Review on monocyte / macrophage functions revealed by intravital imaging during steady state and pathological conditions.
Keywords: two-photon, microscopy, myeloid cells, vasculature, tumors, infection
Abstract
Monocytes and macrophages are a diverse population of innate immune cells that play a critical role in homeostasis and inflammation. These cells are surveillant by nature and closely monitor the vasculature and surrounding tissue during states of health and disease. Given their abundance and strategic positioning throughout the body, myeloid cells are among the first responders to any inflammatory challenge and are active participants in most immune-mediated diseases. Recent studies have shed new light on myeloid cell dynamics and function by use of an imaging technique referred to as intravital microscopy (IVM). This powerful approach allows researchers to gain real-time insights into monocytes and macrophages performing homeostatic and inflammatory tasks in living tissues. In this review, we will present a contemporary synopsis of how intravital microscopy has revolutionized our understanding of myeloid cell contributions to vascular maintenance, microbial defense, autoimmunity, tumorigenesis, and acute/chronic inflammatory diseases.
Introduction
IVM represents a powerful tool to gain mechanistic insights into the dynamics of biologic processes. Over the past 2 decades, the imaging community has pioneered many innovations that have vastly improved our ability to visualize cell behavior in vivo. One IVM approach that has advanced the field considerably is TPM, which was pioneered in 1990 [1]. For standard fluorescence microscopy (e.g., confocal, epifluorescence), a fluorophore is excited by a single photon, causing it to emit light that is collected by a detector. The excitation wavelengths for single photon microscopy are typically within the visible spectrum (390–700 nm). In contrast, TPM relies on short pulses of near-infrared light (680–1300 nm) to excite a fluorophore. At these wavelengths, a single photon does not have sufficient energy to excite the fluorophore. Excitation instead requires the near-simultaneous arrival of 2 photons. This is achieved by focusing pulsed laser light on a small point within the sample, which largely eliminates fluorescent excitation above and below the focal plane. When compared with single photon microscopy, TPM helps to reduce background fluorescence and tissue damage. In addition, near-infrared light penetrates tissue better than visible light, allowing greater imaging depths to be achieved [2]. However, despite the noted benefits of TPM, 1 and 2-photon microscopy are commonly used to perform IVM experiments [3–5].
IVM can be performed by use of single or multiphoton microscopy approaches. General advances in IVM over the past decades include increased temporal resolution, decreased phototoxicity, improved imaging depths, simultaneous acquisition of multiple fluorophores with better resolution, and enhanced image stabilization despite body motion [2, 6, 7]. Intravital imaging improves upon classic in vitro experimentation by enabling study of cellular processes in their natural environments, where molecular gradients, extracellular matrices, cellular densities, and fluid forces remain intact. One of the most advantageous aspects of IVM is the ability to monitor the dynamics of cellular migration and interactions over time in a 3-dimensional microenvironment [7]. In this capacity, IVM has been used extensively over the years to study immune and nonimmune cell populations in diverse fields of study, such as immunology, oncology, developmental biology, and others [8]. The approach has enabled investigators to gain novel insights into the dynamics of many immune cell populations, such as myeloid cells. Macrophages are a type of myeloid cell that were first discovered in the 19th century by Ilya Metchnikoff, who interestingly, was also the first person to conduct an IVM experiment on these cells, accumulating around a rose thorn inserted into a starfish larvae [9, 10]. Monocytes and macrophages are key participants in tissue homeostasis and inflammation [11]. Indeed, after sterile injury or infection, studies have shown that tissue resident macrophages and infiltrating monocyte-derived macrophages can facilitate inflammatory reactions and influence the outcome of a disease [11]. In this review, we will discuss how IVM has been used over the years to shed new light on the biology of monocytes and macrophages.
VASCULAR AND PERIVASCULAR MONOCYTE/MACROPHAGE DYNAMICS
Nonclassical versus classical monocytes
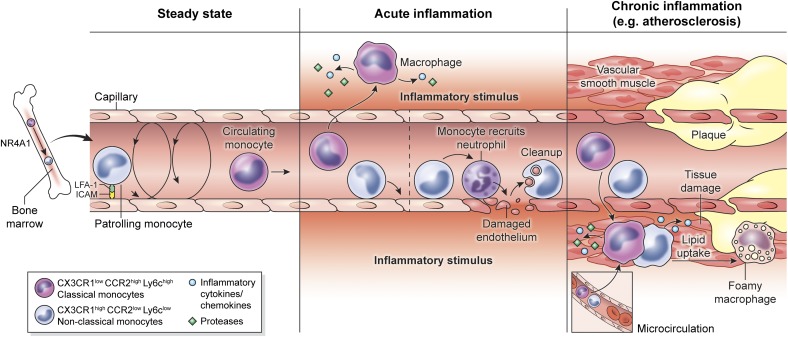
Peripheral blood monocytes are a heterogeneous population of circulating leukocytes. With the use of a murine adoptive transfer system to probe monocyte homing and differentiation in vivo, Geismann et al. [12] identified 2 functional subsets of blood-derived monocytes: classical (CX3CR1loCCR2hiLy6Chi) and nonclassical (CX3CR1hiCCR2loLy6Clo; Fig. 1, left). Corresponding subsets of monocytes were also identified in the blood of humans. Nonclassical monocytes require the transcription factor NR4A1 for their development from proliferating bone marrow precursors (Fig. 1, left) [13]. This represents an important discovery, as it will allow unambiguous manipulation of classical versus nonclassical monocyte populations. Nonclassical monocytes are thought to patrol and maintain the vasculature under steady-state conditions by clearing damaged endothelial cells [14]. Classical monocytes, on the other hand, are sometimes referred to as “inflammatory monocytes,” as a result of their ability to serve as immediate precursors of tissue DCs and macrophages during inflammation [15–17]. These monocytes express the chemokine receptor, CCR2, which participates in their release from the bone marrow and recruitment to sites of inflammation [18]. Following differentiation in inflamed tissues, they can produce TNF-α, iNOS, and ROS that help provide a defense against invading bacterial and parasitic infections [17]. Interestingly, another subset of monocytes with intermediate Ly6c expression was discovered [19] that might represent a transitional state between Ly6chi and Ly6clo monocytes or an entirely distinct monocyte subpopulation altogether. These monocytes are thought to differentiate readily into DCs in the lymph node [20], but the function of these cells in vivo is unknown, and they have not been studied specifically by IVM.
Figure 1. Vascular and perivascular monocyte/macrophage dynamics.
(Left) Blood monocytes in mice are divided into 2 bone marrow-derived subsets: classical monocytes (CX3CR1lowCCR2highLy6Chigh) and nonclassical monocytes (CX3CR1highCCR2lowLy6Clow). The transcription factor, NR4A1, controls bone marrow differentiation of nonclassical monocytes. During steady state, only nonclassical monocytes (also referred to as patrolling monocytes) can be observed crawling along the luminal surface of blood vessels. (Middle) Following induction of acute inflammation, classical monocytes arrest on the vascular lumen, extravasate, and migrate toward the inflammatory stimuli. Nonclassical monocytes can be retained on the vascular lumen, where they have both pro- and anti-inflammatory roles. As sensors of inflammatory stimulus, they trigger the recruitment of activated neutrophils that damage the endothelium. However, they also participate in endothelium cleaning after damage, favoring the resolution of inflammation. (Right) During chronic inflammatory diseases, such as atherosclerosis, both classical and nonclassical monocytes are recruited to vascular plaques and come from the inflamed vessel or adjacent microcirculation. Monocytes can differentiate into macrophages and uptake lipids, thus transforming into foamy cells.
