Abstract
Left ventricular non-compaction is a recently recognized, rare form of cardiomyopathy. It is based on the arrest of endomyocardial morphogenesis during embryogenesis. It was first described in 1984 by Engberding who described it as isolated ‘sinusoids’ within the LV. Right now its prevalence is estimated at 0.014 to 1.3 and 3–4% in heart failure patients. Its clinical manifestations are highly variable, ranging from no symptoms to disabling congestive heart failure, arrhythmias, and systemic thromboemboli. Doppler Echocardiogram is considered the diagnostic procedure of choice and treatment is symptomatic management of its symptoms and complications.
Keywords: Cardiomyopathy, congenital, non-compaction, left ventricle, heart failure
It is a genetic defect, either sporadic or familial, and thus may require screening of asymptomatic relatives of affected patients (1, 2). LVNC (left ventricular non-compaction) is associated with numerous mutations (including E101K mutation in α-cardiac actin [ACTC] gene) (3) and may have genetic overlap with different cardiomyopathic phenotypes including hypertrophic cardiomyopathy (4). It is a pathophysiological process involving the arrest of the normal compaction of the embryonic sponge-like meshwork of interwoven myocardial fibers. Failure of these ‘sinusoids’ to compact between the 5–8 week of embryonic life from the epicardium inward results in a thin, compacted epicardial layer and an extensive non-compacted endocardial layer, with prominent trabeculation and deep recesses that communicate with the left ventricular cavity but not with the coronary circulation (5). This absence of left ventricular compaction leading to extensive left ventricular trabeculation is associated with the development of left ventricular systolic impairment, cardiac arrhythmias, and systemic thromboembolism. Treatment and prognosis differs depending on the extent and ratio of non-compaction and resulting complications.
Case report
We present the case of a 32-year-old Caucasian female with no significant cardiopulmonary history, presenting to the emergency room with recurrent shortness of breath and chest discomfort.
Her shortness of breath had been addressed a few months ago with antibiotics and albuterol inhaler with an underlying diagnosis of pneumonia. However, 2 weeks prior to this admission, she complained that the shortness of breath, which was previously well controlled by the inhalers, seemed to be getting worse. Along with this, she experienced some new dyspnea on exertion, paroxysmal nocturnal dyspnea, and chest pressure-like symptoms.
