Abstract
Left atrial myxomas are rare primary cardiac tumors. Their incidence is estimated to be about 0.1% of total cases. Neurological complications resulting from cardiac myxomas are seen in 20–35% of patients. Transesophageal echocardiogram (TEE) is preferred over transthoracic echocardiogram for evaluation of left atrial myxoma. Three-dimensional (3D) echocardiography ensures better visualization of intracardiac structures. It has been used prior to surgery for diagnostic support in the surgical treatment of cardiac masses. We present a case of a 46-year-old Hispanic male who developed acute ischemic stroke of left frontal lobe and was also found to have multiple ‘silent’ cerebral infarcts in the MRI of the brain. On further workup, he was found to have a left atrial myxoma on 3D TEE. This was resected with the assistance of intra-operative 3D TEE imaging. We present this case to increase awareness and to stress at early evaluation of secondary causes of ischemic cerebrovascular accident, outside the realm of hypercoagulability. This case also exhibits the need for basic cardiac workup in young individuals who present with symptoms of intermittent palpitations or chest pain to minimize significant morbidity or mortality.
Keywords: left atrial myxoma, three-dimensional transesophageal echocardiogram, acute stroke
Primary cardiac tumors are extremely rare. Their incidence is estimated to be about 0.1% of total cases on autopsy (1). Most tumors are found incidentally using imaging techniques such as MRI, two-dimensional (2D) echocardiogram, and/or CT imaging. Neurological complications resulting from cardiac myxomas are seen in 20–35% of patients due to embolization of the tumor which can cause thrombi in the systemic circulation (2, 3). Embolic strokes may occur any time with progression of the tumor. Most left atrial tumors also cause mitral regurgitation, leading to secondary pulmonary hypertension and heart failure (4). Surgical resection is the definitive treatment for a myxoma (5). We present a case of a 46-year-old Hispanic male who had a cerebrovascular accident (CVA) secondary to tumor embolization from a large left atrial myxoma found on three-dimensional (3D) echocardiography imaging.
Case description
A 46-year-old Hispanic male with no known past medical history arrived to the emergency department with his wife presented with slurred speech, confusion, blurry vision, and right upper/lower extremity weakness. Patient's symptoms began 1 day prior to presentation. At the time of presentation, his weakness and blurry vision had improved but his speech and mental status had progressively worsened. He denied any chest pain, shortness of breath, dizziness, lightheadedness, swallowing difficulties, headache, and tingling or numbness in upper or lower extremities. Patient also presented with a history of intermittent palpitations and dyspnea on exertion for the last 1 year, which had been inhibiting him from carrying out his activities of daily living. Patient did not have any significant past surgical history or family history of hypercoagulable state, coronary artery disease, or cardiac tumors. Patient was not on any medications at home. He had no known drug allergies.
On examination, vitals included temperature of 98.6°F, blood pressure of 143/110 mmHg, heart rate of 80 beats/min, respiratory rate of 18/min, and oxygen saturation of 98% on room air. On cardiovascular examination, the patient was found to have a 4/6 systolic murmur heard best at the fifth intercostal space near midclavicular line. Lungs were clear to auscultation bilaterally. Neurological examination revealed expressive aphasia and dysarthria. Furthermore, right upper/lower extremity strength was 4/5 with appropriate tone. No change in light touch or vibratory sensation was noted and reflexes were normal. Additionally, cerebellar signs were absent.
