Abstract
Background:
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease that is becoming a public health problem in recent decades. Obesity and overweight play a key role in NAFLD pathogenesis. Thus, weight loss (especially body fat mass) is one component of therapeutic strategies in NAFLD. Results from experimental studies have shown that garlic (Allium sativum L.) can reduce body weight and body fat mass. However, the effect of garlic on body fat mass and weight in the human population, which is addressed in this study, is still obscure.
Materials and Methods:
In this clinical trial, 110 subjects with NAFLD were randomly assigned to the intervention or the control group. The intervention group received two garlic tablets (containing 400 mg of garlic powder) daily while the control group received placebo tablets. Dietary intake and physical activity of participants were obtained by a validated questionnaire. Body composition was measured by bioelectrical impedance analysis. Data were analyzed using SPSS software version 16.
Results:
In the intervention group, significant reductions were observed in body weight and body fat mass (P < 0.05). We also observed a significant reduction in body weight in the control group, but there were no significant changes in total body water and lean body mass in both groups (P > 0.05). In the intervention group, the percentage change in body weight was significantly greater than the control group (−2.6 vs. −0.7, P = 0.02). No serious side effects associated with the intervention were reported.
Conclusion:
Our trial suggests that garlic supplemfrom experimental studies have shown thatentation can reduce body weight and fat mass among subjects with NAFLD.
Key Words: Body composition, body fat mass, lean body mass, nonalcoholic fatty liver disease
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a term used to describe the lipid accumulation within hepatocytes in the absence of hepatitis viruses and metabolic disorders among nonalcoholic subjects.[1] NAFLD encompasses a wide range of complications from simple steatosis to nonalcoholic steatohepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma.[2] A spectrum of diseases and conditions increases risk of developing or progression of NAFLD such as obesity,[3] type 2 diabetes,[4] hypertension,[5] and hyperlipidemia.[6] The prevalence of NAFLD in obese, diabetic, and dyslipidemic patients has been estimated to be about 80–90%, 30–50%, and 90%, respectively.[7]
Because NAFLD is strongly associated with abdominal obesity, dyslipidemia, and diabetes therapeutic strategies in NAFLD are inexorably linked to modification of them. It has been reported that weight loss improves insulin sensitivity and dyslipidemia, reduces the amount of lipid accumulation in the liver, and may prevent or delay the progression of NAFLD.[8,9,10] Various strategies including lifestyle modifications (caloric restriction, physical increasing), anti-obesity medication, and bariatric surgery are used for weight loss.[11] Nonetheless, sometimes weight loss is not achievable without using dietary supplements.[12]
Garlic (Allium sativum L.) has been used as food and medicinal plant in many countries and cultures for thousands of years. It has traditionally been used to treat respiratory diseases. Current studies also suggest that consumption of garlic can reduce the risk of cardiovascular diseases.[13,14,15] Garlic contains many organosulfur compounds that its biological activities are attributed to them. Evidence from cellular and animal studies suggested that garlic can exert anti-obesity properties.[16,17,18] However, the anti-obesity effect of garlic in the human population is still obscure. This clinical trial was performed to determine the effect of garlic supplementation on body composition among patients with NAFLD, because of a key role of weight loss in NAFLD treatment.
