Abstract
Background/Aim:
Ulcerative colitis (UC) is a type of chronic inflammatory bowel disease with unknown etiology. Several therapeutic strategies such as consumption of medicinal plants have been used for its treatment. The aim of this study was to evaluate healing effects of Calendula officinalis hydroalcoholic extract in experimentally induced UC in rat.
Materials and Methods:
Ninety-six rats, weighing 200 ± 20 g, were randomly divided into eight equal groups. UC induced by 3% acetic acid and oral doses of C. officinalis extract, 1500 and 3000 mg/kg, and enema (gel 10% and 20%) were given. Two groups as positive controls were given asacol (enema) and oral mesalamine. Negative control groups were given normal saline and base gel. On days 3 and 7, intestinal histopathology and weight changes, plus oxidative stress indices including malondialdehyde (MDA) level and myeloperoxidase (MPO) activity were assayed.
Results:
A significant increase in the body weight of rats was seen in the group given C. officinalis extract 3000 mg/kg orally, oral mesalamine, and 20% intracolonic gel form of marigold extract compared with negative control and base gel groups during the experimental period. Acute inflammation and granular atrophy after UC induction were resolved completely completely by both 20% intracolonic gel and 3000 mg/kg orally. An increase in MPO activity and a decrease in MDA level in response to oral and intracolonic gel form of C. officinalis were observed 3 and and 7 days after treatment (P < 0.05).
Conclusion:
Our results indicate that oral and enema forms of hydroalcoholic extract of C. officinalis can be offered as are potential therapeutic agents for UC induced in rats.
Key Words: Histopathology, marigold, oxidative stress, ulcerative colitis
Ulcerative colitis (UC) is an idiopathic inflammatory bowel disease. It is an interaction between including genetic, immunological, reactive oxygen species, and environmental factors, which affects the large intestine. It is clinically characterized by diarrhea, abdominal pain or rectal pain, fever, weight loss, and blood in the stool.[1] Although UC is most commonly detected in young adulthood,[2] the condition can affect patients of any age and both genders. Also this disease affects women slightly more than men.[3] Loftus et al. reported that the incidence of UC is about 10–20 per 106 per year with a prevalence of 100–200 per 106 in Western countries.[4] Overall, the rate of UC is higher in developed countries than in developing countries due to their lifestyle,[5] whereas it is increasing in Iran[6] and Taiwan.[7]
It is suggested that environmental conditions are more effective than genetics. Other factors such as intestinal microflora, mucosal immune response, autoimmune reactions, and especially oxidative stress are also important in the pathophysiology of UC.[8] Medical and surgical therapies are the current modalities for treatment of UC, but surgery is indicated for those who are unresponsive to medical therapy. Although bacterial bowel flora such as Shigella, Clostridium difficile, and Escherichia coli 0157:H7 may be one of the contributing factors in the pathogenesis of chronic mucosal inflammation, antibiotics treatment has no established role in UC.[9]
The use of no effectiveness of usual treatments, the use of alternative medicines, mainly of herbal treatments, for UC is increasing.[10] The effects of many medicinal plants in UC have been investigated in the past decades. These include Strawberry,[11] Vitex negund,[12] licorice,[13,14] Ginkgo biloba,[15] Oroxylum indicu,[1] Origanum onites,[16] and Calendula officinalis[17,18] in rats, Polygonum multiflorum in mice,[19] Fragaria vesca[20] and Moringa olifera in albino rats,[12] Teucrium polium[21] and Calendula Officinalis in dog,[22] and C. officinalis in mice.[23]
C. officinalis, also known as Marigold, is an annual herb native to the Mediterranean region,[24] and an important medicinal plant in the family Asteraceae. In traditional medicine, it is used for the treatment of fever and cancer.[25] It is hypothesized to be effective in treating this disease due to its antioxidant[26,27,28] and anti-inflammatory[29,30] compounds. C. officinalis extract decreases lipid peroxidation and inhibits edema. It is also used for gastrointestinal,[28] gynecological, eye, and skin diseases.[31] The plant is rich in several pharmaceutically active ingredients such as sterols, flavonoids, carotenoids, and glycosides.[32]
This study has been undertaken to compare the effects of oral and enema forms of hydroalcohol extract of C. Officinalis on improvement of acetic acid-induced UC in adult male rats.
