Abstract
Background/Aims:
Tumor recurrence after curative therapy is common for patients with hepatocellular carcinoma (HCC). As fibrosis and chronic inflammation contribute to the progression of HCC, we aimed to identify the predictive value of inflammatory and fibrosis markers for HCC recurrence after curative therapy using radiofrequency ablation (RFA).
Materials and Methods:
We retrospectively reviewed the records of patients with HCC treated with RFA between October 2005 and September 2013. The median duration of follow-up was 40 months (4–95 months). Inflammatory and fibrosis markers and demographic and clinical data were analyzed by Cox proportional hazards model using univariate and multivariate analyses and longitudinal analysis.
Results:
A total of 98 patients were included for analysis. There were 54 cases of HCC recurrence (55.1%). The aspartate aminotransferase-to-platelet ratio index (APRI; 2.3 ± 1.8 vs. 1.3 ± 1.4, P = 0.018) was significantly higher in the recurrence group than in the recurrence-free group. In multivariate analysis, APRI (hazard ratio, 2.64; confidence interval, 1.488–4.714; P = 0.001) was an independent risk factor for tumor recurrence. In particular, patients with APRI >1.38 showed a higher recurrence rate than patients with APRI ≤1.38 (P < 0.001). Longitudinal analysis showed persistently higher APRI values when assessed 12 months after RFA in patients who developed recurrence during follow-up than those who remained recurrence-free.
Conclusions:
These findings show that a high APRI value is associated with HCC recurrence after RFA. Therefore, APRI could play an important role in predicting HCC recurrence after RFA.
Key Words: Aspartate aminotransferase-to-platelet ratio index, hepatocellular carcinoma, radiofrequency ablation
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide, and its incidence is increasing in Asia and in the United States.[1] Its development is linked to the occurrence of a chronic liver disease due to chronic infection of hepatitis B virus (HBV) or hepatitis C virus (HCV), alcohol consumption, and metabolic syndrome. The underlying liver parenchyma is rarely normal and shows various histological changes including inflammation and fibrosis leading to cirrhosis. These histological changes of the underlying liver and the multicentric origin of tumors hamper the possibility of curative treatments including radiofrequency ablation (RFA), partial liver resection, and liver transplantation.[2,3] The cumulative 5-year recurrence rate is approximately 67–80%.[4,5] Furthermore, most patients with HCC are not candidates for surgical resection because of poor hepatic reserve. In this perspective, several minimally invasive techniques were studied, such as RFA, ethanol or acetic acid injection. RFA was gradually replaced by ethanol injection, which is the most widely used percutaneous treatment.[6]
Recurrence of HCC after RFA occurs frequently as a result of local tumor progression (LTP) or intrahepatic distant recurrence (IDR). LTP can occur along the peripheral margin of the ablative lesion when the primary tumor has not been controlled completely after RFA. In contrast, IDR is thought to be the result of the multicentric origin of the HCC.[7] IDR may be related more to systemic factors, such as fibrosis and chronic inflammatory processes, than to local factors.[8] In addition, some noninvasive inflammatory and fibrosis markers may reflect the severity of fibrosis and inflammation in the background liver tissue.[9,10,11] Hence, to determine the appropriate treatment modality and optimal follow-up interval, it is important to identify surrogate markers that can predict HCC recurrence after RFA.
The aim of this study was to evaluate the correlation between inflammatory and fibrosis markers and HCC recurrence after RFA.