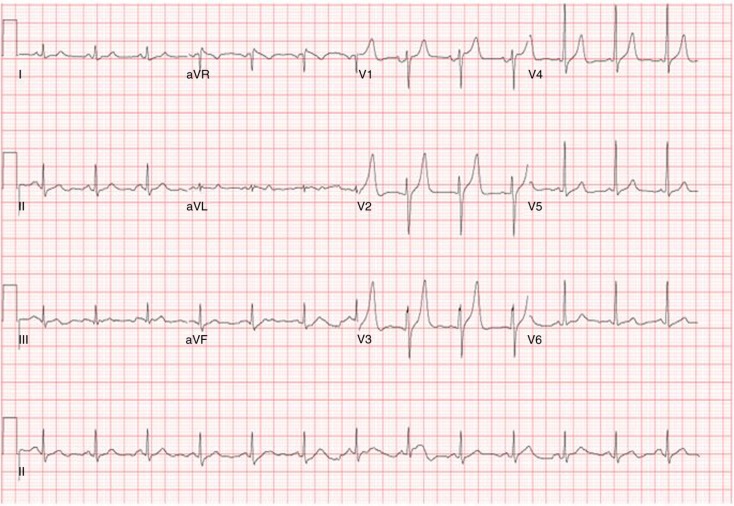
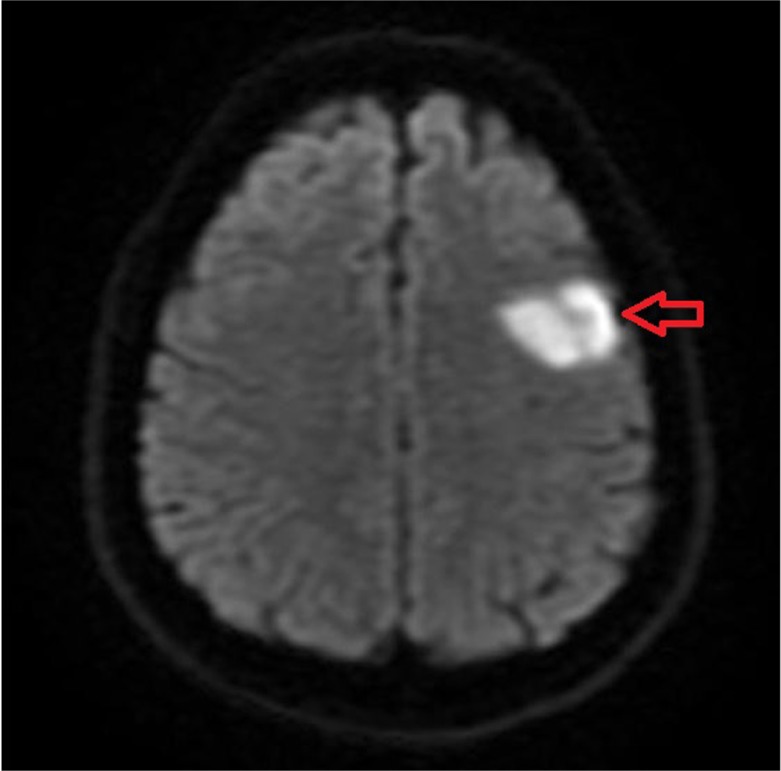
Admission laboratory examination revealed first troponin to be 0.54 and second troponin to be 0.69 (normal <0.3). Electrocardiogram (EKG) on admission shows sinus rhythm with a rate of 80 beats/min and tall t-wave in V2–V3 leads (Fig. 1). Radiograph of chest was normal with no evidence of cardiomegaly or pulmonary edema. Patient underwent a CT of the head without contrast that displayed bifrontal hypodense brain lesion without significant regional mass effect indicative of ischemia. MRI of the brain with and without contrast displayed acute left frontal infarction as well as old right frontal, left pontine, and left cerebellar infarction (Figs. 2 and 3). Patient was not a candidate for TPA, so he was given high-dose aspirin.
Fig. 1.
ECG showing sinus rhythm with a rate of 80 beats/min and tall t-wave in V2–V3 leads.
Fig. 2.
MRI of the brain with and without contrast displayed left frontal lobe infarction (red arrow).
