Abstract
Purpura fulminans (PF) is a descriptive term used to describe a heterogeneous group of disorders characterized by rapidly progressive purpuric lesions that may develop into extensive areas of skin necrosis, and peripheral gangrene. This rare disorder is associated with laboratory evidence of consumptive coagulopathy and is often fatal. PF is usually associated with many infections, most notably with meningococcal, staphylococcal, and streptococcal infections. However, there are very few reports of this entity with spotted fever and scrub typhus from India. Rickettsial infections are an underdiagnosed group of diseases presenting as acute febrile illness, with high mortality in untreated cases. Of the available tests, Weil–Felix is a handy and economical tool for early diagnosis of this fatal disease especially in resource poor settings. We present four infants with PF secondary to rickettsial fever diagnosed by the Weil–Felix test.
Key words: Purpura fulminans, rickettsial infections, Weil–Felix test
INTRODUCTION
Purpura fulminans (PF) is a rare syndrome of intravascular thrombosis and hemorrhagic infarction of the skin that is rapidly progressive and is accompanied by vascular collapse and disseminated intravascular coagulation.[1] Three categories can be identified: Inherited abnormalities of the coagulation system, acute infectious, and idiopathic.[2] Rickettsial infections are a rare cause of PF. In developing countries such as India, the simple, economical Weil–Felix test (WFT) as an initial investigation can guide the clinician in diagnosing and instituting appropriate treatment.[3] We describe four cases of PF secondary to rickettsial infection, and the importance of WFT in timely diagnosis of this often fatal condition.
CASE REPORTS
Case 1
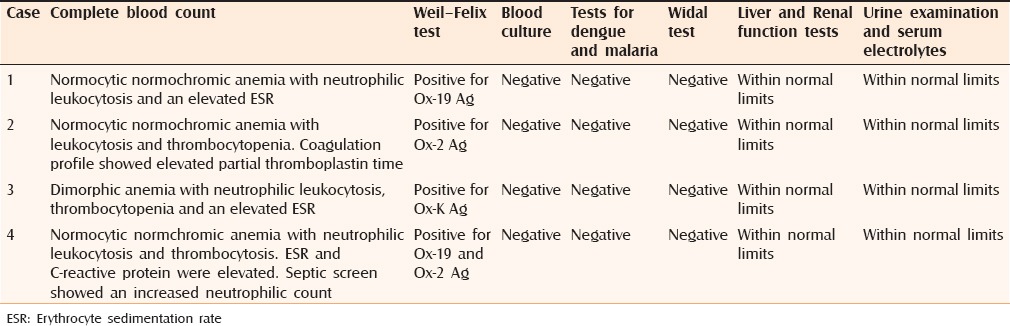
A 6-month-old female infant presented with high-grade fever of 10 days duration associated with a skin rash, which started three days after the onset of fever. The rash was erythematous initially, started over the back and progressed rapidly to involve the limbs and face. The lesions progressed to become black and necrotic with few hemorrhagic blebs. The child progressed to develop multiple ulcers with red to pale granulation tissue covered with necrotic skin [Figure 1]. Reticulate erythema was present over the trunk, palms, and soles. Systemic examination revealed hepatomegaly. On investigation, complete blood count revealed normocytic normochromic anemia with neutrophilic leukocytosis. WFT was positive for Ox-19 antigen. Other relevant investigations were within normal limits [Table 1]. The infant was diagnosed with acute infectious PF secondary to rickettsial infection of the typhus fever group.
Figure 1.
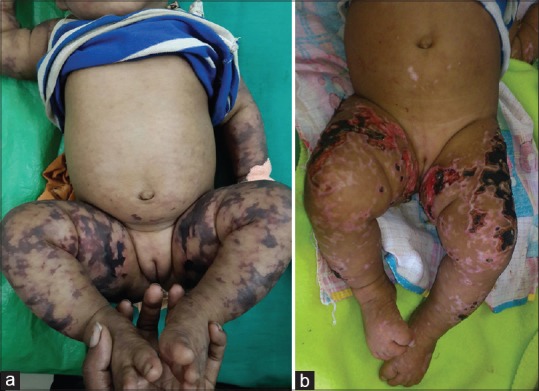
(a) Day 1, stellate sharply defined purpuric patches on the lower and upper limbs. (b) Day 7, necrotic areas of skin with underlying ulcers with red granulation tissue and healing with hypopigmentation
Table 1.
