were quantified with the use of cation-exchange chromatography–HPLC coupled with postcolumn ninhydrin derivatization and quantitation (32) by the Agricultural Experimental Station Chemical Laboratories, University of Missouri-Columbia. We present data for all 10 of these participants instead of the 7 subjects who completed the PSQI questionnaire. The purpose was to document postprandial plasma amino acid responses to the dietary interventions but not to correlate the data to the sleep data. Results are expressed as the total AUC (with 0 μg/mL as the baseline) for 240 min after an acute meal with the use of the trapezoidal rule (33). Because the brain uptake of tryptophan (Trp) and tyrosine (Tyr) depend on the plasma availabilities of other large neutral amino acids (LNAAs) (i.e., Trp, Tyr, phenylalanine, leucine, valine, and isoleucine) (34, 35), we also calculated the Trp AUC, Tyr AUC, Trp AUC:LNAA AUC ratio (Trp:LNAAs), and the Tyr AUC:LNAAs AUC ratio (Tyr:LNAAs).
Studies 1 and 2: power calculations and statistical analyses
Although the indexes of sleep are secondary measurements for both studies 1 and 2, we conducted effect-size calculations for both studies before implementing the PSQI questionnaire.
Study 1
For the within-subject, crossover-designed study, an a priori power calculation was completed for 2 dependent means (correlation was set at 0.5) to detect a difference equal to 1 SD in protein-quantity interventions (α = 0.05; 80% power; 2 tailed). The effect size was one, and the total number of participants needed was 10. Baseline characteristics were assessed with the use of an independent Student’s t test between BP- and SL-protein–source groups. We first assessed the effects of time (repeated variable) and protein source on body mass, BUN, GSS, and subvariables in the PSQI. Second, the effects of the protein quantity (repeated variable) and source on postprandial plasma amino acid concentrations, the GSS, and subvariables in the PSQI were assessed. The baseline GSS (or subvariables) and body mass change from the previous period were used as covariates for analyses of the GSS (or subvariables). Post hoc analyses between protein-quantity interventions were conducted with the use of Bonferroni correction.
Study 2
For the between-subject parallel designed study, an a priori power calculation was completed for 2 independent means to detect a difference equal to 1 SD in protein-quantity interventions (α = 0.05; 80% power; 2 tailed). The effect size was one, and the number of participants needed was 34 (n = 17/group). Baseline characteristics were assessed with the use of an independent Student’s t test between HP- and NP-diet groups. The effects of diet and time on the dependent variables (body mass, BUN, the GSS, and 7 subvariables in the PSQI) were determined via a 2-factor ANOVA with repeated measures of time. Age, sex, and baseline values were used as covariates in all analyses. Residual plots were used to check model assumptions. Post hoc analyses were conducted with the use of Bonferroni correction between the 2 groups and from baseline to the end of intervention for each group.
All data analyses were completed with the use of the Proc Mixed procedure with SAS 9.3 for Windows software (SAS Institute Inc.). Results are presented as least-square means ± SEs unless otherwise noted. Least-square means ± SEs are the model-adjusted means (adjusted on the basis of the covariates and an unbalanced study design) and SEMs from SAS outputs that reflect the statistical results. Significance was determined at P < 0.05.
RESULTS
Participant characteristics at baseline
Study 1
Of 14 participants, the mean ± SE age was 56 ± 3 y, and BMI was 30.9 ± 0.6. There were no differences in these variables between BP and SL groups (data not shown). Baseline indexes of sleep that were measured with the use of the PSQI are shown in Supplemental Table 2.
Study 2
Among the 44 participants who completed the study and were included for data analyses, mean ± SE age was 52 ± 1 y and BMI was 31.4 ± 0.5. No differences were observed in age, BMI, habitual energy and macronutrient intakes, and indexes of sleep between the HP and NP groups (Supplemental Table 2, Supplemental Table 3).
Dietary compliance
Participants in both studies were apparently compliant with the designated diets on the basis of the serum BUN for total protein intake and reduced body mass for ER.