MATERIALS AND METHODS
Study population
This cohort study retrospectively reviewed patients with HCC who underwent curative RFA at our center between October 2005 and September 2013. After a review of medical records, 98 consecutive patients who fulfilled the inclusion criteria were enrolled in this study. Inclusion criteria for performing RFA with or without transarterial chemoembolization (TACE) in patients with HCC were as follows: (1) RFA as the first treatment modality for curative intent; (2) a tumor or tumors visible on computed tomography (CT) or magnetic resonance imaging (MRI) and accessible via the percutaneous route; (3) a single tumor <5 cm in the largest dimension or three tumors or less that are <3 cm each; (4) a grade of A or B in the Child–Pugh (CP) classification of liver function; and (5) no portal venous thrombosis and extrahepatic metastasis. For all patients, proof of HCC malignancy was determined by its characteristic enhancement pattern (hypervascularization in the hepatic arterial phase and washout pattern in the delayed phase) using contrast-enhanced multiphasic CT and/or triphasic MRI. If imaging was inconclusive, the diagnosis was confirmed by ultrasonography-guided biopsy. Diagnosis was supported by the presence of the tumor markers serum α-fetoprotein (AFP) and protein induced by vitamin K absence II (PIVKA II). This study was approved by the institutional review board of the Konkuk University School of Medicine (KUH1010600), and it complies with the standards of the Declaration of Helsinki and current ethical guidelines.
Treatment and follow-up
RFA was performed in all patients with curative intent. All patients were followed-up for at least three months or more, as required. Patients were examined through physical examination, triphasic CT and/or MRI, laboratory tests, and identification of serum tumor markers, such as AFP and PIVKA II, at the initial and follow-up visits. In this study, tumor recurrence was suspected if a new lesion was detected on surveillance imaging study. These were confirmed and diagnosed on a dynamic CT scan or MRI scan. The primary outcome of this study was recurrence-free survival, defined as the time between treatment with RFA and detection of the first intrahepatic recurrence during follow-up, in the case of recurrence, or the last follow-up visit, when there was no HCC recurrence by the time of this analysis. Intrahepatic recurrence was subclassified into two categories: LTP and IDR. LTP was defined as tumor recurrence within 2 cm of the ablation margin, whereas IDR was defined as tumor recurrence beyond 2 cm from the treated margin.[12] Upon confirmation of recurrence, salvage treatments, including RFA, TACE, further surgery, or liver transplantation, were applied where appropriate.
Data collection and calculation of noninvasive inflammatory and fibrosis indices
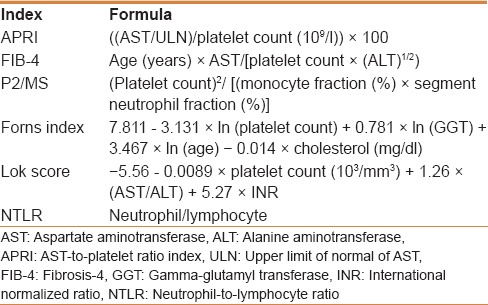
Demographic, clinical, and laboratory data of the study population were collected from electronic medical records and reviewed at the beginning of and during the study period. Demographic variables collected for this study include age, gender, and alcohol consumption. Liver cirrhosis was diagnosed using imaging studies. These findings include hepatic morphologic changes such as medial segment atrophy of left lobe, caudate lobe enlargement, a nodular surface of the liver, and presence of regenerative nodules, splenomegaly, ascites, and varix or collaterals.[13] Next, levels of noninvasive serum inflammatory and fibrosis markers, known to be correlated to the degree of hepatic fibrosis and inflammation, were calculated from the baseline demographic and laboratory data. Detailed formulae for the calculation of the noninvasive serum fibrosis markers, i.e., aspartate aminotransferase (AST)-to-platelet ratio index (APRI), fibrosis-4 score (FIB-4), the Forns index, and the Lok score, as well as the inflammatory markers P2/MS {platelet count2/[monocyte fraction (%) × segment neutrophil fraction (%)]} and neutrophil-to-lymphocyte ratio (NTLR), are presented in Table 1.[9,10,11,14]
Table 1.
