Abstract
Emerging technologies in computer and telecommunication industry has eased the access to computer through telephone. An Interactive Voice/Web Response System (IxRS) is one of the user friendly systems for end users, with complex and tailored programs at its backend. The backend programs are specially tailored for easy understanding of users. Clinical research industry has experienced revolution in methodologies of data capture with time. Different systems have evolved toward emerging modern technologies and tools in couple of decades from past, for example, Electronic Data Capture, IxRS, electronic patient reported outcomes, etc.
Key words: Clinical data management, Interactive Voice Response System, Interactive Web Response System
INTRODUCTION
IxRS is an Interactive Voice/Web Response System, which can be accessed via telephone or web. Users respond/provide their responses via touch-tone key pad of telephone. IxRS is an extremely configurable and customizable system. This empowers sponsors to proactively manage the key aspects of their clinical trials which includes enrolment/randomization, dosing/drug dispensation, clinical supplies, drug inventory management, unblinding, etc.[1]
IxRS is more user friendly and easy to access from anywhere in the world through telephones or web. The system has a feature of automated callouts or audio instructions with 24 × 7 coverage where subject gets prompt about medication that needs to be taken or navigating through different modules that makes the innovation user friendly. This decreases subject's dependability on site staff to a greater extent. This is a shift in change for better site-centric to patient-centric trials. Patient-centric trials are ones in which there is an extreme involvement of subject/patient in a trial that is run and has minimum dependability on site staff. This is one of the examples as part of disruptive innovation that is happening in clinical trial arena. In addition, it has eliminated the risk of biasness at sites by automated randomization, dispensation and unblinding.
Most of the industries such as Banking, Finance, Healthcare, Telecommunication, Teleshopping, and Retail business have opted for IxRS to cater their customers to various solutions which can be accessed from anywhere and most popular example is customer care service.
EVOLUTION OF INTERACTIVE VOICE/WEB RESPONSE SYSTEM IN CLINICAL RESEARCH
Clinical research and pharmaceutical industry has been exponentially grown during the first decade of 21st century in terms of drug discoveries, their development, various medical devices, and other technologies to serve community better in healthcare.[2] This rapid boom has put pharmaceutical industries under tremendous pressure to win the race of getting their drugs in market and getting them patented. This is a key milestone for the sponsors to start getting returns for their huge research investment. Pharma companies are profound from the drug discovery, Investigational New Drug Application, and New Drug Application till the different phases of clinical trial to accelerate the entire process and achieve the key milestones well in advance vis-a-vis the competitors.
In an event to accelerate the drugs’ journey to market and the regulatory guidelines becoming more stringent such as data submission in specific formats (Clinical Data Acquisition Standards Harmonization/Study Data Tabulation Model), adverse event reporting on stipulated timelines, notification of adverse events to specific regulatory bodies in expected timelines; there were instances observed which often led to incorrect randomization/drug dispensation, incorrect recordings of adverse events. These instances put trial subject's safety under risk, also affected the safety and efficacy analysis.
Over the period, there was an obvious rapidity seen in the growth of clinical trials along with technological innovations and integration of tools. This included increase in the number of molecules getting registered for new trials, several therapeutic area trials, medical device trials, bio-similar and bio-betters coming to market which led to increase in the complexity and the global recognition and advancement. In addition, stringent regulatory[3] requirements in terms of safety of patients, data collection/modification, drug supply, submissions to regulatory authorities, have in turn increased the scope of data collection, randomization, safety monitoring, data formatting, documentation, reporting, and analysis.