On presentation, her blood pressure was 90/70 mm Hg, heart rate was 110 bmp, and she was saturating 96% on 2 liters/minute of oxygen. Physical exam revealed mild bi-basal crackles and a S3 on auscultation. She also had elevated Jugular venous pressure (JVP) up to the angle of her jaw.
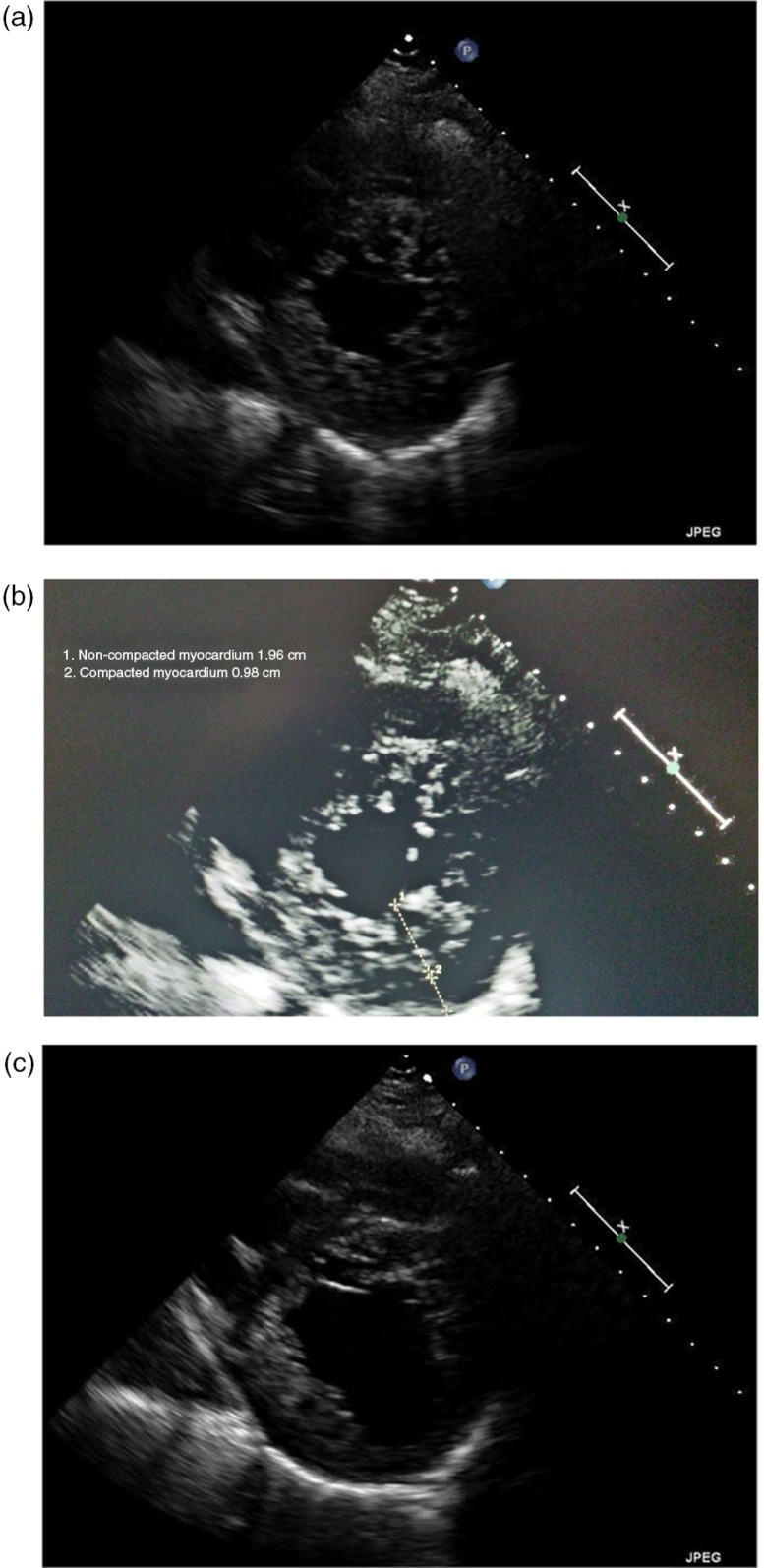
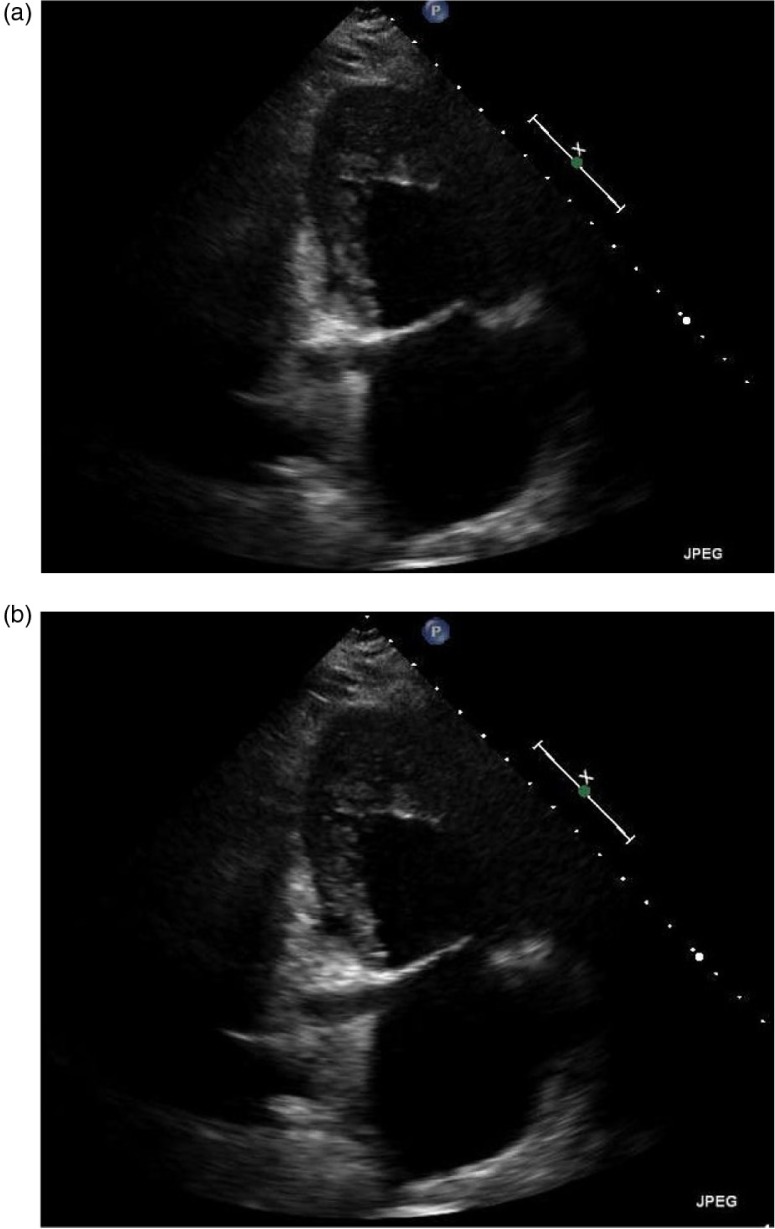
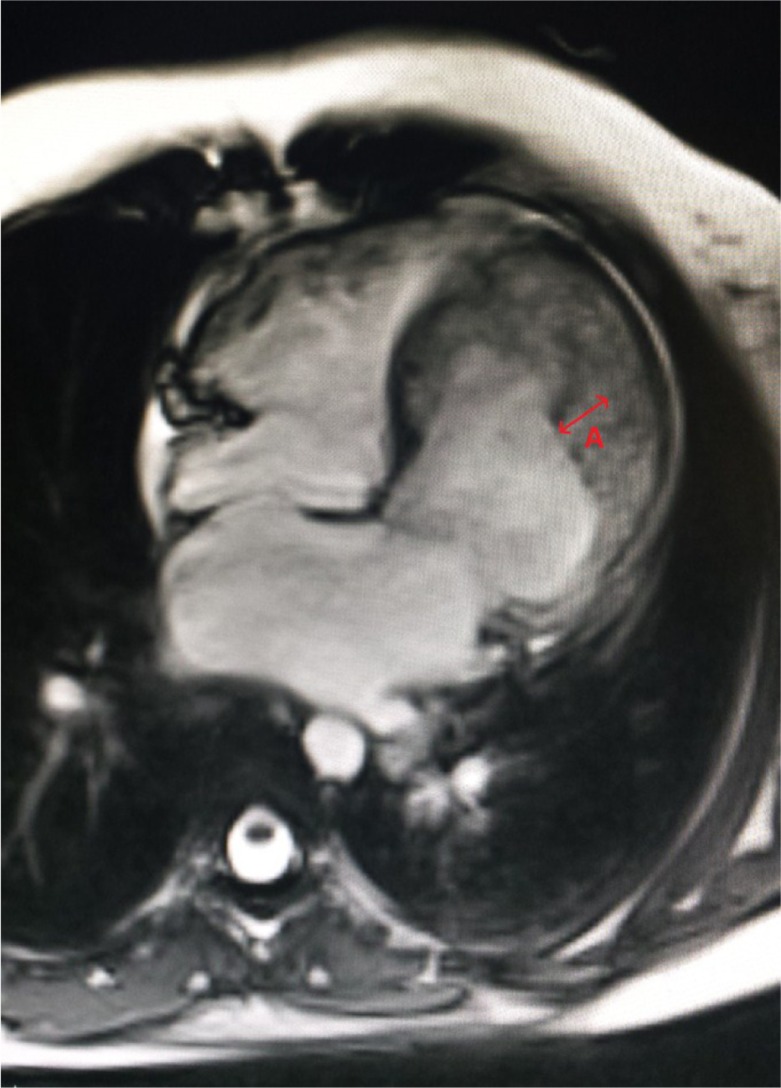
All her labs were within normal limits, except for the Brain natriuretic peptide (BNP) which was elevated to 2,587. An electrocardiogram revealed sinus tachycardia with left atrial enlargement. Due to this presentation, a computed tomography (CT) chest was done that revealed extensive abnormal interstitial markings. Echocardiogram revealed global hypokinesis, with an ejection fraction (EF) of 10%, and severe mitral and tricuspid regurgitation; prominent trabeculations with two distinct myocardial zones (Figs. 1a–c, 2a and b); and a non-compacted to compacted myocardium ratio of 2:1 (Fig. 1b) These findings of non-compaction cardiomyopathy were confirmed with cardiac magnetic resonance imaging (MRI) (Fig. 3).