Steady-state dynamics of blood monocytes
IVM has vastly improved our understanding of how vascular monocytes function in the absence of inflammation. From these studies, the notion of constitutive vascular immune patrolling by monocytes has emerged. In contrast to classical monocytes, nonclassical monocytes crawl along the endothelium of blood vessels during steady-state conditions (Fig. 1, left). This monocytic crawling behavior, also called patrolling, is characterized by long (>200 μm, on average), complex tracks on vascular walls that include U-turns and spirals, which are independent of blood-flow direction. Patrolling monocytes have a slow velocity (10–16 µm/min) when in contact with the vessel wall and constantly move between the bloodstream and endothelial surface, with an average dwell time of 10–20 min [21, 22]. This patrolling primarily depends on the β2 integrin, LFA-1 [14, 21], and has been observed in dermal, heart, cremaster, liver, and mesenteric vessels, as well as in the vascular network of the kidney cortex [13, 21–25], although the frequency of monocyte patrolling varies between tissues. For example, crawling monocytes are more abundant in the dermis and kidney than in the mesentery [14]. In the capillaries of normal kidney glomeruli, monocytes and neutrophils are retained for several minutes, and it is postulated that this basal program of myelomonocytic cell surveillance underlies the susceptibility of the glomerulus to inflammation [25]. Until recently, the function of patrolling monocytes was not understood. Carlin et al. [14] shed light on this important question by showing that monocytes actually scavenge microparticles from the luminal surface of blood vessels during steady-state conditions. These data suggest that nonclassical monocytes are intravascular housekeepers that help participate in the maintenance of vascular endothelium.
Monocyte dynamics during acute inflammation
In addition to studying steady-state conditions, IVM facilitates analysis of monocyte dynamics in the vasculature before and during the onset of tissue inflammation. Nonclassical Ly6Clo monocytes are ideally located to provide immune surveillance of endothelial cells and surrounding tissues, and Carlin et al. [14] provided insights into how these cells respond to an inflammatory stimulus. Administration of a TLR7 agonist, as an inducer of inflammation, resulted in retention (20–25 min on average) of nonclassical monocytes on kidney capillaries. After a few hours, neutrophils were recruited by monocytes in a TLR7-dependent manner, which induced endothelial cell necrosis. The resultant cellular debris was eventually removed by patrolling monocytes (Fig. 1, middle). Interestingly, neutrophil killing and endothelial cell death were abolished in Nr4a1- and CD11a−/− mice, which prevented the development and patrolling of nonclassical monocytes, respectively. These data demonstrate that nonclassical monocytes have intravascular functions during inflammatory as well as steady-state conditions.
During states of inflammation, classical monocytes can also become responsive to the stimulus (Fig. 1, middle). Classical monocytes usually do not adhere to blood vessels under steady-state conditions. Their properties change rapidly in inflamed tissues, and they adopt a rolling behavior along vascular surfaces, usually associated with the Ly6Clo subset in the absence of inflammation. This is often followed by extravasation into the inflamed tissue [22, 26].
Inflammatory monocytes are mobilized to ward off infections, such as Salmonella, but can sometimes participate in disease pathogenesis. Pai et al. [27] monitored the real-time behavior of Ly6C+ monocytes in the vasculature during the development of experimental cerebral malaria. This disease is associated with microcirculatory dysfunction and intravascular leukocyte sequestration. Plasmodium-specific CD8+ T cells are required for the development experimental of cerebral malaria and were shown by IVM to promote adherence of Ly6Chi monocytes to brain vasculature, likely by activating endothelial cells [27]. A similar link between CD8+ T cells and monocytes was observed following CNS viral infection. Intracerebral inoculation of mice with lymphocytic choriomeningitis virus induces fatal immune-mediated meningitis [28, 29]. During disease development, virus-specific CD8+ T cells release chemoattractants that promote extravasation of myelomonocytic cells (monocytes and neutrophils) into the meninges [30]. Examination of the inflamed meninges in symptomatic mice revealed that these secondarily recruited innate immune cells promote vascular breakdown and contribute to fatal disease [30]. Collectively, these data demonstrate that the role played by monocytes in an inflammatory disease process is often dictated by the nature of the initiating stimulus.
Monocyte extravasation into inflamed tissues involves a sequence of steps that include vascular rolling, adhesion, and intraluminal crawling. This process has been studied extensively for many different leukocyte populations [31]. Leukocyte rolling along vascular endothelial cells is mediated by multiple members of the selectin and integrin families, and innate cytokines (IFN-α, TNF-α) can initiate this process, as visualized by IVM [31, 32]. Induction of inflammation by injection of TNF-α induced a different monocytic crawling behavior characterized by short tracks that relied on the integrins macrophage-1 antigen instead of LFA-1, in deep contrast with steady state [24]. Whereas this study did not define the subset of monocyte under investigation, the results nevertheless demonstrate that inflammatory cytokines can affect the crawling behavior of monocytes through modulation of integrin expression. This may, in turn, influence the type of monocytes that extravasate into a tissue during states of inflammation.
A great deal is known about the role of classical monocytes in inflammatory responses, as well as the factors that promote their recruitment [17]. The role of nonclassical monocytes, on the other hand, is less well defined and often debated. Auffray et al. [21] showed by IVM that nonclassical monocytes rapidly extravasated into inflamed tissue within 1 h. Inflammation was induced by aseptic wounding, irritants, or Listeria monocytogenes infection. Interestingly, the infiltrating, nonclassical monocytes provided the only source of TNF-α and IL-1 at this early time-point, and expression subsided within a few hours as the monocytes differentiated into wound-healing macrophages characterized by expression of arginase-1 and the mannose receptor. Nonclassical monocytes thus have the capacity to be proinflammatory (a function normally ascribed to classical monocytes) and are thought to participate in inflammatory disease processes, such as traumatic spinal-cord injury [33] and murine lupus [34, 35]. Nonclassical monocytes also have wound-healing properties and are sometimes derivatives of classical monocytes [36]. A recent IVM study by Dal-Secco et al. [36] demonstrated in a model of liver injury that classical CCR2hiCX3CR1lo monocytes initially surrounded the damaged area and then converted into nonclassical CCR2loCX3CR1hi monocytes that participated in tissue repair. This conversion was induced by IL-4 and IL-10, demonstrating that the local tissue milieu can foster reprogramming of classical monocytes to promote wound healing.
Monocyte dynamics during chronic diseases
Atherosclerosis.
Atherosclerosis is a disease process that results in arterial thickening and inflammation, which can ultimately give rise to heart disease or stroke, as a result of decreased blood flow and damage of the affected vessel, with complications including rupture of the plaque and thrombotic vessel occlusion (Fig. 1, right) [37]. This process is exceedingly complex and not entirely understood but is thought to involve retention of LDLs in endothelial cells comprising vessel walls. LDL particles are susceptible to oxidation and can promote the recruitment of monocytes, leading to alterations in permeability and vascular damage. Although many cell types, including DCs, T cells, endothelial cells, smooth muscle cells, and others, have been linked to atherosclerosis, the chronic recruitment of classical monocytes to the developing plaque and their differentiation into macrophages are involved in disease progression (Fig. 1, right) [38].
IVM has helped uncover how myelomonocytic cells contribute to the development of atherosclerotic lesions. For example, ApoE−/− mice are often used as a model of human atherosclerosis [39–41]. Visualization of monocytes (along with potential neutrophils) in ApoE−/− mice through expression of GFP under the lysozyme M promoter [42] revealed recruitment of these innate immune cells to the periphery of vascular plaques within the aorta [43]. It was also shown that microvessels associated with advanced atherosclerotic lesions can serve as a portal for myelomonocytic cell entry into plaques (Fig. 1, right) [44]. Eriksson [44] monitored recruitment of myelomonocytic cells into the advanced lesions of ApoE−/− mice at 12–24 mo of age by IVM and revealed that these cells were associated with plaque venules rather than arterioles or capillaries, demonstrating that venules can become a primary entry for monocytes and neutrophils during atherosclerosis.
A combination of techniques, including IVM, has been used to unravel the functions of classical and nonclassical monocytes during the development of atherosclerosis [45]. Nonclassical Ly6Clo monocytes infiltrate atherosclerotic lesions less frequently than Ly6Chi monocytes, and their accumulation relies on CCR5 instead of CX3CR1 [45]. Whereas there has been some controversy regarding the role of nonclassical monocytes in disease progression, 2 recent studies addressed this question by reconstituting ApoE−/− and Ldlr−/− mice with bone marrow from Nr4a1−/− mice [46, 47]. In both murine atherosclerosis models, deletion of Nr4a1 resulted in enhanced lesions associated with macrophage polarization toward a proinflammatory phenotype. These data suggest that nonclassical monocytes have a protective role during atherosclerosis. However, additional studies are required to determine whether these monocytes limit lesion development in the early phase of disease by cleaning the vasculature or by promoting vascular repair and reducing inflammation after vessels become damaged.