With the growing rate of clinical trials containing massive data and patient pool across the globe, the sponsors have been involuntarily forced to recalibrate their conventional way of conducting trials and to opt for new innovative and more normalized way of conducting clinical trials.[4] Telecommunication industry along with information technology came up with various innovative technologies to cater to the needs of different industries. An Interactive Voice Response System (IVRS) is one of the technologies developed and customized to enhance the quality of healthcare services. Tailored programs in computer (Information Technology) can be accessed through telephone (Telecommunication) by user around the clock. Computer-based applications such as randomizer and drug inventory management system used for kit generation are easily accessed through telephone with the use of IVRS. Moreover, as a backup, the web-based Interactive Web Response (IWR) system was developed. IVR was more commonly used in healthcare viz. IWR as it could be accessed from any corner of the world without internet and computer. Thus, this innovative eClinical solution, IxRS, from telecommunication industry was acquainted with healthcare and clinical research industry. Being a potential innovative technology, IxRS has proven its efficiency in clinical research. In the early first decade of 21st century, the use of IVRS was limited only with few government services such as railway in India. This was because of lack of technology and infrastructure within interior parts of the country. During the last 15 years, the various new technologies such as mobiles and internet reached beyond metro cities and into interior part of India such as small towns and developing cities. IVRS has been implemented by different industries such as telecom service providers, banking, and customer care services for product-based companies to capture the market.[5] Over the period of time, IxRS has evolved to great extent and helped in overcoming many challenges, some of the challenges that were addressed are:[1]
Adaptive trial design
In prospectively planned events of amendments in clinical trials, IxRS can be customized to implement the dynamic randomization and complex dosing algorithms efficiently. Automated randomization through IxRS eases process of assigning subjects to different treatment arms and proper dispensation of drug per treatment arm following the dosing algorithm.
Global trials
IxRS extends major contribution in the success of Global clinical trial in addition to adaptive trials challenges encountered by industry due to globalization of clinical trials such as remote recruitment, randomization, and drug dispensation.
Clinical Outcome Assessments and Patient-Reported Outcomes
Successful collection of Patient-Reported Outcomes (PRO) can be governed by IxRS. PRO or Clinical Outcome Assessments is modern technique of data collection from patients and physician against their response to diary questionnaires. IxRS along with handheld electronic patient reported outcomes device is used to capture data and store it in databases for analysis prior to regulatory submission.
Randomization/stratification
Randomization of subjects in various strata is based upon predefined criteria. Stratified randomization refers to situation in which strata are constructed based on the values of prognostic variables, and a randomization scheme is performed separately within each stratum.
Multiple dosing/weight based dosage
For pediatric studies, complex dosing algorithm is considered and here dosing is based upon different criteria such as weight of subject, age in months, and special consideration toward vaccination schedules. In pediatric age group, dosing is given based on body weight or body surface area. It is based on an understanding of drug pharmacokinetic (PK) that is directly proportional to body size, i.e. PK parameters increases in proportion with increasing body size. These complex dosing algorithms can be implemented in IxRS and used in studies for proper drug dispensation.[6]
Product inventory management
Product inventory management includes product supply/re-supply to site, electronic proof of receipt, product status/condition (damaged and on-hold), etc. Implementing IxRS for product inventory management overcomes challenges faced during shipment of drugs such as drugs not reaching respective sites on time, their condition on arrival at site (s), special consideration to injectable as they need to be shipped and stored at specific temperature throughout their transfer/transport till it can be administered to patient, and automated product resupply at respective sites (s).
As a general practice, IxRS is not implemented in all clinical research studies. The sponsor takes decision on the implementation of Electronic Data Capture (EDC) system, IxRS, and other eClinical systems in a particular study based upon several parameters. The sponsor identifies the implementation of IxRS in a study after considering some of the points outlined below.
Consulting with study team members such as clinical study manager, study biostatistician, clinical supply chain management representative
Complexity of randomization, including stratification
Complexity of product management
Integration with other systems
Number of geographical regions or clinical sites
Study budget
Timelines.