MATERIALS AND METHODS
Participants
This randomized, double-blind, placebo-controlled clinical trial was performed to investigate the effect of garlic supplementation on body composition among NAFLD volunteers. All subjects were recruited from Metabolic Liver Disease Research Center in Isfahan University of Medical Sciences, Isfahan, Iran. The appropriate sample size was estimated using the suggested formula for parallel clinical trials. We considered type 1 error of 5% (α = 0.05), type 2 error of 20% (β = 20, power = 80%), and body fat mass as a key variable. On the basis of a previous study,[19] the variance of body fat mass was 3 kg. We also considered 1.7 kg as the difference in mean (d) of body fat mass. The result showed that the study needed 45 subjects in each group. To allow participants dropping out during the study, 110 patients were selected for study. The inclusion criteria of the study were: Men and women aged between 20 and 70 years, alanine aminotransferase and aspartate aminotransferase (AST) ≥40 U/L and newly diagnosed NAFLD by ultrasonography. The exclusion criteria were: Pregnancy and lactation, smoking subjects, allergic to garlic, use of supplements, alcohol, anti-hypertensive medications, anti-obesity medication, statins and metformin, kidney disease, hyperthyroidism and hypothyroidism, chronic heart failure, hemochromatosis, cholestasis, Wilson's disease, and cirrhosis.[20] This study was approved by the Research Council and Ethics Committee of Isfahan University of Medical Sciences and was registered on Iranian Registry of Clinical Trials website (IRCT2014110819853N1).
Study design
In this 15-week clinical randomized trial, participants were randomly assigned to intervention or control group using the stratified blocked randomization method. All participants, assistant, and researcher were blinded to the treatment assignment throughout the study until the main analyses were completed. A trained person, who was not blinded to the intervention, performed the randomized allocation and assigned participants to interventions. Based on previous studies,[21,22] the intervention group received 400 mg garlic powder tablets (coated tablets contain 1.5 mg allicin, Amin Pharmaceuticals Co., Isfahan, Iran) twice a day while the control group received placebo (coated tablets contain starch and microcrystalline cellulose). Placebo was produced in School of Pharmacy, Isfahan University of Medical Sciences, Iran. All patients received one tablet after breakfast and one tablet after dinner for a 15-week period. Placebo tablets were in the same shape and appearance as the garlic tablets. Participant adherence was measured by tablet counting at each visit and phone call. Poor compliance was defined as <80% of expected tablets taken.[23] Both groups were advised to the common dietary and behavioral recommendations during follow-up. The participants were not allowed to eat more than two garlic cloves per week.
Anthropometric assessment
In this study, a trained person measured height and body composition under the standard protocols in participants with light clothes and without shoes at the beginning and the end of the study. Body composition was measured by bioelectrical impedance analysis (BIA) using the Body Composition Analyzer (Jawon IOI 353, Korea). According to the methods of using BIA, patients were advised to be hydrated; avoid exercising in the previous 4–6 h and consuming tea or caffeine in the previous 24 h.[24,25] Body mass index (BMI) was calculated as weight (kg) divided by height (m2). We used the same machine and instrument in order to avoid inter-instrument variation in all measurements.
Dietary intake assessment
Dietary intake was assessed by a 3-day food record (1-week end and 2 work days) at baseline and the end of the study, in order to assess dietary variation. The amount of each food item was calculated using household measures. Then food records were analyzed using the Nutritionist IV software (version 7.0; N-squared Computing, Salam, OR, USA).
Physical activity assessments
The International Physical Activity Questionnaire (IPAQ) short form was used to estimate physical activity level. IPAQ was taken of each participant at baseline, during the study period, and the end of the study by a trained person. After calculating metabolic equivalent (MET) value for each person, participants were stratified into three categories (low, moderate, and high levels of physical activity) according to the guidelines for data processing and analysis of the IPAQ.
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software (version 16, SPSS Inc., Chicago, IL, USA). The normality of variable distribution was tested using the Kolmogorov–Smirnov test. Paired t-test used to assess the within-group comparisons; Independent-samples Student's t-test used to assess the between-group comparisons; and Chi-square used to assess the nominal or ordinal variables. The treatment effects were evaluated through the difference in percentage changes between the two groups using analysis of covariance. Results are shown as mean ± standard deviation (SD). The P < 0.05 was considered as statistically significant.