Study 1
According to the prescribed energy-restricted diet (mean ± SE: 1614 ± 74 kcal/d), 10%, 20%, and 30% of energy intakes from protein would provide ∼40, 81, and 121 g protein/d, respectively. With the use of calculations of protein intakes that were determined on a baseline body mass basis (mean ± SE: 85.3 ± 2.8 kg), intakes were ∼0.47, 0.95, and 1.42 g · kg−1 · d−1, respectively.
Fasting serum BUN was not different between BP and SL groups at baseline or at the end of the 10%, 20%, or 30% protein feeding periods (Supplemental Figure 1A). At the end of the three 4-wk feeding periods, BUN was progressively higher with higher protein intake. Body mass progressively decreased over time independent of the dietary protein source (Supplemental Figure 2A).
Study 2
Fasting serum BUN was not different between HP and NP groups at baseline but was higher in the HP group during the 16-wk period of ER (Supplemental Figure 1B). Both groups lost body mass over time with no difference in responses between HP and NP groups (Supplemental Figure 2B).
Postprandial plasma amino acids concentrations (study 1)
Overall, a higher dietary protein intake increased circulating Trp and Tyr concentrations (Supplemental Table 4). For Trp, consumption of a meal with 30% protein resulted in a higher composite postprandial response than when meals with 20% or 10% protein were consumed. However, there were no differences in postprandial Trp:LNAAs between protein-intake trials. For Tyr, higher protein consumption resulted in higher postprandial responses. The Tyr:LNAA ratio was not influenced by protein intake. The protein source did not influence these variables.
GSS
The consumption of a higher quantity of protein during ER resulted in lower (better) GSSs in both studies.
Study 1
At baseline, 8 participants (57%) were classified as poor sleepers (GSS >5 au), but 7 (50%), 7 (50%), and 2 (14%) subjects were classified as poor sleepers at the end of the 10%, 20%, and 30% protein–intake periods, respectively.
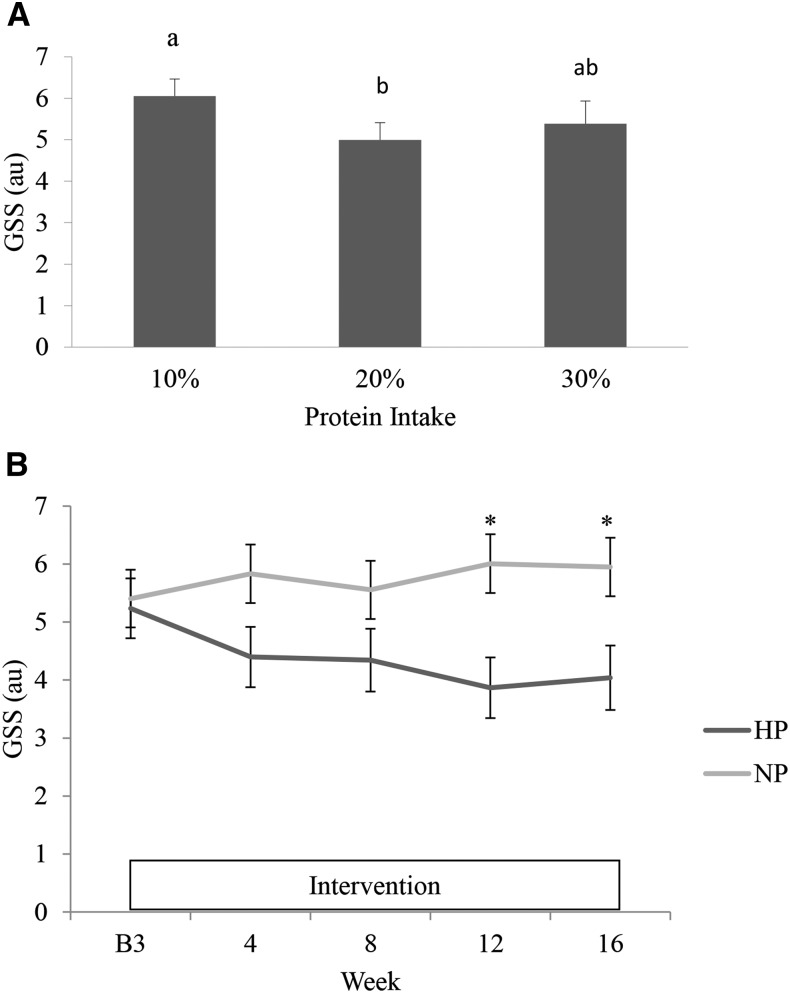
There was no difference in GSSs between BP and SL groups (data not shown). In all participants, the GSS was higher when 10% protein was consumed than when 20% protein was consumed (Figure 3A). The consumption of 30% protein did not further improve the GSS.