Investigative profile of the patients
Case 2
A 12-month-old female infant born of a second-degree consanguineous marriage presented with highgrade fever associated with chills of eight days duration. History of vomiting and loose stools was present. An erythematous skin rash began on the fifth day of fever over the trunk and rapidly progressed to involve the face and extremities with a rapid color change to black. On examination, black necrotic patches overlying ulcers were present over the gluteal region, lower limbs, trunk, and finger tips [Figure 2]. Systemic examination revealed no abnormality.
Figure 2.
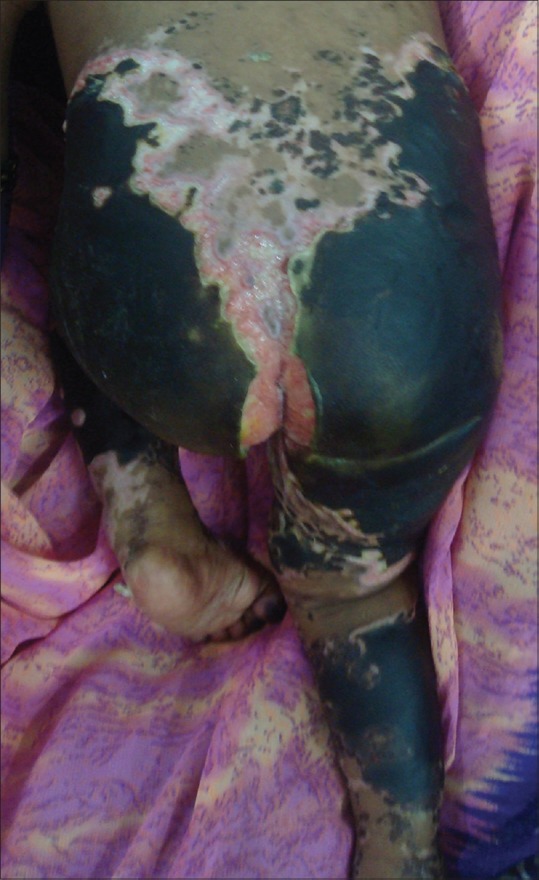
Sloughing necrotic gangrenous skin with underlying ulcers with granulation tissue over the lower trunk, gluteal region, thighs, and legs
Investigations revealed anemia with leukocytosis and thrombocytopenia. Coagulation profile showed elevated partial thromboplastin time. WFT was positive for Ox-2 antigen. Other investigations were normal [Table 1]. A diagnosis of acute PF secondary to rickettsial infection of the spotted fever group was made.
Case 3
A 7-month-old female baby born to a consanguineous couple presented with fever of eight days duration associated with a purpuric rash of two days duration, which began over the face and rapidly progressed to involve the trunk and extremities. On cutaneous examination, sharply demarcated purpuric patches with necrotic changes were present over the upper limb, buttocks, and lower limb [Figure 3]. Systemic examination was normal.
Figure 3.