Classical monocytes, on the other hand, are thought to exacerbate the pathogenesis of atherosclerosis. Classical monocytes express CCR2, and deletion of this receptor was shown to decrease development of atherosclerotic lesions in ApoE−/− mice on a high-fat diet [48, 49]. Relative to the nonclassical subset, classical monocytes adhere to endothelium and enter progressive lesions more efficiently. Upon lesion entry, these monocytes differentiate into macrophages and acquire proinflammatory functional properties before transitioning into foam cells, which are macrophages that have engulfed lipids (fats) within atherosclerotic plaques (Fig. 1, right) [45]. Foam cells are not inherently pathogenic, but their presence serves as a sign of the vascular disease process underway.
As monocytes are barometers of atherosclerosis and can also influence lesion development [45], it is important to monitor their real-time dynamics and decipher the mechanisms that enable them to interact with atherosclerotic vessels [38]. Studies have shown that platelet activation at the lesion site can contribute to the pathogenesis of atherosclerosis. Injection of activated platelets increased monocyte adhesion to the vasculature and enhanced lesion size in ApoE−/− mice [50]. The platelets were shown to facilitate this process through delivery of chemokines, such as CCL5 and CXCL4. Genetic modulation of platelets was also shown to influence lesion development [51].
DVT.
IVM has proven particularly useful in visualizing some of the earliest events during inflammation, most notably, leukocyte rolling and adhesion on inflamed blood vessels. During the development of vascular diseases, such as atherosclerosis, the real-time information provided by IVM can help distinguish between cause and effect, as monocytes can actively participate in a disease or simply serve as a barometer for alterations in tissue homeostasis. Other vascular diseases have benefited from IVM. For example, DVT is a vascular disease that results from the formation of a venous clot (often in the legs) that can dislodge and travel to the lungs, causing a pulmonary embolism—a potentially life-threatening situation. Von Brühl et al. [52] recently developed a new murine model of DVT by restricting blood flow in the inferior vena cava. This induced the formation of small thrombi, beginning at 6–12 h, which triggered the recruitment of inflammatory monocytes and neutrophils to the vessel wall, revealed by IVM. Myelomonocytic cell recruitment began at 1 h and increased steadily over time. Recruitment was dependent on endothelial P-selectin and release of the coagulant, tissue factor, by myelomonocytic cells. Interestingly, DVT did not develop when tissue factor was deleted specifically from myelomonocytic cells. In addition, it was shown that platelets and neutrophil extracellular traps were required for DVT, supporting a complex interplay among monocytes, neutrophils, and platelets in the development of this potentially fatal disease.
AD.
AD is a chronic neurodegenerative disease that is associated with the formation of plaques and neurofibrillary tangles in the brain [53]. A hallmark feature of the disease is the accumulation of Aβ, which is thought to stem from impaired elimination of the neurotoxic Aβ peptide. As amyloid deposition is extracellular, myeloid cells have the opportunity to participate in its cleanup. Aβ is commonly deposited in the cerebral vasculature of AD patients, and a recent study used intravital TPM to determine whether circulating monocytes could clear vascular Aβ in a transgenic murine model of AD (APPswe/PS1+/−) [54]. With the use of TPM, it was revealed that patrolling monocytes were attracted to and crawled along the lumen of Aβ+ veins, acquired Congo red+ (an amyloid dye) aggregates, and eventually re-entered the bloodstream. Interestingly, this monocyte behavior was not observed on Aβ+ arteries or blood vessels without Aβ. Venous surveillance for Aβ was linked to nonclassical Ly6C+ monocytes through generation of APP/PS1 chimeras, reconstituted with Nr4a1−/− bone marrow. Transcription-factor Nr4a1−/− selectively removed patrolling Ly6Clo monocytes, which over time, markedly increased Aβ deposition in the hippocampus and cortex. These data demonstrate that nonclassical monocytes play an important role in the removal of Aβ from cerebral vasculature. CCR2+ classical monocytes can also play a neuroprotective role during AD, and there is some evidence to support that these cells become dysfunctional over time, which may facilitate disease progression [55]. There is, however, still much to learn about the dynamics of classical and nonclassical monocytes during the course of AD.
MACROPHAGE INTERACTIONS IN VIVO
In addition to illuminating myeloid cell dynamics in vasculature, IVM facilitates study of these cells in tissue parenchyma during physiologic and pathophysiological conditions. The advantage of the use of this approach is that interactions between macrophages and their surroundings can be monitored in real time, which can help us to understand better the direct contribution of these cells to various biologic processes. Another advantage of using IVM to study tissue macrophages is that their activity can be experimentally modulated and then monitored in vivo. This offers the interesting possibility of developing therapeutics that modify macrophage interactions and functions as they participate in inflammatory disease processes. However, it is important first to map out all of the relevant interactions by IVM. In the following sections, we discuss how IVM studies have informed us about parenchymal macrophage interactions with immune and nonimmune cells.
Macrophage phagocytosis
Bacteria.
The role of macrophages in phagocytosis and clearance of host and microbial-derived products has been studied extensively by use of many different approaches [11]. IVM has helped uncover some interesting macrophage interactions in vivo that enhance phagocytic activity. Whereas platelets play an important role in hemostasis, studies have also demonstrated that they are important participants in immune responses. Platelets were shown to secrete antimicrobial compounds and to enhance the functions of monocytes and neutrophils [56]. Bacteria, such as MRSA and Bacillus cereus, are known to induce platelet aggregation within minutes of infection. Wong et al. [57] recently uncovered the in vivo relevance of this phenomenon by performing an IVM study of liver Kupffer cells, which are tissue-resident macrophages strategically positioned in liver sinusoids, where they trap and phagocytose pathogens in circulation [58]. Under steady-state conditions, circulating platelets expressing GPIb (or CD42) interact transiently with sinusoidal Kupffer cells via surface-expressed vWF [57]. This results in "touch-and-go" platelet-Kupffer cell interactions. Following infection, bacteria were rapidly captured by Kupffer cells, inducing platelet aggregation and bacterial encasement (Fig. 2A). The transition of platelets from touch-and-go interactions to stable aggregation on Kupffer cells following infection was mediated by GPIIb (platelets) and vWF (Kupffer cells). These stable interactions were important for the host defense against B. cereus, as deficiency in platelet aggregation increased bacterial spread, liver injury, and mortality. Thus, platelets appear to cooperate with liver-resident macrophages to facilitate uptake and control of bacteria.
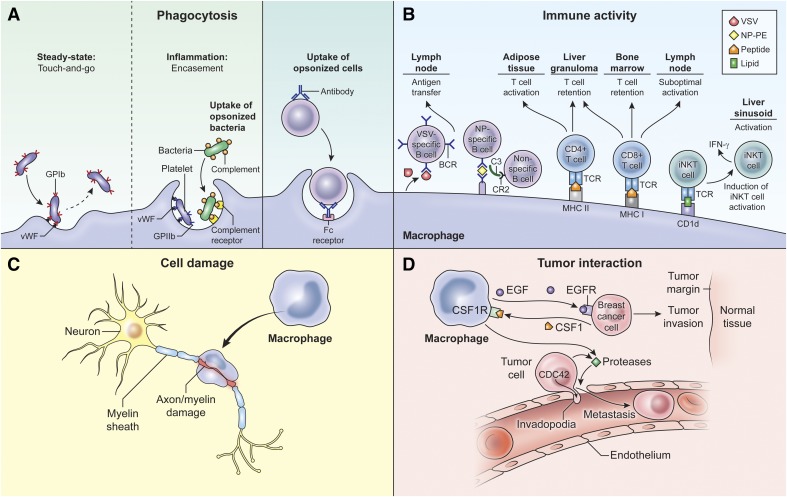
Figure 2. Macrophage interactions with immune and nonimmune cells.
Macrophages are highly malleable and can play different roles during inflammation. (A) Liver Kupffer cells have been visualized interacting with platelets, either in short-term contacts (touch-and-go) or in inflammation-induced, long-term contacts that facilitate encasement of captured bacteria. Macrophages can also engulf opsonized cells, such as antibody-coated lymphoma cells. (B) Macrophages are present in lymphoid and nonlymphoid tissues, where they modulate lymphocyte activation. In the lymph node, SCS macrophages capture antigens (e.g., VSV or NP-PE) and transfer them to specific and non-specific B cells (VSV or NP antigens). In the adipose tissue, macrophages promote cognate T cell activation, and within liver granulomas infected by BCG and Leishmania donovani, Kupffer cells foster retention of pathogenic CD4+ and CD8+ T cells. Kupffer cells can also directly activate iNKT cells in liver sinusoids following Borrelia burgdorferi infection. (C) During CNS autoimmune disease, studies have shown that infiltrating macrophages can induce myelin/axonal damage. (D) Macrophages also promote cancer cell invasion at the tumor margin, and macrophages that reside in the deeper perivascular regions can attract tumor cells, promoting intravasation and metastasis.