Formulae for noninvasive serum fibrosis and inflammatory indices
Statistical analysis
Comparison between groups was performed using the Student's t-test for continuous variables with normal distribution and the Chi-square tests for categorical variables. The area under the receiver operating characteristic (AUROC) curve of inflammatory and fibrosis indices was calculated to predict HCC recurrence. The Youden index was calculated to select the optimal cut-off value for stratifying patients with a high risk of tumor recurrence. Recurrence curves were plotted by using the Kaplan–Meier method, and the difference between groups was assessed with the log-rank test. Factors independently related to recurrence were tested by using a Cox proportional hazards regression analysis adjusted for variables. Comparisons of continuous repeated measures were performed with a paired t-test for variables with normal distribution and with the Wilcoxon signed rank test for variables with non-normal distribution. A P < 0.05 was considered statistically significant. For the statistical analysis, SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was used.
RESULTS
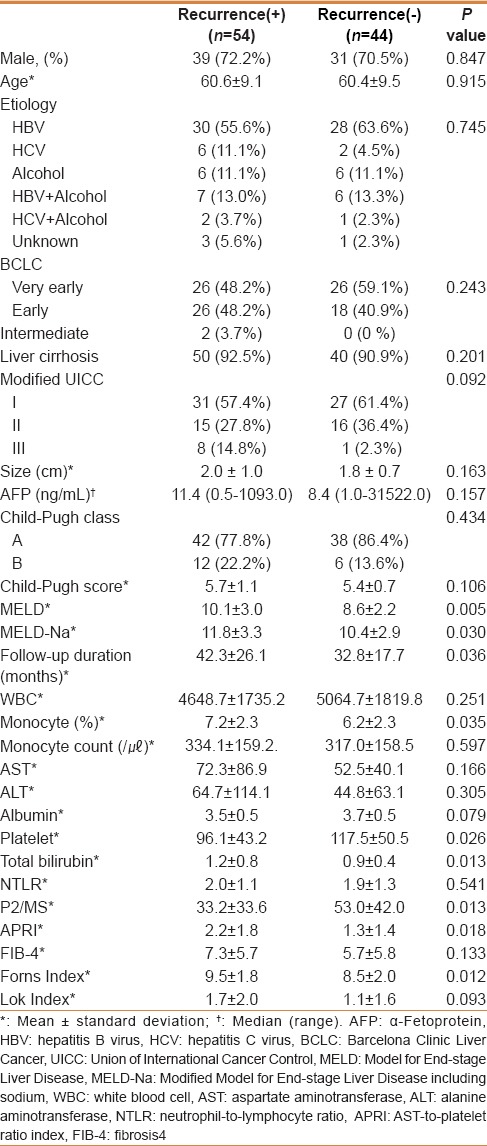
Patient characteristics and long-term outcome
The baseline demographic features and clinical characteristics are listed in Table 2. A total of 98 patients were included for analysis. The median follow-up time among all patients was 40 months (range, 4–95 months). The average patient age was 60.5 years (standard deviation, 9.2). Seventy patients (71.4%) were male and the most common etiology was hepatitis B. According to the imaging studies, 8 patients were considered chronic liver disease, and 90 were considered liver cirrhosis. Tumor was classified according to the modified Union for International Cancer Control (mUICC) classification (stage I, II, and III).[15] The number of patients with mUICC stage I, II, and III were 58 (59.1%), 31 (31.6%), and 9 (9.1%), respectively. Recurrence of HCC was detected in 54 patients (55.1%) over the entire duration of follow-up. The Model for End-stage Liver Disease (MELD) score (10.1 ± 3.0 vs. 8.6 ± 2.2, P = 0.005), APRI (2.2 ± 1.8 vs. 1.3 ± 1.4, P = 0.018), and Forns index (9.5 ± 1.8 vs. 8.5 ± 2.0, P = 0.012) were significantly higher, whereas P2/MS (33.2 ±33.6 vs. 53.0 ± 42.0, P = 0.013) was significantly lower in the recurrence group than in the recurrence-free group [Table 2]. IDR occurred in 43 of 54 patients (79.6%) during the follow-up period. Mortality was observed in three patients in the recurrence group and in four patients in the recurrence-free group.
Table 2.