MATERIALS AND METHODS
Plant materials
C. officinalis was collected from Abadeh of Fars province in Iran (GPS coordinates: 31.160833,52.650556) during the spring season. All plants were in the flowering stage and the taxonomic identification of each plant was confirmed by a Specialized Botanist. The plant also matched the digital herbarium of Botanical Garden and Botanical Museum Berlin-Dahlem, Freie University, Berlin (http://herbarium.bgbm.org/object/BW16687040 and http://ww2.bgbm.org/herbarium/(Barcode: B -W 16687 -02 0/Image Id: 397152). This plant also was recorded in the herbarium of Pharmacology Department, Shiraz University of Medical Sciences, Shiraz, Iran, as herbarium number: 763.
Preparation of hydroalcoholic extract
Hydroalcoholic extract was prepared from C. officinalis flowers by Pharmacology Department of Shiraz University of Medical Sciences according to the previous reports.[33,34,35,36,37,38,39,40,41] C. officinalis flowers were collected from Fars province (southwest of Iran). Flowers were shade-dried in the laboratory at ambient temperature of 25–30°C and relative humidity. Then, dried flowers were powdered in a mechanical grinder and soaked in adequate volume of ethanol: Water (80:20) solution for 72 h using percolation method. The extract was filtered and evaporated in a rotary evaporator under reduced pressure then dried in temperature of 50°C for 72 h and stored at −20°C.
Animals and housing
Totally 96 Sprague–Dawley male rats, weighing 200 ± 20 g, were obtained from Laboratory Animal Center of Shiraz University of Medical Sciences. The animals were housed in a restricted-access room under controlled environment (22 ± 1°C, 55% relative humidity, and 12:12 h light–dark cycle) for seven days as acclimation with access to water and feed ad libitum. The study protocol was approved by the Ethics Committee of Shiraz University of Medical Sciences (registration number: 90-5077).
UC induction
Acetic acid was used for induction of UC according to a previous report.[36] All rats were fasted overnight and induction was done using a soft catheter, where the tip of the catheter inserted up to 8 cm proximal to the anus after removing distal stools. UC was induced by transrectal administration of 1 mL of 3% acetic acid. The rats were maintained in head down position for 30 s to prevent leakage.
Experimental grouping
Rats were randomly divided into eight equal groups and different treatments were applied daily as following:
Group I (negative control 1) received 1 ml/kg of normal saline orally
Group II (negative control 2) received 1 ml/kg of base gel intracolonic
Group III (positive control 1) received 10 mg/kg of asacol intracolonic
Group IV (positive control 2) received 10 mg/kg of mesalamine orally
Group V received the 10% C. officinalis extract in the gel form, intracolonic
Group VI received the 20% C. officinalis extract in the gel form, intracolonic
Group VII received 1500 mg/kg of C. officinalis extract orally
Group VIII received 3000 mg/kg of C. officinalis extract orally.
The 1500 and 300 mg/kg oral form of C. officinalis extract were given other than food in the form of syrup by gavage every morning, at 8 o’clock.
Base gel was (100 g) produced by dissolving 1 g of carboxymethyl cellulose and 9 g glycin in 90 mL of deionized water. Their weight was also recorded daily, and percentage of weight change was calculated using the following formula:
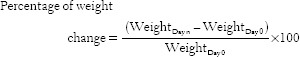
Myeloperoxidase activity
On the 3rd day after starting the treatment, half of the animals from each group were randomly selected to take blood samples directly from their heart under inhaled anesthesia in order to measure the activity of myeloperoxidase (MPO) in the serum. After blood sampling, the animals were euthanized for histopathological study. The study same procedure was followed on the 7th day after starting the treatment. MPO activity can be measured in tissues by assays using hydrogen peroxide and o-dianisidine dihydrochloride as substrates.[37]
Malondialdehyde level
Obtained tissues were stored under liquid nitrogen and then tissue malondialdehyde (MDA) assessment was performed. In this procedure the measurement of thiobarbituric acid reactive substances in PBS tissue homogenate was performed,[38] because MDA is one of the end products of lipid peroxidation (LPO), and the extent of LPO is most frequently measured by estimating MDA levels.[39] There are different indicators for oxidative stress that show tissue lipid peroxidation. Due to available laboratory instruments, MPO and MDA (LPO indicators) were evaluated.
Histopathological evaluation
Half of the animals from each group were randomly selected and euthanized by ether inhalation on day 3 after induction of colitis and half of them after 7 days. Abdomens were opened using a surgical scissor. Colonic tissue was studied histopathologically as described in previous reports.[40,41,42,43,44] For each rat, the distal 8 cm portion of the colon was immediately removed,[36] free of surrounding fat, and fixed in 10% buffered formalin for histopathological examination. Formalin-fixed tissues were processed routinely and embedded in paraffin. Blocks were cut at 5 μm, and sections were stained with hematoxylin–eosin. All sections were studied and photographed by a light microscope.