FIGURE 3.
Least-square mean ± SE GSSs at the end of each protein-intake–periods in study 1 (A) and during B3 and weeks 4, 8, 12, and 16 in study 2 for the HP group (dark gray) and the NP group (light gray) (B). Bonferroni method was used for post hoc analyses, differences between 10%, 20%, and 30% protein-intake periods were shown with letters (A) and between NP and HP were shown with asterisks (B). au, arbitrary unit; B3, baseline week 3; GSS, global sleep score; HP, high protein; NP, normal protein.
Study 2
At the end of baseline and intervention weeks 4, 8, 12, and 16, the numbers of poor sleepers in the HP group were 8 (38%), 6 (29%), 5 (24%), 5 (24%), and 4 (19%), whereas in the NP group, 7 (30%), 9 (39%), 12 (52%), 10 (43%), and 9 (39%) participants were poor sleepers.
At baseline, GSSs were comparable between HP and NP groups. However, during the period of weight loss, the GSS response was different between groups (Figure 3B) (P-group-by-time interaction = 0.035). Post hoc analyses indicated that GSSs were lower in the HP group than in the NP group at intervention weeks 12 (3.9 ± 0.5 compared with 6.0 ± 0.5 au, respectively) and 16 (4.0 ± 0.6 compared with 5.9 ± 0.5 au, respectively).
Other variables from the PSQI
Protein intake did not influence other variables except for the use of sleeping medication in study 2.
Study 1
Sleep duration was not influenced by the protein source or intake. The 7 subvariables of PSQI were not influenced by the protein source or intake (Supplemental Table 5A).
Study 2
Sleep duration was not influenced by ER or protein intake (Supplemental Table 3B). The use of sleeping medication changed over time differently between groups (Supplemental Table 3B) (P-group-by-time interaction = 0.033). Compared with at baseline, the use of sleeping medication increased in the NP group (P = 0.003) and did not change in the HP group. The other 6 subvariables of the PSQI were not influenced by protein intake (Supplemental Table 5B).
DISCUSSION
Because two-thirds of the US population are overweight or obese (36), an understanding of the effects of dietary energy and macronutrients on sleep quality or duration in groups of people with excess weight may prove clinically important. Indexes of sleep have been suggested to be associated with numerous metabolic dysfunctions including obesity, type 2 diabetes, hypertension, and cardiovascular diseases (1–6). A subjective sleep score by the PSQI was also suggested to be associated with the metabolic syndrome (37). Our studies provide evidence to strengthen the 2015 Dietary Guidelines Advisory Committee statements that “indices of sleep” may be a “mediator or moderator” (12) of dietary effects on metabolic health risks and emphasize the importance of including sleep-variable measurements in dietary studies that aim for weight loss or an improvement of metabolic health to elucidate proper mechanisms.
In both studies 1 and 2, as was consistent with our hypothesis, the GSS was lower (better) after the higher-protein diets were consumed than after the lower-protein diets were consumed during ER-induced weight loss. To our knowledge, the results from study 2, which is the longest-term (16-wk) randomized controlled trial to assess the effect of protein intake on sleep, concur with those from study 1 and provide novel information on the time course of the improvements in sleep after higher dietary protein was consumed. Strengths of the 2 studies included controlling and objectively documenting adherence to the prescribed diets both for energy intake (body mass changes) and total protein intake (differences in BUN concentrations).