RESULTS
In our study, eight participants in the intervention group were excluded: Travel (n = 1), change in phone number (n = 2), poor compliance (n = 3), and use of medicine (n = 2). Four patients in the control group were also excluded: Pregnancy (n = 1), change in phone number (n = 1), and poor compliance (n = 2). Finally, 98 participants (47 patients in the intervention group and 51 patients in the control group) completed the trial and were included in the analysis [Figure 1]. No serious side effects were reported by participants.
Figure 1.
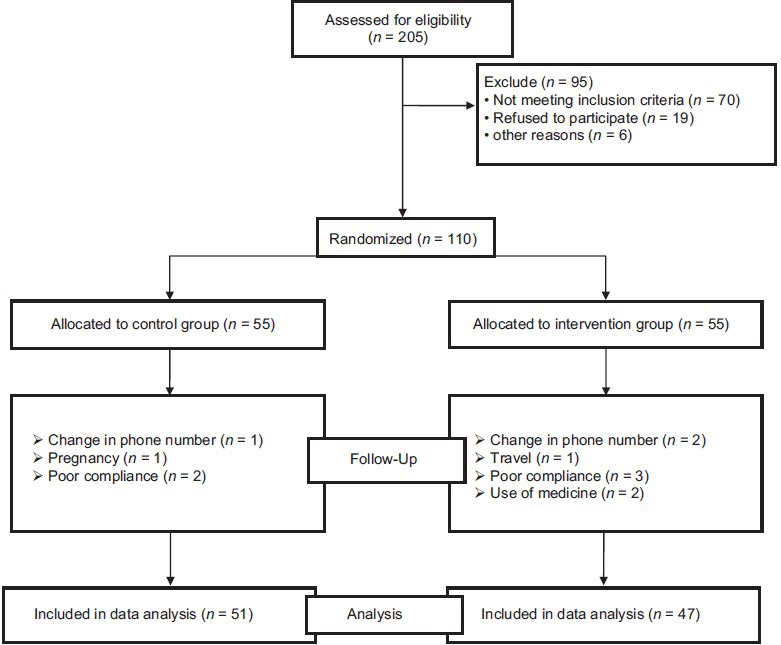
Study procedure and volunteers randomization
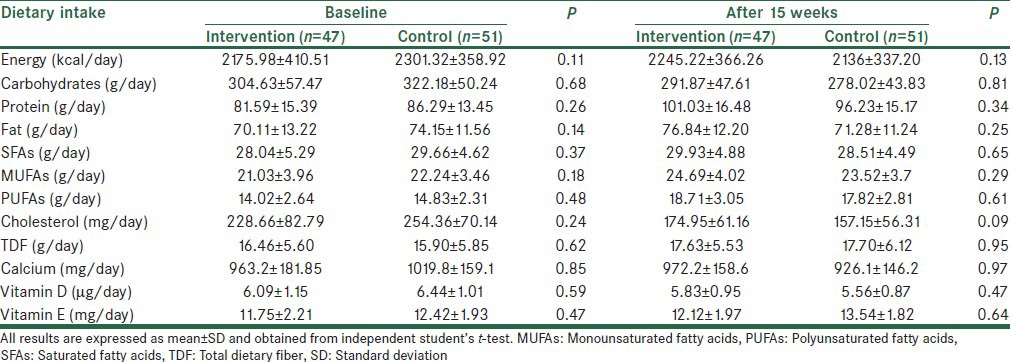
The mean ± SD age, BMI, and weight at the study baseline were 45.2 ± 11.2 years, 29.1 ± 6.1 kg/m2, and 81.1 ± 14.7 kg, respectively. Baseline means of age, weight, and BMI did not differ significantly between the two groups, but the mean height in the control group was significantly greater than the intervention group. There were no significant differences in physical activity, fatty liver grade, education level, and gender between the two groups [Table 1]. Based on dietary records, no significant differences in the mean intake of energy and nutrients were seen between the two groups at baseline and also after 15 weeks [Table 2]. We did not observe a significant change in nutrients in both groups during the study period (P < 0.05). During the study period, energy intake in the intervention did not have significant changes, whereas a significant reduction was seen in energy intake in control (+69.24 ± 513.64 kcal/day vs. −162.67 ± 549.01 kcal/day, P = 0.03). Also, based on mean physical activity, there were no significant differences between the two groups (intervention group: 2.59 ± 0.48 MET h/day vs. control group: 2.73 ± 0.42 MET h/day; P = 0.14).
