Abstract
Inflammatory bowel disease (IBD), including Crohn's disease (CD) and ulcerative colitis (UC), is a chronic inflammatory disorder characterized by alternating phases of clinical relapse and remission. The etiology of IBD remains largely unknown, although a combination of patient's immune response, genetics, microbiome, and environment plays an important role in disturbing intestinal homeostasis, leading to development and perpetuation of the inflammatory cascade in IBD. As chronic intestinal inflammation is associated with the formation of reactive oxygen and reactive nitrogen species (ROS and RNS), oxidative and nitrosative stress has been proposed as one of the major contributing factor in the IBD development. Substantial evidence suggests that IBD is associated with an imbalance between increased ROS and decreased antioxidant activity, which may explain, at least in part, many of the clinical pathophysiological features of both CD and UC patients. Hereby, we review the presently known oxidant and antioxidant mechanisms involved in IBD-specific events, the animal models used to determine these specific features, and also the antioxidant therapies proposed in IBD patients.
Key Words: Animal models, antioxidants, Crohn's disease, lipid peroxidation, reactive oxygen species, ulcerative colitis
INTRODUCTION
Inflammatory bowel disease (IBD), including its two major forms, Crohn's disease (CD) and ulcerative colitis (UC), is a chronic inflammatory disorder characterized by alternative phases of clinical relapse and remission. CD can affect any part of the gastrointestinal tract, whereas UC involves only rectum and colon. Although the exact etiology of IBD remains uncertain, a combination of patient's immune response, genetics, microbiome, and environment plays an important role in the development and perpetuation of the inflammatory cascade in both CD and UC patients.[1]
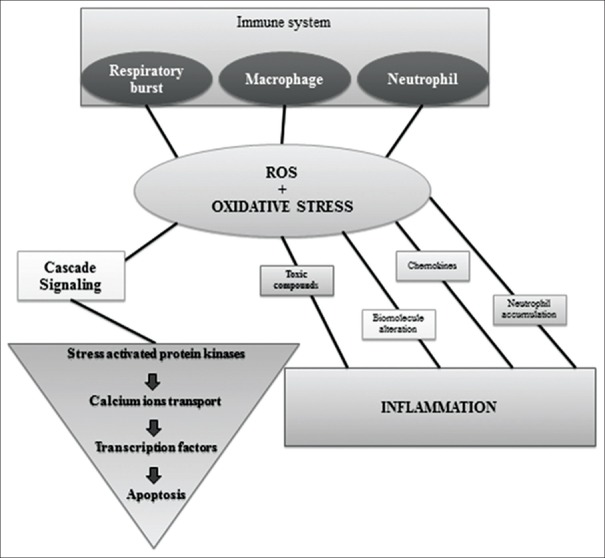
Oxidative stress, among the immune-regulatory factors, has been proposed as one of the major mechanism involved in the pathophysiology of IBD.[2] It has been established that chronic intestinal inflammation is associated with overproduction of both reactive oxygen and reactive nitrogen species (ROS and RNS)[3] leading to oxidative and nitrosative stress, respectively, which has been implicated in several human diseases, including IBD. Substantial evidence suggests that IBD is associated with an imbalance between ROS and antioxidant activity which creates oxidative stress as the result of either ROS overproduction or a decreased antioxidant activity[4] [Figure 1]. ROS include superoxide (O2), nitric oxide (NO), hydroxyl radical (−OH), hydroperoxyl radical (O2H), hydrogen peroxide (H2O2), and singlet oxygen (O2).[5,6] Furthermore, it has been shown that 1%–2% of the electrons traveling through the respiratory chain leak out and interact with free oxygen leading to superoxide and hydrogen peroxide,[7,8] which further generates the hydroxyl radicals when iron ions are found in excess in reaction medium.[9,10,11,12,13,14] Superoxide can also be produced through oxidases’ action in postischemic tissues,[15,16] soluble oxidases in phagocytic cells,[11] and can be the forerunner for other ROS. Once they are formed, this reactive species begins to interact with the molecular complexes inducing cellular oxidative damage. Under physiological conditions, their generation is controlled by the antioxidant system, which consists of enzymes such as superoxide dismutase (SOD), catalase, and glutathione peroxidases (GPXs) and their antioxidant substrates (such as glutathione and α-tocopherol).[17] Generated in abnormally high levels, ROS have destructive effects which can affect lipids, proteins, and nucleic acids by causing fragmentation products and cross-linked metabolism products[18,19] that can lead to lipid peroxides formation, enzymatic dysfunction, and DNA strand break products.[20] These destructive effects can be removed by antioxidant balance, which acts like free radical scavengers or cellular oxidation inhibitors,[17,21] which can be categorized in plasma and intracellular antioxidant mechanisms.[1,5,22]
Figure 1.
The origin of ROS and effects of ROS accumulation
It has also been shown that oxidative stress plays an important role in gastrointestinal diseases. Some studies have shown that inflammation is a key component of gastrointestinal diseases generally, starting with gastroesophageal reflux disease,[23,24,25] gastritis, Helicobacter pylori gastric infections,[26,27,28,29,30] and ending with IBD[31,32,33] and small bowel disease.[34,35,36,37,38] As many of gastrointestinal (GI) tract diseases cause or are caused by an exacerbated anti-inflammatory response, it is almost undoubtable that oxidative stress mechanisms are disturbed. To create a reactive potential for neutrophils, for example, an increased production of ROS might be necessary. Thus, several studies have shown high production of oxygen-derived free radicals, which led to esophageal epithelial cell injury and worsen esophageal mucosal damage in patients with gastroesophageal reflux disease.[23] An increased sensitivity to oxygen peroxide, increased lipid peroxidation, and decreased superoxide anion radical levels were also observed in patients with gastric reflux disease.[39,40,41] In H. pylori-induced gastritis, ROS are usually released from activated neutrophils. This is considered to be one of the main mechanisms of H. pylori-induced gastric mucosal injury, as H. pylori exhibits robust chemotactic activity for neutrophils.[27] Free radicals can be released in both IBD patients and animal models of colitis.[42] Activated neutrophils and macrophages are responsible for ROS or RNS generation, and the levels of ROS can be correlated with the severity of inflammatory changes in the colonic mucosa[31] Also, decreased blood and mucosal levels of plasma antioxidants (vitamins A, C, E, and β-carotene) were observed in patients having CD.[22,43,44,45,46,47,48,49,50,51] In addition, it has been shown that IL-1 and TNFα cytokines can be inhibited by antioxidants administered to IBD patients.[52] Also, vitamins such as C and E can inhibit oxidative stress in human intestinal smooth muscle cells of Crohn's bowels.[53]
The main cellular antioxidant enzymes are catalase, SOD, and GPX.[1,5] More than that, CD patients show decreased main cellular antioxidant enzymes (SOD and GPX) activities in intestinal mucosa.[54,55,56,57,58,59,60] Nitric oxide synthase (NOS) is thought to be the main producer of nitric oxide (NO) in UC, the induction of colonic NOS may be involved in the mucosal vasodilation and increased vascular permeability of active UC, and could also contribute to the impaired motility that accompanies toxic dilatation.[42] Small bowel injury induced by nonsteroidal anti-inflammatory drugs is accompanied by an apoptotic signal via oxidative stress mitochondrial pathway. Thus, it has been shown to significantly increase intracellular ROS production.[37,61]
According to Mayo Clinic,[62] IBD involves chronic inflammation of all or part of the digestive tract, but it primarily includes UC and CD. UC is an inflammatory bowel disease that causes long-lasting inflammation and sores in the inner most lining of colon and rectum. CD is an IBD that causes inflammation of digestive tract lining that spreads deep into affected tissues. Thus, IBD's characteristic inflammation can affect different areas of the digestive tract—the large intestine, small intestine, or both. Separately from classic IBDs are regarded collagenous colitis and lymphocytic colitis due to their specific and slightly special features.
MOLECULAR MECHANISMS INVOLVING OXIDATIVE STRESS IN IBD
There are several known indirect evidences that suggest that the chronically inflamed intestine may be subjected to considerable oxidative stress and thus susceptible to oxidative injury, especially conceding that an inflammatory process induces oxidative stress and reduces cellular antioxidant capacity.[5] Also, overproduced free radicals react with cell membrane fatty acids and proteins permanently impairing their function. In addition, free radicals can lead to mutation and DNA damage.[4] First, it is well known that phagocytes are activated by certain pro-inflammatory mediators such as LTB4 or platelet activating factor (PAF) to release large amounts of potentially cytotoxic reactive oxygen metabolites into the interstitial compartment.[63] Enhanced synthesis of LTB4 and PAF has been demonstrated in mucosal samples obtained from patients with active IBD.[64] Secondly, there are several reports that have demonstrated that phagocytic leukocytes obtained from patients with active IBD respond to various proinflammatory stimuli with enhanced reactive oxygen metabolism when compared to cells obtained from healthy volunteers.[65]
Also, high NOS activity has been found in experimental models of colitis and in the intestinal mucosa of patients with IBD.[66] High levels of the end products of NO metabolism, nitrates, and nitrites were found in the plasma, feces, and colonic lumen of IBD patients.[67]
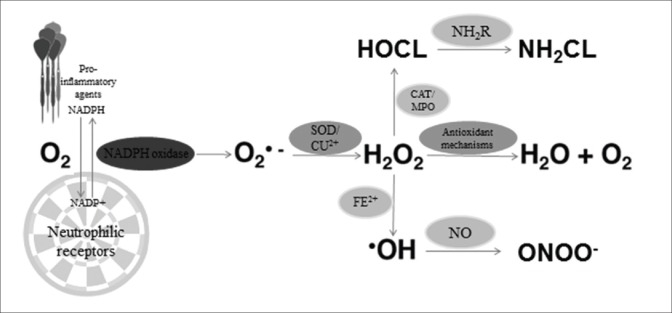
It seems that a dramatic increase of oxygen consumption can be observed during proinflammatory agent's interaction with neutrophil-specific receptors [Figure 2].
Figure 2.