Infected erythrocytes.
Plasmodium parasites are the causative agent of malaria in humans, and infection during pregnancy can induce severe maternal and fetal complications, including death. The parasites infect erythrocytes, which then move throughout the circulatory system and adhere in different tissues. Infection during pregnancy is particularly problematic, as infected erythrocytes adhere to the placenta. To gain insights into this process, de Moraes et al. [59] conducted an IVM study, in which they inoculated pregnant mice with fluorescently tagged Plasmodium berghei-infected erythrocytes and monitored interactions with the placenta at gestational d 18. This study revealed that blood flow within the developing placenta varies from low to high, and infected erythrocytes preferentially accumulated within areas of low blood flow. Some of these infected cells were then targeted for phagocytic uptake by macrophages and unidentified fetal-derived cells. This likely served as a mechanism to control the parasite, but the role of the macrophages in this disease process was not determined.
The ability of macrophages to engage in phagocytosis is one of their defining features, and it is now clear, based on IVM and other studies that macrophages can help control infections [11, 60]. Control of a parasite, such as Plasmodium, is aided by macrophage phagocytosis in filtration organs, such as the spleen and liver. In addition, circulating monocytes must survey the vascular surface in search of adherent erythrocytes containing parasite. During experimental cerebral malaria, monocyte surveillance of CNS blood vessels is even enhanced as a consequence of endothelial cell activation [27]. However, there is much still to learn about monocyte/macrophage dynamics during complex infectious disease processes, such as malaria.
Tumor cells.
Another function that macrophages perform well is antibody-dependent phagocytosis (Fig. 2A). mAb have long been used to deplete circulating cell populations, including tumor cells. In fact, administration of therapeutic antibodies has become a particularly useful way to treat certain types of cancer. For example, anti-CD20 antibody is clinically approved to treat B cell malignancies [61]. To gain insights into the mechanism underlying B cell depletion, an IVM study was performed by use of a murine model of Burkitt lymphoma [62]. Within minutes of anti-CD20 injection, circulating B cells were arrested on Kupffer cells located in the liver sinusoids. This was followed by engulfment and degradation of the tumor cells. A similar observation was made by IVM using another murine tumor model, where tumor cells, opsonized by a mAb, were eliminated by Kupffer cells in a FcγR-dependent manner, thus preventing metastasis [63]. Both studies demonstrate that Kupffer cells are perfectly poised in the liver to acquire and rapidly degrade opsonized cellular material in circulation.
Damaged tissue.
Tissue injury often induces an innate immune reaction, referred to as sterile immunity (as a result of the absence of an infection). Sterile immune reactions are driven by “danger signals,” released by injured cells [64–66]. Tissue-resident and blood-derived macrophages are usually active participants in sterile immune reactions. A wound-healing response to damage is designed to be beneficial and promote tissue repair; however, sterile immune responses can sometimes become maladaptive, causing further damage. IVM was used recently to document the dynamics of a sterile immune reaction in the living brain, following a small focal contusion injury [66, 67]. Microglia are brain-resident macrophages that continually scan their extracellular surroundings during steady-state conditions (Supplemental Movie 1) and are known to respond to extracellular nucleotides, such as ATP, ADP, and UDP, following tissue injury [68–71]. Following focal brain injury, microglia transformed into different morphologic configurations, resembling jellyfish or honeycombs (Supplemental Movie 1) [67]. These transformations were linked to purinergic receptor signaling and were designed, in part, to structurally support the damaged glial limitans, which provide a barrier between the meninges and brain parenchyma. Jellyfish microglia also showed clear evidence of phagocytic activity after repositioning to the damaged glial limitans (Supplemental Movie 1). These data demonstrate that microglia can reshape their morphology and initiate a neuroprotective, sterile immune response within minutes of brain injury.
Microglia and macrophages participate in other CNS injury responses as well. For example, traumatic spinal-cord injury can result in severe functional deficits, as axons die back from the lesion epicenter and glial scar forms. Axons initially die back as an intrinsic reaction to damage, but additional injury can occur over the ensuing days or weeks, as a result of infiltration of blood-derived immune cells that include monocyte-derived macrophages [72]. Myeloid cells can be neuroprotective or toxic, depending on their state of differentiation, and numerous studies have focused on strategies to promote beneficial immune cell activity following spinal-cord injury, especially through modulation of myeloid cells [72–75]. Current dogma suggests that infiltrating classical monocytes can exacerbate spinal lesions, whereas nonclassical monocytes are neuroprotective and promote proper wound healing. With the use of CX3CR1gfp/+ CCR2rfp/+ dual reporter mice [76], Evans et al. [77] performed an IVM study to track the infiltration and anatomic position of different blood-derived monocyte populations in relation to axonal dieback following spinal-cord injury. CCR2+ (classical) and CX3CR1+ (nonclassical) monocytes were recruited to the lesion site over the days following spinal-cord injury. It was also determined that CX3CR1+ myeloid cells were associated more frequently with dying axons than CCR2+ cells, suggesting that nonclassical macrophages contribute to axonal dieback (Fig. 2C); however, a cause-and-effect relationship was not established in this study. More dynamic studies are required to determine how best to modulate myeloid cell activity in the injured CNS. Fluorescent reporter mice provide useful tools to track myeloid cell dynamics in relation to damage, but it is also important to link this information to distinct macrophage functions and states of differentiation over time.
Macrophage APC and immune cell interactions
Lymph node.
DCs are professional APCs considered essential for the development of optimal T cell responses in secondary lymphoid tissues [78]. However, studies have shown that specialized macrophage populations can also participate in the generation of adaptive immune responses [11], and IVM has proven particularly useful in elucidating how macrophages participate in these responses. A number of IVM studies have focused on lymph nodes because of the ease with which this secondary lymphoid tissue can be imaged. Lymph nodes are designed to prevent dissemination of pathogens that infect peripheral tissues. Specialized macrophages are now known to capture antigens that drain through lymphatic vessels and then facilitate immune responses through interactions with CD8+ and CD4+ T and B cells.
Lymph nodes are encased in a fibrous capsule, and just beneath this capsule is a space referred to as the SCS that receives draining lymphatic fluid and is inhabited by specialized macrophages. SCS macrophages have low phagocytic activity but are good at capturing certain types of particulate antigens, as well as viruses, and displaying them on their cell surface [79]. Some have referred to SCS macrophages as cellular “flypaper,” as a result of their ability to capture and display lymph-borne pathogens [79, 80]. To demonstrate this function, an IVM study of the popliteal lymph node was performed following injection of VSV into the footpad [80]. Interestingly, SCS macrophages captured VSV within minutes of injection and then transferred cell surface-bound virus through the SCS floor to B cells residing in the underlying follicles (Fig. 2B). In the absence of SCS macrophages, increased viral dissemination and decreased B cell activation were observed, demonstrating that these cells help control viruses by limiting their spread and promoting humoral immunity.
A similar capture and transfer mechanism was observed by TPM following in vivo generation of immune complexes [81, 82]. In these studies, immune complexes were generated by passively administering polyclonal antibodies against PE and then injecting PE subcutaneously. PE is a fluorophore, which permitted visualization of the immune complexes. TPM revealed that SCS macrophages in the lymph node captured the immune complexes and transferred them to underlying B cells through 2 different mechanisms. Nonspecific B cells obtained immune complexes from the processes of SCS macrophages through a mechanism dependent on the CR. These complexes were then transferred by B cells to follicular DCs. Antigen-specific B cells used their BCR also to obtain immune complexes from SCS macrophages and then migrated to the T cell area of the lymph zone to initiate a germinal center reaction (Fig. 2B). These studies show that SCS macrophages play a critical role in the development of humoral immune responses.