Statistical analysis
The data were expressed as means ± SD. Differences between groups were carried out by one-way ANOVA and Tukey HSD post hoc test. Pathological data were evaluated using Mann–Whitney test. All statistical analyses were done by SPSS software (Version 20) and Excel Office program (Version 10). Differences at P < 0.05 were considered significant.
RESULTS
Hydroalcoholic extraction of the dried flowers of C. officinalis yielded 18.19 g dried extract in 100 g of dried powder. Therefore, the efficiency of our extraction procedure was 18.19%. Significant difference was seen in weight change between all treatment groups in comparison with negative control and base gel groups at the end of experiment (P < 0.05). the most statistical differences were observed in C. officinalis extract at a dose of 3000 mg/kg orally, mesalamine (oral) and 20% intracolonic gel form of marigold extract, respectively [Figure 1]. Changes in histopathological features of the colonic tissue after induction of UC and 7 days of treatment by C. officinalis extract as a dose of as 20% intracolonic gel and 3000 mg/kg orally are presented in Figure 2. Also, Table 1 shows histopathological assessment of colonic tissue after different treatments post-induction of UC in rats on days 3 and 7. Acute inflammation and granular atrophy after UC induction were resolved completely by both 20% intracolonic gel and 3000 mg/kg orally. Histopathological scoring varied and includes no damage, mild, moderate, and severe damage with a score of 0–4, respectively. MPO activity of different groups in 3 and 7 days after treatment is presented in Figure 3. MPO activity on 3 days after administration of C. officinalis extract at 1500 and 3000 mg/kg orally, 10%, 20% intracolonic gel form, asacol (enema), and mesalamine (oral) significantly decreased (P < 0.05) when compared with negative control groups 1 and 2. Also MPO activity on day 3 PT in groups, which had received 20% intracolonic gel form and 3000 mg/kg orally revealed a significant increase in comparison to asacol (P < 0.05). MPO activity on 7 days after administration of C. officinalis extract (3000 mg/kg) orally, 20% intracolonic gel form, and mesalamine significantly decreased as compared with both the negative control groups similar to that of administration of asacol, oral form of C. officinalis extract (1500 mg/kg) and 10% intracolonic gel form (P < 0.05). Also, there was a significant increase for MPO activity on day 7. PT in groups were given 20% intracolonic gel forms when compared with mesalamine, asacol, 20% intracolonic gel forms and C. officinalis extract as 3000 mg/kg orally (P < 0.05).
Figure 1.
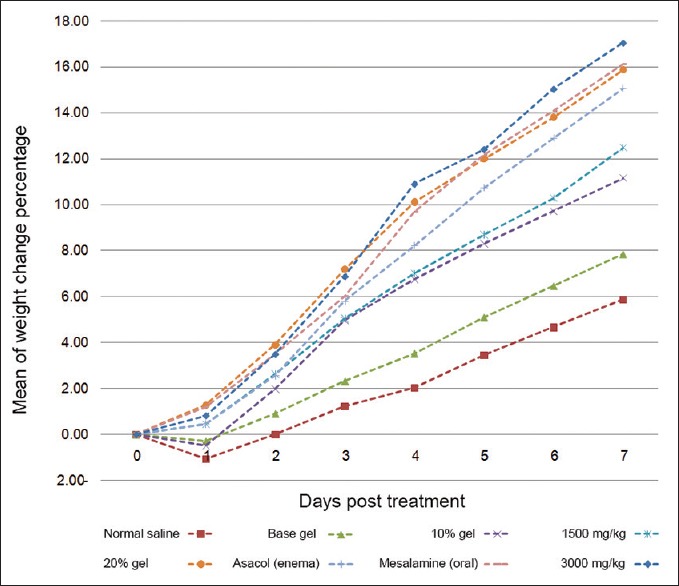
Percentage of mean weight changes in different groups during seven days after ulcerative colitis induction
Figure 2.