Neutrophilic oxidant mechanism (explanations in text)
In this way, probably the NADPH oxidase from the neutrophil membrane shows an increase in activity consuming oxygen and facilitating signal-receptor interaction.[68] At the same time, oxygen using reactions get to the inevitable production of instable ROS, such as superoxide anion, which results in hydrogen peroxide and oxygen. The end products further interact with active ions of iron, generating another ROS, the hydroxyl radical. Other studies have shown that the production of hydroxyl radical via neutrophil activity is very low. It seems that the enzymatic complex of catalase–myeloperoxidase is consuming most of the hydrogen peroxide, which can no longer interact with ionic iron or oxygen.[69] More than that, most of the ionic iron is preserved in the cell chelated to proteins (transferring, lactoferrin, and ferritin).[70]
Apart from its reducing potential, myeloperoxidase seems to have oxidation potential for chloride ions by hydrogen peroxide yielding a highly cytotoxic neutrophil system.[71] It has been also shown that hypochlorous acid is much more toxic than common ROS and can easily react with sulfhydryl, amino acids, nitrogenous compounds, pyridine nucleotides, but not with peroxide polyunsaturated lipids.[72,73]
Furthermore, the N-chloramine products contain oxidizing equivalents too, also toxic and extremely instable, influencing membrane permeability.[72] They can also interact with intracellular compounds resulting in chloramine, which is significantly more toxic than hypochlorous acid for bacteria and certain eukaryotic cells.[73] These suggest that neutrophil oxidants may be important in the pathogenesis of the depressed intestinal contractility mechanism. Furthermore, because several oxidants can interact with DNA molecules, it is almost indubitable that they may be mutagens and therefore explaining the possible link between chronic inflammation and neoplastic transformation.[72]
Although there were conflicting results, it seems that IBD patients show excessive oxidant activities compared with control subjects, interestingly, more significantly in CD patients. In this way, in reaction to oxidative stress, tissues often respond producing antioxidants, but persistent oxidative stress can deplete body antioxidant resources and overtake the ability to produce more and therefore leading to lower antioxidant levels. Also, interestingly enough, the antioxidant levels are showing important changes in IBD patients.
The majority of studies that examined the relationship between IBD severity and oxidative stress were not able to show significant correlation.[57,74,75,76,77,78] On the other hand, some studies show that[79] oxidized glutathione concentrations increase in active UC. Also, lipid peroxidation and antioxidant activity seem to significantly change in CD patients[80] suggesting that both can be used as CD severity markers, but only considered together and not separately.
Apart from the innate immunity, the one consisted in neutrophils, macrophages, and other phagocytes, which is activated by the interaction of microbial agents and specific receptors,[81] the adaptive immune system is higher in specificity although closely connected.[82] The main contributors in IBD pathology are the T-helper cells. Due to pro-inflammatory release, Th-1 and Th-2 cells are being activated in producing high amounts of interferon (which cause CD-like pathogenesis) and several types of interleukins (which cause UC-like pathogenesis)[83] By these findings, it has been proved that Th-17 also intervenes in IBD pathology through local tissue destruction and interleukin production.[84] Further on, the inflammation gets uncontrolled due to disruption of epithelial barrier and increased bowel permeability.
It is well known that the intestinal epithelial barrier is in epithelium cells specialized in absorption and secretion,[85] which are kept together by different types of cellular junctions. When these junctions are disrupted, epithelial permeability increases, and immune response arises consequently to intestinal inflammation.[81] In this way, some cytokines and interleukins interact in epithelial junctions clearly leading to increased permeability.[86]
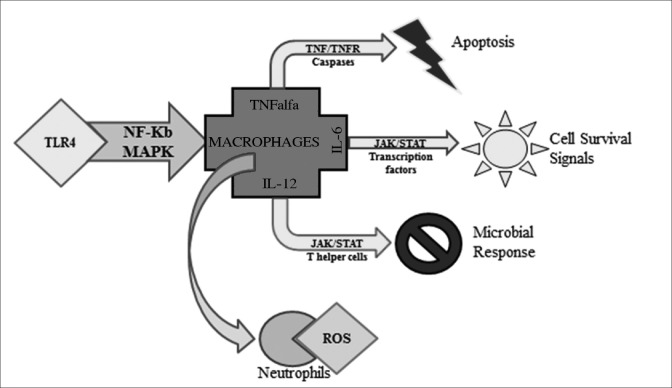
Presently, it is considered that IBD is a multifactorial disease. In other words, IBD can be caused by a multitude of factors closely related or closely interacting in order to trigger immune responses via pro-inflammatory molecule production. These factors can be environmental, biological, or even genetic, but the exact way of interaction is yet unknown. As Monteleone and coworkers highlighted,[87] several differences have been determined in CD and UC inflammatory profiles. Moreover, Pedersen and coworkers[88] showed that inflammatory cells are regulated by different pathways involved in inflammation [Figure 3] and that the pro-inflammatory mediators released through these pathways result in the progression of disease.
Figure 3.
Different regulation of immune response pathways
Toll-like receptor 4 from the receptor presenting gut cells is activated by microbial agents. TLR4further activate nuclear factor NF-kB and mitogen-activated protein kinases (MAPK), which determine TNFα, IL6, and IL12 secretion by the macrophages. TNFα can play the role of a proapototic signal. IL6 activated transcription through JAK/STAT pathway and leads to cell survival signals production. IL12 also through JAK/STAT pathway leads to helper T cells activation, which secrete interferon gamma. Through JAK/STAT pathway again, IFNγ leads to gut microbial response.[88,89,90] At the same time, macrophages activate neutrophils and together produce a large quantity of ROS as an inflammatory response.
DYNAMICS OF THE MOST RELEVANT OXIDATIVE STRESS MARKERS IN IBD
As stated before, IBD is observed in two tissue distinguished variants: CD which affects any region of the gastrointestinal tract in a discontinuous and transmural manner and UC restricted to the surface mucosa of the colon, in particular the rectum. Dysfunctional immune regulation is believed to be the main cause of these abnormalities. Among the immune regulatory factors, the highest levels appear in ROS concentrations. This leads to either initiation or propagation of the disease. As mentioned before, there are several reasons why ROS are indispensable in cells’ lives due to mediatory purposes in normal cellular processes.
In fact, in IBD, oxidative damage can be evaluated by investigating either of the oxidant or antioxidant markers: SOD, CAT, GPX, uric acid, melatonin, transitional metals binding proteins, and vitamins, as previously mentioned. Therefore lipid and protein oxidation can be assessed by CAT activity dynamics, protein thiols levels determination, or antioxidant system status.[91] Lipid peroxidation can be also evaluated by malondialdehyde and conjugated dienes levels.
In this way, it is generally believed that IBD is characterized by chronic or relapsing inflammation. The uncontrolled and sustained host immune response is coupled with extensive inflammatory burst in the intestine with the presence of inflammatory cells and oxidative stress markers.
Many of these findings were brought to light in years of research. For example, studies on colonic biopsies showed that oxidative stress can be correlated to disease activity and that the main sites of ROS production are phagocytic leukocytes during their massive infiltration of the intestinal mucosa in inflammation. Furthermore, there were studies that looked into the oxidants and antioxidants balance in IBD and have shown poor antioxidant status. Also, in randomized controlled trials, antioxidants such as vitamin E, vitamin C, fish oil, beta-carotene, or selenium were tested for antioxidant adjuvant potential.
In this way, the group led by Lih-Brody et al.[92] was one of the first that showed significant correlations between IBD and oxidative stress. They observed increased reactive oxygen intermediates (ROIs), protein carbonyl contents (POPs), DNA oxidation products such as 8-hydroxy-2’-deoxyguanosine (8-OHdG), and iron, and decreased copper and Cu-Zn SOD activity in CD biopsies. Also, for UC, there was an increase in ROIs, POPs, and iron in inflamed tissue as compared to controls, whereas decreased zinc and copper were observed. By all these, the aforementioned authors concluded that an imbalance in the formation of ROS and antioxidant micronutrients may be important in the pathogenesis of the tissue injury in IBD and may provide a rationale for therapeutic modulation with antioxidants.
Also, Chiarpotto et al.[93] demonstrated even more connections between oxidative stress and IBD by evaluating TGFβ1 expression in human colon adenocarcinoma. As CD is often regarded as precancerous, parallel studies were conducted in adenocarcinoma and CD patients, by investigating standard oxidative markers and TGFβ1 ARNm. The authors observed that malondialdehyde and 4-hydroxynonenal levels were significantly lower in controls than in cancer patients and significantly higher in CD patients. These results suggested that there is an association between oxidative damage and fibrogenic cytokine expression in the human intestine. However, it seems that further studies are needed to conclusively prove the correlation between the two events.
There were some studies that came to endorse Chiarpotto's assumption many years later.[80] Regarding the oxidative stress status, nitric oxide (NO), and TGF-1 were shown to be crucial in CD, Rezaie et al.[80] determined total antioxidant activity (TAC), specific antioxidants, and lipid peroxidation levels in human subjects’ saliva. Twenty-nine CD patients were included demonstrating that in CD patients TAC, albumin, and uric acid levels were decreased as compared with control subjects.
Also, TGF-1 was significantly increased in CD patients as compared to healthy subjects, whereas NO levels were increased by fourfold and LPO concentration by fivefold. In this way, it seems that LPO and TAC levels correlate with disease severity, which underlines the importance of oxidative stress in the pathogenesis of CD.
Another growth factor was investigated in the light of oxidative stress in IBD in 2004 by Jahanshahi et al.[94] The existence of oxidative stress and nitrosative stress was examined by highlighting alterations in epidermal growth factor (EGF) secretion in saliva of IBD patients. In this way, the authors found that excessive NO production is present in saliva of both CD and UC patients, but only saliva of CD patients is oxidative stressed. Moreover, EGF secretion is normal in UC patients, although CD patients showed a signiflcant increase in salivary EGF levels.
In addition, PDGF-BB factor was also tested by Krzystek-Korpacka et al.[95] showing that PDGF was increased exclusively in active IBD. Regarding the disease marker state, plasma-PDGF better reflected oxidative stress, whereas serum-PDGF reflected inflammation and angiogenesis. It seems that IBD is associated with an increase in platelet-stored and circulating PDGF. However, PDGF as an active-IBD marker was not better than currently applied C-reactive protein and erythrocyte sedimentation rate.