Table 1.
Comparison of baseline characteristics between the two groups
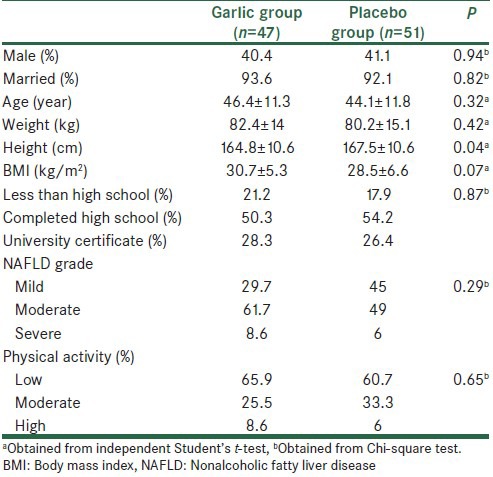
Table 2.
Comparison of dietary intake between the two groups
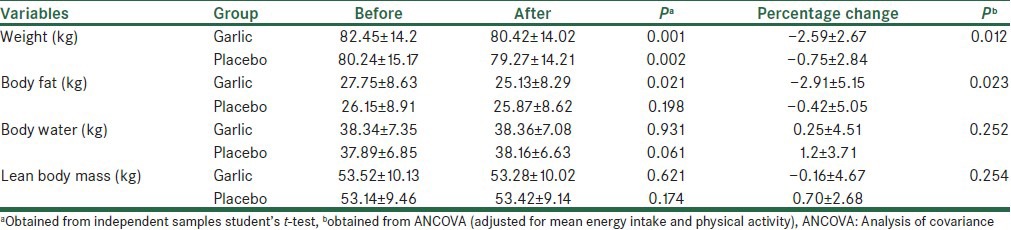
The mean ± SD of anthropometric variables are shown in Table 3. The comparison between the two groups showed no significant differences in baseline of these variables (P < 0.05). At the end of the study, we observed a significant reduction in body weight in both groups and a significant reduction in body fat mass in the intervention group, but there were no significant changes in other variables. The comparison of the percentage changes of anthropometric variables between the two groups showed that in the intervention group, patients had a significantly greater decrease in body weight (−2.59 ± 2.67 vs. −0.75 ± 2.84 kg, P = 0.01) and body fat mass (−2.91 ± 5.1 vs. −0.42 ± 5.05 kg, P = 0.02).
Table 3.
Changes in anthropometric values among control and garlic groups (mean±SD)
DISCUSSION
The results of our study demonstrated that a 15-week garlic supplementation among NAFLD patients significantly decreased body fat mass and body weight without any significant changes in lean body mass. To the best of our knowledge, this study is the first clinical trial published about the effect of garlic on body composition in both genders.
In the present study, there were significant reductions in body weight not only in the garlic group but also in the placebo group. It seems that the observed reduction in body weight among the control group may be due to significant reductions in energy intake. In garlic group, although energy intake was unchanged, weight changes in the garlic group were significantly greater than the placebo group. These observations imply that garlic supplementation successfully reduced body weight. Consistent with this, Lee et al. showed that adding garlic to diet-induced obese mice suppressed body weight gain.[18]
We also observed no significant changes in lean body mass after a 15-week garlic supplementation. The weight loss is often accompanied by a loss of lean body mass, which has deleterious metabolic consequences.[26,27] Therefore, this finding demonstrated that garlic could have beneficial effects on lean body mass. In a clinical study, Seo et al. reported that the aged garlic extract reduced body weight and lean body mass in postmenopausal women after a 12-week intervention.[28] There are several differences between our study and aforesaid report such as gender, supplement, and study duration. It also seems that their observations cannot be reliable due to the small sample size (placebo; n = 6 and age intake; n = 8).