Fig. 1.
(a–c) Para-sternal short axis views showing the non-compacted layer with numerous trabeculations. Figure 1b illustrates the two separate layers.
Fig. 2.
(a and b) Apical 2 chamber view revealing prominent myocardial trabeculations and deep intertrabecular recesses communicating with the left ventricular cavity.
Fig. 3.
Cardiac MRI: four chamber view with some degree of motion artifact. Red arrow represents the non-compacted myocardium.
She was initially started on carvedilol and lisinopril 20 mg daily, but these were discontinued due to episodes of hypotension. After confirmation of the diagnosis, she underwent a right heart catheterization and automatic implantable cardioverter defibrillator (AICD) placement for primary prevention. She was subsequently started on digoxin and metoprolol succinate 50 mg daily for the heart failure (HF) and warfarin for prophylactic anticoagulation with a goal International normalised ratio (INR) of 2–3. Eventually, a low dose of Lasix 20 mg daily was added to optimize her medical management. Since her initial diagnosis, the patient has returned to the emergency room multiple times with volume overload due to medication non-compliance. Each time after being optimized, she returned to her baseline functional status where she could perform her activities of daily living without significant difficulty.
Discussion
Epidemiology
Due to lack of awareness, low prevalence, ongoing research regarding its cause, and clinical course and treatment, the current incidence of this entity is 0.05% in adults (6). As expected its prevalence in patients with HF is higher; 3–4% (7, 8). Both sexes are affected equally in the sporadic form of LVNC (9) and it is more prevalent among the black population compared with the white population (10). However, with increasing awareness, medical literature, and improving diagnostic techniques, the prevalence of LVNC is bound to increase.
Pathophysiology
During the early embryonic phase, the myocardium consists of a meshwork of loosely woven myocardial fibers separated by deep recesses. These recesses link the myocardium with the left ventricular cavity, to provide blood supply to the myocardium. Between the 5th and the 8th week of life, the coronary circulation develops and the sinusoidal mesh-like myocardium gradually compacts from epicardium inward and the recesses are reduced to capillaries (1, 11, 12). The exact trigger for this phenomenon is still unknown but is thought to involve VEGF (vascular endothelial growth factor) and angiopoietin (9). Failure of these ‘sinusoids’ to compact results in a thin, compacted epicardial layer and an extensive non-compacted endocardial layer (5, 6, 9). Arrest of this normal endomyocardial morphogenesis may be associated with other congenital heart diseases and coronary artery abnormalities (6). Left ventricle is uniformly affected, while right ventricular involvement is reported in less than half of the patients.
Histologically, failure of this compaction process results in deep intratrabecular spaces that communicate with the left ventricular cavity in isolated non-compaction and with coronary circulation in non-compaction associated with other congenital heart diseases (13).
Both sporadic and familial forms of non-compaction have been described. Various genes have been described in the familial form of the disease, including mutations in G4.5 gene on Xq28 (14) and cardiac specific CSX gene (15). Due to such heavy genetic linkage, screening and genetic counseling is recommended in first degree relatives (13).
Clinical features
Patients with LVNC may have non-specific and heterogeneous presentation ranging from asymptomatic to severe cardiac dysfunction. Although genetic in nature, age of onset is also quite variable and may occur at an advanced age. Three major issues related to this disorder are arrhythmias, systemic embolizations, and HF. The later being the commonest presentation.
Over two-thirds of the patients in the largest series with LVNC had symptomatic HF (13), and in the cohort described by Chin et al. (5), 63% patients were reported to have depressed EF. Ventricular dysfunction may be systolic or diastolic. Diastolic dysfunction has been linked to abnormal relaxation and restrictive filling due to hyper trabeculation (16). Whereas myocardial alteration causing micro vascular subendocardial perfusion defects even with normal coronaries may contribute to the ventricular systolic dysfunction and arrhythmias (1). These perfusion defects have been described in LVNC using MRI (17), Positron emission tomography (PET) (18) and Scintigraphy with thallium-201 (12).
Arrhythmias including atrial fibrillation in 25% of adults (6, 13), paroxysmal supraventricular tachycardia (PSVT) and complete heart blocks have been described in literature (6, 12). Ventricular tachyarrhythmias and sudden cardiac deaths have been described in nearly 40% of cases (19). However, this evidence is controversial as the largest series of pediatric patients showed no cases of ventricular tachycardia (VT) or sudden cardiac death (12).