Baseline characteristics of patients with and without tumor recurrence
ROC curve for determination of cut-off for indices
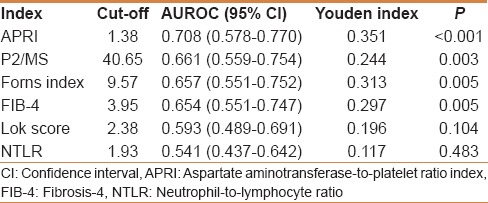
The AUROC for APRI, P2/MS, the Forns index, FIB-4, the Lok score, and NTLR were 0.708, 0.661, 0.657, 0.654, 0.593, and 0.541, respectively. With a cut-off value of 1.38, the sensitivity and specificity of APRI were 66.6% and 68.5%, respectively, and the Youden index was 0.351. The diagnostic accuracy of the six variables used to distinguish between patients with and without risk for HCC recurrence is indicated by receiver operating characteristic (ROC) analysis, as shown in Table 3. APRI had the highest AUROC amongst the six variables.
Table 3.
Comparison of area under receiver operating characteristic curve for prediction of tumor recurrence
Risk factors for HCC recurrence after RFA
Univariate analysis showed that APRI ≥1.38, P2/MS ≤40.65, Forns index ≥9.57, MELD, and CP scores were significantly associated with recurrence-free survival. In a multivariate analysis with the Cox proportional hazards model, APRI [hazard ratio (HR), 2.64; confidence interval (CI), 1.488–4.714; P = 0.001] was the only significant predictive factor for tumor recurrence [Table 4].
Table 4.
Independent risk factors associated with tumor recurrence after radiofrequency ablation for hepatocellular carcinoma identified by multivariate analysis using a stepwise Cox hazard regression model
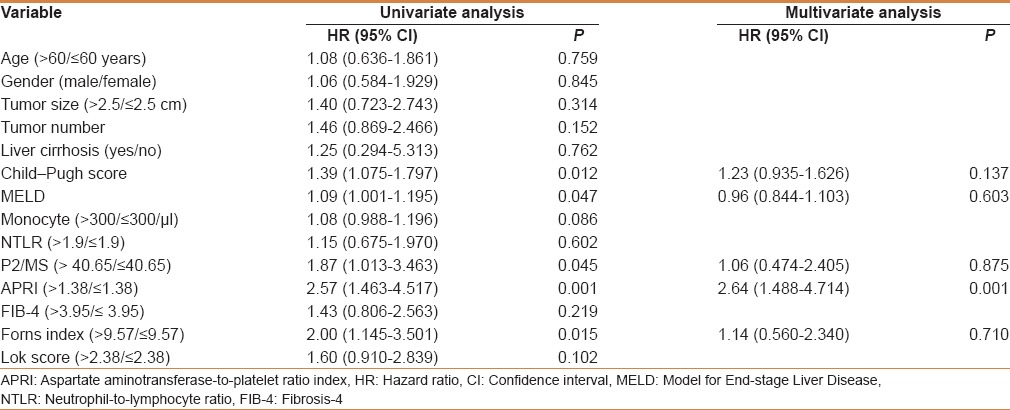
Recurrence analysis based on the score risk categories
The cumulative probability obtained in a Kaplan–Maier analysis of HCC recurrence was significantly different between the two risk categories as APRI ≥1.38 and APRI <1.38, as illustrated in Figure 1. Kaplan–Meier analysis of the data showed significantly shorter cumulative recurrence-free survival in patients with APRI ≥1.38 (P < 0.001).
Figure 1.