Our results are inconsistent with those of Karl et al. (27) who reported that young normal and overweight military personnel did not have improved sleep quality after the consumption of 2.4 (high; n = 12) compared with 1.6 (moderate; n = 14) compared with 0.8 (low, n = 13) g protein · kg−1 · d−1 during a 21-d period of weight loss (30% ER and 10% physical activity). Our study 1 protein-specific intervention periods were conducted in about the same time frame (4 wk) as in this parallel-design study but used a within-subject crossover design that may have had more statistical power. Indeed, the changes in the sleep quality index (measured with the use of the Karolinska Sleep Diary, whereby a lower score represents better sleep quality) were 0.5 compared with 0.1 compared with −0.3 au in the groups with low-, moderate-, and high-protein intakes, respectively, which suggested that high protein intake may be beneficial. However, caution is warranted when our results are compared with those of Karl et al. (27) because of the weight-status and age differences of the participants in the 2 studies. Sleep problems are generally more likely to occur in people with higher BMI (38) and older age (39). Thus, we studied groups of people who may have been more likely to have had poor sleep quality (supported by a group mean GSS <5 au at baseline) and to have been positively affected by interventions [such as higher protein intake (23)] to improve sleep. In addition, except for ER, Karl et al. (27) designed a 10% energy deficit as physical activity. This designation may have masked the effect of protein intakes on sleep because acute and regular physical activity were suggested to improve both subjective sleep and objective sleep (40).
In both studies 1 and 2, the energy contents of the diets with different amounts of protein were equalized by manipulating carbohydrate intakes but keeping fat intakes the same. Thus, we could not exclude the possibility that lower carbohydrate intake in the trials with 20% protein (1.5 g · kg−1 · d−1) may have contributed to better GSSs than the trials with 10% protein (0.8 g · kg−1 · d−1). We described both studies with respect to protein intake because the magnitude of protein intake doubled from 10% to 20%, but carbohydrate only decreased about one-seventh from 65% to 55%. Results from an acute-feeding study showed that more carbohydrate consumption before bedtime may increase postprandial insulin secretion, which preferentially assists Trp rather than LNAAs being transported through the blood-brain barrier (41). This implies greater synthesis of serotonin and melatonin and, thus, better sleep (6, 19, 42, 43). However, short-term dietary studies have not provided consistent results. Afaghi et al. (44) reported that healthy adults had increased slow wave sleep and decreased rapid eye movement sleep when they consumed a very low carbohydrate diet (<1% of energy from carbohydrate) for 1 or 2 d than when a control diet (72% of energy from carbohydrate) was consumed (44). Whether a shift from rapid eye movement sleep to slow-wave sleep translates into better sleep or not is unclear. The common understanding is that slow-wave sleep is restorative and promotes anabolic processes of the body (45).
Although higher protein intake resulted in greater postprandial plasma amino acid responses, these results are not intended to infer on the mechanisms by which higher protein intake may improve sleep. The mechanism of the effect of protein on sleep after acute feeding may be related to Trp, Tyr, and the synthesis of brain neurotransmitters (serotonin, melatonin, and dopamine) (19, 46–50). Plasma Trp:LNAA and Tyr:LNAA ratios have been predictors of brain Trp and Tyr concentrations, respectively, in rat studies (51–53). It was previously shown that high-protein diets produce more LNAAs than Trp and Tyr. The higher LNAAs compete with Trp or Tyr for the blood-brain barrier transporter, thereby leading to reduced brain serotonin or dopamine synthesis (41). Therefore, it was proposed that the consumption of higher-protein meals may not promote sleep acutely. Our results showed otherwise; 30% compared with 20% compared with 10% protein intakes did not change the Trp:LNAA and Tyr:LNAAs ratios after an acute meal. In a 4-wk intervention study conducted in rhesus monkeys, the authors reported that a higher-protein diet induced higher 24-h plasma and cerebrospinal fluid Trp and serotonin metabolites (54). Thus, acclimated protein intake may have modified the body’s ability to produce or remove brain Trp and serotonin in a yet-to-be-determined way. To assess the long-term effects of protein intake on the plasma amino acids profile and synthesis of brain neurotransmitters, measurements during sleep are needed.