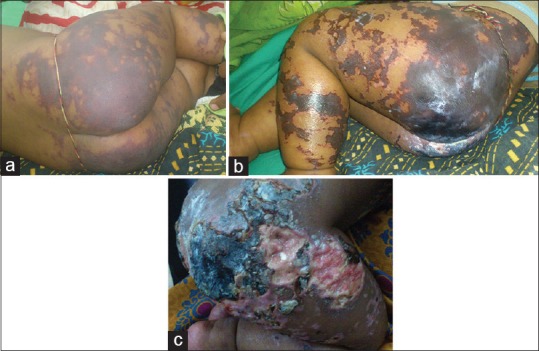
(a) Day 1, erythematous to purplish well-defined confluent patches over the gluteal region, thighs, and legs. (b) Day 4, black infarcted areas of skin over the buttocks and lower limb. (c) Day 10, ulcers with slough and pale red granulation tissue with overlying gangrenous areas of skin on the gluteal region and thighs
Investigations revealed dimorphic anemia with neutrophilic leukocytosis, thrombocytopenia, and an elevated erythrocyte sedimentation rate (ESR). WFT was positive for Ox-K Ag. Other investigations were within normal limits [Table 1]. The infant was diagnosed with acute PF secondary to infection with rickettsial scrub typhus group.
The infants were managed in the pediatric intensive care unit and were started on doxycycline, amikacin, and a third-generation cephalosporin. Doxycyline is economical and the drug of choice for all rickettsial diseases in patients of all ages, even during pregnancy. It was instituted at a dose of 2.2 mg/kg b.d. for a duration of 10 days. The other antibiotics were started to provide wider coverage and to prevent secondary infections due to significant tissue loss. Blood transfusion and supportive therapy were instituted. The patients showed improvement and were referred to plastic surgery for better management of the extensive tissue loss.
Case 4
A 4-year-old male child presented with fever and painful skin lesions on upper and lower limbs of 12 days duration. The skin lesions began on the lower limbs and rapidly evolved to form black necrotic areas. History of joint pain was present [Figure 4]. On examination, there were stellate brownish black necrotic patches on the upper and lower extremities. Hepatomegaly was present.
Figure 4.
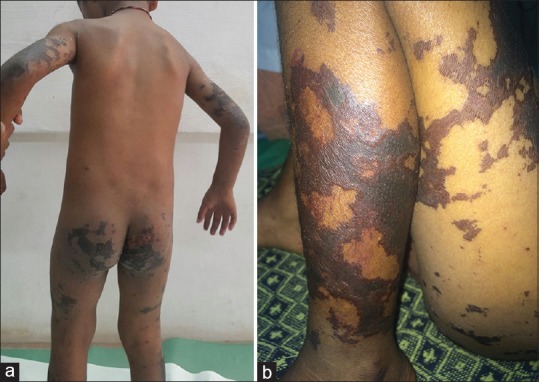
(a) Stellate brownish patches present over the gluteal region, upper and lower limbs. (b) Well-defined black necrotic areas of skin over the thighs and legs
Investigations showed normocytic normochromic anemia with neutrophilic leukocytosis. Platelet count, ESR and C-reactive protein were elevated. The septic screen showed neutrophilia. WFT was positive for Ox-2 and Ox-19 Ag. Other relevant investigations were normal [Table 1]. The patient was diagnosed with acute PF secondary to rickettsial spotted fever group and was treated with netilmicin, piperacillin, and doxycycline. Supportive, nutritional, and fluid therapy were instituted. The patient recovered and the lesions healed with hypopigmentation.