The Kaplan-Meier curve of two hepatocellular carcinoma groups with different aspartate aminotransferase (AST)-to-platelet ratio index values. In particular, patients with an AST-to-platelet ratio index (APRI) > 1.38 had significantly higher recurrence rates than patients with an APRI ≤ 1.38 (P = 0.001). The P-values were calculated by using the log-rank test
Factor associated with local and distant intrahepatic recurrence
Among the 54 patients with tumor recurrence, 11 were diagnosed with LTP and 43 with IDR. Multivariate analysis of patients with LTP showed that a maximal tumor size longer than 2.5 cm (HR, 2.068; CI, 1.022–4.184; P = 0.043) was an independent risk factor associated with LTP after RFA. It also showed that a high APRI (HR, 2.82; CI, 1.475–5.411; P = 0.001) was an independent risk factor for IDR.
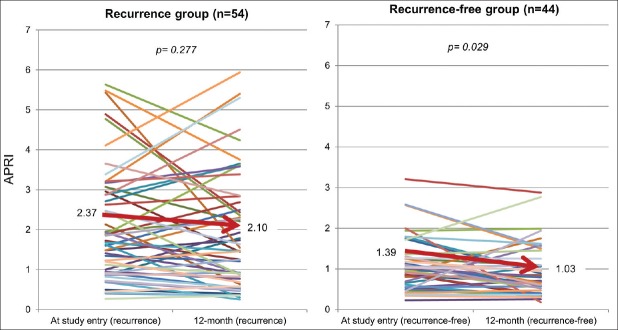
Longitudinal change of APRI values during follow-up
The average APRI values assessed 12 months after RFA in patients who developed HCC recurrence (n = 54) were persistently higher than those who remained recurrence-free over the complete follow-up period. In addition, APRI values in patients who were recurrence-free (n = 44) decreased significantly by the last follow-up [Figure 2].
Figure 2.
Longitudinal trends of average aspartate aminotransferase (AST)-to-platelet ratio index values. This graph shows the changes of the individual AST-to-platelet ratio index (APRI) values on recurrence group and on recurrence-free group in all patients from the study entry to the 12-month follow-up. The bold line indicates the trend of the average APRI value on recurrence group (A) and recurrence-free group (B)
DISCUSSION
In this study, we found that a high APRI value was associated with HCC recurrence after RFA. In addition, APRI values in patients who remained recurrence-free (n = 44) were significantly decreased during the follow-up period. Our data suggest that APRI could play an important role in predicting HCC recurrence after percutaneous ablation.
APRI has primarily been studied in patients with HCV infection, human immunodeficiency virus and HCV co-infections, or alcoholic liver disease.[16,17] APRI may predict HCC risk as was seen in a cohort of Japanese patients with non-alcoholic fatty liver disease.[18] A recent study reported that APRI may be a marker of HCC risk in HBV patients in cirrhosis-dependent and -independent manners.[19] It is known that patients with advanced fibrosis have lower production of thrombopoietin and a higher degree of portal hypertension, leading to lower production and higher sequestration of platelets.[20] Moreover, impaired sinusoidal clearance of AST in patients with advanced hepatic fibrosis also leads to a relative increase in serum AST levels, causing an increase in APRI.[21] We found that high APRI values were associated with HCC recurrence after RFA. This is consistent with a Taiwanese study where APRI seems to be a predictive marker for HCC recurrence after RFA.[22] Another Taiwanese study also found that APRI could serve as a reliable predictive marker for recurrence and survival in patients undergoing surgical resection.[23] In addition, there was a significant correlation between APRI and liver fibrosis in chronic hepatitis B and C patients with HCC.[24] APRI may be an important indicator for cirrhosis, the most important factor for the development of HCC in patients with chronic hepatitis.
However, a study from Greece showed that although APRI is significantly associated with the presence of cirrhosis, it could not be used to predict the severity of liver fibrosis.[21] Such results can be partially explained by the fluctuating pattern of AST in patients with chronic hepatitis. AST levels may differ according to sinusoidal clearance and mitochondrial injury. Despite the contradictory result, the Greek study still supported the potential usefulness of APRI in the evaluation of liver disease. However, no study has assessed the role of serial changes in APRI in predicting the risk of HCC recurrence after RFA. In the present study, we found a significant correlation between the baseline and follow-up APRI and the risk of developing HCC in a longitudinally persistent manner.