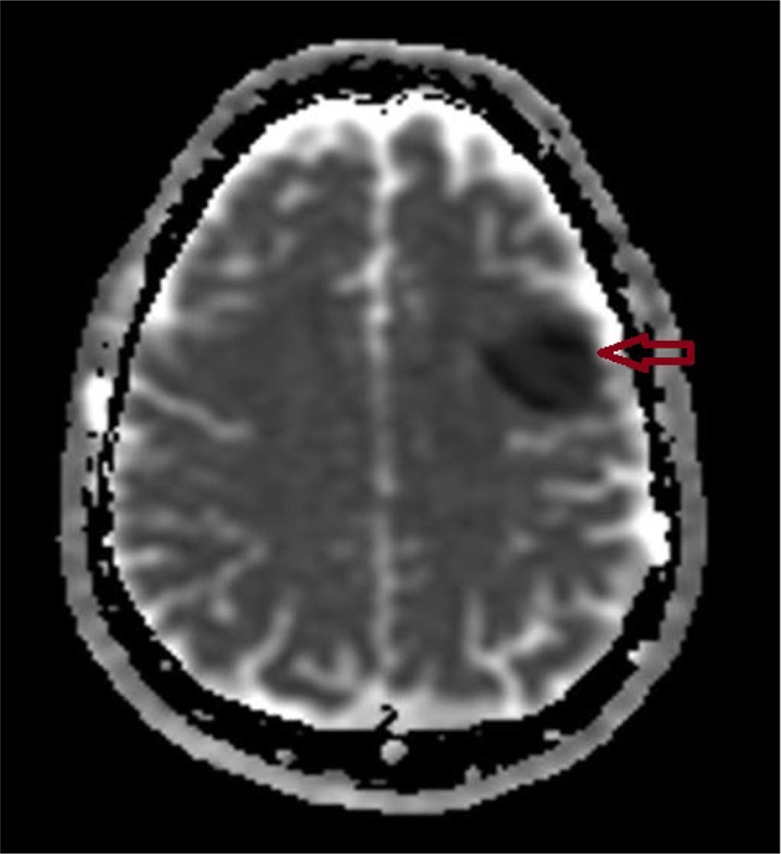
Fig. 3.
MRI of the brain (ADTC mapping) showing acute infarction at the corresponding area of left frontal lobe when compared to Fig. 2 (arrow).
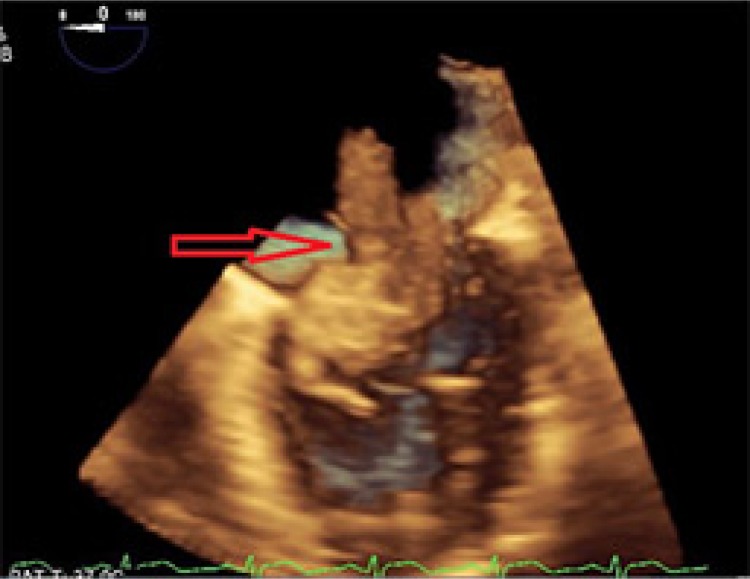
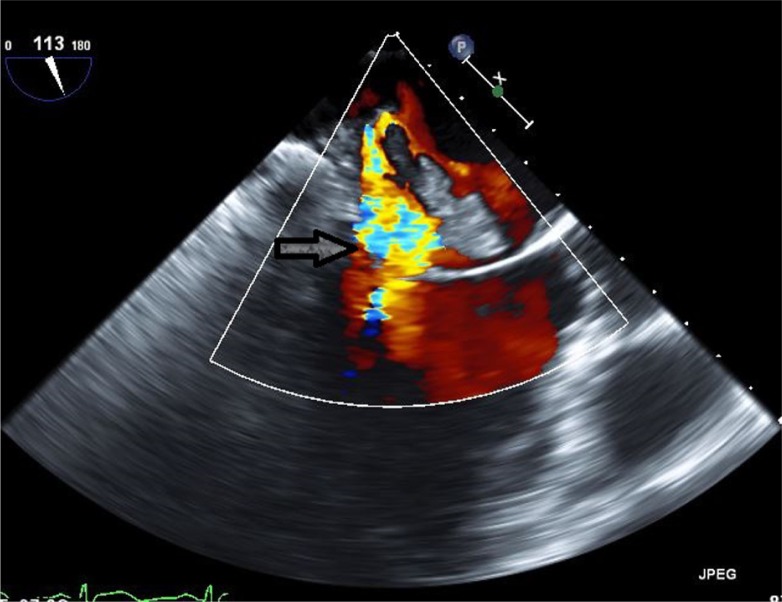
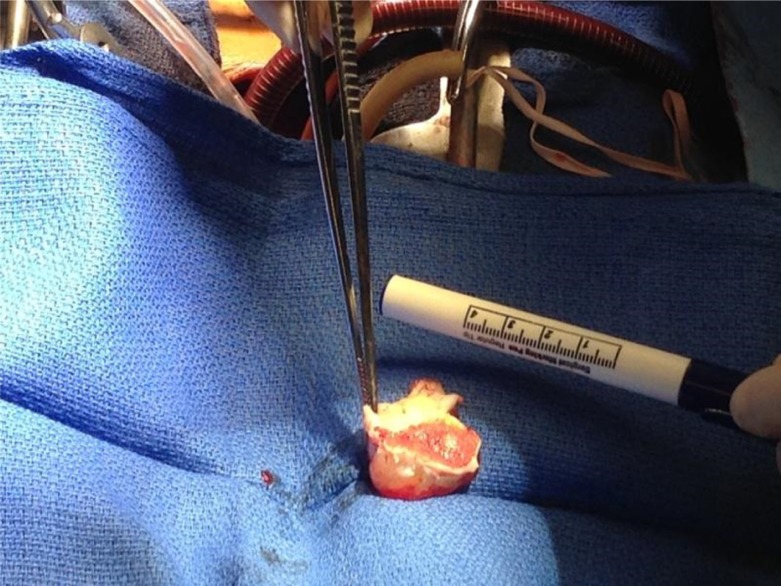
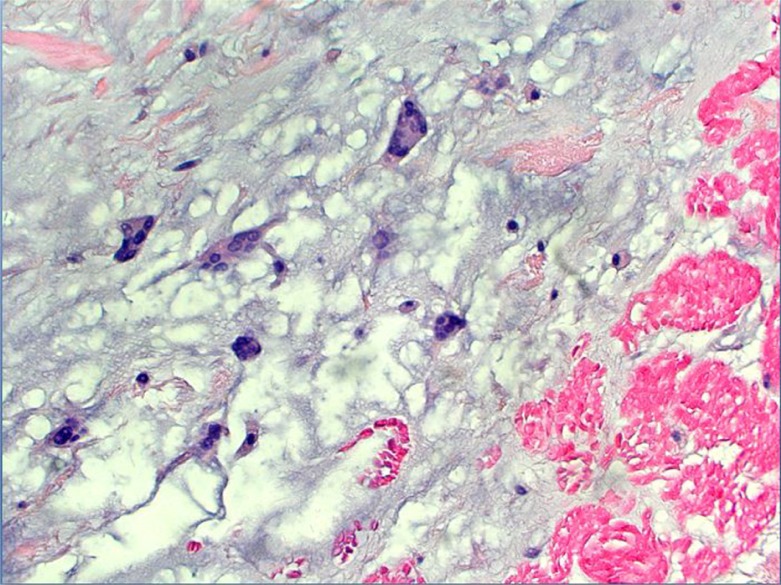
Work-up for secondary causes of CVA included 3D transesophageal echocardiogram (TEE), which displayed a very large highly mobile left atrial myxoma attached to the lower atrium and mitral annulus area. The myxoma transversed the mitral valve and encroached on the left ventricle, leading to severe mitral regurgitation (Figs. 4 and 5). The patient underwent an emergent excision of the myxoma with mitral valve ring annuloplasty. The mass was found to be 4.5 cm×4 cm horizontally (Fig. 6). Cytopathological analysis of the mass revealed it to be a benign atrial myxoma (Fig. 7). Patient was transferred to the cardiothoracic unit and was observed for a period of 48 h. The patient did not have any postoperative complications and recovered without any neurological deficits. The patient was started on Coumadin and was told to follow-up with cardiothoracic surgery as an outpatient. He subsequently recovered from his right-sided weakness as evidenced during his routine follow-up at the cardiothoracic surgery clinic.