Another important finding in our study was a significant reduction in body fat mass after garlic supplementation without significant changes in energy intake. Although there are limited studies in this context, Joo et al. reported that dietary supplementation with garlic significantly reduced adipose tissue mass in rats fed a high-fat diet.[29]
In the adipocytes, fat is accumulated due to excess energy intake. White adipose tissue (WAT) is the main storage for reserve energy in the form of TG. In contrast, brown adipose tissue (BAT) plays an important role in using the triglyceride (TG) to produce heat. Thermogenesis in BAT is induced via the sympathetic nervous system.[30] In rats, it has been reported that garlic and its derivatives enhanced the sympathetic nervous system activation through increasing the secretion of norepinephrine.[31,32] The anti-obesity action associated with garlic may refer to its thermogenic properties. In support to this suggestion, it has been reported that garlic and its derivatives increased the oxygen consumption.[33] Some studies also suggested that anti-obesity effect of garlic and its derivatives might be mediated at least partially by other mechanisms. Keophiphath et al. indicated that 1,2-vinyldithiin (a garlic-derived organosulfur) suppressed human preadipocyte differentiation and lipid accumulation in differentiated preadipocyte by inhibiting gene expression of peroxisome proliferator-activated receptor gamma.[34] Yang et al. reported that ajoene (another garlic-derived organosulfur) could induce apoptosis in 3T3-L1 adipocytes through the activation of mitogen-activated protein kinases and fragmentation of DNA.[35] Joo et al. also showed that garlic caused an increase in the fecal triglyceride by decreasing intestinal triglyceride absorption.[29]
Several points should be considered as strengths of the current study such as the stratified blocked randomization design, equal macro and micronutrient distribution between two groups, high compliance rate (more than 93%) among patients completing the study, and adequate sample size and study duration.
We should mention several limitations. In the current study, we used BIA as a noninvasive, safe, portable, and rapid method for assessment of body composition while BIA is not the gold-standard measure of body composition. Also, using BIA to measure body fat mass has a major limitation due to changes in total body water during the study.[24,25] We did not observe changes in total body water during the study, so this did not limit our findings. Another limitation of this study was that we could not precisely assess dietary intake throughout the trial. So, these might have been affected our results.
CONCLUSION
This study demonstrated that a 15-week garlic supplementation could decrease body fat mass among NAFLD patients. Therefore, garlic may reduce the amount of fat in the liver and prevent or delay the progression of NAFLD. Further studies with stronger design, longer periods are necessary.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank all participants and assistants in this research.