Whereas SCS macrophages are well equipped to display surface antigens and elicit a B cell response, they are less capable of eliciting optimal T cell activation. TPM studies involving peptide-pulsed DCs or soluble antigens to stimulate immunity have demonstrated that T cell priming occurs in deeper areas of the lymph node around structures referred to as high endothelial venules [83–85]. However, this mode of priming is not universally applicable to all adaptive immune responses. Following infection with VSV or VV, examination of the inguinal lymph node by TPM revealed that both pathogens localized to the SCS, targeting macrophages and DCs [86, 87]. Interestingly, naïve CD8+ T cells relocated from the deeper lymph node to just beneath the SCS, where they interacted with and were primed by DCs. T cell interactions with DCs were favored by release of the chemokine CCL5 [87]. Inhibition of CCL5 or depletion of DCs resulted in CD8+ T cell interactions with SCS macrophages, resulting in suboptimal activation (Fig. 2B). These data demonstrate that T cell priming can occur beneath the SCS following viral infection, and optimal activation is achieved through interactions with DCs rather than macrophages.
Similar to VV and VSV, infection with the intracellular protozoan parasite Toxoplasma gondii results in pathogen accumulation in the SCS region of draining lymph nodes, localizing to SCS macrophages and DCs [88, 89]. IVM studies have revealed that antigen-specific CD8+ T cells move to the infected area and engage in stable interactions. Antigen-experienced memory CD8+ T cells were found to relocate more rapidly to the SCS than naïve T cells, giving them an enhanced ability to protect the host upon rechallenge [88]. These interactions, however, were not entirely protective. IVM studies demonstrated evidence of parasite transfer from infected APCs to CD8+ T cells following engagement, and blockade of T cell egress from the lymph node reduced parasite dissemination [88]. The SCS is designed to capture lymph-borne pathogens and trigger adaptive immune responses but can sometimes promote pathogen transfer and dissemination to the detriment of the host. Even this remarkable scavenging system can be exploited by a well-adapted pathogen.
Liver.
The liver is a highly vascularized tissue with fenestrated endothelium, which facilitates filtration of blood-derived antigens. It is now widely recognized as a lymphoid organ, although there is still much to learn about immune cell dynamics in this compartment [90, 91]. Kupffer cells are particularly interesting to study, as they are tissue-resident macrophages that reside in the luminal walls of liver sinusoids and are perfectly poised to survey circulating blood. IVM has been used to monitor these cells in several different models of infectious disease.
Control of certain infections, such as Mycobacterium, requires the formation of an inflammatory structure, referred to as a granuloma. These structures serve as sites of focal inflammation and help prevent pathogen dissemination by promoting sustained interactions between innate and adaptive immune cells. Intravenous inoculation of Mycobacterium bovis BCG induces a protective granulomatous response in the liver within 2 wk of infection. Egen et al [92] discovered that within 1 min of infection, BCG was associated with Kupffer cells in liver sinusoids, demonstrating the efficiency with which these cells capture circulating pathogens. These BCG-containing Kupffer cells persisted for several weeks and relocated to nucleate granulomas in the liver parenchyma. Pathogen-specific CD4+ T cells were rapidly recruited to and retained within these structures, where they interacted primarily with macrophages (Fig. 2B). These data demonstrate that liver and blood-derived macrophages play an important role in defining the architecture of granulomas.
Similar observations were made in the liver following intravenous inoculation of the protozoan parasite, L. donovani [93]. The hepatic response to L. donovani involves formation of granulomas, and imaging studies revealed that the granuloma core consists primarily of Kupffer cells that migrate from surrounding sinusoids and interact directly with infiltrating, parasite-specific CD8+ T cells. Thus, granulomas formed against L. donovani are similar to those observed following BCG infection, in that they are comprised of MHC I/II-expressing Kupffer cells that promote pathogen-specific T cell retention.
In general, Kupffer cells are highly efficient at capturing blood-borne pathogens and coordinating CD8+ and CD4+ T cell responses. Studies have shown that Kupffer cells can also elicit responses from iNKT cells (Fig. 2B), which are a T cell population that expresses a limited TCR repertoire and recognizes lipid (instead of peptide) antigens presented by CD1d—a nonclassical MHC molecule. Some pathogens express lipid antigens that are presented by CD1d. One relevant example is the spirochete responsible for human Lyme disease, B. burgdorferi. An IVM study was performed to determine how the liver coordinates a defense against a B. burgdorferi infection [94]. Following infection, Kupffer cells were highly efficient at capturing most spirochetes within just a few hours. This was followed by CD1d-mediated antigen presentation and recruitment of iNKT cells, which were shown to cluster around and interact directly with Kupffer cells. This clustering behavior was dependent on CXCR3 signaling and CD1d. In the absence of iNKT cells or Kupffer cells, B. burgdorferi was able to spread to joints (a secondary target) more efficiently and enter the liver parenchyma. Collectively, these data demonstrate that Kupffer cells are efficient at initiating an adaptive immune defense against many different pathogens. However, some pathogens, such as P. berghei (a malaria-inducing parasite), have been shown to circumvent the Kupffer cell defense system and enter the liver parenchyma [95].
Bone marrow.
The bone marrow is a primary lymphoid organ responsible for hematopoiesis. In addition to generating immune cells, the bone marrow is capable of initiating an adaptive immune response against blood-borne antigens [96]. Milo et al. [97] recently used TPM to examine the generation of a CD8+ T cell response in the bone marrow following injection of a soluble antigen into the blood supply. The bone marrow was found to contain a population of highly motile, naïve CD8+ T cells. Following injection of a soluble antigen, CD8+ T cells specific to the antigen were shown to form clusters around APCs and stably arrest within a few hours, which eventually resulted in priming over the ensuing days. Interestingly, T cell responses in the bone marrow persisted in the absence of DCs, raising the possibility that macrophages were capable of initiating priming. This would differ from requirements upheld in other secondary lymphoid tissues (e.g., spleen and lymph nodes), where DCs are primarily responsible for optimal T cell priming.
Adipose tissue.
Obesity resulting from a high-fat diet can induce inflammation in adipose tissue that is mediated, in part, by macrophages and T cells [98]. Chronic inflammation in adipose tissue can ultimately give rise to metabolic abnormalities and insulin resistance. Previous studies have shown that local interactions between APCs and T cells are necessary to sustain adipose inflammation during states of obesity. For example, MHC II-deficiency reduces inflammation in adipose tissue, as well as insulin resistance in mice fed a high-fat diet [99]. As adipose tissue-resident macrophages express MHC II and are thought to participate in the inflammatory process, Cho et al. [100] used IVM to study their interactions with T cells. In mice fed a high-fat diet for 20 wk, CD4+ T cells were shown to interact directly with APCs in adipose tissue following injection of a cognate antigen. A role for macrophages was established by specifically deleting MHC II from the myeloid cell lineage, which reduced infiltration of CD4+ T cells and blood-derived macrophages, as well as insulin resistance in these mice. The current model based on these data is that tissue-resident macrophages induce CD4+ T cell activation in adipose tissue, promoting recruitment of blood-derived APCs that help sustain the inflammatory response.
Lung.
The lung, similar to the heart, is a particularly challenging tissue to image in real-time, as a result of motion artifacts, spherical aberrations induced by air, and light scattering caused by RBCs. Several groups, however, have developed strategies to circumvent these problems [26, 101–104]. For example, organ stabilization can be achieved by gluing tissue to a glass coverslip or a stabilizing ring [26, 105], by use of suction [101, 106] or by working with isolated, pump-perfused tissues [106]. In addition, respiration can be controlled and monitored by use of artificial ventilation systems and image acquisition timed to match chest movements, providing another level of stabilization [104]. In a recent IVM study, Fiole et al. [103] were able to visualize alveolar macrophages and DCs in the lung following infection with Bacillus anthracis spores. Interestingly, alveolar macrophages were shown to capture inhaled spores and then interact with DCs. Whereas the importance of these contacts was not established, they suggest a role for macrophage–DC interactions in the defense against B. anthracis. One possibility is that these interactions facilitate transfer of antigen. Now that the hurdle of imaging the lung by IVM has been overcome, further studies are required to determine the role played by resident macrophages in various inflammatory processes.
CNS.