Moreover, Pelli et al. in 1999,[96] continued to investigate oxidative stress in IBD by aiming to determine whether excessive lipid peroxidation plays a crucial role in IBD pathology. This is one of the few studies that were concentrated on slightly different oxidative markers such as breath alkanes. On 20 IBD patients breath alkanes were determined by standard procedures resulting in high concentrations of ethane, propane, and pentane, but not butane and isoprene. By these findings, it is clear that an excess of lipid peroxidation is probably an important pathogenetic factor in IBDs, and this may be accessed through a noninvasive method. Moreover, just a year later, Koch et al.[97] conducted a complex study in order to determine whether oxidative stress is involved in UC pathology. Considering this, the authors hypothesized that diminished total antioxidant capacity in active UC would be associated with increased colonic lipid peroxidation. Moreover, they found that depletion of glutathione in UC may be a specific disorder rather than a secondary defect attributable to global oxidative stress. And, at the same time, they were among the first and few who postulated that nonspecific antioxidant supplements appear unlikely to be beneficial in the treatment of UC. In addition, a parallel study also reported altered lipid profiles, lipoprotein composition, and oxidant and antioxidant levels in child CD patients.[98]
Also, D’Odorico et al.[99] were the first to demonstrate the importance of antioxidant depletion through increased free radical peripheral leukocyte DNA damage and decreased plasma antioxidant defenses found in malnourished IBD patients. In their IBD food antioxidant intake study, they observed that antioxidant concentrations were significantly reduced in IBD patients, particularly in those with active disease. Also, 8-OHdG concentrations were significantly increased, but no correlations were found between antioxidant and 8-OHdG concentrations. Moreover, carotenoid concentrations were significantly reduced in malnourished IBD patients probably because protein, fruit, and vegetable intakes of IBD patients were significantly lower than those of controls.
Today's standard biochemical oxidative stress markers were tested on IBD also by Tuzun et al.,[100] who used IBD patients to determine correlations between oxidative stress markers and tissue injury. They found that plasma GPx levels of the patients group were significantly higher than the control group, whereas there was no significant difference between patients and controls in view of plasma levels of MDA. Also, it seems that in IBD patients there is an antioxidant capacity despite oxidative stress through high levels of GPx and normal plasma MDA levels.
Moreover, Forrest et al.[76] considered another correlation between oxidative stress markers and IBD features by assuming that purines and kynurenines may be involved in the regulation of neuronal activity and therefore gut motility and secretion. Thus, purines and kynurenines were analyzed in IBD patients along with levels of tryptophan and its metabolites, but the end products were unchanged in all patients. Still, the levels of kynurenine and kynurenic acid were significantly elevated in patients with IBD as compared to control subjects. They also analyzed serum lipid peroxidation products that were significantly elevated when compared to control subjects, suggesting the presence of increased oxidative stress consistent with inflammatory activity. In this way, it seems that the elevated level of kynurenic acid may represent either a compensatory response to elevated activation of enteric neurons, or a primary abnormality, which induces a compensatory increase in gut activity. Moreover, it seems that this correlation may indicate a role for kynurenine modulation of glutamate receptors in the symptoms of IBD.
Also, this correlation was investigated further by Ciorba et al.[101] based on Clarke et al's.[102] assumption too. The latter postulated that several gastrointestinal diseases including IBD and malignancy are associated with elevated expression of indoleamine 2,3 dioxygenase-1 (IDO1) that initiates tryptophan catabolism and generates several bioactive kynurenine-based metabolites.
Also, based on Forrest et al.'s study, which showed that serum kynurenine: tryptophan ratio is elevated in both active CD and in colon cancer, suggesting this measurement may prove useful as a disease biomarker, Ciorba et al. showed that IDO1 activity significantly shapes gastrointestinal disease pathophysiology and severity and measures of IDO1 activity may be useful as a disease biomarker. Thus the manipulation of IDO1 activity has great potential as treatment for both inflammatory and malignancy associated gastrointestinal disease.
Also, Sampietro et al.[103] got even further in assumptions and tried to connect oxidative stress with postoperative shock and to show whether oxidative stress lowers in intensity after a correctional surgery is applied in complicated IBD cases. In this way, the study evaluated basal peroxidative state, peroxidative state after stimulation with copper sulfate, lag time of plasma peroxidation susceptibility, plasma levels of vitamin E and A, C reactive protein, erythrocyte sedimentation rate, and Crohn's disease activity index before and after surgery. Thus, it seems that peroxidative plasma levels, as well as inflammatory indices significantly reduced 2 months and 1 year after surgery. Also, basal levels of peroxidation and antioxidant scavengers seem to be deregulated, whereas there seems to be a correlation between basal thiobarbituric acid reactive substances, lag-time, and erythrocyte sedimentation rate with C reactive protein. Moreover, it seems that the imbalance between pro- and antioxidant mechanisms is due to chronic gut inflammation and an excess of lipid peroxidation is probably an important pathogenetic factor.
In addition, Barbosa et al.[104] were one of the first that begun to explore the antioxidant potential of different compounds as oxidative stress diminishing agents. In this way, in their study they aimed to verify the blood oxidative stress in patients with UC and determine whether the association of sulfasalazine to fish oil omega-3 fatty acids is more effective than isolated use of sulfasalazine to reduce the oxidative stress. They observed mild changes, with the exception of erythrocyte sedimentation rate, which decreased with both treatments. Moreover, controversial findings were made in oxidative stress markers’ levels, since besides increased SOD activity, levels of plasma malondialdehyde, erythrocyte lipid peroxidation, and catalase were not different from those in the control group.
Also, phagocytic leukocytes were clearly found to be involved in IBD oxidative stress, but new approaches suppose that oxidative stress causes and factors get even further. In this way, Rana et al.[105] stated that oxidative stress contributes to severity of UC, but the status of erythrocyte antioxidant defense remains unknown. So, it seems that oxidative stress and antioxidant levels in erythrocytes of UC patients may be in a close correlation. Thus, on an 80 UC patients study, these authors investigated the levels of lipid peroxidation (LPO), reduced glutathione (GSH), catalase, and superoxide dismutase (SOD). It seems that increased levels of LPO, SOD, and catalase and decreased level of GSH vouch for oxidative stress's significant role in pathophysiology of UC. Furthermore, the levels of LPO, GSH, catalase, and SOD remained same during different disease activities.
Also, series of studies conducted by Hatoum et al.[106,107] and Binion and Rafiee[108] got even further with assumptions on microvascular dysfunction connected to chronic IBD. Using in vitro video microscopy to assess endothelium-dependent vasodilation in response to acetylcholine and fluorescence microscopy to detect oxyradicals, they found that IBD-involved microvessels generated significantly higher levels of ROS as compared with control and uninvolved IBD vessels. It seems that human intestinal microvessels from chronically inflamed IBD show microvascular endothelial dysfunction, characterized by loss of NO-dependent dilation that may contribute to reduced perfusion, poor wound healing, and maintenance of chronic inflammation.
Another perspective on IBD oxidative stress mechanisms were also offered by Kruidenier et al.[109] based on Zelko et al.'s[110] genetic study. Thus, Kruidenier et al. discuss how SOD isoforms are differently expressed in IBD patients. In this way, it seems that although the three human isoforms of superoxide dismutase (SOD), copper/zinc (Cu/Zn)-SOD, manganese (Mn)-SOD, and extracellular (EC)-SOD, form the primary endogenous defense against oxidative stress, their expression levels and cellular localization in IBD mucosa might be controversial. Mucosal SOD isoform expression has been found to be differentially modified in IBD patients. The Mn-SOD protein levels were increased in both active and remission IBD, whereas the Cu/Zn-SOD content decreased with inflammation levels. EC-SOD, found in much reduced quantities, had the tendency to be decreased in IBD patients. Thus, the authors, managed to prove their hypothesis observing that Mn-SOD and Cu/Zn-SOD were both predominantly expressed in intestinal epithelial cells and Mn-SOD secreting cells were considerably increased in IBD, whereas Cu/Zn-SOD expression in epithelial cells was less affected. Moreover, Cu/Zn-SOD and Mn-SOD were present in neutrophils and macrophages, and EC-SOD was mainly localized in small vessels, stromal cells, and neutrophils, but not affected by inflammation. They also found that the activity of Mn-SOD did not concordantly increase with the immunological assessments, and postulated that a proportion of the Mn-SOD in IBD is present in an enzymatically inactive form. Through these changes, IBD is proven to be a complex disease affecting and being affected by the mucosal antioxidant cascade. Moreover, the fact that gene expression may be modified by IBD effects could give some valuable clues regarding the genetic component of IBD itself.
THE RELEVANCE OF ANIMAL MODELS FOR THE STUDY OF OXIDATIVE STRESS IN IBD
Since the exact etiology of idiopathic IBD is not known, many types of animal models have been developed to study this disease over the past decades. Thus, as the purpose was to find a viable IBD treatment in humans, most of the animal models were chosen and designed to mimic as close as possible the clinical and histological features.
Most of these models are based either on chemical induction, immune cell transfer, or gene targeting [Table 1]. Therefore, mouse models are broadly designed according to the defect in mucosal immunity, which is believed to be most important for the onset of the disease. Thus, a model can mimic defects in epithelial integrity or permeability, defects in innate immune cells or defects in cells of the adaptive immune system.[111]
Table 1.
Studies on oxidative stress status in several types of IBD animal models
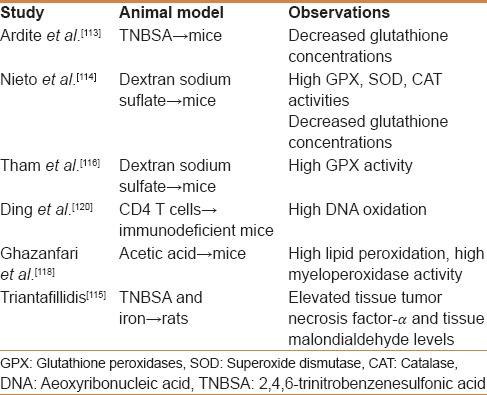
Moreover, many attempts to create an ideal experimental model of IBD through toxic chemicals administration have been carried out but these are still not ideal animal models. It seems that the complicated genetic, immunologic, environmental, and physiologic factors that form the pathophysiology of IBD cannot be reproduced simultaneously.[112]
In this way, most of the studies used TNBS–ethanol, DSS, or acetic acid to induce colitis in mice or rats.
Thus, Ardite et al.[113] used TNBS in 50% ethanol in rats to determine the functional role of mucosal glutathione showing that the interaction of TNBS with GSH led to almost instantaneous disappearance of GSH, whereas the reductive metabolism of TNBS by GSSG reductase generated ROS. Also, the authors observed that GSH levels in TNBS-ethanol-treated rats recovered by 1-2 weeks, an effect that was accounted for an increase of g-glutamylcysteine synthetase (g-GCS). Thus, TNBS-induced IBD model showed two independent mechanisms of injury, GSH depletion and ROS generation, both being required for the manifestation of mucosal injury. The authors also suggested that GSH precursors may be of relevance in the acute relapse of IBD.