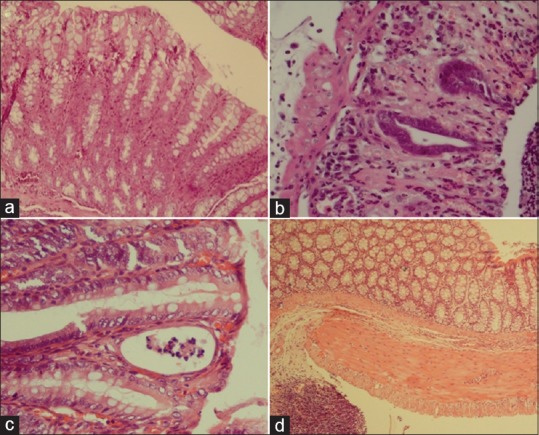
Histopathological changes associated with experimental ulcerative colitis induction and treatment with Calendula officinalis extract. (a), healthy colon; (b), acute inflammation and granular atrophy in response to acetic acid 3%; (c), 20% gel of C. officinalis extract in enema rout; (d), 3000 mg/kg of C. officinalis extract, orally (H and E, ×400)
Table 1.
Histopathologic assessment of colonic tissue after treatment of oral and enema forms of hydroalcohol extract of marigold post-induction of UC in rats in the third and seventh days (mean±SD, n=6)
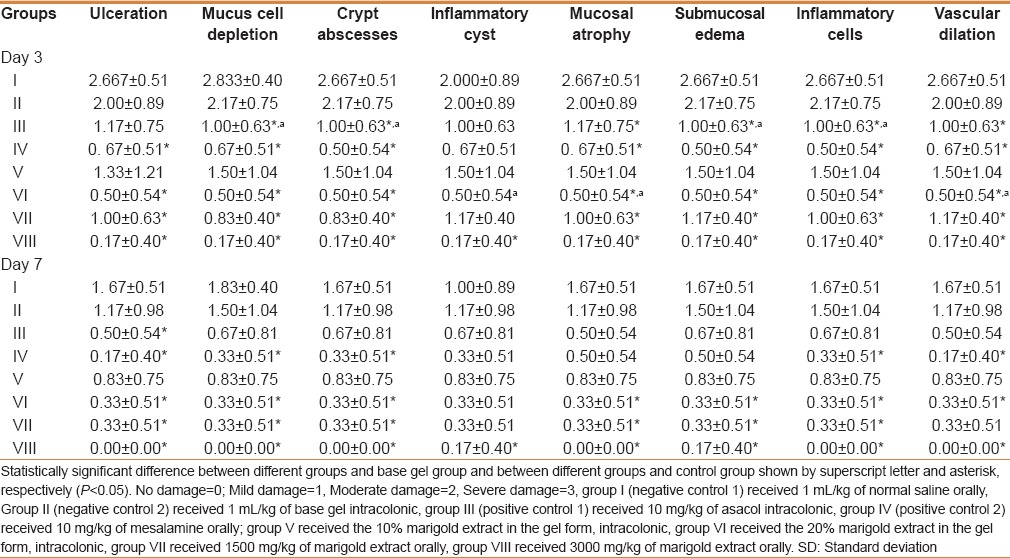
Figure 3.
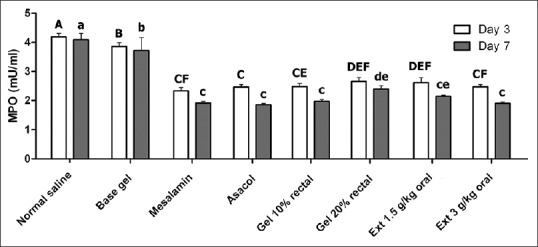
Changes of myeloperoxidase (MPO) activity in different groups at 3 and 7 days after induction of ulcerative colitis. Significant differences between groups at day 3 and 7 were indicated by different capital and lowercase letters, respectively (P < 0.05)
MDA level changes in different groups on 3rd and 7th day after treatment are presented in Figure 4. As demonstrated, MDA level in base gel treatment group was significantly lower than normal saline-treated group on both 3rd and 7th days after treatment (P < 0.05). Also, MDA levels on on the 3rd and 7th days after administration of C. officinalis extract orally in both doses, 10% intracolonic gel form, asacol (enema), and mesalamine (orally) significantly decreased (P < 0.05) when compared with both negative control groups (P < 0.05). Other significant differences between different treatment groups are presented in Figure 4.
Figure 4.