REFERENCES
- 1.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–19. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 2.Zivkovic AM, German JB, Sanyal AJ. Comparative review of diets for the metabolic syndrome: Implications for nonalcoholic fatty liver disease. Am J Clin Nutr. 2007;86:285–300. doi: 10.1093/ajcn/86.2.285. [DOI] [PubMed] [Google Scholar]
- 3.Pang Q, Zhang JY, Song SD, Qu K, Xu XS, Liu SS, et al. Central obesity and nonalcoholic fatty liver disease risk after adjusting for body mass index. World J Gastroenterol. 2015;21:1650–62. doi: 10.3748/wjg.v21.i5.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: The dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 5.Patel S, Lawlor DA, Ferreira DL, Hughes AD, Chaturvedi N, Callaway M, et al. The association of nonalcoholic fatty liver disease with central and peripheral blood pressure in adolescence: Findings from a cross-sectional study. J Hypertens. 2015;33:546–52. doi: 10.1097/HJH.0000000000000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatrath H, Vuppalanchi R, Chalasani N. Dyslipidemia in patients with nonalcoholic fatty liver disease. Semin Liver Dis. 2012;32:22–9. doi: 10.1055/s-0032-1306423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155–61. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 8.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–98. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 9.Yki-Järvinen H. Nutritional modulation of nonalcoholic fatty liver disease and insulin resistance: Human data. Curr Opin Clin Nut Metab Care. 2010;13:709–14. doi: 10.1097/MCO.0b013e32833f4b34. [DOI] [PubMed] [Google Scholar]
- 10.Kramer CK. Weight loss is a useful therapeutic objective. Can J Cardiol. 2015;31:211–5. doi: 10.1016/j.cjca.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki A, Nitta H, Otsuka K, Umemura A, Baba S, Obuchi T, et al. Bariatric surgery and non-alcoholic fatty liver disease: Current and potential future treatments. Front Endocrinol (Lausanne) 2014;5:164. doi: 10.3389/fendo.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stern JS, Peerson J, Mishra AT, Sadasiva Rao MV, Rajeswari KP. Efficacy and tolerability of a novel herbal formulation for weight management. Obesity (Silver Spring) 2013;21:921–7. doi: 10.1002/oby.20211. [DOI] [PubMed] [Google Scholar]
- 13.Rivlin RS. Historical perspective on the use of garlic. J Nutr. 2001;131(3 Suppl):951S–4. doi: 10.1093/jn/131.3.951S. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Yao HP, Huang FF, Wu W, Gao Y, Chen ZB, et al. Allicin, a major component of garlic, inhibits apoptosis in vital organs in rats with trauma/hemorrhagic shock. Crit Care Med. 2008;36:3226–32. doi: 10.1097/CCM.0b013e31818f2103. [DOI] [PubMed] [Google Scholar]
- 15.Sobenin IA, Pryanishnikov VV, Kunnova LM, Rabinovich YA, Martirosyan DM, Orekhov AN. The effects of time-released garlic powder tablets on multifunctional cardiovascular risk in patients with coronary artery disease. Lipids Health Dis. 2010;9:119. doi: 10.1186/1476-511X-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim EJ, Lee DH, Kim HJ, Lee SJ, Ban JO, Cho MC, et al. Thiacremonone, a sulfur compound isolated from garlic, attenuates lipid accumulation partially mediated via AMPK activation in 3T3-L1 adipocytes. J Nutr Biochem. 2012;23:1552–8. doi: 10.1016/j.jnutbio.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Han CY, Ki SH, Kim YW, Noh K, Lee da Y, Kang B, et al. Ajoene, a stable garlic by-product, inhibits high fat diet-induced hepatic steatosis and oxidative injury through LKB1-dependent AMPK activation. Antioxid Redox Signal. 2011;14:187–202. doi: 10.1089/ars.2010.3190. [DOI] [PubMed] [Google Scholar]
- 18.Lee MS, Kim IH, Kim CT, Kim Y. Reduction of body weight by dietary garlic is associated with an increase in uncoupling protein mRNA expression and activation of AMP-activated protein kinase in diet-induced obese mice. J Nutr. 2011;141:1947–53. doi: 10.3945/jn.111.146050. [DOI] [PubMed] [Google Scholar]