Also, Nieto et al.[114] used also used TNBS in order to determine impairments in antioxidant defense system. This way they reproduced UC symptoms by intrarectal administration in rats and observed that enzymatic markers of colon injury showed higher activities in rats group with UC. Higher concentrations of prostaglandin E2 and leukotriene B4 were also observed in TNBS-treated animals and decreased after second week of treatment. Moreover, all antioxidant enzyme activities were higher at one and two weeks after treatment; however, a significant decrease in total glutathione content was also observed. Thus, it seems that the deficiency in glutathione of this animal model could be a target for new therapies to treat UC. In addition, the latest TNBS models experimental study[115] aimed to investigate the influence of orally administered iron in normal gut mucosa and mucosa of animals with TNBS colitis. In this way, the group of Triantafillidis used 159 Wistar rats grouped in 10 experimental cohorts, observing that moderate and high iron supplementation induced inflammation in the healthy colon and increased the activity of the experimentally induced TNBS colitis and concluded that iron can induce colonic inflammation and aggravate TNBS colitis.
In addition, DSS-induced colitis models were also used. In this way, Tham et al.[116] used DSS-treated mice in order to evaluate GPx activity in IBD. As it has been previously shown, endogenously generated oxidants have been implicated in IBD. All plasma glutathione peroxidase (GPx) activity in humans is attributable to E-GPx. Therefore, on DSS-treated mice, E-GPx levels were increased by more than 60% compared with control group. As the major source of plasma GPx is the kidney, the mentioned authors suggested that the inflammatory injury in the intestine elicits an increase in E-GPx in the plasma that is associated with an increase in E-GPx mRNA in the kidney. Also, in IBD-susceptible designed mice, DSS administration results in chronic colitis, while combined with genotoxic colon carcinogen azoxymethane results in colorectal cancer following inflammation.[117] Moreover, Tanaka et al. conclude that the DSS/AOM model is a useful tool in studying the mechanisms that connect inflammation to colon cancer while also being important for identifying xenobiotics with modifying effects.
Acetic acid-induced colitis was also used as a pathogenesis model in mice and rats. Thus, Ghazanfari et al.[118] and Ghafari et al.[119] evaluated the effects of several essential oils on IBD using acetic acid as an inducer of colitis symptoms in mice. The authors observed that lipid peroxidation and myeloperoxidase activity significantly increased in acetic acid-treated mice in comparison to the normal group. In addition, both lipid peroxidation and myeloperoxidase activity were reduced by essential oils administration more efficiently than prednisolone, the classic therapeutic compound. Of course, proper clinical investigation should be carried out to confirm the activity in human disease.
In addition, immune system engineered models were also designed in order to investigate macrophages, neutrophils, and phagocytic cells involvement and transformation during IBD active episodes and relapses. Thus, Ding et al.[120] reported DNA oxidative and nitrosative damage on modified CD4 T cells transferred to severe combined immunodeficient female mice. 8-oxodG, 8-Nitroguanine, iNOS, proliferating cells nuclear antigen, and p53 protein were highly present or expressed in gut epithelial cells. Moreover, pathological findings of this mouse model showed severe inflammation in colon tissues, and were similar to IBD patients. Mucus production and presence in feces and bloody diarrhea was observed in DBCB-induced UC models (dinitrochlorobenzene). Dinitrochlorobenzene can bind to tissue proteins eliciting a cell-mediated immune response with signiflcantly higher levels of CD4+ CD29+ cells.[121] Moreover, this model can be also used as an excellent apoptosis model in IBD possibly in experiments on regeneration capacity of different compounds. However, unfortunately this model was not used to investigate oxidative stress in IBD.
Other animal models untested for oxidative stress refer to bacterial induction of colitis. Mizoguchi et al.[122] and Papanikolaou et al.[123] observed that Salmonella typhimurium and Salmonella dublin-induced colitis on mice shows inflammation similar to histopathological characteristics to human UC, including epithelial crypt loss, erosion, and neutrophil inflltration. On the other hand, invasive E. coli strain adheres to both small and large intestinal epithelial cells[124] inducing colonic inflammation only in mild epithelial damaged mice (DSS treatment).[125] Also, Nagatani et al.[126] used a similar model in order to test chitin microparticles for anti-inflammatory potential. They observed that it ameliorates colonic intestinal inflammation, by blocking the interaction of bacterial-derived factors with host.
IS ANTIOXIDANT THERAPY RELEVANT IN IBD?
Considering the strong evidence that ROS is increased in IBD, it could be possible for the antioxidant therapy to be useful as a treatment alongside classical medication. In fact, the oldest and most commonly used drug for IBD, 5-amino salicylic acid, also has ROS scavenging capabilities.[127] Since then, many trials have been conducted in order to determine whether antioxidant therapy can be efficient in IBD. Conclusively, more reports agreed that the complex oxidant mechanisms and unknown physiopathology of IBD makes quite hard to evaluate antioxidant therapy in terms of efficiency in IBD treatment.
For example, a study performed by Nagatani et al.[126] on IBD stable patients shows high oxidative stress in placebo treated patients and decreasing values in antioxidant therapy patients. Although the disease was stable in all patients, some of them showed remissions during treatment, but a correlation in remission occurrence and treatment were not clear.[128]
Also, other studies revealed no significant results due to controversial antioxidants active doses,[129,130] whereas other reports evaluated antioxidant efficiency during remission and observed that SOD activity signiflcantly increased, but GPx activity was reduced after antioxidant treatment.[131] In addition, there are some studies that were not able to report any significant differences between placebo treatment and antioxidant treatment due to closely similar clinical results.[132] Furthermore, all clinical trials used far smaller medium potency antioxidants doses, a fact that again questions their effectiveness in chronic disease, such as IBD.
Given what is shown in the present review, an obvious question arises: Is antioxidant administration of any relevance as therapy adjuvant or even as therapy on IBD patients? Skeptical opinions arise on the new antioxidant therapies tested on bolder animal models and shown to be potentially efficient in IBD-like symptoms treatment due to many risks associated with these compounds being tested on human subjects.
In this way, Oz et al.[133] reported three structurally dissimilar antioxidants that provided protection in DSS-induced colitis rodent models, supporting a possible role for antioxidant therapy in IBD patients. They measured weight, colon length and damage, hematocrits, serum amyloid A, TNFα, IL10, and reduced and oxidized glutathione in the control and DSS groups and S-adenosylmethionine, green tea polyphenols, and 2-(R, S)-n-propylthiazolidine-4(R)-carboxylic acid treated groups. Importantly, all of the IBD markers improved after antioxidant treatment. In addition, the same authors speculated that some of the tested compounds may have other properties such as additive or synergistic effects that need further exploration.
Moreover, two years later, Oz et al. group compared two cysteine prodrugs and a glutathione delivery agent, by observing that both prodrugs and delivery agent efficiently treated DSS-induced colitis in mice. In this way, GSH levels were depleted in DSS animals, and normalized with prodrug treatment. Moreover, IBD biomarkers showed high levels in colitis animals and improved with therapy. Also, molecular tests showed macrophages implications in lamina propria events, which were attenuated by prodrug administration.
Also, another green tea antioxidant compound (epigallocatechin-3gallate) was tested on different colitis model mice by various groups and found to be efficient.[134,135,136] Promising results were also obtained by testing other strong antioxidants such as pyrolidine-dithiocarbamate (PDTC),[137] 2,3,5,4’-tetrahydroxystilbene-2-O-beta-d-glucoside (THSG),[138] partenolide,[139] or synthetic compounds such as dihydroxymethylepoxyquinomicin (DHMEQ)[140] derived from the natural epoxyquinomicin antibiotic or MitoQ,[141] a synthetic compound designed to accumulate in mitochondria in order to block lipid peroxidation. Furthermore, some studies report native medication such as Ayurvedic formulations (Triphala extract)[142] to be possibly efficient in antioxidant therapy of IBD.
In addition, animal studies were considered a starting point for human studies, clinical trials, and randomized controlled trials. Due to the need for further research after animal testing and before therapeutic human administration, many studies were conducted to find key antioxidant compounds to be used on IBD patients, such as selenium, zinc, vitamin C, vitamin E, l-glutamine, vitamin B12 or even probiotics such as Acidophillus or Bifidobacteria-based drugs.[143] Conclusively, no tangible results were obtained.
In this way, some of the latest studies refer to polyphenols’ antioxidant potential in IBD patients.[144] Polyphenols have been acknowledged to be antioxidant and anti-inflammatory and therefore, have been proposed as an alternative to prevent or treat chronic inflammatory diseases.[145,146] Animal studies proved that polyphenols can effectively modulate intestinal inflammation through cell signaling pathways modulation. However, it seems that knowledge regarding the use of polyphenols in managing human IBD is still far lacunar, and further clinical studies should bring more solid evidence of their beneficial effects.
Another interesting approach is connected to the influence of triterpenoids present in apple peel in gene expression associated with IBD study.[147] Using T84 colon carcinoma cells, the group of Mueller et al. studied various pentacyclic triterpenoids from apple peel in order to see modifications in three speciflc inflammation-associated marker genes (TNFα, IL-8, IP-10) and in the synthesis of certain pro-inflammatory proteins. Thus, IP-10 expression was inhibited by all three polyphenols, TNFα was slightly affected and the IL-8 level was increased. Also, some polyphenols reduced the synthesis of IP-10, while sICAM-1, IL-23, and GROa were slightly repressed. Thus, it seems that triterpenoids present in apple peel may be implicated in the anti-inflammatory properties of apple constituents, suggesting that these compounds could be relevant as possible adjuvants in IBD treatment.
Moreover, Triandafillidis et al.[115] postulated that melatonin can be used as an antioxidant and antiapoptotic agent in IBD. In this way, it seems that based on several animal studies, this group assumed that melatonin plays an important role in regulating epithelial functions and can reduce bacterial translocation and its antiapoptotic effect, therefore reducing the extent of mucosal damage.