Another study-design factor to consider is the predominant protein source. In study 1, we did not observe differences in sleep quality and duration between BP and SL groups. In study 2, milk-protein concentrate was used to achieve higher protein intake. Milk protein contains mainly casein (80%) and whey (20%) proteins (55). The whey-derived α-lactalbumin was suggested to improve sleep quality or related outcomes in both animals and humans because of its high content of Trp (56–58). In both study 1 and 2, we observed consistent improvements in indexes of sleep, which inferred that the consumption of a higher protein quantity from a variety of protein sources improves sleep, but more research is warranted.
The results from the 2 studies presented have clinical relevance for sleep problems. First, the improvements in sleep with higher-protein diets occurred as a large percentage of participants shifted from being categorized by the PSQI questionnaire as poor sleepers to good sleepers. Second, as regards weight loss, the general consensus is that weight loss increases sleep duration and improves sleep quality (59–61). However, in study 2, sleep only improved in the HP group, whereas it was unchanged in the NP group after weight loss. These results suggest that the composition of a diet may affect weight-loss-induced improvements in sleep and that, for overweight and obese adults, the consumption of 0.8 g protein · kg−1 · d−1 is not enough to promote weight-loss–induced improvements in sleep. Finally, in study 2, the observation that the use of a sleeping medication increased over time in the NP group but was not changed in the HP group may have indicated that higher protein intake not only improved sleep but the means used to promote sleep. We make this statement cautiously because our studies did not include objective measurements of sleep quality (e.g., actigraphy or polysomnography) or sleep-related medication use.
There were limitations of these 2 randomized controlled trials. First, for study 1, sleep measurements were only made in a subgroup of 14 participants, which limited our ability to detect differences between protein sources. Second, indexes of sleep were secondary measurements in both studies. Therefore, we did not set inclusion and exclusion criteria that were specific for sleep. Third, self-reported measures such as the PSQI are subject to biases. Results from this study need to be confirmed with the use of objective measurements of sleep. In addition, the habitual dietary intakes reported for study 2 were also self-reported, which may not have been accurate (62). However, the habitual dietary intake data were presented for descriptive purposes only and did not affect the outcomes of interest, which were measured after the subjects had acclimated to the prescribed, controlled diets. Finally, as previously mentioned, we did not have plasma amino acid measurements during sleep, which prevented us from exploring relations to sleep variables.
In conclusion, the results from these 2 randomized controlled diet studies indicate the middle-aged and older overweight and moderately obese adults may improve sleep by maintaining higher protein intakes when dieting to lose weight.
Acknowledgments
We thank Janet Green, who coordinated the logistics of the 2 studies.
The authors’ responsibilities were as follows—JZ, JEK, and CLHA: conducted the research; JZ, JEK, NC, and WWC: analyzed the data; JZ and WWC: designed the research, wrote the manuscript, and had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. WWC was a member of the National Dairy Council Whey Protein Advisory Panel while the research was being conducted. The other authors reported no conflicts of interest related to the study.
Footnotes
Abbreviations used: au, arbitrary unit; BP, beef and pork; BUN, blood (serum) urea nitrogen; ER, energy restriction; GSS, global sleep score; HP, high protein; LNAA, large neutral amino acid; NP, normal protein; PSQI, Pittsburgh Sleep Quality Index; SL, soy and legume.