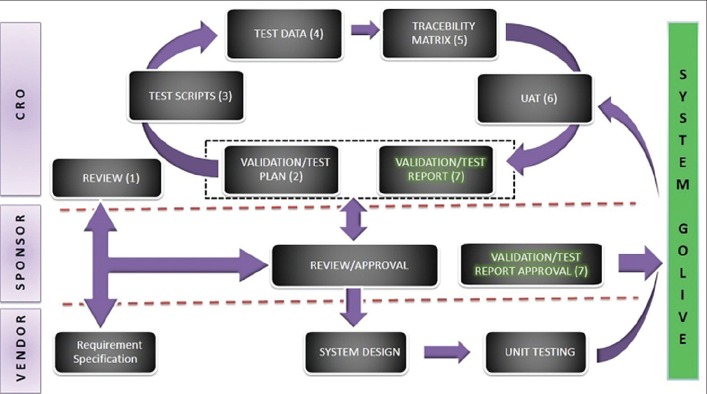
Once decision on deploying IxRS for a study is confirmed, sponsor/Clinical Research Organizations (CROs) approach preferred clinical service provider with clinical study protocol and case report form to get different modules such as enrolment, randomization, and drug dispensation of study that needs to be incorporated in IxRS. Service provider with the help of protocol, requirement specification, and inputs from study team builds different modules in an IxRS. These modules get thoroughly tested/validated by sponsor/CRO as per their predefined validation process and with required supporting documentation. Validation of IxRS is one of the important steps in IxRS development. IxRS once live will be accessed at multiple sites globally, so it becomes critically important to make sure that the IxRS is working as per the expected requirements listed in protocol and specification. Failure in meeting requirements may directly affect the safety of participating subjects. After successful completion of testing, the IxRS is declared live and patient recruitment can begin at different sites. Figure 1 shows the generic process flow followed across industry.
Figure 1.
Interactive Voice/Web Response System generic process
ADVANTAGES OF INTERACTIVE VOICE/WEB RESPONSE SYSTEM
Beyond its ease to access, operate, and round the clock availability, IxRS has many other advantages which recommends its deployment into complex clinical trials, clinical trials with large number of patients, with different treatment arms, and with complex dosing schedules. Few of them are listed below:[7]
Its scalability enables to recruit and manage large number of patients
Its robustness helps to have multiple studies ongoing at single time point
Real-time response from systems enables assigning unique numbers to patients, for example, enrollment/randomization and dispensation
With technological evolution, easy integration with other eClinical systems enabling key data points flowing from multiple systems into one.
Easy deployment irrespective of trial type, number of subjects, number of sites, therapeutic areas.
CLINICAL STUDY MODULES COVERED
Leading eClinical solution providers from biopharmaceuticals with innovative technologies and integrations have enhanced the implementation of IxRS. This in turn is assisting in improving the efficiency of data collection and management of this data when it comes to final analysis and submission. Different modules listed below can be implemented effectively through IxRS. Choice of selection of modules may vary from sponsor to sponsor.
Site management
This module can be used to activate or deactivate the sites, to set a predefined drug supply to sites. This module is accessed by the sponsor.
Cohort management
This module is used to open or close cohorts based upon the set conditions defined in protocol. In addition, the sponsor can update the cohort target based on the requirement.
Product management
Using this module, the sponsor can update expiration date of product that will be shipped to sites.
Enrolment/randomization
Randomization is the process of assigning trial subjects to treatment or control groups using an element of chance to determine the assignments to reduce bias.[8] IxRS with the help of randomizer tool deployed by service provider, to enroll/randomize the patients into different treatments arms as defined in protocol, generates the unique enrolment/randomization number for each patient. Based on randomization number, the patient receives appropriate treatment in the trial as per the randomization plan.
Dosing/drug dispensation
Dosing algorithms are built in IxRS to dispense investigational drug to patients as per the protocol defined dosing schedule and treatment arm. The patient receives a unique kit number or box number after drug dispensation call. Earlier Investigators had to assign kits manually to subjects while performing drug dispensation.
Electronic proof of receipt
Receipt of drug at sites can be confirmed through this module. In addition, the condition of drug received and its timeframe can be mentioned during this call.