Microglia are the most abundant CNS macrophages and are primarily responsible for tending to the parenchyma (Supplemental Movie 1) [107]. These cells are derived from primitive myeloid precursors in the yolk sac during embryonic development and self renew during adulthood [108–110]. The meninges, perivascular spaces, and choroid plexus, on the other hand, are surveyed by specialized bone marrow-derived macrophages (Supplemental Movie 1) [111]. These cells turn over more regularly than microglia and are replaced by hematopoietic cells [112]. The meninges and choroid plexus are immunologically different from the CNS parenchyma and in many ways, resemble a peripheral tissue in scavenging potential. As cerebral spinal fluid made by the choroid plexus passes through the CNS ventricular system, this elaborate network of bone marrow-derived macrophages is responsible for capturing antigenic material and mounting a response. Macrophages are usually among the first innate immune cells to respond to pathogens that enter the CNS. The presence of macrophages in the meninges and fluid spaces also provides an explanation for why CNS immune responses often begin in the meninges before moving into the parenchyma [29, 111].
In addition to mounting a defense against pathogens, CNS macrophages can contribute to disease. EAE is commonly studied as an animal model of human multiple sclerosis. The disease is initiated by immunizing rodents with CNS myelin components or by adoptively transferring myelin-specific CD4+ T cells. IVM has yielded some important insights into how meningeal phagocytes participate in the development of this autoimmune disease process [113, 114]. With the use of an adoptive transfer model of EAE, Bartholomäus et al. [113] examined the interactions of fluorescently tagged MBP-specific CD4+ T cells in the days leading up to the development of overt symptoms. IVM revealed that the autoreactive CD4+ T cells first scanned the luminal surface of meningeal blood vessels in the spinal cord before extravasating into the meningeal space. Following entry, the MBP-specific T cells interacted with MHC II-expressing phagocytes in meninges and perivascular spaces, which resulted in translocation of NFAT to the T cell nucleus [114]. These data demonstrate that relatively short-duration interactions with meningeal phagocytes can activate autoreactive CD4+ T cells. This event precedes T cell entry into the CNS parenchyma and the development of overt disease. More IVM studies are needed to determine the relative contributions of CNS-resident versus blood-derived macrophages to autoimmune diseases, such as EAE. Static imaging studies suggest that classical monocyte-derived macrophages play a role in myelin stripping during the parenchymal phase of disease (Fig. 2C) [115].
In summary, tissue-resident and blood-derived macrophages have the ability to influence immune and nonimmune cell populations throughout the body (see Table 1). The nature and consequence of these interactions are usually dictated by the initiating stimulus, which can range from pathogens to damaged tissue. As representatives of the innate immune surveillance program, macrophages must decode these different stimuli and attempt to elicit a productive biologic response. Given the diversity of stimuli to which macrophages are exposed, sometimes mistakes are made and initially well-intended responses can function to the detriment of the host. The maladaptive interactions between macrophages and tumor cells provide an excellent example of this phenomenon and will be described in more detail below.
TABLE 1.
Consequences of macrophage interactions in vivo
| Macrophages visualized | Interacting cell | Consequence of contact | Location | Event-triggering contact | Time-point | Reference |
|---|---|---|---|---|---|---|
| F4/80+ Kupffer cells | CD49b+ platelets | Platelet encasement of bacteria and engulfment | Liver | B. cereus or MRSA infection | 1–10 min postinfection | [57] |
| F4/80+ Kupffer cells | Antibody-coated B16F10 tumor cells | Phagocytosis and digestion | Liver | Tumor cells injected into antibody-treated mice | 24 h after tumor cell injection | [63] |
| CX3CR1-GFP+ macrophages/microglia | Thy1-YFP+ axons | Axon dieback and phagocytosis | Spinal cord | Traumatic spinal-cord injury | 5 and 22 d after injury | [77] |
| VSV+ SCS macrophages | VSV-specific B cells | Antigen transfer and B cell activation | Popliteal lymph node | VSV virus infection | 5–30 min postinfection | [80] |
| Fluorescent dextran+ macrophages | OT-I CD8+ T cells (naïve) | Suboptimal CD8+ T cell activation | Inguinal lymph node | VV infection | 6–8 h postinfection | [87] |
| CD11c-YFP+ SCS macrophages | OT-I CD8+ T cells (memory) | Parasite invasion of CD8+ T cells | Mesenteric lymph node | T. gondii infection | 5 h postinfection | [88] |
| LysM-GFP+ Kupffer cells | OT-II CD4+ T cells (effector) | CD4+ T cell retention in the granuloma | Liver granulomas | M. bovis BCG infection | 1–3 wk postinfection | [92] |
| Nanobead+ Kupffer cells | OT-I CD8+ T cells (effector) | Antigen presentation to CD8+ T cells | Liver granulomas | L. donovani infection | 14–21 d postinfection | [93] |
| F4/80+ Kupffer cells | CXCR6-GFP iNKT cells | iNKT cell activation | Liver sinusoids | B. burgdorferi infection | 24 h postinfection | [94] |
| CD11c-mCherry+ adipose tissue macrophages | OT-II CD4+ T cells (naïve) | CD4 T cell activation | Adipose tissue | OVA injection | 2 h after injection | [100] |
| Fluorescent dextran+ macrophages | CX3CR1-GFP+ DCs | Unknown | Lung | Inhalation of B. anthracis spores | 5 h postinfection | [103] |
| Fluorescent dextran+ macrophages | MBP-specific CD4+ T cells (effectors) | Antigen presentation and activation of CD4+ T cells | Spinal-cord meninges | Intravenous injection of 5 × 106 MBP-specific CD4+ T cells | 2.5 d posttransfer | [113] |
| Fluorescent dextran+ macrophages | Tumor cells | Comigration | Mammary tumors | Injection of MTLn3 carcinoma cells | 3 wk after injection | [124] |
| Dextran+, LysM-GFP+, or c-fms-GFP+ macrophages | Tumor cells | Intravasation | Mammary tumors | Spontaneous carcinoma | 16- to 18-wk-old mice | [118] |
YFP, Yellow fluorescent protein; OT-I, OVA -specific CD8+ TCR transgenic cells; OT-II, OVA-specific CD4+ TCR transgenic cells.
Macrophage–tumor cell interactions
Tumors are exceedingly complex, given their diverse cellular origins and mechanisms of growth, invasion, and metastasis. To complicate matters further, studies have shown that tissue-resident and blood-derived macrophages can actually promote (instead of impede) tumorigenesis [116–122]. One of the more convincing early studies revealed, in a murine model of spontaneous mammary carcinogenesis, that mutation in M-CSF-1 delayed development of invasive, metastatic lung carcinomas [116]. Pulmonary metastasis was restored following transgenic reconstitution of CSF-1. In a more recent study, Qian et al. [120] extended upon this observation, by showing that classical CCR2+ granzyme 1+ monocytes are recruited to sites of pulmonary metastases but not the originating mammary tumor. This differential migration pattern was linked to expression of CCL2 by the pulmonary metastases. Importantly, CCL2 blockade reduced metastasis and extended survival, demonstrating that inflammatory monocytes can promote extravasation of tumor cells into the lung. Monocytes achieved this by releasing vascular endothelial growth factor A. These data indicate that targeting monocytes/macrophages might provide some therapeutic benefit for certain types of cancer.
Macrophage dynamics and functions are quite diverse, which gives them many different ways to influence tumorigenesis [119, 121]. Macrophages can suppress tumor-specific immunity, enhance angiogenesis, and promote tumor metastasis and invasion. IVM has been instrumental in shedding light on some of the ways that macrophages benefit tumors [122, 123]. In mammary tumor models, macrophages are often found at the leading edge of the tumor. These macrophages were imaged by TPM and shown to migrate in association with tumor cells [118] (Fig. 2D). This pattern of comigration was dependent on macrophage-derived EGF and tumor cell-derived CSF-1, which created a paracrine signaling loop between the 2 cell populations [117, 118]. Macrophages were important for coordinated migration, as depletion of these cells with clodronate markedly reduced streams of motile tumor cells by 90% [124]. Macrophage–tumor cell interactions in perivascular areas deep within the tumor also promoted tumor cell intravasation (entry into the blood supply)—a prerequisite for metastasis (Fig. 2D). A recent TPM study uncovered that tumor cell contact with macrophages induced formation of a degradative, actin-rich protrusion in tumor cells (referred to as invadopodia) that was dependent on RhoA signaling [125]. The tumor cells used these structures to degrade the basal lamina overlying blood vessels and facilitate intravasation. Thus, macrophages at a tumor margin and deep within the tumor can interact with tumor cells and promote tumorigenesis directly.