Previous studies showed that tumor factors and liver functional reserves determine the long-term outcomes of HCC patients undergoing RFA.[5,7] We used an RFA cohort to validate the power of the six noninvasive parameters in predicting recurrence. APRI had a fair degree of accuracy with an area under the curve (AUC) of 0.708 for HCC recurrence after RFA. Notably, the predictive power of APRI for the risk of HCC recurrence remained significant, even after adjusting for cirrhosis. This is consistent with analysis of the observed change in APRI during the follow-up period. Persistently higher APRI values were observed during the follow-up in the recurrence group (n = 54). However, APRI values in recurrence-free patients (n = 44) decreased significantly during follow-up. These results suggest that APRI is suitable for the prediction of HCC recurrence during follow-up. It is uncertain whether reduction of APRI correlates with an improvement of fibrosis stage. In this study, the AST level decreased significantly and the platelet count did not change significantly during the follow-up period in the recurrence group. APRI seems to be an indicator for fibrosis (both AST and platelet count), and may also reflect biochemical responses to hepatocarcinogenesis (the AST component). To differentiate the effects of the etiology of cirrhosis and control for potential confounding factors, we restricted the analysis to HBV patients treated with antiviral drugs during follow-up. No significant difference was observed in the number of patients who received antiviral therapy between the two groups in our cohort. Although this reduced our sample size, the same general pattern was observed. It has been reported that antiviral therapy can ameliorate liver inflammation, reduce fibrosis, and improve the liver functional reserve in patients with chronic viral hepatitis.[25] However, whether antiviral therapy can also prevent recurrence of viral hepatitis-related HCC after RFA remains controversial.[26,27] In a recent study, long-term therapy with antiviral therapy did not eliminate the risk of HCC in patients with chronic hepatitis, especially those with pre-existing cirrhosis.[28] The effect of antiviral therapy on recurrence in patients undergoing RFA could not be evaluated in this study and requires further validation in prospective studies.
In this study, recurrence was further divided into two patterns: LTP and IDR. The pathogenesis of local recurrence is associated with residual tumor cells after RFA therapy, leading to re-growth of the primary tumor.[29,30] IDR may be related more to systemic factors than to local factors. In this study, maximal tumor size is an important risk factor for LTP recurrence. IDR occurred more frequently than LTP in our cohort. We found that a high APRI value was significantly associated with an increased risk of recurrence in a multivariate analysis adjusting for all major variables. This association between APRI and recurrence was strengthened for IDR. Based on our findings, it is clear that APRI does not solely indicate cirrhosis, and further research is required to elucidate the specific oncogenic mechanisms. Therefore, potential risk factors indicative of hepatocarcinogenesis, including inflammation, fibrosis, APRI, and maximal tumor size, should be considered significant when predicting HCC recurrence.