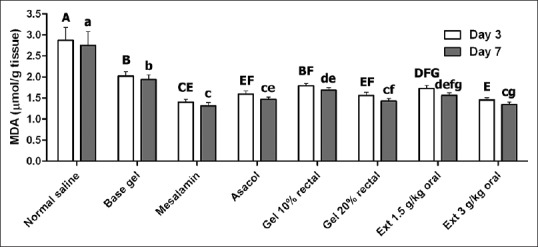
Changes of malondialdehyde (MDA) level in different groups at 3 and 7 days after induction of ulcerative colitis. Significant differences between groups at day 3 and 7 were indicated by different capital and lowercase letters, respectively (P < 0.05)
DISCUSSION
In the present study, the healing effects of C. officinalis extract as oral and intracolonic administration in acid-induced UC were evaluated and compared with normal saline, base gel, and standard treatments. Our results demonstrated that there were clear differences between groups that received hydroalcohol extract of C. officinalis with negative control groups, although they were similar to positive control groups.
UC increases the inflammatory cells and free radicals with severe oxidative stress. MPO and MDA, inflammatory mediators, are two factors to evaluate oxidative stress. MPO is used as a marker enzyme for measuring polymorphonuclear leukocytes and has been used as a quantitative index of inflammation in several tissues, including the intestine.[45] MDA and MPO are indicators of lipid peroxidation. So the high amount of MPO and MDA is due to the severity of inflammation, lipid peroxidation, and tissue damage.[46] Measuring of MPO activity and MDA level showed that C. officinalis extract in the two forms of oral and transrectal administration and in different doses on is more effective than negative control and base gel groups in decreasing of inflammation in acetic acid-induced UC but similar to the asacol (transrectal)-treated group. In the present study, by using of C. officinalis extract orally and intracoloniclly, the decrease in the MPO and MDA activity occurs with the decrease in lipid peroxidation. This may be due to decline in the inflammation and neutrophil accumulation in the inflamed intestine. These findings were in agreement with results of Preethi et al.[30] that C. officinalis extract by oral administration acted as anti-inflammatory in reduction of paw edema and inflammation in mice and confirmed by Preethi et al.[27] that the oral administration of C. officinalis extract acts as antioxidant inhibited lipid peroxidation in mice tissues and also scavenged free radicals.
C. officinalis extract possessed flavonoids, flavoxanthin, saponins, triterpiniod esters, and auroxanthin, and that by blocking the arachidonic acid pathway and inhibiting mechanisms of MPO and MDA, it induces its anti-inflammatory and antioxidant properties.[26,27,29,30,32,47] Due to these, C. officinalis extract could prevent colitis. Triterpenoids, by increasing the transcriptional activity of nuclear factor erythroid-derived 2)-like 2 (NRF2), which induces the formation of superoxide dismutase and catalase, keep tissues from oxidative stress.[12]
Histopathological study showed that C. officinalis extract could induce a significant improvement in healing of UC while that of oral (3000 mg/kg) and gel form (20%, trans-rectal) had similar effects with mesalamine (oral) due to high proliferation of epithelial cells. In negative control and base gel groups, reduction of epithelial cells, granulation tissues, and healing were significantly obvious when compared with others. Histological changes of this study in control groups were in agreement with results of Tanideh et al.[11] in rats and Mehrabani et al.[22] in dogs with acetic acid-induced UC, respectively. The histopathological analyses of UC also give the evidence of the increased healing potential of the C. officinalis extract due to its ability to decrease acute phase proteins. In addition, some histopathological factors such as cyst, inflammatory cells, edema, and abscess in group were given 3000 mg/kg of C. officinalis extract (oral form) were at least among all experimental groups, which approved that of authority to reduce inflammation. Vasodilation in negative control and base gel groups was more than others, whereas it was at least in group were given 3000 mg/kg of C. officinalis extract (oral form). Also this showed the potency of C. officinalis extract to degrade inflammation due to its flavonoids[48] and triterpiniod[47] compounds.
Microscopic findings such as mucosal and submucosal erosion, acute inflammation, and granular epithelial necrosis were seen in colon tissues of negative control groups more than others. But those which received the extract, inflammation was low and erosion was limited to the mucosa. Interestingly, healing effects of transrectal gel form (20%) and oral administration (3000 mg/kg) of C. officinalis extract were significantly more in comparison to control groups similar to mesalamine orally. Mehrabani et al.[22] reported similar results after administration of C. officinalis that caused significant mucosal healing in dogs with UC.
CONCLUSION
This study demonstrated that the daily application of C. officinalis extract in gel form or by oral administration can relieve the UC induced by acetic acid in the colon of rat. Results for tissue MDA level and MPO activity plus histopathological evaluations indicated a reduction in inflammation. Studies are required to assess if this agent would be of potential benefit in humans.