CONCLUSIONS
Monocytes and macrophages provide the body with an elaborate cellular sentinel program and participate in everything from basic tissue cleanup to complex diseases, such as atherosclerosis and autoimmunity. One of the most important roles played by macrophages based on their position is the scavenging of materials that enter bodily fluids. As examples, blood, lymph, and cerebral spinal fluid all have specialized macrophages monitoring their content. During steady-state conditions, their task is to survey and remove unwanted waste; however, a pathogen will trigger an entirely different macrophage program designed to sequester/degrade the invader, alarm the host, and facilitate adaptive immunity through specific cell–cell interactions (Table 1) [126]. These basic functions are generally protective but can sometimes become maladaptive, especially when myeloid cells are directed against chronically degenerating tissue, self-antigens, or tumors. In these situations, monocytes and macrophages have been shown to do more harm than good, opening the possibility of therapeutically suppressing their activity. The challenge with therapeutic manipulation of myeloid cells is deciding which subset to target. Thus far, 2 generic-subset monocytes have been defined (classical vs. nonclassical), and studies have shown that they can have opposing roles, even in the very same disease process. The contribution of monocytes/macrophages to an inflammatory process can also shift over time, making it even more important to link the actual functions of these cells to the manifesting disease biology.
IVM has proven instrumental in capturing the real-time dynamics of myeloid cells operating under diverse homeostatic and inflammatory conditions. Reliance on conventional, single time-point assays (immunohistochemistry, gene/protein expression assays, etc.) alone could not have led to the discovery of patrolling monocytes, dynamic microglia (Supplemental Movie 1), or the local influence of macrophages on pathogen-specific T cells and tumor cells. During the process of tumor metastasis, for example, dynamic imaging studies unveiled that macrophages and tumor cells comigrate toward vessels, which promotes tumor release into the bloodstream. This is an especially important IVM observation with obvious clinical implications. A continued use of IVM, in combination with various functional readouts, should vastly improve our understanding of myeloid cells during states of health and disease. There are now a number of fluorescent probes that allow detection of basic macrophage functions, such as production of ROS, lysosomal proteases, metalloproteinases, and cathepsins (Fig. 3). The monitoring of macrophage activity by IVM should allow researchers to link in vivo myeloid cell dynamics to their functional state. This would represent an improvement over the simple delineation of myeloid cells into subsets based on surface markers alone. At this point, there is no doubt that IVM has revolutionized our understanding of monocytes/macrophages, and with continued developments in this field [127–129], it should be possible to someday fully unlock the mysteries of these remarkably malleable immune sentinels.
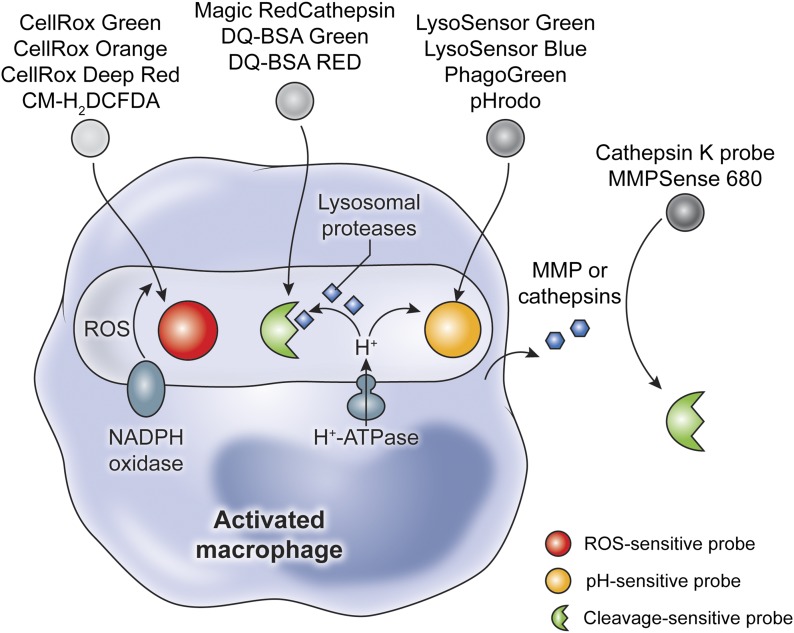
Figure 3. Fluorescent probes used to illuminate macrophage functions.
Following activation, macrophages generate ROS as part of their oxidative burst, which can be monitored by use of ROS-sensitive probes. Degradation of pathogens and remodeling of extracellular matrix are performed by pH-activated lysosomal proteases and matrix metalloproteinases (MMPs), respectively. Probes that generate fluorescence upon cleavage are used to monitor such activities. Finally, the fate of many internalized pathogens is determined by acidification of the macrophage phagosome. Acidification itself (H+) can be visualized by use of pH-sensitive dyes. All of these activities can be associated with macrophages by use of myeloid reporter mice (CX3CR1-GFP, CSF1R-GFP, or LysM-GFP) or in vivo macrophage labeling.
AUTHORSHIP
R.R. and D.B.M. designed the review and wrote the manuscript.
Supplementary Material
Acknowledgments
This work was supported by the U.S. National Institutes of Health (NIH) intramural program. The authors thank Ethan Tyler in the NIH Medical Arts Design Section for his help with the illustrations.
Glossary
- −/−
deficient
- Aβ
amyloid β
- AD
Alzheimer's disease
- ApoE
apolipoprotein E
- APP
amyloid precursor protein
- BCG
bacillus Calmette-Guérin
- CR
complement receptor
- DC
dendritic cell
- DVT
deep vein thrombosis
- EAE
experimental autoimmune encephalomyelitis
- EGF
epidermal growth factor
- GPI/IIb
glycoprotein 1/2b
- iNKT cell
invariant NK T cell
- IVM
intravital microscopy
- LDLR
LDL receptor
- Ly6C
lymphocyte antigen 6C
- LysM
lysozyme M
- MBP
myelin basic protein
- MMP
matrix metalloproteinase
- MRSA
methicillin-resistant Staphylococcus aureus
- NP
4-hydroxy-3-nitrophenylacetyl
- NR4A1
nuclear receptor subfamily 4, group A, member 1
- PS1
presenilin-1
- rfp
red fluorescent protein
- ROS
reactive oxygen species
- SCS
subcapsular sinus
- TPM
2-photon laser-scanning microscopy
- VSV
vesicular stomatitis virus
- VV
vaccinia virus
- vWF
von Willebrand factor
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1.Denk W., Strickler J. H., Webb W. W. (1990) Two-photon laser scanning fluorescence microscopy. Science 248, 73–76. [DOI] [PubMed] [Google Scholar]
- 2.Herz J., Zinselmeyer B. H., McGavern D. B. (2012) Two-photon imaging of microbial immunity in living tissues. Microsc. Microanal. 18, 730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cahalan M. D., Parker I. (2008) Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs. Annu. Rev. Immunol. 26, 585–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittet M. J., Weissleder R. (2011) Intravital imaging. Cell 147, 983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephens D. J., Allan V. J. (2003) Light microscopy techniques for live cell imaging. Science 300, 82–86. [DOI] [PubMed] [Google Scholar]
- 6.Bullen A., Friedman R. S., Krummel M. F. (2009) Two-photon imaging of the immune system: a custom technology platform for high-speed, multicolor tissue imaging of immune responses. Curr. Top. Microbiol. Immunol. 334, 1–29. [DOI] [PubMed] [Google Scholar]
- 7.Tang J., van Panhuys N., Kastenmüller W., Germain R. N. (2013) The future of immunoimaging—deeper, bigger, more precise, and definitively more colorful. Eur. J. Immunol. 43, 1407–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weigert R., Porat-Shliom N., Amornphimoltham P. (2013) Imaging cell biology in live animals: ready for prime time. J. Cell Biol. 201, 969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmalstieg F. C. Jr., Goldman A. S. (2008) Ilya Ilich Metchnikoff (1845–1915) and Paul Ehrlich (1854–1915): the centennial of the 1908 Nobel Prize in Physiology or Medicine. J. Med. Biogr. 16, 96–103. [DOI] [PubMed] [Google Scholar]
- 10.Gordon S. (2008) Elie Metchnikoff: father of natural immunity. Eur. J. Immunol. 38, 3257–3264. [DOI] [PubMed] [Google Scholar]
- 11.Epelman S., Lavine K. J., Randolph G. J. (2014) Origin and functions of tissue macrophages. Immunity 41, 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geissmann F., Jung S., Littman D. R. (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82. [DOI] [PubMed] [Google Scholar]