- 19.Nyamathi A, Sinha S, Ganguly KK, Ramakrishna P, Suresh P, Carpenter CL. Impact of protein supplementation and care and support on body composition and CD4 count among HIV-infected women living in rural India: Results from a randomized pilot clinical trial. AIDS Behav. 2013;17:2011–21. doi: 10.1007/s10461-013-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: Prospective cohort study. BMJ. 2004;328:983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner B, Mølgaard C, Marckmann P. Effect of garlic (Allium sativum) powder tablets on serum lipids, blood pressure and arterial stiffness in normo-lipidaemic volunteers: A randomised, double-blind, placebo-controlled trial. Br J Nutr. 2004;92:701–6. doi: 10.1079/bjn20041255. [DOI] [PubMed] [Google Scholar]
- 22.Mader FH. Treatment of hyperlipidaemia with garlic-powder tablets. Evidence from the German Association of General Practitioners’ multicentric placebo-controlled double-blind study. Arzneimittelforschung. 1990;40:1111–6. [PubMed] [Google Scholar]
- 23.Braam RL, van Uum SH, Lenders JW, Thien T. Bromide as marker for drug adherence in hypertensive patients. Br J Clin Pharmacol. 2008;65:733–6. doi: 10.1111/j.1365-2125.2007.03068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis – Part I: Review of principles and methods. Clin Nutr. 2004;23:1226–43. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Bioelectrical impedance analysis in body composition measurement: National Institutes of Health Technology Assessment Conference Statement. Am J Clin Nutr. 1996;64(3 Suppl):524S–32. doi: 10.1093/ajcn/64.3.524S. [DOI] [PubMed] [Google Scholar]
- 26.Hunter GR, Byrne NM, Sirikul B, Fernández JR, Zuckerman PA, Darnell BE, et al. Resistance training conserves fat-free mass and resting energy expenditure following weight loss. Obesity (Silver Spring) 2008;16:1045–51. doi: 10.1038/oby.2008.38. [DOI] [PubMed] [Google Scholar]
- 27.Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: Implications for sarcopenic obesity. Nutr Rev. 2010;68:375–88. doi: 10.1111/j.1753-4887.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- 28.Seo DY, Lee SR, Kim HK, Baek YH, Kwak YS, Ko TH, et al. Independent beneficial effects of aged garlic extract intake with regular exercise on cardiovascular risk in postmenopausal women. Nutr Res Pract. 2012;6:226–31. doi: 10.4162/nrp.2012.6.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joo H, Kim CT, Kim IH, Kim Y. Anti-obesity effects of hot water extract and high hydrostatic pressure extract of garlic in rats fed a high-fat diet. Food Chem Toxicol. 2013;55:100–5. doi: 10.1016/j.fct.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 30.Yonezawa T, Kurata R, Hosomichi K, Kono A, Kimura M, Inoko H. Nutritional and hormonal regulation of uncoupling protein 2. IUBMB Life. 2009;61:1123–31. doi: 10.1002/iub.264. [DOI] [PubMed] [Google Scholar]
- 31.Oi Y, Kawada T, Kitamura K, Oyama F, Nitta M, Kominato Y, et al. Garlic supplementation enhances norepinephrine secretion, growth of brown adipose tissue, and triglyceride catabolism in rats. J Nutr Biochem. 1995;6:250–5. [Google Scholar]
- 32.Oi Y, Kawada T, Shishido C, Wada K, Kominato Y, Nishimura S, et al. Allyl-containing sulfides in garlic increase uncoupling protein content in brown adipose tissue, and noradrenaline and adrenaline secretion in rats. J Nutr. 1999;129:336–42. doi: 10.1093/jn/129.2.336. [DOI] [PubMed] [Google Scholar]
- 33.Unce DU, Tiryaki G, Nmez S, Unce ML. Effects of garlic on aerobic performance. Turk J Med Sci. 2000;30:557–61. [Google Scholar]
- 34.Keophiphath M, Priem F, Jacquemond-Collet I, Clément K, Lacasa D. 1, 2-vinyldithiin from garlic inhibits differentiation and inflammation of human preadipocytes. J Nutr. 2009;139:2055–60. doi: 10.3945/jn.109.105452. [DOI] [PubMed] [Google Scholar]
- 35.Yang JY, Della-Fera MA, Nelson-Dooley C, Baile CA. Molecular mechanisms of apoptosis induced by ajoene in 3T3-L1 adipocytes. Obesity (Silver Spring) 2006;14:388–97. doi: 10.1038/oby.2006.52. [DOI] [PubMed] [Google Scholar]