Footnotes
Source of Support: Nil
Conflict of Interest: The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
REFERENCES
- 1.Joshi SV, Vyas BA, Shah PD, Shah DR, Shah SA, Gandhi TR. Protective effect of aqueous extract of Oroxylum indicum Linn. (root bark) against DNBS-induced colitis in rats. Indian J Pharmacol. 2011;43:656–61. doi: 10.4103/0253-7613.89821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanauer SB. Inflammatory bowel disease: Epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12(Suppl 1):S3–9. doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein CN, Blanchard JF, Rawsthorne P, Wajda A. Epidemiology of Crohn's disease and ulcerative colitis in a central Canadian province: A population-based study. Am J Epidemiol. 1999;149:916–24. doi: 10.1093/oxfordjournals.aje.a009735. [DOI] [PubMed] [Google Scholar]
- 4.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 5.Aghazadeh R, Zali M, Bahari A, Amin K, Ghahghaie F, Firouzi F. Inflammatory bowel disease in Iran: A review of 448 cases. Arch Iran Med. 2004;7:210–6. doi: 10.1111/j.1440-1746.2005.03905.x. [DOI] [PubMed] [Google Scholar]
- 6.Shayesteh AA, Saberifirozi M, Abedian S, Sebghatolahi V. Epidemiological, demographic, and colonic extension of ulcerative colitis in Iran: A systematic review. Middle East J Dig Dis. 2013;5:29–36. [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CY, Lee KT, Tzu-Chi LC, Lai WT, Huang YB. Epidemiology and disease burden of ulcerative colitis in Taiwan: A nationwide population-based study. Value Health Reg Issues. 2013;2:127–34. doi: 10.1016/j.vhri.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Ajlouni Y, Shennak M. Ulcerative colitis. In: Shennak MM, editor. Ulcerative Colitis from Genetics to Complications. InTec; 2012. pp. 12–18. [Google Scholar]
- 9.Turunen UM, Färkkilä MA, Hakala K, Seppälä K, Sivonen A, Ogren M, et al. Long-term treatment of ulcerative colitis with ciprofloxacin: A prospective, double-blind, placebo-controlled study. Gastroenterology. 1998;115:1072–8. doi: 10.1016/s0016-5085(98)70076-9. [DOI] [PubMed] [Google Scholar]
- 10.Rahimi R, Mozaffari S, Abdollahi M. On the use of herbal medicines in management of inflammatory bowel diseases: A systematic review of animal and human studies. Dig Dis Sci. 2009;54:471–80. doi: 10.1007/s10620-008-0368-x. [DOI] [PubMed] [Google Scholar]
- 11.Tanideh N, Baseri FA, Jamshidzadeh A, Ashraf MJ, Kuhi O, Mehrabani D. The healing effect of Strawberry extract on acetic acid-induced ulcerative colitis in rat. World Appl Sci J. 2014;31:281–8. [Google Scholar]
- 12.Das S, Kanodia L. Effect of ethanolic extract of leaves of Vitex negundo L. on acetic acid induced colitis in Albino rats. Asian J Pharm Clin Res. 2013;6:138–41. doi: 10.4103/0253-7613.117728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takhshid MA, Mehrabani D, Ai J, Zarepoor M. The healing effect of licorice extract in acetic acid-induced ulcerative colitis in rat model. Comp Clin Pathol. 2012;21:1139–44. [Google Scholar]
- 14.Zargari-Samadnejad A, Mehrvarz S, Mohammadi-Samani S, Allizadeh-Naeini M, Tanideh N. Healing effect of Licorice extract in acetic acid-induced ulcerative colitis in rat. Res Pharmaceutical Sci. 2012;7:S837. [Google Scholar]
- 15.Mustafa A, El-Medany A, Hagar HH, El-Medany G. Ginkgo biloba attenuates mucosal damage in a rat model of ulcerative colitis. Pharmacol Res. 2006;53:324–30. doi: 10.1016/j.phrs.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Dundar E, Olgun EG, Isiksoy S, Kurkcuoglu M, Baser KH, Bal C. The effects of intra-rectal and intra-peritoneal application of Origanum onites L. essential oil on 2,4,6-trinitrobenzenesulfonic acid-induced colitis in the rat. Exp Toxicol Pathol. 2008;59:399–408. doi: 10.1016/j.etp.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Ozkol H, Tülüce Y, Koyuncu I. Subacute effect of cigarette smoke exposure in rats: Protection by pot marigold (Calendula officinalis L.) extract. Toxicol Ind Health. 2011;28:3–9. doi: 10.1177/0748233711401263. [DOI] [PubMed] [Google Scholar]