REFERENCES
- 1.Wu Y, Zhai L, Zhang DF. Sleep duration and obesity among adults: a meta-analysis of prospective studies. Sleep Med 2014;15:1456–62. [DOI] [PubMed] [Google Scholar]
- 2.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2010;33:414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 2011;32:1484–92. [DOI] [PubMed] [Google Scholar]
- 4.Dean DA, Wang R, Jacobs DR, Duprez D, Punjabi NM, Zee PC, Shea S, Watson K, Redline S. A systematic assessment of the association of polysomnographic indices with blood pressure: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015;38:587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broussard J, Brady MJ. The impact of sleep disturbances on adipocyte function and lipid metabolism. Best Pract Res Clin Endocrinol Metab 2010;24:763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaput JP. Sleep patterns, diet quality and energy balance. Physiol Behav 2014;134:86–1. [DOI] [PubMed] [Google Scholar]
- 7.Brunner EJ, Wunsch H, Marmot MG. What is an optimal diet? Relationship of macronutrient intake to obesity, glucose tolerance, lipoprotein cholesterol levels and the metabolic syndrome in the Whitehall II study. Int J Obes Relat Metab Disord 2001;25:45–53. [DOI] [PubMed] [Google Scholar]
- 8.Liese AD, Schulz M, Moore CG, Mayer-Davis EJ. Dietary patterns, insulin sensitivity and adiposity in the multi-ethnic Insulin Resistance Atherosclerosis Study population. Br J Nutr 2004;92:973–84. [DOI] [PubMed] [Google Scholar]
- 9.Cecchini M, Sassi F, Lauer JA, Lee YY, Guajardo-Barron V, Chisholm D. Tackling of unhealthy diets, physical inactivity, and obesity: health effects and cost-effectiveness. Lancet 2010;376:1775–84. [DOI] [PubMed] [Google Scholar]
- 10.Nettleton JA, Polak JF, Tracy R, Burke GL, Jacobs DR Jr. Dietary patterns and incident cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr 2009;90:647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwingshackl L, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 2015,115:780-800.e5. [DOI] [PubMed] [Google Scholar]
- 12.US Department of Agriculture and US Department of Health and Human Services. Scientific report of the 2015 Dietary Guidelines Advisory Committee. Part D. Chapter 3: Individual diet and physical activity behavior change. [Internet]. [cited 2015 Jul 15]. Available from: http://www.health.gov/dietaryguidelines/2015-scientific-report/.
- 13.Penev PD. Update on energy homeostasis and insufficient sleep. J Clin Endocrinol Metab 2012;97:1792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hursel R, Rutters F, Gonnissen HK, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation in healthy men on energy expenditure, substrate oxidation, physical activity, and exhaustion measured over 48 h in a respiratory chamber. Am J Clin Nutr 2011;94:804–8. [DOI] [PubMed] [Google Scholar]
- 15.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci USA 2013;110:5695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr 2010;91:1550–9. [DOI] [PubMed] [Google Scholar]
- 17.Spaeth AM, Dinges DF, Goel N. Effects of Experimental Sleep Restriction on Weight Gain, Caloric Intake, and Meal Timing in Healthy Adults. Sleep 2013;36:981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr 2009;89:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peuhkuri K, Sihvola N, Korpela R. Diet promotes sleep duration and quality. Nutr Res 2012;32:309–19. [DOI] [PubMed] [Google Scholar]
- 20.Grandner MA, Jackson N, Gerstner JR, Knutson KL. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite 2013;64:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zadeh SS, Begum K. Comparison of nutrient intake by sleep status in selected adults in Mysore, India. Nutr Res Pract 2011;5:230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato-Mito N, Sasaki S, Murakami K, Okubo H, Takahashi Y, Shibata S, Yamada K, Sato K. The midpoint of sleep is associated with dietary intake and dietary behavior among young Japanese women. Sleep Med 2011;12:289–94. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi M, Uemura H, Katsuura-Kamano S, Nakamoto M, Hiyoshi M, Takami H, Sawachika F, Juta T, Arisawa K. Relationship of dietary factors and habits with sleep-wake regularity. Asia Pac J Clin Nutr 2013;22:457–65. [DOI] [PubMed] [Google Scholar]