Emergency unblinding
This module is used for unblinding of subjects in case of any serious adverse event either by subject number or by kit number assigned. This module can only be accessed by sponsor and statistician.
Product inventory adjustment
Drug supply management to sites can be governed through this module. Once quantity of drug goes beyond defined number at site, required quantity will be automatically shipped to respective site (s) to make sufficient availability of drugs at site.
INTERACTIVE VOICE/WEB RESPONSE SYSTEM VALIDATION
As a technology, IxRS is very much robust and user friendly for end users; however, at backend, it has very complex programming codes written for each and every module that is been built. These IxRS modules need to be thoroughly validated both at programming stage by service providers and from end user point of view by the sponsor or CRO. Validation of IxRS becomes a vital step as there is direct impact on patient randomization, drug dispensation, and unblinding, which are critical modules for any study from safety, regulatory, and business perspective. High quality validation is, therefore, critical before deploying IxRS.
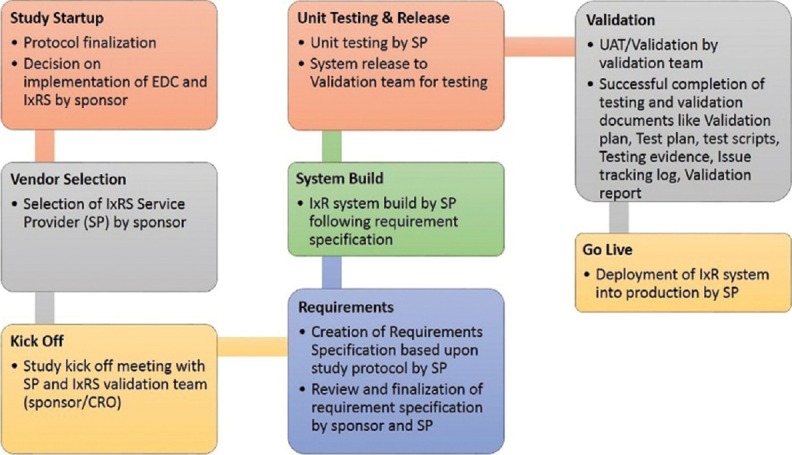
Figure 2 elaborates one of the processes followed for IxRS validation by sponsors. Food and Drug Administration (FDA) has outlined few regulations[9] and recommendations for computerized systems used in clinical research. Some of the regulations and recommendations have been listed below:
Figure 2.
Interactive Voice/Web Response System validation process
-
FDA Regulatory Requirements:
- 21 CFR part 11
- 21 CFR 812.140 (a)
- 21 CFR 812.140 (b)
- All 21 CFR part 812.
-
FDA guidance, May 2007:
- Training of personnel
- Internal/external security safeguards
- Source documentation and retention
- Other system features
- Standard operating procedures
- Instructions for user to perform direct entry of data
- Maintenance of audit trails
- Validation documentation
- Backups and rollback procedure.
INTERACTIVE VOICE/WEB RESPONSE SYSTEM, A CENTRAL HUB FOR ECLINICAL SYSTEMS
IxRS acts as central hub for other systems used in clinical research. IxRS can be integrated with various eClinical systems such as Clinical Trial Management System, EDC, and Drug Supply Management System to automate data transfer process for key variables/data points which are collected through IxRS such as screening ID, randomization number, and kit number.
Figure 3 illustrates integration process and variables/data points integrated across different systems from IxRS.[10]
Figure 3.