Fig. 4.
Three-dimensional transesophageal echocardiogram showing large highly mobile left atrial myxoma attached to the lower atrium and mitral annulus area (red arrow).
Fig. 5.
Three-dimensional transesophageal echocardiogram Doppler image showing severe mitral regurgitation (arrow).
Fig. 6.
Left atrial resected mass measuring 4.5 cm×4 cm in horizontal dimension.
Fig. 7.
Left atrial myxoma with mesenchymal cells within myxoid matrix (hematoxylin and eosin, 200×).
Discussion
We present this case to increase awareness and to stress at early evaluation of secondary causes of ischemic CVA, outside the realm of hypercoagulability. In most cases, valvular dysfunction including mitral regurgitation is apparent after the myxoma is removed; but in the aforementioned case, the myxoma was large enough to have transversed the mitral valve leading to significant regurgitation prior to its removal (6). Additionally, the size of this mass is also to be appreciated as it is one of the largest that has been seen in most published cases. As per our literature search, this is the third case of preoperative mitral regurgitation in a patient with left atrial myxoma who presented with CVA.
Atrial myxoma is a common benign cardiac tumor that is detected often in younger adults with stroke or transient ischemic attack (1 in 250) than in older patients (1 in 750) (3). The incidence of myxoma is 0.5% per million every year (4). The etiology of atrial myxoma is currently unknown, although most cases are sporadic and about 10% have an autosomal-dominant genetic pattern. Nearly 20% of cases are asymptomatic upon admission and discovered as an incidental finding (7). Others present with a wide range of clinical entities including ischemic stroke, syncope, unspecified headaches, and seizures (2). Recent studies have reported that 9–22% of patients with atrial myxomas suffered from embolic stroke (2, 8). Often patients present with recurrent stroke, leading to significant morbidity and mortality (7). In patients who presented with ischemic strokes, it is important to note that the tumor emboli are not related to size of the myxoma, rather, are related to the mobility and friability of the mass itself (4, 5, 8).
TEE is preferred over transthoracic echocardiogram (TTE) for the evaluation of left atrial myxoma. The sensitivity of TEE is 100% for diagnosis of cardiac tumors (9, 10). Cardiac MRI may be useful in determining tumor size, attachment, and mobility. This may aid in surgical resection, which should not be deferred even in asymptomatic cases discovered incidentally (11).
Three-dimensional echocardiography was developed in the 1970s, as a method for measuring ventricular volume (12). Three-dimensional echocardiography with transesophageal transducers and digital technology ensures better visualization of intracardiac structures. It has been used prior to surgery and in the operating room for diagnostic support in the surgical treatment of cardiac masses. It has also been of assistance in evaluation and surgical repair of mitral, aortic, and tricuspid valvular diseases, and in congenital heart diseases, such as correction of interatrial septal defects and interventricular septal defects (13). In this case, the 3D reconstruction of images allowed for a better anatomical detailing of the mass in regard to its fixating pedicle in the interatrial septum and thus made it feasible to determine the extent of surgical resection required.
In evaluation of secondary causes of multiple cerebral infarcts in a young individual, left atrial myxoma should be considered in the differential diagnosis. Our patient had intermittent palpitations and dyspnea on exertion for a year prior to presentation, and no workup was initiated to determine the etiology. On MRI imaging, the patient was noted to have prior old strokes that were undiagnosed due to the lack of symptoms experienced by the patient. Although our patient recovered without any residual weakness or deficit, this case exhibits a need for basic cardiac workup like EKG and/or 2D echocardiogram in young individuals who present with such symptoms, in order to minimize significant morbidity or mortality.