- 18.Parente LM, Andrade MA, Brito LA, Moura VM, Miguel MP, Lino-Júnior Rde S, et al. Angiogenic activity of Calendula officinalis flowers L. in rats. Acta Cir Bras. 2011;26:19–24. doi: 10.1590/s0102-86502011000100005. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Zhao L, Han T, Chen S, Wang J. Protective effects of 2,3,5,4’-tetrahydroxystilbene-2-O-beta-d-glucoside, an active component of Polygonum multiflorum Thunb, on experimental colitis in mice. Eur J Pharmacol. 2008;578:339–48. doi: 10.1016/j.ejphar.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Kanodia L, Borgohain M, Das S. Effect of fruit extract of Fragaria vesca L. on experimentally induced inflammatory bowel disease in Albino rats. Indian J Pharmacol. 2011;43:18–21. doi: 10.4103/0253-7613.75660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehrabani D, Bahrami F, Hosseini SV, Ashraf MJ, Tanideh N, Rezaianzadeh A, et al. The healing effect of Teucrium polium in acetic acid-induced ulcerative colitis in the dog as an animal model. Middle East J Dig Dis. 2012;4:40–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Mehrabani D, Ziaei M, Hosseini SV, Ghahramani L, Bananzadeh AM, Ashraf MJ, et al. The effect of Calendula officinalis in therapy of acetic acid induced ulcerative colitis in dog as an animal model. Iran Red Crescent Med J. 2011;13:884–90. [PMC free article] [PubMed] [Google Scholar]
- 23.Preethi KC, Siveen KS, Kuttan R, Kuttan G. Inhibition of metastasis of B16F-10 melanoma cells in C57BL/6 mice by an extract of Calendula officinalis L flowers. Asian Pac J Cancer Prev. 2010;11:1773–9. [PubMed] [Google Scholar]
- 24.Fonseca YM, Catini CD, Vicentini FT, Nomizo A, Gerlach RF, Fonseca MJ. Protective effect of Calendula officinalis extract against UVB-induced oxidative stress in skin: Evaluation of reduced glutathione levels and matrix metalloproteinase secretion. J Ethnopharmacol. 2010;127:596–601. doi: 10.1016/j.jep.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Krag K. Cambridge, Massachusetts, United States: Harvard University; 1976. Plants Used as Contraceptives by the North American Indians: An Ethnobotanical Study: Senior Honors Thesis; pp. 87–99. [Google Scholar]
- 26.Albulescu M, Alexa N, Gojan C. Calendula officinalis flowers, source of extracts with antioxidant activity. Ser Chem. 2004;13:169–70. [Google Scholar]
- 27.Preethi KC, Kuttan G, Kuttan R. Antioxidant Potential of an Extract of Calendula officinalis. Flowers in vitro. and in vivo. Pharm biol. 2006;44:691–7. [Google Scholar]
- 28.Yoshikawa M, Murakami T, Kishi A, Kageura T, Matsuda H. Medicinal flowers. III. Marigold. (1): Hypoglycemic, gastric emptying inhibitory, and gastroprotective principles and new oleanane-type triterpene oligoglycosides, calendasaponins A, B, C, and D, from Egyptian Calendula officinalis. Chem Pharm Bull (Tokyo) 2001;49:863–70. doi: 10.1248/cpb.49.863. [DOI] [PubMed] [Google Scholar]
- 29.Meenatchisundaram S, Parameswari G, Subbraj T, Suganya T, Michael A. Note on pharmacological activities of Calendula officinalis L. Ethnobot Leaflets. 2009;13:51–4. [Google Scholar]
- 30.Preethi KC, Kuttan G, Kuttan R. Anti-inflammatory activity of flower extract of Calendula officinalis Linn. and its possible mechanism of action. Indian J Exp Biol. 2009;47:113–20. [PubMed] [Google Scholar]
- 31.Chakraborthy GS. Antimicrobial activity of the leaf extracts of Calendula officinalis Linn. J Herb Med Toxicol. 2008;2:65–6. [Google Scholar]
- 32.Epididymis ligation: A minimally invasive technique for preparation of teaser rams. Pietta P, Bruno A, Mauri P, Rava A. Separation of flavonol-2-O-glycosides from Calendula officinalis and Sambucus nigra by high-performance liquid and micellar electrokinetic capillary chromatography. J Chromatogr. 1992;593:165–70. doi: 10.1016/0021-9673(92)80282-y. [DOI] [PubMed] [Google Scholar]