DISCUSSION
PF is a life-threatening disorder characterized by sudden progressive cutaneous hemorrhage and necrosis.[4] Three forms have been classified: neonatal, idiopathic, and acute infectious.[1]
Neonatal PF generally presents within 72 h of birth but may present in later months of life.[2] The purpuric lesions are distributed mainly over the perineal region and flexors of thighs and abdomen.[1] Protein C mutations or inherited deficiency of protein S or antithrombin III may lead to neonatal PF.[1]
Idiopathic or postinfectious PF characteristically occurs one to three weeks after an acute infectious process. The disorder is more common in young children, and varicella and streptococcal infections are the most common antecedents.[5] After appearing to recover from an otherwise uncomplicated childhood illness, patients suddenly develop extensive areas of purpura, principally affecting the buttocks and lower limbs. The disease may progress rapidly to cause extensive areas of skin necrosis and gangrene of the limbs or digits. Thromboembolic complications may subsequently occur.[6,7] The pathogenesis involves acute transient decrease in protein C, protein S, or antithrombin III levels.[1]
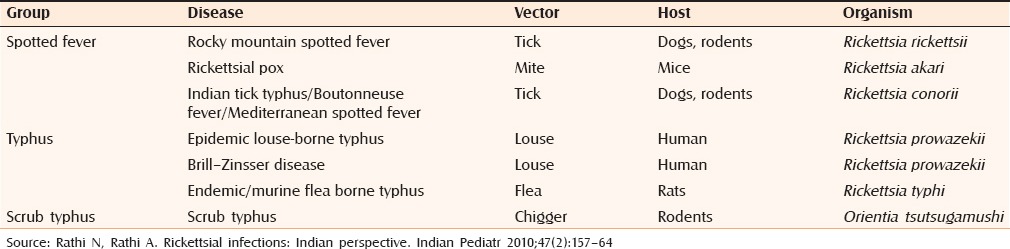
The most common form, acute infectious PF, occurs in the context of acute bacterial sepsis including, Staphylococcus aureus, Streptococci, Hemophilus influenza, Haemophilus aegypticus, and Pseudomonas aeruginosa.[8] Rickettsial infections have been occasionally associated with it. Among the rickettsiae, R. conorii, R. rickettsii, and R. australis have produced fatal PF.[9] [Table 2][10] Indian tick typhus has been described as an etiological factor for PF from various parts of India.[9] Scrub typhus has been rarely associated with it.[9] The rickettsial rash is usually macular, maculopapular, petechial, or palpable purpura resembling vasculitis, and typically involves the palms and soles. A classic triad of fever, rash, and history of tick exposure is often cited.[11]
Table 2.
The rickettsial group of infections
The laboratory features are suggestive of consumption coagulopathy, with low concentrations of fibrinogen, clotting factors and platelets, and prolonged prothrombin and partial thromboplastin time. Fibrin degradation products tend to be raised and concentrations of protein C, S, and antithrombin III are reduced.[1]
Tests available to diagnose rickettsiosis are culture, molecular tests, and serology including immunofluorescence and WFT. Specific investigations like immunoflourescence, Western blot or polymerase chain reaction are expensive and not readily available.[3] Because of logistics and other constraints, WFT proves to be handy and affordable for the peripheral areas.[12]
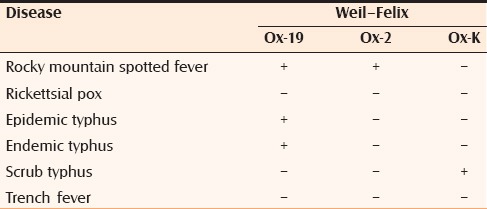
WFT relies on the principle of agglutination of antibodies that are formed during rickettsial infection with certain strains of Proteus vulgaris (Ox-2, Ox-19, Ox-K)[13] [Table 3]. The WFT has a sensitivity of 33% and specificity of 46% and has been evaluated to be useful in developing countries as the first diagnostic step in rickettsial diseases.[3] Either a fourfold rise in the agglutinin titer in paired sera or a single titer of more than or equal to 1:320 is considered diagnostic for infection with these febrile agents. The results of the WFT of the patients included in the case-series are tabulated in table 1. One of the major limitations of WFT is the cross-reactivity among several rickettsial species.[3]
Table 3.
Interpretation of the Weil–Felix test
Doxycycline is the drug of choice for Spotted Fever Group (SFG) rickettsiosis, the other drugs commonly used are chloramphenicol, macrolides, and rifampicin. Antibiotic susceptibility does not vary much among the different species and hence exact species identification is not essential.[14]
CONCLUSION
Rickettsial fever is seldom diagnosed in India, probably due to a low index of suspicion. There have been very few reports of rickettsioses in children from the southern Indian states. Underdiagnosed and misdiagnosed rickettsial infections are an important public health problem leading to an increased morbidity and mortality in patients with PF. This case-series highlights that rickettsial infections may be more prevalent than documented in south India and need to be considered in the etiological workup of children with PF. WFT, although not sensitive is relatively specific and is inexpensive and thus may be used as an initial investigation to substantiate the diagnosis of rickettsial infections along with clinical correlation.