- 24.Santana AA, Pimentel GD, Romualdo M, Oyama LM, Santos RV, Pinho RA, de Souza CT, Rodrigues B, Caperuto EC, Lira FS. Sleep duration in elderly obese patients correlated negatively with intake fatty. Lipids Health Dis 2012;11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacey JH, Hawkins C, Crisp AH. Effects of dietary protein on sleep E.E.G. in normal subjects. Adv Biosci 1978;21:245–7. [PubMed] [Google Scholar]
- 26.Lindseth G, Lindseth P, Thompson M. Nutritional effects on sleep. West J Nurs Res 2013;35:497–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karl JP, Thompson LA, Niro PJ, Margolis LM, McClung JP, Cao JJ, Whigham LD, Combs GF Jr, Young AJ, Lieberman HR, et al. Transient decrements in mood during energy deficit are independent of dietary protein-to-carbohydrate ratio. Physiol Behav 2015;139:524–31. [DOI] [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 29.Institute of Medicine. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington (DC): National Academies Press; 2005. [Google Scholar]
- 30.Morse MH, Haub MD, Evans WJ, Campbell WW. Protein requirement of elderly women: nitrogen balance responses to three levels of protein intake. J Gerontol A Biol Sci Med Sci 2001;56:M724–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring) 2007;15:421–9. [DOI] [PubMed] [Google Scholar]
- 32.Association of Official Agricultural Chemists INTERNATIONAL. Official methods of analysis amino acids analysis complete amino acid profile (AAP) - item 72. Association of Analytical Communities, Gaithersburg (MD): 17th edition, 2006. [Google Scholar]
- 33.Fluckey JD, Hickey MS, Brambrink JK, Hart KK, Alexander K, Craig BW. Effects of resistance exercise on glucose tolerance in normal and glucose-intolerant subjects. J Appl Physiol (1985) 1994;77:1087–92. [DOI] [PubMed] [Google Scholar]
- 34.Fernstrom JD, Wurtman RJ. Brain serotonin content: increase following ingestion of carbohydrate diet. Science 1971;174:1023–5. [DOI] [PubMed] [Google Scholar]
- 35.Fernstrom JD. Large neutral amino acids: dietary effects on brain neurochemistry and function. Amino Acids 2013;45:419–30. [DOI] [PubMed] [Google Scholar]
- 36.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jennings JR, Muldoon MF, Hall M, Buysse DJ, Manuck SB. Self-reported sleep quality is associated with the metabolic syndrome. Sleep 2007;30:219–23. [DOI] [PubMed] [Google Scholar]
- 38.Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, Walsleben JA, Finn L, Enright P, Samet JM. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002;162:893–900. [DOI] [PubMed] [Google Scholar]
- 39.Unruh ML, Redline S, An MW, Buysse DJ, Nieto FJ, Yeh JL, Newman AB. Subjective and objective sleep quality and aging in the sleep heart health study. J Am Geriatr Soc 2008;56:1218–27. [DOI] [PubMed] [Google Scholar]
- 40.Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW. The effects of physical activity on sleep: a meta-analytic review. J Behav Med 2015;38:427–49. [DOI] [PubMed] [Google Scholar]
- 41.Wurtman RJ, Wurtman JJ, Regan MM, McDermott JM, Tsay RH, Breu JJ. Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios. Am J Clin Nutr 2003;77:128–32. [DOI] [PubMed] [Google Scholar]
- 42.Halson SL. Sleep in elite athletes and nutritional interventions to enhance sleep. Sports Med 2014;44(Suppl 1):S13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bravo R, Matito S, Cubero J, Paredes SD, Franco L, Rivero M, Rodriguez AB, Barriga C. Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly humans. Age (Dordr) 2013;35:1277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Afaghi A, O’Connor H, Chow CM. Acute effects of the very low carbohydrate diet on sleep indices. Nutr Neurosci 2008;11:146–54. [DOI] [PubMed] [Google Scholar]
- 45.Adam K. Sleep as a restorative process and a theory to explain why. Prog Brain Res 1980;53:289–305. [DOI] [PubMed] [Google Scholar]