- 33.Hafezi H, Vahdati A, Sepehrimanesh M. Effect of Satureja khuzestanica Jamzad extract on serum lipid profile, blood glucose level and body weight gain in diabetes mellitus: A Rattus norvegicus model. Comp Clin Pathol. 2014 [Google Scholar]
- 34.Koohi-Hosseinabadi O, Moini M, Safarpoor A, Derakhshanfar A, Sepehrimanesh M. Effects of dietary Thymus vulgaris extract alone or with atorvastatin on the liver, kidney, heart, and brain histopathological features in diabetic and hyperlipidemic male rats. Comp Clin Pathol. 2015 [Google Scholar]
- 35.Hajhashemi V, Ghannadi A, Hajiloo M. Analgesic and anti-inflammatory effects of Rosa damascena hydroalcoholic extract and its essential oil in animal models. Iran J Pharm Res. 2010;9:163–8. [PMC free article] [PubMed] [Google Scholar]
- 36.Tanideh N, Nematollahi SL, Hosseini SV, Hosseinzadeh M, Mehrabani D, Safarpour A, et al. The healing effect of Hypericum perforatum extract on acetic acid-induced ulcerative colitis in rat. Ann Colorectal Res. 2014;2:e25188. [Google Scholar]
- 37.Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemie myocardium. J Pharmacol Methods. 1985;14:157–67. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- 38.Subbarao KV, Richardson JS, Ang LC. Autopsy samples of Alzheimer's cortex show increased peroxidation in vitro. J Neurochem. 1990;55:342–5. doi: 10.1111/j.1471-4159.1990.tb08858.x. [DOI] [PubMed] [Google Scholar]
- 39.Lata H, Ahuja GK, Narang AP, Walia L. Effect of immobilisation stress on lipid peroxidation and lipid profile in rabbits. Indian J Clin Biochem. 2004;19:1–4. doi: 10.1007/BF02894248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajaian H, Nazifi S, Mansourian M, Sepehrimanesh M, Poorbaghi SL, Ghezelbash A. Pathological changes associated with experimental salinomycin toxicosis in calves. Online J Vet Res. 2008;12:15–22. [Google Scholar]
- 41.Sadeghi-Nejad B, Shiravi F, Ghanbari S, Alinejadi M, Zarrin M. Antifungal activity of Satureja khuzestanica (Jamzad) leaves extracts. Jundishapur J Microbiol. 2007;3:36–40. [Google Scholar]
- 42.Sepehrimanesh M, Azarpira N, Saeb M, Nazifi S, Kazemipour N, Koohi O. Pathological changes associated with experimental 900-MHz electromagnetic wave exposure in rats. Comp Clin Pathol. 2014;23:1629–31. [Google Scholar]
- 43.Tafti AK, Nazifi S, Rajaian H, Sepehrimanesh M, Poorbaghi SL, Mohtarami S. Pathological changes associated with experimental salinomycin toxicosis in sheep. Comp Clin Pathol. 2008;17:255–8. [Google Scholar]
- 44.Tamadon A, Nikahval B, Sepehrimanesh M, Mansourian M, Naeini AT, Nazifi S. Epididymis ligation: A minimally invasive technique for preparation of teaser rams. Vet Surg. 2010;39:121–7. doi: 10.1111/j.1532-950X.2009.00617.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhou H, Mineshita S. The effect of berberine chloride on experimental colitis in rats in vivo and in vitro. J Pharmacol Exp Ther. 2000;294:822–9. [PubMed] [Google Scholar]
- 46.Blair-Johnson M, Fiedler T, Fenna R. Human myeloperoxidase: Structure of a cyanide complex and its interaction with bromide and thiocyanate substrates at 1.9 A resolution. Biochemistry. 2001;40:13990–7. doi: 10.1021/bi0111808. [DOI] [PubMed] [Google Scholar]
- 47.Della Loggia R, Tubaro A, Sosa S, Becker H, Saar S, Isaac O. The role of triterpenoids in the topical anti-inflammatory activity of Calendula officinalis flowers. Planta Med. 1994;60:516–20. doi: 10.1055/s-2006-959562. [DOI] [PubMed] [Google Scholar]
- 48.Jiménez-Medina E, Garcia-Lora A, Paco L, Algarra I, Collado A, Garrido F. A new extract of the plant Calendula officinalis produces a dual in vitro effect: Cytotoxic anti-tumor activity and lymphocyte activation. BMC Cancer. 2006;6:119. doi: 10.1186/1471-2407-6-119. [DOI] [PMC free article] [PubMed] [Google Scholar]