Acknowledgements
This case report was supported by Internal Medicine Residency Program at St. Francis Medical Center. We thank our colleagues from cardiothoracic and cardiovascular division who provided insight and expertise that greatly assisted in the case preparation. We thank Dr. Sara Wallach-Chairman and Program Director of Internal Medicine Residency Program for assistance particularly in journal selection, and Dr. Charles Kososky for comments that greatly improved the manuscript of the case report. We are also immensely grateful to Dr. Kulpreet Barn for his comments on an earlier version of the manuscript. At last, we thank internal medicine residents at St Francis Medical Center for their great work ethics and excellent role in patient care.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.Figueroa-Torres Y, Martínez-Ojeda JA, Franqui-Rivera H, Martínez-Toro J. Benign cardiac neoplasms: The experience at the Cardiovascular Center of Puerto Rico and the Caribbean. P R Health Science J. 2008;27:373–6. [PubMed] [Google Scholar]
- 2.Ekinci EI, Donnan GA. Neurological manifestations of cardiac myxoma: A review of the literature and report of cases. Intern Med J. 2004;34:243–9. doi: 10.1111/j.1444-0903.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- 3.Hart RG, Albers GW, Koudstaal PJ. Cardioembolic stroke. In: Ginsberg MD, Bogousslavsky J, editors. Cerebrovascular disease: Pathophysiology, diagnosis and management. London: Blackwell Science; 1998. pp. 1392–429. [Google Scholar]
- 4.Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine. 2001;80:159–72. doi: 10.1097/00005792-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Hitoshi Hirose, Youdelman BA, Entwistle JW. Stroke from a large left atrial myxoma. Open Cardiovasc Med J. 2008;2:115–17. doi: 10.2174/1874192400802010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitlock R, Evans R, Lonn E, Teoh K. Giant left atrial myxoma and associated mitral valve pathology. J Cardiothorac Vasc Anesth. 2007;21:103–5. doi: 10.1053/j.jvca.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Kessab R, Wehbe L, Badaoui G, el Asmar B, Jebara V, Ashoush R. Recurrent cerebrovascular accident: Unusual and isolated manifestation of myxoma of the left atrium. J Med Liban. 1999;47:246–50. [PubMed] [Google Scholar]
- 8.Aggarwal SK, Barik R, Sarma TC, Iyer VR, Sai V, Mishra J, et al. Clinical presentation and investigation findings in cardiac myxoma: New insights from the developing world. Am Heart J. 2007;154:1102–7. doi: 10.1016/j.ahj.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 9.Engberding R, Daniel WG, Erbel R, Kasper W, Lestuzzi C, Curius JM, et al. Diagnosis of heart tumours by transesophageal echocardiography: A multicentre study in 154 patients. Eur Heart J. 1993;14:1223–8. doi: 10.1093/eurheartj/14.9.1223. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi K, Koide Y. Role of intraoperative transesophageal echocardiography in detecting masked mitral regurgitation during left atrial myxoma surgery. J Anesth. 2014;29:134–7. doi: 10.1007/s00540-014-1885-8. [DOI] [PubMed] [Google Scholar]
- 11.Reddy DB, Jena A, Venugopal P. Magnetic resonance imaging (MRI) in evaluation of left atrial masses: An in vitro and in vivo study. J Cardiovasc Surg (Torino) 1994;35:289–94. [PubMed] [Google Scholar]
- 12.Roelandt JRT, Yao J, Karsprzak JD. Three-dimensional echocardiography. Curr Opin Cardiol. 1998;13:386–98. doi: 10.1097/00001573-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 13.De Simone R, Glombitza G, Vahl CF, Meiner HP, Hagl S. Three-dimensional Doppler: Techniques and clinical applications. Eur Heart J. 1999;20:619–27. doi: 10.1053/euhj.1998.1342. [DOI] [PubMed] [Google Scholar]