Thromboembolic events may be related to the development of thrombi in the extensively trabeculated ventricle complimented by reduced systolic function. Concomitant atrial fibrillation, if present, may also add to the risk (6, 16). The occurrence of these events ranged from 21 to 38% (5, 6, 13).
An association with some form of neuromuscular disorder has also been described in 82% of patients (20, 21).
Differential diagnosis
Dilated cardiomyopathy, hypertensive heart disease, apical hypertrophic cardiomyopathy, infiltrative cardiomyopathy, eosinophilic endomyocardial disease, aberrant chordate tendineae, localized left ventricular hypertrophy, left-ventricular thrombi cardiac metastasis, endocardial fibroelastosis, eosinophilic endomyocardial disease, and Barth syndrome.
Diagnosis
Diagnosis is based purely on structural features seen on imaging. Quantification and diagnosis of the disorder is not easy. Similarity with other defects, as well as non-specific clinical manifestations, makes the diagnosis of non-compaction difficult. These difficulties in the diagnosis may account for the difference in the prevalence reported by various studies.
2D transthoracic echocardiogram is most commonly used technique for diagnosis of LVNC as well as follow-up. Characteristic echocardiographic findings of non-compaction are multiple, prominent myocardial trabeculations and deep intertrabecular recesses communicating with the LV cavity in the absence of any other cardiac lesions (Figs. 1a, b, c, and 2a). Color Doppler imaging demonstrates blood flow through these deep recesses in continuity with the ventricular cavity.
Various criteria have been proposed, but the commonly criteria used in clinical practice were established by Jenni et al. (22). These include: 1) appearance of at least four prominent trabeculations and deep intertrabecular recesses; 2) appearance of blood flow from the ventricular cavity into the intertrabecular recesses as visualized by Color Doppler imaging; 3) the segments of non-compacted myocardium mainly involving the apex and the inferior-mid and lateral-mid of the left ventricular wall and typically show a two-layered structure with an end systolic ratio greater than two between the non-compacted subendocardial layer and the compacted subepicardial layer; 4) absence of coexisting cardiac abnormalities.
MRI provides good correlation with echocardiography for localization and extent of non-compaction and especially useful in cases with poor echocardiographic image quality. Studies have shown that cardiac magnetic resonance (CMR) is superior to 2D echocardiography (ECHO) in regards to the number of segments that can be analyzed (especially during assessment of anterior, anterolateral and inferolateral segments) and the evaluation of the extent of the two-layered structure (23). The criteria for diagnosis by CMR were proposed by Petersen et al. (24). The ratio of non-compacted myocardium to compacted myocardium must be greater than 2.3:1 during the diastole.
Prognosis
Like hypertrophic cardiomyopathy, it is best for patients with LVCN to avoid heavy physical exertion, strenuous activities and pregnancy. Prognosis is individualized, mainly based on the degree of impaction. Some patient may remain asymptomatic and may be identified on routine screening or as an accidental finding. While others show early clinical signs leading to early death (25). In a study consisting of LVNC in the pediatric population the mortality rate associated with the condition was 12.8%. Cardiac arrhythmias and ventricular dysfunction were associated with highest risk (26). Like our patient, these high-risk candidates will require early, aggressive interventions including an AICD and evaluation for cardiac transplant (25).
Treatment
Treatment is directed toward the three common implications of LVNC.
HF here is treated similar to other causes of HF with reduced ejection fraction (HFrEF). Beta blockers, ACE inhibitors, diuretic, digoxin may be used to manage the systolic and diastolic dysfunction (25). Patients with end stage or refractory HF will need a heart transplant. So far, only six cases of LVNC leading to heart transplant have been reported (27). Data from Europe and America reported a 4- to 6-year combined mortality or transplantation rate of ~50–60% (13, 25).
Given the risk for sudden cardiac death and arrhythmias annual Holter monitoring may be done. Additionally, EP studies and anti-arrhythmic therapies may be warranted depending on the presentation. AICD placement, bi-ventricular pacemakers may help reduce SCD and have a role in patients with reduced EF and prolonged intraventricular conduction (25).
Long-term anticoagulation is recommended in all cases regardless of symptoms or the presence of intracardiac thrombus (6, 13, 25, 28).
Finally, echocardiographic screening is recommended in first degree relatives along with neuromuscular evaluations due to non-cardiac associations of LVNC (5, 13).