CONCLUSION
In conclusion, we demonstrated that a high APRI value is associated with HCC recurrence after RFA. Regardless of cirrhosis, APRI is higher in cases of HCC recurrence. We recommend careful monitoring and long-term follow-up of patients with high APRI values after percutaneous ablation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–27. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762–73. doi: 10.1002/hep.23725. [DOI] [PubMed] [Google Scholar]
- 3.Rahbari NN, Mehrabi A, Mollberg NM, Muller SA, Koch M, Buchler MW, et al. Hepatocellular carcinoma: Current management and perspectives for the future. Ann Surg. 2011;253:453–69. doi: 10.1097/SLA.0b013e31820d944f. [DOI] [PubMed] [Google Scholar]
- 4.Kim YS, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: Analysis of the pattern and risk factors. Eur J Radiol. 2006;59:432–41. doi: 10.1016/j.ejrad.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Zytoon AA, Ishii H, Murakami K, El-Kholy MR, Furuse J, El-Dorry A, et al. Recurrence-free survival after radiofrequency ablation of hepatocellular carcinoma. A registry report of the impact of risk factors on outcome. Jpn J Clin Oncol. 2007;37:658–72. doi: 10.1093/jjco/hym086. [DOI] [PubMed] [Google Scholar]
- 6.Gervais DA, Arellano RS. Percutaneous tumor ablation for hepatocellular carcinoma. AJR Am J Roentgenol. 2011;197:789–94. doi: 10.2214/AJR.11.7656. [DOI] [PubMed] [Google Scholar]
- 7.Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–7. [PubMed] [Google Scholar]
- 8.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–7. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 9.Wang GY, Yang Y, Li H, Zhang J, Jiang N, Li MR, et al. A scoring model based on neutrophil to lymphocyte ratio predicts recurrence of HBV-associated hepatocellular carcinoma after liver transplantation. PLoS One. 2011;6:e25295. doi: 10.1371/journal.pone.0025295. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Kwak MS, Lee JH, Chung GE, Yu SJ, Jung EU, Kim BH, et al. Low P2/MS value is an independent risk factor for early recurrence of hepatocellular carcinoma after radio frequency ablation therapy. Hepatogastroenterology. 2011;58:147–52. [PubMed] [Google Scholar]
- 11.Zarski JP, Sturm N, Guechot J, Paris A, Zafrani ES, Asselah T, et al. ANRS HCEP 23 Fibrostar Group. Comparison of nine blood tests and transient elastography for liver fibrosis in chronic hepatitis C: The ANRS HCEP-23 study. J Hepatol. 2012;56:55–62. doi: 10.1016/j.jhep.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Chung GE, Kim W, Lee JH, Kim YJ, Yoon JH, Lee JM, et al. Negative hepatitis B envelope antigen predicts intrahepatic recurrence in hepatitis B virus-related hepatocellular carcinoma after ablation therapy. J Gastroenterol Hepatol. 2011;26:1638–45. doi: 10.1111/j.1440-1746.2011.06777.x. [DOI] [PubMed] [Google Scholar]
- 13.Saygili OB, Tarhan NC, Yildirim T, Serin E, Ozer B, Agildere AM. Value of computed tomography and magnetic resonance imaging for assessing severity of liver cirrhosis secondary to viral hepatitis. Eur J Radiol. 2005;54:400–7. doi: 10.1016/j.ejrad.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Attallah AM, Omran MM, Farid K, El-Bendary M, Emran TM, Albannan MS, et al. Development of a novel score for liver fibrosis staging and comparison with eight simple laboratory scores in large numbers of HCV-monoinfected patients. Clin Chim Acta. 2012;413:1725–30. doi: 10.1016/j.cca.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 15.Ueno S, Tanabe G, Nuruki K, Hamanoue M, Komorizono Y, Oketani M, et al. Prognostic performance of the new classification of primary liver cancer of Japan (4 th edition) for patients with hepatocellular carcinoma: A validation analysis. Hepatol Res. 2002;24:395–403. doi: 10.1016/s1386-6346(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 16.Lieber CS, Weiss DG, Morgan TR, Paronetto F. Aspartate aminotransferase to platelet ratio index in patients with alcoholic liver fibrosis. Am J Gastroenterol. 2006;101:1500–8. doi: 10.1111/j.1572-0241.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- 17.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 18.Kawamura Y, Arase Y, Ikeda K, Seko Y, Imai N, Hosaka T, et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107:253–61. doi: 10.1038/ajg.2011.327. [DOI] [PubMed] [Google Scholar]