Also, in 2012, Alhouayek and Muccioli[148] reviewed endocannabinoid system potential in IBD therapy, providing an interesting background for a further study in 2013[149] that used a nonpsychotropic plant cannabinoid in order to experiment anti-IBD potential. In this way, it seems that anandamide and 2-arachidonoylglycerol are endogenous bioactive lipids that bind to and activate the cannabinoid receptors, and together with the enzymes responsible for their biosynthesis and degradation constitute the endocannabinoid system (ECS), which is involved in gut homeostasis, modulating gastrointestinal motility, visceral sensation, and inflammation, as well as being recently implicated in IBD pathogenesis. Furthermore, Borelli et al.[149] showed that canabigerol attenuated murine colitis, reduced nitric oxide production in macrophages, and reduced ROS formation in intestinal epithelial cells.
CONCLUSIONS
IBD is a multifactorial disease that involves many genetic, clinical, physiological, and environmental features. Furthermore, it seems that animal model studies have proved that there are many mechanisms implicated in IBD pathogenesis and collectively, they show an important contribution of oxidative stress and oxidant mechanisms in IBD determinism. Most of the animal studies were to confirm or contradict some hypotheses that show key correlations between ROS and IBD characteristic inflammation. All these findings offered a starting point for treatment development by using chemical compounds with antioxidant potential. On the other side, there were many to postulate that even natural compounds, ions or vitamins, can successfully compete with chemicals in order to treat or accompany the drug therapy. However, animal modeling has also shown several drawbacks such as the impossibility of developing a model that recapitulates the entire IBD clinical features at the same time. For obvious reasons, human subject testing was significantly limited; therefore antioxidant therapy further remains a controversy. In this way, whether antioxidant adjuvant or therapy can be used as an IBD treatment will still be a valuable question through the years to come.
Financial support and sponsorship
Nil.
Conflicts of interest
None to declare, except for the author Balmuş Ioana-Miruna who is now co-funded by the European Social Fund through Sectorial Operational Programme Human Resources Development 2007-2013, project number POSDRU/187/1.5/S/155397, project title “Towards a New Generation of Elite Researchers through Doctoral Scolarships.
REFERENCES
- 1.Baumgart DC, Carding SR. Inflammatory bowel disease: Cause and immunobiology. Lancet. 2007;369:1627–40. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 2.Borody TJ, Campbell J, Torres M, Nowak A, Leis S. Reversal of idiopathic thrombocytopenic purpura [ITP] with fecal microbiota transplantation [FMT] Am J Gastroenterol. 2011;106:S352. [Google Scholar]
- 3.Wendland BE, Aghdassi E, Tam C, Carrier J, Steinhart AH, Wolman SL, et al. Lipid peroxidation and plasma antioxidant micronutrients in Crohn disease. Am J Clin Nutr. 2001;74:259–64. doi: 10.1093/ajcn/74.2.259. [DOI] [PubMed] [Google Scholar]
- 4.Halliwell B. Antioxidants and human disease: A general introduction. Nut Rev. 1997;55:S44–52. doi: 10.1111/j.1753-4887.1997.tb06100.x. [DOI] [PubMed] [Google Scholar]
- 5.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 6.Rice-Evans C, Burdon R. Free radical-lipid interactions and their pathological consequences. Prog Lipid Res. 1993;32:71–110. doi: 10.1016/0163-7827(93)90006-i. [DOI] [PubMed] [Google Scholar]
- 7.Frei B, Stocker R, Ames BN. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci U S A. 1998;85:9748–752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas SR, Davies MJ, Stocker R. Oxidation and antioxidation of human low-density lipoprotein and plasma exposed to 3-morpholinosydnonimine and reagent peroxynitrite. Chem Res Toxicol. 1998;11:484–94. doi: 10.1021/tx970173a. [DOI] [PubMed] [Google Scholar]
- 9.Knutson MD, Walter PB, Ames BN, Viteri FE. Both iron deficiency and daily iron supplements increase lipid peroxidation in rats. J Nutr. 2000;130:621–8. doi: 10.1093/jn/130.3.621. [DOI] [PubMed] [Google Scholar]
- 10.Oldenburg B, van Berge Henegouwen GB, Rennick D, van Asbeck BS, Koningsberger JC. Iron supplementation affects the production of pro-inflammatory cytokines in IL-10 deficient mice. Eur J Clin Invest. 2000;30:505–10. doi: 10.1046/j.1365-2362.2000.00650.x. [DOI] [PubMed] [Google Scholar]
- 11.Carrier J, Aghdassi E, Platt I, Cullen J, Allard JP. Effect of oral iron supplementation on oxidative stress and colonic inflammation in rats with induced colitis. Aliment Pharmacol Ther. 2001;15:1989–99. doi: 10.1046/j.1365-2036.2001.01113.x. [DOI] [PubMed] [Google Scholar]
- 12.Carrier J, Aghdassi E, Cullen J, Allard JP. Iron supplementation increases disease activity and vitamin E ameliorates the effect in rats with dextran sulfate sodium-induced colitis. J Nutr. 2002;132:3146–50. doi: 10.1093/jn/131.10.3146. [DOI] [PubMed] [Google Scholar]
- 13.Barollo M, D’Inca M, Scarpa M, Medici V, Cardin R, Bortolami R, et al. Effects of iron manipulation on trace elements level in a model of colitis in rats. World J Gastroenterol. 2005;11:4396–9. doi: 10.3748/wjg.v11.i28.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erichsen K, Milde AM, Arslan G, Helgeland L, Gudbrandsen OA, Ulvik RJ, et al. Low-dose oral ferrous fumarate aggravated intestinal inflammation in rats with DSS-induced colitis. Inflamm Bowel Dis. 2005;11:744–8. doi: 10.1097/01.mib.0000174374.83601.86. [DOI] [PubMed] [Google Scholar]
- 15.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–63. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 16.Cañas PE. The role of xanthine oxidase and the effects of antioxidants in ischemia reperfusion cell injury. Acta Physiol Pharmacol Ther Latinoam. 1999;49:13–20. [PubMed] [Google Scholar]
- 17.Krinsky NI. Mechanism of action of biological antioxidants. Proc Soc Exp Biol Med. 1992;200:248–54. doi: 10.3181/00379727-200-43429. [DOI] [PubMed] [Google Scholar]
- 18.Machlin LJ, Bendich A. Free radical tissue damage: Protective role of antioxidant nutrients. FASEB J. 1987;1:441–5. [PubMed] [Google Scholar]
- 19.Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–9. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 20.Floyd RA. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990;4:2587–97. [PubMed] [Google Scholar]
- 21.Alzoghaibi MA. Concepts of oxidative stress and antioxidant defense in Crohn's disease. World J Gastroenterol. 2013;19:6540–7. doi: 10.3748/wjg.v19.i39.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez-Banares F, Abad-Lacruz A, Xiol X, Gine JJ, Dolz C, Cabre E, et al. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol. 1989;84:744–8. [PubMed] [Google Scholar]
- 23.Wetscher GJ, Perdikis G, Kretchmar DH, Stinson RG, Bagchi D, Redmond EJ, et al. Esophagitis in Sprague-Dawley rats is mediated by free radicals. Dig Dis Sci. 1995;40:1297–305. doi: 10.1007/BF02065542. [DOI] [PubMed] [Google Scholar]
- 24.Olliver JR, Hardie LJ, Gong Y, Dexter S, Chalmers D, Harris KM, et al. Risk factors, DNA damage, and disease progression in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev. 2005;14:620–5. doi: 10.1158/1055-9965.EPI-04-0509. [DOI] [PubMed] [Google Scholar]
- 25.Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, et al. Oxidative damages are critical in pathogenesis of reflux esophagitis: Implication of antioxidants in its treatment. Free Radic Biol Med. 2005;30:905–15. doi: 10.1016/s0891-5849(01)00472-5. [DOI] [PubMed] [Google Scholar]
- 26.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–31. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 27.Mai UE, Perez-Perez GI, Allen JB, Wahl SM, Blaser MJ, Smith PD. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J Exp Med. 1992;175:517–25. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.An international association between Helicobacter pylori infection and gastric cancer. The EUROGAST Study Group. Lancet. 1993;341:1359–62. [PubMed] [Google Scholar]
- 29.Davies GR, Simmonds NJ, Stevens TR, Sheaff MT, Banatvala N, Laurenson IF, et al. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut. 1994;35:179–85. doi: 10.1136/gut.35.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farinati F, Cardin R, Libera GD, Rugge M, Herszènyi L, Di Mario F, et al. Determinants for the development of chronic atrophic gastritis and intestinal metaplasia in the stomach. Eur J Cancer Prev. 1995;4:181–6. doi: 10.1097/00008469-199504000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Seril DN, Liao J, Yang G, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: Studies in humans and animal models. Carcinogenesis. 2003;24:353–62. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- 32.D’Incà R, Cardin R, Benazzato L, Angrima I, Martines D, Sturniolo GC. Oxidative DNA damage in the mucosa of ulcerative colitis increases with disease duration and dysplasia. Inflamm Bowel Dis. 2004;10:23–7. doi: 10.1097/00054725-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Damiani CR, Benetton CA, Stoffel C, Bardini KC, Cardoso VH, Di Giunta G, et al. Oxidative stress and metabolism in animal model of colitis induced by dextran sulfate sodium. J Gastroenterol Hepatol. 2007;22:1846–51. doi: 10.1111/j.1440-1746.2007.04890.x. [DOI] [PubMed] [Google Scholar]