- 46.España RA, Scammell TE. Sleep neurobiology from a clinical perspective. Sleep 2011;34:845–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernstrom JD, Wurtman RJ. Brain serotonin content: physiological regulation by plasma neutral amino acids. 1971. Obes Res 1997;5:377–80. [DOI] [PubMed] [Google Scholar]
- 48.Wurtman RJ, Larin F, Mostafapour S, Fernstrom JD. Brain catechol synthesis: control by brain tyrosine concentration. Science 1974;185:183–4. [DOI] [PubMed] [Google Scholar]
- 49.Rye DB, Jankovic J. Emerging views of dopamine in modulating sleep/wake state from an unlikely source: PD. Neurology 2002;58:341–6. [DOI] [PubMed] [Google Scholar]
- 50.Chiu N-T, Lee B-F, Yeh TL, Chen PS, Lee IH, Chen KC, Yang YK. Relationship between striatal dopamine transporter availability and sleep quality in healthy adults. Mol Imaging Biol 2011;13:1267–71. [DOI] [PubMed] [Google Scholar]
- 51.Gustafson JM, Dodds SJ, Burgus RC, Mercer LP. Prediction of brain and serum free amino acid profiles in rats fed graded levels of protein. J Nutr 1986;116:1667–81. [DOI] [PubMed] [Google Scholar]
- 52.Peters JC, Harper AE. Adaptation of rats to diets containing different levels of protein: effects on food intake, plasma and brain amino acid concentrations and brain neurotransmitter metabolism. J Nutr 1985;115:382–98. [DOI] [PubMed] [Google Scholar]
- 53.Fernstrom JD, Hirsch NJ. Rapid repletion of brain serotonin in malnourished corn-fed rats following L-tryptophan injection. Life Sci 1975;17:455–63. [DOI] [PubMed] [Google Scholar]
- 54.Grimes MA, Cameron JL, Fernstrom JD. Cerebrospinal fluid concentrations of tryptophan and 5-hydroxyindoleacetic acid in Macaca mulatta: diurnal variations and response to chronic changes in dietary protein intake. Neurochem Res 2000;25:413–22. [DOI] [PubMed] [Google Scholar]
- 55.Bendtsen LQ, Lorenzen JK, Bendsen NT, Rasmussen C, Astrup A. Effect of dairy proteins on appetite, energy expenditure, body weight, and composition: a review of the evidence from controlled clinical trials. Adv Nutr 2013;4:418–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Minet-Ringuet J, Le Ruyet PM, Tomé D, Even PC. A tryptophan-rich protein diet efficiently restores sleep after food deprivation in the rat. Behav Brain Res 2004;152:335–40. [DOI] [PubMed] [Google Scholar]
- 57.Markus CR, Jonkman LM, Lammers JH, Deutz NE, Messer MH, Rigtering N. Evening intake of alpha-lactalbumin increases plasma tryptophan availability and improves morning alertness and brain measures of attention. Am J Clin Nutr 2005;81:1026–33. [DOI] [PubMed] [Google Scholar]
- 58.Markus CR, Olivier B, Panhuysen GE, Van Der Gugten J, Alles MS, Tuiten A, Westenberg HG, Fekkes D, Koppeschaar HF, de Haan EE. The bovine protein alpha-lactalbumin increases the plasma ratio of tryptophan to the other large neutral amino acids, and in vulnerable subjects raises brain serotonin activity, reduces cortisol concentration, and improves mood under stress. Am J Clin Nutr 2000;71:1536–44. [DOI] [PubMed] [Google Scholar]
- 59.Chaput JP, Drapeau V, Hetherington M, Lemieux S, Provencher V, Tremblay A. Psychobiological impact of a progressive weight loss program in obese men. Physiol Behav 2005;86:224–32. [DOI] [PubMed] [Google Scholar]
- 60.Verhoef SP, Camps SG, Gonnissen HK, Westerterp KR, Westerterp-Plantenga MS. Concomitant changes in sleep duration and body weight and body composition during weight loss and 3-mo weight maintenance. Am J Clin Nutr 2013;98:25–31. [DOI] [PubMed] [Google Scholar]
- 61.Alfaris N, Wadden TA, Sarwer DB, Diwald L, Volger S, Hong P, Baxely A, Minnick AM, Vetter ML, Berkowitz RI, et al. Effects of a 2-year behavioral weight loss intervention on sleep and mood in obese individuals treated in primary care practice. Obesity (Silver Spring) 2015;23:558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Archer E, Pavela G, Lavie CJ. The Inadmissibility of What We Eat in America and NHANES Dietary Data in Nutrition and Obesity Research and the Scientific Formulation of National Dietary Guidelines. Mayo Clin Proc 2015;90:911–26. [DOI] [PMC free article] [PubMed] [Google Scholar]