Financial support and sponsorship
JJM Medical College, Davangere, Karnataka.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Talwar A, Kumar S, Gopal MG, Nandini AS. Spectrum of purpura fulminans: Report of three classical prototypes and review of management strategies. Indian J Dermatol Venereol Leprol. 2012;78:228. doi: 10.4103/0378-6323.93655. [DOI] [PubMed] [Google Scholar]
- 2.Adcock DM, Bronza JP, Marlar RA. Proposed classification and pathologic mechanism of purpura fulminans and skin necrosis. Semin Thromb Hemost. 1990;16:333–40. doi: 10.1055/s-2007-1002686. [DOI] [PubMed] [Google Scholar]
- 3.Mittal V, Gupta N, Bhattacharya D, Kumar K, Ichhpujani RL, Singh S, et al. Serological evidence of rickettsial infections in Delhi. Indian J Med Res. 2012;135:538–41. [PMC free article] [PubMed] [Google Scholar]
- 4.Darmstadt GL. Acute infectious purpura fulminans: Pathogenesis and medical management. Pediatr Dermatol. 1998;15:169–83. doi: 10.1046/j.1525-1470.1998.1998015169.x. [DOI] [PubMed] [Google Scholar]
- 5.Francis RB., Jr Acquired purpura fulminans. Semin Thromb Hemost. 1990;16:310–25. doi: 10.1055/s-2007-1002684. [DOI] [PubMed] [Google Scholar]
- 6.Levin M, Eley BS, Louis J, Cohen H, Young L, Heyderman RS. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355–63. doi: 10.1016/s0022-3476(95)70063-3. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen P, Reynaud J, Pouzol P, Munzer M, Richard O, Francois P. Varicella and thrombotic complications associated with transient protein C and protein S deficiencies in children. Eur J Pediatr. 1994;153:646–49. doi: 10.1007/BF02190684. [DOI] [PubMed] [Google Scholar]
- 8.Levin M, Eley B, Faust S. Purpura fulminans. In: Irvine A, Hoeger P, Yan A, editors. Textbook of Pediatric Dermatology. 3rd ed. Vol. 2. West Sussex, UK: WB Publication; 2011. pp. 162.1–162.16. [Google Scholar]
- 9.Jain D, Viswanathan S, Ramasamy C. A life threatening rash, an unexpected cause. Case Rep Dermatol Med 2014. 2014:146251. doi: 10.1155/2014/146251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rathi N, Rathi A. Rickettsial infections: Indian perspective. Indian Pediatr. 2010;47:157–64. doi: 10.1007/s13312-010-0024-3. [DOI] [PubMed] [Google Scholar]
- 11.Parola P, Fenollar F, Badiaga S, Brouqui P, Raoult D. First documentation of Rickettsia conorii infection (strain Indian tick typhus) in a Traveler. Emerg Infect Dis. 2001;7:909–10. doi: 10.3201/eid0705.017527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamarasu K, Malathi M, Rajagopal V, Subramani K, Jagadeeshramasamy D, Mathai E. Serological evidence for wide distribution of spotted fevers and typhus fever in Tamil Nadu. Indian J Med Res. 2007;126:128–30. [PubMed] [Google Scholar]
- 13.Bhalwar R, Tilak R, Rao MK, Tilak VW. Surveillance of scrub typhus in the fringe around Pune: Potential for transmission does exist. MJAFI. 2003;59:117–20. doi: 10.1016/S0377-1237(03)80054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kundavaram A, Francis NR, Jude AJ, Varghese GN. Acute infectious purpura fulminans due to probable spotted fever. J Postgrad Med. 2014;60:198–99. doi: 10.4103/0022-3859.132345. [DOI] [PubMed] [Google Scholar]