Conclusion
In conclusion, high index of suspicion is required, which will need to be confirmed by ECHO or CT. Early diagnosis and treatment is required as these patients can have fatal outcomes from the arrhythmias, thromboembolic phenomenon, and HF. Aggressive management is required for high-risk patients that include symptomatic treatment as well as AICD placement and possible transplant, if feasible. In spite of medical advancements, the overall prognosis for these patients does not offer much hope.
Acknowledgements
We would like to thank the Department of Medicine and Cardiology for their continued support.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Disclosure
None of the authors have any financial or personal bias that would inappropriately compromise the publication of this work.
Consent
Informed consent was obtained from the patient and his family for educational use of the below-mentioned data and no personal patient information has been disclosed.
References
- 1.Aragona P, Badano LP, Pacileo G, Pino GP, Sinagra G, Zachara E. Isolated left ventricular non-compaction. Ital Heart J Suppl. 2005;6(10):649–59. [PubMed] [Google Scholar]
- 2.Murphy RT, Thaman R, Blanes JG, Ward D, Sevdalis E, Papra E, et al. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur Heart J. 2005;26(2):187–92. doi: 10.1093/eurheartj/ehi025. [DOI] [PubMed] [Google Scholar]
- 3.Monserrat L, Hermida-Prieto M, Fernandez X, Rodríguez I, Dumont C, Cazón L, et al. Mutation in the alpha-cardiac actin gene associated with apical hypertrophic cardiomyopathy, left ventricular non-compaction, and septal defects. Eur Heart J. 2007;28(16):1953–61. doi: 10.1093/eurheartj/ehm239. [DOI] [PubMed] [Google Scholar]
- 4.Kelley-Hedgepeth A, Towbin JA, Maron MS. Images in cardiovascular medicine. Overlapping phenotypes: Left ventricular noncompaction and hypertrophic cardiomyopathy. Circulation. 2009;119(23):e588–9. doi: 10.1161/CIRCULATIONAHA.108.829564. [DOI] [PubMed] [Google Scholar]
- 5.Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82(2):507–13. doi: 10.1161/01.cir.82.2.507. [DOI] [PubMed] [Google Scholar]
- 6.Ritter M, Oechslin E, Sütsch G, Attenhofer C, Schneider J, Jenni R. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc. 1997;72(1):26–31. doi: 10.4065/72.1.26. [DOI] [PubMed] [Google Scholar]
- 7.Kovacevic-Preradovic T, Jenni R, Oechslin EN, Noll G, Seifert B, Attenhofer Jost CH. Isolated left ventricular noncompaction as a cause for heart failure and heart transplantation: A single center experience. Cardiology. 2009;112(2):158–64. doi: 10.1159/000147899. [DOI] [PubMed] [Google Scholar]
- 8.Patrianakos AP, Parthenakis FI, Nyktari EG, Vardas PE. Noncompaction myocardium imaging with multiple echocardiographic modalities. Echocardiography. 2008;25(8):898–900. doi: 10.1111/j.1540-8175.2008.00708.x. [DOI] [PubMed] [Google Scholar]
- 9.Zambrano E, Marshalko SJ, Jaffe CC, Hui P. Isolated noncompaction of the ventricular myocardium: Clinical and molecular aspects of a rare cardiomyopathy. Lab Invest. 2002;82(2):117–22. doi: 10.1038/labinvest.3780404. [DOI] [PubMed] [Google Scholar]
- 10.Kohli SK, Pantazis AA, Shah JS, Adeyemi B, Jackson G, McKenna WJ, et al. Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: Time for a reappraisal of diagnostic criteria? Eur Heart J. 2008;29(1):89–95. doi: 10.1093/eurheartj/ehm481. [DOI] [PubMed] [Google Scholar]
- 11.Dusek J, Ostádal B, Duskova M. Postnatal persistence of spongy myocardium with embryonic blood supply. Arch Pathol. 1975;99(6):312–17. [PubMed] [Google Scholar]
- 12.Ichida F, Hamamichi Y, Miyawaki T, Ono Y, Kamiya T, Akagi T, et al. Clinical features of isolated noncompaction of the ventricular myocardium: Long-term clinical course, hemodynamic properties, and genetic background. J Am Coll Cardiol. 1999;34(1):233–40. doi: 10.1016/s0735-1097(99)00170-9. [DOI] [PubMed] [Google Scholar]