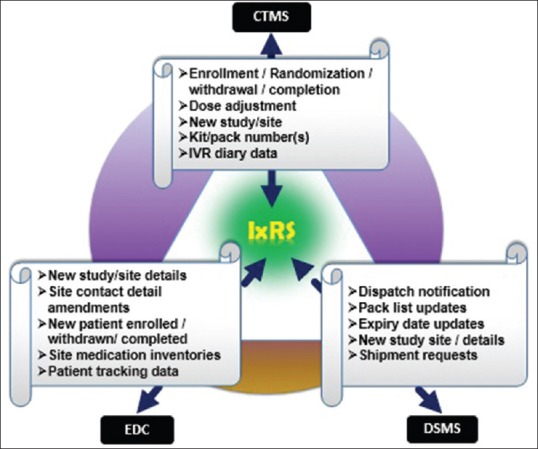
Interactive Voice/Web Response System integration with eClinical systems
CONCLUSION
Pharma companies need to opt for emerging and innovative technologies in clinical research to compete in global competition to utilize maximum benefits for patent filed before its expiry. Implementation of IxRS into clinical studies can accelerate trial by remote recruitment, appropriate randomization, and automated product management. With evolving technologies, IxRS can be easily implemented into studies and can be accessed remotely through telecommunicating devices such as phone, mobile, and IPad even from the areas where internet facilities are not available. Hence, IxRS can become indistinct choice for most flexible, user-friendly, and robust tool for the pharma companies.
IxRS has boomed pharma industry and clinical trials started implementing IxRS to large extent. IxRS service providers were dreaded by other technological innovations such as EDC systems which were likely to replace the IxRS. However, today we can see rapid growth in the use of IxRS in clinical trials.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
I am grateful and would like to acknowledge the expertise and guidance provided by Dr. Nimita Limaye, Mr. Deven Babre and Mr. Sharad Sharma that greatly supported this article.
REFERENCES
- 1.Bedford J. The Renaissance in IVR/IWR Systems. [Last accessed on 2015 Sep]. Available from: http://www.almacgroup.com/wp-content/uploads/IVRIWRSytems1.pdf .
- 2.Using Interactive Voice Response (IVR) System for Clinical Trials by IVRS Development. [Last accessed on 2015 Nov 03]. Available from: http://www.ivrsdevelopment.com/ivrs_clinical_trial_management.htm .
- 3.Regulatory Requirements Become More Complex as Clinical Trials Increase. [Last accessed on 2015 Nov 03]. Available from: http://www.centerwatch.com/news-online/article/2064/regulatory-requirements-become-morecomplex-as-clinical-trials-increase#sthash.hJlaRLHq.lhcYtLXt.dpbs .
- 4.Lee H, Friedman ME, Cukor P, Ahern D. Interactive Voice Response System (IVRS) in Health Care Services. [Last accessed on 2015 Nov 03]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14688763 . [DOI] [PubMed]
- 5.IVRS Technology in India is Evolving – Chat with Expert Uttam Pegu. [Last accessed on 2015 Nov 03]. Available from: http://www.exotel.in/blog/ivrstechnology-in-india-is-evolving-uttam-pegu/
- 6.Drug Dosing Based on Weight and Body Surface Area. [Last accessed on 2015 Nov 03]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22711238 .
- 7.IXRS by Almac Group. [Last accessed on 2015 Nov 03]. Available from: http://www.almacgroup.com/clinical-technologies/ixrs/
- 8.ICH Harmonised Tripartite Guideline, Guideline for Good Clinical Practice, E6(R1) [Last accessed on 2015 Nov 03]. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf .
- 9.Helfgott J. Computerized Systems Used in Medical Device Clinical Investigations. [Last accessed on 2015 Nov 03]. Available from: http://www.fda.gov/downloads/Training/CDRHLearn/UCM176477.pdf .
- 10.Byrom B. An Integrated Approach to Successful eClinical Trials. https://www.google.co.in/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&cad=rja&uact= 8&ved=0CCUQFjABahUKEwi00oTLi_bIAhVOwo4KHRzjABs&url=http%3A%2F%2Fwww.ccra.org.uk%2Fmeetings%2F20051101%2Fclinphone.ppt&usg=AFQjCNHQJp-lymUpmqWdCskfmvwYiYUADQ&bvm=bv.106379543,d.c2E .