- 34.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–5. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 35.Omatsu T, Naito Y, Handa O, Mizushima K, Hayashi N, Qin Y, et al. Reactive oxygen species-quenching and anti-apoptotic effect of polaprezinc on indomethacin-induced small intestinal epithelial cell injury. J Gastroenterol. 2005;45:692–702. doi: 10.1007/s00535-010-0213-9. [DOI] [PubMed] [Google Scholar]
- 36.Adebayo D, Bjarnason I. Is non-steroidal anti-inflammatory drug (NSAID) enteropathy clinically more important than NSAID gastropathy? Postgrad Med J. 2006;82:186–91. doi: 10.1136/pgmj.2005.039586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chattopadhyay I, Bandyopadhyay U, Biswas B, Maity P, Banerjee RK. Indomethacin inactivates gastric peroxidase to induce reactive-oxygen-mediated gastric mucosal injury and curcumin protects it by preventing peroxidase inactivation and scavenging reactive oxygen. Free Radic Biol Med. 2006;40:1397–408. doi: 10.1016/j.freeradbiomed.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 38.Lim YJ, Lee JS, Ku YS, Hahm KB. Rescue strategies against non-steroidal anti-inflammatory drug-induced gastroduodenal damage. J Gastroenterol Hepatol. 2009;24:1169–78. doi: 10.1111/j.1440-1746.2009.05929.x. [DOI] [PubMed] [Google Scholar]
- 39.Wetscher GJ, Hinder RA, Bagchi D, Hinder PR, Bagchi M, Perdikis G, et al. Reflux esophagitis in humans is mediated by oxygen-derived free radicals. Am J Surg. 1997;170:552–7. doi: 10.1016/s0002-9610(99)80014-2. [DOI] [PubMed] [Google Scholar]
- 40.Erbil Y, Türkoglu U, Barbaros U, Balik E, Olgac V, Kaya H, et al. Oxidative damage in an experimentally induced gastric and gastroduodenal reflux model. Surg Innov. 2005;12:219–25. doi: 10.1177/155335060501200306. [DOI] [PubMed] [Google Scholar]
- 41.Dvorak K, Payne CM, Chavarria M, Ramsey L, Dvorakova B, Bernstein H, et al. Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: Relevance to the pathogenesis of Barrett's oesophagus. Gut. 2007;56:763–71. doi: 10.1136/gut.2006.103697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boughton-Smith NK, Evans SM, Hawkey CJ, Cole AT, Balsitis M, Whittle BJ, et al. Nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Lancet. 1993;342:338–40. doi: 10.1016/0140-6736(93)91476-3. [DOI] [PubMed] [Google Scholar]
- 43.Kuroki F, Iida M, Tominaga M, Matsumoto T, Hirakawa K, Sugiyama S, et al. Multiple vitamin status in Crohn's disease. Correlation with disease activity. Dig Dis Sci. 1993;38:1614–8. doi: 10.1007/BF01303168. [DOI] [PubMed] [Google Scholar]
- 44.Kuroki F, Iida M, Tominaga M, Matsumoto T, Hirakawa K, Fujishima M. Is vitamin E depleted in Crohn's disease at initial diagnosis? Dig Dis. 1994;12:248–54. doi: 10.1159/000171459. [DOI] [PubMed] [Google Scholar]
- 45.Bousvaros A, Zurakowski D, Duggan C, Law T, Rifai N, Goldberg NE, et al. Vitamins A and E serum levels in children and young adults with inflammatory bowel disease: Effect of disease activity. J Pediatr Gastroenterol Nutr. 1998;26:129–35. doi: 10.1097/00005176-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Schoon EJ, Müller MC, Vermeer C, Schurgers LJ, Brummer RJ, Stockbrügger RW. Low serum and bone vitamin K status in patients with longstanding Crohn's disease: Another pathogenetic factor of osteoporosis in Crohn's disease? Gut. 2001;48:473–7. doi: 10.1136/gut.48.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abreu MT, Kantorovich V, Vasiliauskas EA, Gruntmanis U, Matuk R, Daigle K, et al. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn's disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut. 2004;53:1129–36. doi: 10.1136/gut.2003.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tajika M, Matsuura A, Nakamura T, Suzuki T, Sawaki A, Kato T, et al. Risk factors for vitamin D deficiency in patients with Crohn's disease. J Gastroenterol. 2004;39:527–33. doi: 10.1007/s00535-003-1338-x. [DOI] [PubMed] [Google Scholar]
- 49.Reimund JM, Arondel Y, Escalin G, Finck G, Baumann R, Duclos B. Immune activation and nutritional status in adult Crohn's disease patients. Dig Liver Dis. 2005;37:424–31. doi: 10.1016/j.dld.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 50.Ghishan FK, Kiela PR. Advances in the understanding of mineral and bone metabolism in inflammatory bowel diseases. Am J Physiol Gastrointest Liver Physiol. 2005;300:G191–201. doi: 10.1152/ajpgi.00496.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waśko-Czopnik D, Paradowski L. The influence of deficiencies of essential trace elements and vitamins on the course of Crohn's disease. Adv Clin Exp Med. 2012;21:5–11. [PubMed] [Google Scholar]
- 52.Reimund JM, Allison AC, Muller CD, Dumont S, Kenney JS, Baumann R, et al. Antioxidants inhibit the in vitro production of inflammatory cytokines in Crohn's disease and ulcerative colitis. Eur J Clin Invest. 1998;28:145–50. doi: 10.1046/j.1365-2362.1998.00257.x. [DOI] [PubMed] [Google Scholar]
- 53.Alzoghaibi MA, Walsh SW, Willey A, Fowler AA, 3rd, Graham MF. Linoleic acid, but not oleic acid, upregulates the production of interleukin-8 by human intestinal smooth muscle cells isolated from patients with Crohn's disease. Clin Nutr. 2003;22:529–35. doi: 10.1016/s0261-5614(03)00083-9. [DOI] [PubMed] [Google Scholar]
- 54.Buffinton GD, Doe WF. Altered ascorbic acid status in the mucosa from inflammatory bowel disease patients. Free Radic Res. 1995;22:131–43. doi: 10.3109/10715769509147535. [DOI] [PubMed] [Google Scholar]
- 55.Geerling BJ, Badart-Smook A, Stockbrügger RW, Brummer RJ. Comprehensive nutritional status in patients with long-standing Crohn disease currently in remission. Am J Clin Nutr. 1998;67:919–26. doi: 10.1093/ajcn/67.5.919. [DOI] [PubMed] [Google Scholar]
- 56.Rath HC, Caesar I, Roth M, Schölmerich J. Nutritional deficiencies and complications in chronic inflammatory bowel diseases. Med Klin (Munich) 1998;93:6–10. doi: 10.1007/BF03045033. [DOI] [PubMed] [Google Scholar]
- 57.Geerling BJ, v Houwelingen AC, Badart-Smook A, Stockbrügger RW, Brummer RJ. The relation between antioxidant status and alterations in fatty acid profile in patients with Crohn disease and controls. Scand J Gastroenterol. 1999;34:1108–16. doi: 10.1080/003655299750024913. [DOI] [PubMed] [Google Scholar]
- 58.Filippi J, Al-Jaouni R, Wiroth JB, Hébuterne X, Schneider SM. Nutritional deficiencies in patients with Crohn's disease in remission. Inflamm Bowel Dis. 2006;12:185–91. doi: 10.1097/01.MIB.0000206541.15963.c3. [DOI] [PubMed] [Google Scholar]
- 59.Hengstermann S, Valentini L, Schaper L, Buning C, Koernicke T, Maritschnegg M, et al. Altered status of antioxidant vitamins and fatty acids in patients with inactive inflammatory bowel disease. Clin Nutr. 2008;27:571–8. doi: 10.1016/j.clnu.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Valentini L, Schaper L, Buning C, Hengstermann S, Koernicke T, Tillinger W, et al. Malnutrition and impaired muscle strength in patients with Crohn's disease and ulcerative colitis in remission. Nutrition. 2008;24:694–702. doi: 10.1016/j.nut.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 61.Farrugia G, Balzan R. The Proapoptotic Effect of Traditional and Novel Nonsteroidal Anti-Inflammatory Drugs in Mammalian and Yeast Cells. Oxid Med Cell Longev. 2013 doi: 10.1155/2013/504230. 504230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colombel JF, Loftus EV, Jr, Tremain WJ, Egan LJ, Harmsen WS, Schleck CD, et al. The safety profile of infliximab in patients with Crohn's disease: The Mayo Clinic experience in 500 patients. Gastroenterology. 2004;126:19–31. doi: 10.1053/j.gastro.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 63.Ingraham LM, Coates TD, Allen JM, Higgins CP, Baehner RL, Boxer LA. Metabolic, membrane and functional responses of human polymorphonuclear leukocytes to platelet-activating factor. Blood. 1982;59:1259–66. [PubMed] [Google Scholar]
- 64.Sharon P, Stenson WF. Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology. 1984;86:453–60. [PubMed] [Google Scholar]
- 65.Kitahora T, Suzuki K, Asakura H, Yoshida T, Suematsu M, Watanabe M, et al. Active oxygen species generated by monocytes and polymorphonuclear leukocytes in Crohn's disease. Dig Dis Sci. 1988;33:951–5. doi: 10.1007/BF01535990. [DOI] [PubMed] [Google Scholar]
- 66.Kimura H, Hokari R, Miura S, Shigematsu T, Hirokawa M, Akiba Y, et al. Increased expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in colonic mucosa of patients with active ulcerative colitis. Gut. 1998;42:180–7. doi: 10.1136/gut.42.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oudkerk Pool M, Bouma G, Visser JJ, Kolkman JJ, Tran DD, Meuwissen SG, et al. Serum nitrate levels in ulcerative colitis and Crohn's disease. Scand J Gastroenterol. 1995;30:784–8. doi: 10.3109/00365529509096328. [DOI] [PubMed] [Google Scholar]
- 68.Tauber AI, Babior BM. Neutrophil oxygen reduction: The enzymes and the products. Adv Free Radical Biol Med. 1985;1:265–307. [Google Scholar]
- 69.Winterbourn CC. Myeloperoxidase as an effective inhibitor of hydroxyl radical production. Implications for the oxidative reactions of neutrophils. J Clin Invest. 1986;78:545–50. doi: 10.1172/JCI112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Britigan BE, Coffman TJ, Buettner GR. Spin trapping evidence for the lack of significant hydroxyl radical production during the respiration burst of human phagocytes using a spin adduct resistant to superoxide-mediate destruction. J Biol Chem. 1990;265:2650–6. [PubMed] [Google Scholar]