- 13.Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: A distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. 2000;36(2):493–500. doi: 10.1016/s0735-1097(00)00755-5. [DOI] [PubMed] [Google Scholar]
- 14.Ichida F, Tsubata S, Bowles KR, Haneda N, Uese K, Miyawaki T, et al. Novel gene mutations in patients with left ventricular noncompaction or Barth syndrome. Circulation. 2001;103(9):1256–63. doi: 10.1161/01.cir.103.9.1256. [DOI] [PubMed] [Google Scholar]
- 15.Pauli RM, Scheib-Wixted S, Cripe L, Izumo S, Sekhon GS. Ventricular noncompaction and distal chromosome 5q deletion. Am J Med Genet. 1999;85(4):419–23. [PubMed] [Google Scholar]
- 16.Agmon Y, Connolly HM, Olson LJ, Khandheria BK, Seward JB. Noncompaction of the ventricular myocardium. J Am Soc Echocardiogr. 1999;12(10):859–63. doi: 10.1016/s0894-7317(99)70192-6. [DOI] [PubMed] [Google Scholar]
- 17.Soler R, Rodríguez E, Monserrat L, Alvarez N. MRI of subendocardial perfusion deficits in isolated left ventricular noncompaction. J Comput Assist Tomogr. 2002;26(3):373–5. doi: 10.1097/00004728-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Junga G, Kneifel S, Von Smekal A, Steinert H, Bauersfeld U. Myocardial ischaemia in children with isolated ventricular non-compaction. Eur Heart J. 1999;20(12):910–16. doi: 10.1053/euhj.1998.1398. [DOI] [PubMed] [Google Scholar]
- 19.Rigopoulos A, Rizos IK, Aggeli C, Kloufetos P, Papacharalampous X, Stefanadis C, et al. Isolated left ventricular noncompaction: An unclassified cardiomyopathy with severe prognosis in adults. Cardiology. 2002;98(1–2):25–32. doi: 10.1159/000064677. [DOI] [PubMed] [Google Scholar]
- 20.Stöllberger C, Finsterer J, Blazek G. Left ventricular hypertrabeculation/noncompaction and association with additional cardiac abnormalities and neuromuscular disorders. Am J Cardiol. 2002;90(8):899–902. doi: 10.1016/s0002-9149(02)02723-6. [DOI] [PubMed] [Google Scholar]
- 21.Stöllberger C, Finsterer J, Blazek G, Bittner RE. Left ventricular non-compaction in a patient with becker's muscular dystrophy. Heart. 1996;76(4):380. doi: 10.1136/hrt.76.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart. 2001;86(6):666–71. doi: 10.1136/heart.86.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thuny F, Jacquier A, Jop B, Giorgi R, Gaubert J-Y, Bartoli J-M, et al. Assessment of left ventricular non-compaction in adults: Side-by-side comparison of cardiac magnetic resonance imaging with echocardiography. Arch Cardiovasc Dis. 2010;103(3):150–9. doi: 10.1016/j.acvd.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH, et al. Left ventricular non-compaction: Insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46(1):101–5. doi: 10.1016/j.jacc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 25.Weiford BC, Subbarao VD, Mulhern KM. Noncompaction of the ventricular myocardium. Circulation. 2004;109(24):2965–71. doi: 10.1161/01.CIR.0000132478.60674.D0. [DOI] [PubMed] [Google Scholar]
- 26.Brescia ST, Rossano JW, Pignatelli R, Jefferies JL, Price JF, Decker JA, et al. Mortality and sudden death in pediatric left ventricular noncompaction in a tertiary referral center. Circulation. 2013;127(22):2202–8. doi: 10.1161/CIRCULATIONAHA.113.002511. [DOI] [PubMed] [Google Scholar]
- 27.Conraads V, Paelinck B, Vorlat A, Goethals M, Jacobs W, Vrints C. Isolated non-compaction of the left ventricle: A rare indication for transplantation. J Heart Lung Transplant. 2001;20(8):904–7. doi: 10.1016/s1053-2498(01)00264-9. [DOI] [PubMed] [Google Scholar]
- 28.Jenni R, Oechslin EN, van der Loo B. Isolated ventricular non-compaction of the myocardium in adults. Heart. 2007;93(1):11–15. doi: 10.1136/hrt.2005.082271. [DOI] [PMC free article] [PubMed] [Google Scholar]