- 19.Hann HW, Wan S, Lai Y, Hann RS, Myers RE, Patel F, et al. Aspartate aminotransferase to platelet ratio index as a prospective predictor of hepatocellular carcinoma risk in patients with chronic hepatitis B virus infection. Gastroenterol Hepatol. 2015;30:131–8. doi: 10.1111/jgh.12664. HYPERLINK “http://www.ncbi.nlm.nih.gov/pubmed/?term=AST+to+platelet+ratio+index+as+a+prospective+predictor+of+hepatocellular+carcinoma+risk+in+patients+with+chronic+HBV+infection” \o “Journal of gastroenterology and hepatology.” . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peck-Radosavljevic M. Thrombocytopenia in liver disease. Can J Gastroenterol. 2000;14(Suppl D):60–6D. doi: 10.1155/2000/617428. [DOI] [PubMed] [Google Scholar]
- 21.Chrysanthos NV, Papatheodoridis GV, Savvas S, Kafiri G, Petraki K, Manesis EK, et al. Aspartate aminotransferase to platelet ratio index for fibrosis evaluation in chronic viral hepatitis. Eur J Gastroenterol Hepatol. 2006;18:389–96. doi: 10.1097/00042737-200604000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Kao WY, Chiou YY, Hung HH, Chou YH, Su CW, Wu JC, et al. Risk factors for long-term prognosis in hepatocellular carcinoma after radiofrequency ablation therapy: The clinical implication of aspartate aminotransferase-platelet ratio index. Eur J Gastroenterol Hepatol. 2011;23:528–36. doi: 10.1097/MEG.0b013e328346d529. [DOI] [PubMed] [Google Scholar]
- 23.Hung HH, Su CW, Lai CR, Chau GY, Chan CC, Huang YH, et al. Fibrosis and AST to platelet ratio index predict post-operative prognosis for solitary small hepatitis B-related hepatocellular carcinoma. Hepatol Int. 2010;4:691–9. doi: 10.1007/s12072-010-9213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin CS, Chang CS, Yang SS, Yeh HZ, Lin CW. Retrospective evaluation of serum markers APRI and AST/ALT for assessing liver fibrosis and cirrhosis in chronic hepatitis B and C patients with hepatocellular carcinoma. Intern Med. 2008;47:569–75. doi: 10.2169/internalmedicine.47.0595. [DOI] [PubMed] [Google Scholar]
- 25.Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, et al. Chronic Hepatitis B Guideline Working Party of the Asian-Pacific Association for the Study of the Liver. Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2008 update. Hepatol Int. 2008;2:263–83. doi: 10.1007/s12072-008-9080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janssen HL, Arif A. Averting hepatocellular carcinoma in chronic hepatitis B with antiviral therapy: Tipping the balance or not yet? Gastroenterology. 2014;147:24–6. doi: 10.1053/j.gastro.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 27.Lim YS, Han S, Heo NY, Shim JH, Lee HC, Suh DJ. Mortality, liver transplantation, and hepatocellular carcinoma among patients with chronic hepatitis B treated with entecavir vs lamivudine. Gastroenterology. 2014;147:152–61. doi: 10.1053/j.gastro.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 28.Papatheodoridis GV, Manolakopoulos S, Touloumi G, Vourli G, Raptopoulou-Gigi M, Vafiadis-Zoumbouli I, et al. Virological suppression does not prevent the development of hepatocellular carcinoma in HBeAg-negative chronic hepatitis B patients with cirrhosis receiving oral antiviral (s) starting with lamivudine monotherapy: Results of the nationwide HEPNET. Greece cohort study. Gut. 2011;60:1109–16. doi: 10.1136/gut.2010.221846. [DOI] [PubMed] [Google Scholar]
- 29.Lam VW, Ng KK, Chok KS, Cheung TT, Yuen J, Tung H, et al. Risk factors and prognostic factors of local recurrence after radiofrequency ablation of hepatocellular carcinoma. J Am Coll Surg. 2008;207:20–9. doi: 10.1016/j.jamcollsurg.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Nouso K, Matsumoto E, Kobayashi Y, Nakamura S, Tanaka H, Osawa T, et al. Risk factors for local and distant recurrence of hepatocellular carcinomas after local ablation therapies. J Gastroenterol Hepatol. 2008;23:453–8. doi: 10.1111/j.1440-1746.2007.05120.x. [DOI] [PubMed] [Google Scholar]