- 71.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–76. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 72.Thomas EL, Grisham MB, Jefferson MM. Myeloperoxidase-dependent effect of amines on functions of isolated neutrophils. J Clin Invest. 1983;72:441–54. doi: 10.1172/JCI110992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grisham MB. Effect of 5-aminosalicylic acid on ferrous sulfate-mediated damage to deoxyribose. Biochem Pharmacol. 1990;39:2060–3. doi: 10.1016/0006-2952(90)90630-4. [DOI] [PubMed] [Google Scholar]
- 74.Genser D, Kang MH, Vogelsang H, Elmadfa I. Status of lipidsoluble antioxidants and TRAP in patients with Crohn's disease and healthy controls. Eur J Clin Nutr. 1999;53:675–9. doi: 10.1038/sj.ejcn.1600764. [DOI] [PubMed] [Google Scholar]
- 75.Erichsen K, Hausken T, Ulvik RJ, Svardal A, Berstad A, Berge RK. Ferrous fumarate deteriorated plasma antioxidant status in patients with Crohn's disease. Scand J Gastroenterol. 2003;38:543–8. doi: 10.1080/00365520310000771. [DOI] [PubMed] [Google Scholar]
- 76.Forrest CM, Gould SR, Darlington LG, Stone TW. Levels of purine, kynurenine and lipid peroxidation products in patients with inflammatory bowel disease. Adv Exp Med Biol. 2003;527:395–400. doi: 10.1007/978-1-4615-0135-0_46. [DOI] [PubMed] [Google Scholar]
- 77.Rezaie A, Khalaj S, Shabihkhani M, Nikfar S, Zamani M , et al. Study on the Correlations among Disease Activity Index and Salivary Transforming Growth Factor-β1 and Nitric Oxide in Ulcerative Colitis Patients. An NY Acad Sci. 2007;1095:305–14. doi: 10.1196/annals.1397.034. [DOI] [PubMed] [Google Scholar]
- 78.Koutroubakis IE, Malliaraki N, Dimoulios PD, Karmiris K, Castanas E, Kouroumalis EA. Decreased total and corrected antioxidant capacity in patients with inflammatory bowel disease. Dig Dis Sci. 2004;49:1433–7. doi: 10.1023/b:ddas.0000042242.22898.d9. [DOI] [PubMed] [Google Scholar]
- 79.Holmes EW, Yong SL, Eiznhamer D, Keshavarzian A. Glutathione content of colonic mucosa: Evidence for oxidative damage in active ulcerative colitis. Dig Dis Sci. 1998;43:1088–95. doi: 10.1023/a:1018899222258. [DOI] [PubMed] [Google Scholar]
- 80.Rezaie A, Ghorbani F, Eshghtork A, Zamani MJ, Dehghan G, Taghavi B, et al. Alterations in salivary antioxidants, nitric oxide, and transforming growth factor-beta1 in relation to disease activity in Crohn's disease patients. Ann N Y Acad Sci. 2006;1091:110–22. doi: 10.1196/annals.1378.060. [DOI] [PubMed] [Google Scholar]
- 81.Pastorelli L, De Salvo C, Mercado JR, Vecchi M, Pizarro TT. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: Lessons learned from animal models and human genetics. Front Immunol. 2013;4:280. doi: 10.3389/fimmu.2013.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6–21. doi: 10.3748/wjg.v20.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang YZ, Li YY. Inflammatory bowel disease: Pathogenesis. World J Gastroenterol. 2014;20:91–9. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siegmund B, Zeitz M. Innate and adaptive immunity in inflammatory bowel disease. World J Gastroenterol. 2011;17:3178–83. doi: 10.3748/wjg.v17.i27.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beisner J, Stange EF, Wehkamp J. Innate antimicrobial immunity in inflammatory bowel diseases. Expert Rev Clin Immunol. 2010;6:809–18. doi: 10.1586/eci.10.56. [DOI] [PubMed] [Google Scholar]
- 86.McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:100–13. doi: 10.1002/ibd.20539. [DOI] [PubMed] [Google Scholar]
- 87.Monteleone I, Vavassori P, Biancone L, Monteleone G, Pallone F. Immunoregulation in the gut: Success and failures in human disease. Gut. 2003;50(Suppl 3):III60–4. doi: 10.1136/gut.50.suppl_3.iii60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pedersen J, Coskun M, Soendergaard C, Salem M, Nielsen OH. Inflammatory pathways of importance for management of inflammatory bowel disease. World J Gastroenterol. 2014;20:64–77. doi: 10.3748/wjg.v20.i1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Coskun M, Olsen J, Seidelin JB, Nielsen OH. MAP kinases in inflammatory bowel disease. Clin Chim Acta. 2011;412:513–20. doi: 10.1016/j.cca.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 90.Coskun M, Salem M, Pedersen J, Nielsen OH. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol Res. 2013;76:1–8. doi: 10.1016/j.phrs.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 91.Bouzid D, Gargouri B, Mansour RB, Amouri A, Tahri N, Lassoued S, et al. Oxidative stress markers in intestinal mucosa of Tunisian inflammatory bowel disease patients. Saudi J Gastroenterol. 2013;19:131–5. doi: 10.4103/1319-3767.111956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078–86. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 93.Chiarpotto E, Scavazza A, Leonarduzzi G, Camandola S, Biasi F, Teggia PM, et al. Oxidative damage and transforming growth factor beta 1 expression in pretumoral and tumoral lesions of human intestine. Free Radic Biol Med. 1996;22:889–94. doi: 10.1016/s0891-5849(96)00481-9. [DOI] [PubMed] [Google Scholar]
- 94.Jahanshahi G, Motavasel V, Rezaie A, Hashtroudi AA, Daryani NE, Abdollahi M. Alterations in antioxidant power and levels of epidermal growth factor and nitric oxide in saliva of patients with inflammatory bowel diseases. Dig Dis Sci. 2004;49:1752–7. doi: 10.1007/s10620-004-9564-5. [DOI] [PubMed] [Google Scholar]
- 95.Krzystek-Korpacka M, Neubauer K, Matusiewicz M. Platelet-derived growth factor-BB reflects clinical, inflammatory and angiogenic disease activity and oxidative stress in inflammatory bowel disease. Clin Biochem. 2009;42:1602–9. doi: 10.1016/j.clinbiochem.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 96.Pelli MA, Trovarelli G, Capodicasa E, De Medio GE, Bassotti G. Breath alkanes determination in ulcerative colitis and Crohn's disease. Dis Colon Rectum. 1999;42:71–6. doi: 10.1007/BF02235186. [DOI] [PubMed] [Google Scholar]
- 97.Koch TR, Yuan LX, Stryker SJ, Ratliff P, Telford GL, Opara EC. Total antioxidant capacity of colon in patients with chronic ulcerative colitis. Dig Dis Sci. 2000;45:1814–9. doi: 10.1023/a:1005517824877. [DOI] [PubMed] [Google Scholar]
- 98.Levy E, Rizwan Y, Thibault L, Lepage G, Brunet S, Bouthillier L, et al. Altered lipid proflle, lipoprotein composition, and oxidant and antioxidant status in pediatric Crohn disease. Am J Clin Nutr. 2000;71:807–15. doi: 10.1093/ajcn/71.3.807. [DOI] [PubMed] [Google Scholar]
- 99.D’Odorico A, Bortolan S, Cardin R, D’Inca’ R, Martines D, Ferronato A, et al. Reduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel disease. Scand J Gastroenterol. 2001;36:1289–94. doi: 10.1080/003655201317097146. [DOI] [PubMed] [Google Scholar]
- 100.Tuzun A, Erdil A, Inal V, Aydin A, Bağci S, Yeşilova Z, et al. Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease. Clin Biochem. 2002;35:569–72. doi: 10.1016/s0009-9120(02)00361-2. [DOI] [PubMed] [Google Scholar]
- 101.Ciorba MA. Kynurenine pathway metabolites: Relevant to vitamin B-6 deficiency and beyond. Am J Clin Nutr. 2013;98:863–4. doi: 10.3945/ajcn.113.072025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clarke G, Fitzgerald P, Cryan JF, Cassidy EM, Quigley EM, Dinan TG. Tryptophan degradation in irritable bowel syndrome: Evidence of indoleamine 2,3-dioxygenase activation in a male cohort. BMC Gastroenterol. 2009;9:6. doi: 10.1186/1471-230X-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sampietro GM, Cristaldi M, Cervato G, Maconi G, Danelli P, Cervellione R, et al. Oxidative stress, vitamin A and vitamin E behaviour in patients submitted to conservative surgery for complicated Crohn's disease. Dig Liver Dis. 2010;34:696–701. doi: 10.1016/s1590-8658(02)80020-2. [DOI] [PubMed] [Google Scholar]
- 104.Barbosa DS, Cecchini R, El Kadri MZ, Rodríguez MA, Burini RC, Dichi I. Decreased oxidative stress in patients with ulcerative colitis supplemented with flsh oil omega-3 fatty acids. Nutrition. 2003;19:837–42. doi: 10.1016/s0899-9007(03)00162-x. [DOI] [PubMed] [Google Scholar]
- 105.Rana SV, Sharma S, Prasad KK, Sinha SK, Singh K. Role of oxidative stress and antioxidant defence in ulcerative colitis patients from north India. Indian J Med Res. 2014;139:568–71. [PMC free article] [PubMed] [Google Scholar]
- 106.Hatoum OA, Binion DG, Otterson MF, Gutterman DD. Acquired microvascular dysfunction in inflammatory bowel disease: Loss of nitric oxide-mediated vasodilation. Gastroenterology. 2003;125:58–69. doi: 10.1016/s0016-5085(03)00699-1. [DOI] [PubMed] [Google Scholar]
- 107.Hatoum OA, Binion DG, Miura H, Telford G, Otterson MF, Gutterman DD. Role of hydrogen peroxide in ACh-induced dilation of human submucosal intestinal microvessels. Am J Physiol Heart Circ Physiol. 2005;288:H48–54. doi: 10.1152/ajpheart.00663.2004. [DOI] [PubMed] [Google Scholar]
- 108.Binion DG, Rafiee P. Is Inflammatory bowel disease a vascular disease? Targeting angiogenesis improves chronic inflammation in inflammatory bowel disease. Gastroenterology. 2009;136:400–3. doi: 10.1053/j.gastro.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 109.Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: Semi-quantiflcation, localization, and association with mucosal antioxidants. J Pathol. 2003;201:28–36. doi: 10.1002/path.1409. [DOI] [PubMed] [Google Scholar]
- 110.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–49. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 111.Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev. 2007;59:1073–83. doi: 10.1016/j.addr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 112.Hoffmann JC, Pawlowski NN, Kühl AA, Höhne W, Zeitz M. Animal models of inflammatory bowel disease: An overview. Pathobiology. 2002-2003;70:121–30. doi: 10.1159/000068143. [DOI] [PubMed] [Google Scholar]
- 113.Ardite E, Sans M, Panés J, Romero FJ, Piqué JM, Fernández-Checa JC. Replenishment of glutathione levels improves mucosal function in experimental acute colitis. Lab Invest. 2000;80:735–44. doi: 10.1038/labinvest.3780077. [DOI] [PubMed] [Google Scholar]
- 114.Nieto N, Torres MI, Fernández MI, Girón MD, Ríos A, Suárez MD, et al. Experimental ulcerative colitis impairs antioxidant defense system in rat intestine. Dig Dis Sci. 2000;45:1820–7. doi: 10.1023/a:1005565708038. [DOI] [PubMed] [Google Scholar]
- 115.Triantafillidis JK, Douvi G, Agrogiannis G, Patsouris E, Gikas A, Papalois AE. Effect of mesalamine and prednisolone on TNBS experimental colitis, following various doses of orally administered iron. Biomed Res Int 2014. 2014 doi: 10.1155/2014/648535. 648535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tham DM, Whitin JC, Cohen HJ. Increased expression of extracellular glutathione peroxidase in mice with dextran sodium sulfate-induced experimental colitis. Pediatr Res. 2002;51:641–6. doi: 10.1203/00006450-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 117.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–73. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ghazanfari G, Minaie B, Yasa N, Nakhai LA, Mohammadirad A, Nikfar S, et al. Biochemical and histopathological evidences for beneflcial effects of Satureja Khuzestanica Jamzad essential oil on the mouse model of inflammatory bowel diseases. Toxicol Mech Methods. 2007;16:365–72. doi: 10.1080/15376520600620125. [DOI] [PubMed] [Google Scholar]
- 119.Ghafari H, Yasa N, Mohammadirad A, Dehghan G, Zamani MJ, Nikfar S, et al. Protection by Ziziphora clinopoides of acetic acid-induced toxic bowel inflammation through reduction of cellular lipid peroxidation and myeloperoxidase activity. Hum Exp Toxicol. 2006;25:325–32. doi: 10.1191/0960327105ht626oa. [DOI] [PubMed] [Google Scholar]
- 120.Ding X, Hiraku Y, Ma N, Kato T, Saito K, Nagahama M, et al. Inducible nitric oxide synthase-dependent DNA damage in mouse model of inflammatory bowel disease. Cancer Sci. 2005;96:157–63. doi: 10.1111/j.1349-7006.2005.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jiang XL, Cui HF. A new chronic ulcerative colitis model produced by combined methods in rats. World J Gastroenterol. 2000;6:742–6. doi: 10.3748/wjg.v6.i5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mizoguchi E. Chitinase 3-like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology. 2006;130:398–411. doi: 10.1053/j.gastro.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 123.Papanikolaou IS, Psilopoulos DI, Liatsos C, Lazaris AC, Petraki K, Mavrogiannis C. Salmonella colitis or inflammatory bowel disease? A case demonstrating overlapping of clinical, endoscopic and pathologic features. Annual of Gastroenterology. 2001;14:65–9. [Google Scholar]
- 124.Jensen SR, Fink LN, Nielsen OH, Brynskov J, Brix S. Ex vivo intestinal adhesion of Escherichia coli LF82 in Crohn's disease. Microb Pathog. 2011;51:426–31. doi: 10.1016/j.micpath.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 125.Low D, Tran HT, Lee IA, Dreux N, Kamba A, Reinecker HC, et al. Chitin-binding domains of Escherichia coli chiA mediates interactions with intestinal epithelial cells in mice with colitis. Gastroenterology. 2013;145:602–12.e9. doi: 10.1053/j.gastro.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nagatani K, Wang S, Llado V, Lau CW, Li Z, Mizoguchi A, et al. Chitin microparticles for the control of intestinal inflammation. Inflamm Bowel Dis. 2013;18:1698–710. doi: 10.1002/ibd.22874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Miles AM, Grisham MB. Antioxidant properties of aminosalicylates. Methods Enzymol. 1994;234:555–72. doi: 10.1016/0076-6879(94)34128-1. [DOI] [PubMed] [Google Scholar]
- 128.Aghdassi E, Wendland BE, Steinhart AH, Wolman SL, Jeejeebhoy K, Allard JP. Antioxidant vitamin supplementation in Crohn's disease decreases oxidative stress: A randomized controlled trial. Am J Gastroenterol. 2003;98:348–53. doi: 10.1111/j.1572-0241.2003.07226.x. [DOI] [PubMed] [Google Scholar]
- 129.Trebble TM, Arden NK, Wootton SA, Calder PC, Mullee MA, Fine DR, et al. Fish oil and antioxidants alter the composition and function of circulating mononuclear cells in Crohn disease. Am J Clin Nutr. 2004;80:1137–44. doi: 10.1093/ajcn/80.5.1137. [DOI] [PubMed] [Google Scholar]
- 130.Trebble TM, Stroud MA, Wootton SA, Calder PC, Fine DR, Mullee MA, et al. High-dose flsh oil and antioxidants in Crohn's disease and the response of bone turnover: A randomised controlled trial. Br J Nutr. 2005;94:253–61. doi: 10.1079/bjn20051466. [DOI] [PubMed] [Google Scholar]
- 131.Geerling BJ, Badart-Smook A, van Deursen C, van Houwelingen AC, Russel MG, Stockbrügger RW, et al. Nutritional supplementation with N-3 fatty acids and antioxidants in patients with Crohn's disease in remission: Effects on antioxidant status and fatty acid proflle. Inflamm Bowel Dis. 2000;6:77–84. doi: 10.1097/00054725-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 132.Seidner DL, Lashner BA, Brzezinski A, Banks PL, Goldblum J, Fiocchi C, et al. An oral supplement enriched with flsh oil, soluble flber, and antioxidants for corticosteroid sparing in ulcerative colitis: A randomized, controlled trial. Clin Gastroenterol Hepatol. 2004;3:358–69. doi: 10.1016/s1542-3565(04)00672-x. [DOI] [PubMed] [Google Scholar]
- 133.Oz HS, Chen TS, McClain CJ, de Villiers WJ. Antioxidants as novel therapy in a murine model of colitis. J Nutr Biochem. 2005;16:297–304. doi: 10.1016/j.jnutbio.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 134.Abboud PA, Hake PW, Burroughs TJ, Odoms K, O’Connor M, Mangeshkar P, et al. Therapeutic effect of epigallocatechin-3-gallate in a mouse model of colitis. Eur J Pharmacol. 2008;579:411–7. doi: 10.1016/j.ejphar.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 135.Ran ZH, Chen C, Xiao SD. Epigallocatechin-3-gallate ameliorates rats colitis induced by acetic acid. Biomed Pharmacother. 2008;62:189–96. doi: 10.1016/j.biopha.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 136.Brückner M, Westphal S, Domschke W, Kucharzik T, Lügering A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J Crohns Colitis. 2012;6:226–35. doi: 10.1016/j.crohns.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 137.Hagar HH, Medany A, El Eter E, Araf M. Ameliorative effect of pyrrolidinedithiocarbamate on acetic acid-induced colitis in rats. Eur J Pharmacol. 2007;554:69–77. doi: 10.1016/j.ejphar.2006.09.066. [DOI] [PubMed] [Google Scholar]
- 138.Wang X, Zhao L, Han T, Chen S, Wang J. Protective effects of 2,3,5,4’-tetrahydroxystilbene-2-O-beta-d-glucoside, an active component of Polygonum multiflorum Thunb, on experimental colitis in mice. Eur J Pharmacol. 2008;578:339–48. doi: 10.1016/j.ejphar.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 139.Zhao ZJ, Xiang JY, Liu L, Huang XL, Gan HT. Parthenolide, an inhibitor of the nuclear factor-κB pathway, ameliorates dextran sulfate sodium-induced colitis in mice. Int Immunopharmacol. 2012;12:169–74. doi: 10.1016/j.intimp.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 140.Funakoshi T, Yamashita K, Ichikawa N, Fukai M, Suzuki T, Gotoa R, et al. A novel NF-κB inhibitor, dehydroxymethylepoxyquinomicin, ameliorates inflammatory colonic injury in mice. J Crohns Colitis. 2012;6:215–25. doi: 10.1016/j.crohns.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 141.Dashdorj A, Jyothi KR, Lim S, Jo A, Nguyen MN, Ha J, et al. Mitochondria-targeted antioxidant MitoQ ameliorates experimental mouse colitis by suppressing NLRP3 inflammasome-mediated inflammatory cytokines. BMC Med. 2013;11:178. doi: 10.1186/1741-7015-11-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Koppikar SJ, Jagtap SD, Devarshi PP, Jangle NM, Awad VB, Wele AA, et al. Triphala, an Ayurvedic formulation improves the antioxidant status on TNBS induced IBD in rats. Eur J Integr Med. 2014;6:646–56. [Google Scholar]
- 143.Ringel Y, Quigley EM, Lin HC. Using probiotics in gastrointestinal disorders. Am J Gastroenterol Suppl. 2012;1:34–40. [Google Scholar]
- 144.Biasi F, Astegiano M, Maina M, Leonarduzzi G, Poli G. Polyphenol supplementation as a complementary medicinal approach to treating inflammatory bowel disease. Curr Med Chem. 2014;18:4851–65. doi: 10.2174/092986711797535263. [DOI] [PubMed] [Google Scholar]
- 145.Shapiro H, Singer P, Halpern Z, Bruck R. Polyphenols in the treatment of inflammatory bowel disease and acute pancreatitis. Gut. 2007;56:426–35. doi: 10.1136/gut.2006.094599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Romier B, Schneider YJ, Larondelle Y, During A. Dietary polyphenols can modulate the intestinal inflammatory response. Nutr Rev. 2007;67:363–78. doi: 10.1111/j.1753-4887.2009.00210.x. [DOI] [PubMed] [Google Scholar]
- 147.Mueller D, Triebel S, Rudakovski O, Richling E. Influence of triterpenoids present in apple peel on inflammatory gene expression associated with inflammatory bowel disease (IBD) Food Chem. 2013;139:339–46. doi: 10.1016/j.foodchem.2013.01.101. [DOI] [PubMed] [Google Scholar]
- 148.Alhouayek M, Muccioli GG. The endocannabinoid system in inflammatory bowel diseases: From pathophysiology to therapeutic opportunity. Trends Mol Med. 2012;18:615–25. doi: 10.1016/j.molmed.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 149.Borrelli F, Fasolino I, Romano B, Capasso R, Maiello F, Coppola D, et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem Pharmacol. 2013;85:1306–16. doi: 10.1016/j.bcp.2013.01.017. [DOI] [PubMed] [Google Scholar]