- 13.Hanna R. N., Carlin L. M., Hubbeling H. G., Nackiewicz D., Green A. M., Punt J. A., Geissmann F., Hedrick C. C. (2011) The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat. Immunol. 12, 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlin L. M., Stamatiades E. G., Auffray C., Hanna R. N., Glover L., Vizcay-Barrena G., Hedrick C. C., Cook H. T., Diebold S., Geissmann F. (2013) Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 153, 362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auffray C., Fogg D. K., Narni-Mancinelli E., Senechal B., Trouillet C., Saederup N., Leemput J., Bigot K., Campisi L., Abitbol M., Molina T., Charo I., Hume D. A., Cumano A., Lauvau G., Geissmann F. (2009) CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J. Exp. Med. 206, 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serbina N. V., Jia T., Hohl T. M., Pamer E. G. (2008) Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 26, 421–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi C., Pamer E. G. (2011) Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 11, 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serbina N. V., Pamer E. G. (2006) Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317. [DOI] [PubMed] [Google Scholar]
- 19.Sunderkötter C., Nikolic T., Dillon M. J., Van Rooijen N., Stehling M., Drevets D. A., Leenen P. J. (2004) Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 172, 4410–4417. [DOI] [PubMed] [Google Scholar]
- 20.Gordon S., Taylor P. R. (2005) Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964. [DOI] [PubMed] [Google Scholar]
- 21.Auffray C., Fogg D., Garfa M., Elain G., Join-Lambert O., Kayal S., Sarnacki S., Cumano A., Lauvau G., Geissmann F. (2007) Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670. [DOI] [PubMed] [Google Scholar]
- 22.Girgis N. M., Gundra U. M., Ward L. N., Cabrera M., Frevert U., Loke P. (2014) Ly6Chigh monocytes become alternatively activated macrophages in schistosome granulomas with help from CD4+ cells. PLoS Pathog. 10, e1004080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W., Nava R. G., Bribriesco A. C., Zinselmeyer B. H., Spahn J. H., Gelman A. E., Krupnick A. S., Miller M. J., Kreisel D. (2012) Intravital 2-photon imaging of leukocyte trafficking in beating heart. J. Clin. Invest. 122, 2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumagin R., Prizant H., Lomakina E., Waugh R. E., Sarelius I. H. (2010) LFA-1 and Mac-1 define characteristically different intralumenal crawling and emigration patterns for monocytes and neutrophils in situ. J. Immunol. 185, 7057–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devi S., Li A., Westhorpe C. L., Lo C. Y., Abeynaike L. D., Snelgrove S. L., Hall P., Ooi J. D., Sobey C. G., Kitching A. R., Hickey M. J. (2013) Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat. Med. 19, 107–112. [DOI] [PubMed] [Google Scholar]
- 26.Kreisel D., Nava R. G., Li W., Zinselmeyer B. H., Wang B., Lai J., Pless R., Gelman A. E., Krupnick A. S., Miller M. J. (2010) In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc. Natl. Acad. Sci. USA 107, 18073–18078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pai S., Qin J., Cavanagh L., Mitchell A., El-Assaad F., Jain R., Combes V., Hunt N. H., Grau G. E., Weninger W. (2014) Real-time imaging reveals the dynamics of leukocyte behaviour during experimental cerebral malaria pathogenesis. PLoS Pathog. 10, e1004236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang S. S., McGavern D. B. (2008) Lymphocytic choriomeningitis infection of the central nervous system. Front. Biosci. 13, 4529–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGavern D. B., Kang S. S. (2011) Illuminating viral infections in the nervous system. Nat. Rev. Immunol. 11, 318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J. V., Kang S. S., Dustin M. L., McGavern D. B. (2009) Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature 457, 191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt S., Moser M., Sperandio M. (2013) The molecular basis of leukocyte recruitment and its deficiencies. Mol. Immunol. 55, 49–58. [DOI] [PubMed] [Google Scholar]
- 32.Higashiyama M., Hokari R., Kurihara C., Ueda T., Nakamura M., Komoto S., Okada Y., Watanabe C., Kawaguchi A., Nagao S., Miura S. (2010) Interferon-α increases monocyte migration via platelet-monocyte interaction in murine intestinal microvessels. Clin. Exp. Immunol. 162, 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donnelly D. J., Longbrake E. E., Shawler T. M., Kigerl K. A., Lai W., Tovar C. A., Ransohoff R. M., Popovich P. G. (2011) Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. J. Neurosci. 31, 9910–9922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amano H., Amano E., Santiago-Raber M. L., Moll T., Martinez-Soria E., Fossati-Jimack L., Iwamoto M., Rozzo S. J., Kotzin B. L., Izui S. (2005) Selective expansion of a monocyte subset expressing the CD11c dendritic cell marker in the Yaa model of systemic lupus erythematosus. Arthritis Rheum. 52, 2790–2798. [DOI] [PubMed] [Google Scholar]
- 35.Santiago-Raber M. L., Amano H., Amano E., Baudino L., Otani M., Lin Q., Nimmerjahn F., Verbeek J. S., Ravetch J. V., Takasaki Y., Hirose S., Izui S. (2009) Fcgamma receptor-dependent expansion of a hyperactive monocyte subset in lupus-prone mice. Arthritis Rheum. 60, 2408–2417. [DOI] [PubMed] [Google Scholar]
- 36.Dal-Secco D., Wang J., Zeng Z., Kolaczkowska E., Wong C. H., Petri B., Ransohoff R. M., Charo I. F., Jenne C. N., Kubes P. (2015) A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J. Exp. Med. 212, 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Libby P., Ridker P. M., Hansson G. K. (2011) Progress and challenges in translating the biology of atherosclerosis. Nature 473, 317–325. [DOI] [PubMed] [Google Scholar]
- 38.Taqueti V. R., Jaffer F. A. (2013) High-resolution molecular imaging via intravital microscopy: illuminating vascular biology in vivo. Integr. Biol. (Camb). 5, 278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plump A. S., Smith J. D., Hayek T., Aalto-Setälä K., Walsh A., Verstuyft J. G., Rubin E. M., Breslow J. L. (1992) Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 71, 343–353. [DOI] [PubMed] [Google Scholar]
- 40.Zhang S. H., Reddick R. L., Piedrahita J. A., Maeda N. (1992) Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 258, 468–471. [DOI] [PubMed] [Google Scholar]
- 41.Jawien J. (2012) The role of an experimental model of atherosclerosis: apoE-knockout mice in developing new drugs against atherogenesis. Curr. Pharm. Biotechnol. 13, 2435–2439. [PubMed] [Google Scholar]
- 42.Faust N., Varas F., Kelly L. M., Heck S., Graf T. (2000) Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood 96, 719–726. [PubMed] [Google Scholar]
- 43.Rotzius P., Soehnlein O., Kenne E., Lindbom L., Nystrom K., Thams S., Eriksson E. E. (2009) ApoE(−/−)/lysozyme M(EGFP/EGFP) mice as a versatile model to study monocyte and neutrophil trafficking in atherosclerosis. Atherosclerosis 202, 111–118. [DOI] [PubMed] [Google Scholar]
- 44.Eriksson E. E. (2011) Intravital microscopy on atherosclerosis in apolipoprotein E-deficient mice establishes microvessels as major entry pathways for leukocytes to advanced lesions. Circulation 124, 2129–2138. [DOI] [PubMed] [Google Scholar]
- 45.Swirski F. K., Weissleder R., Pittet M. J. (2009) Heterogeneous in vivo behavior of monocyte subsets in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 29, 1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamers A. A., Vos M., Rassam F., Marinković G., Kurakula K., van Gorp P. J., de Winther M. P., Gijbels M. J., de Waard V., de Vries C. J. (2012) Bone marrow-specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circ. Res. 110, 428–438. [DOI] [PubMed] [Google Scholar]
- 47.Hanna R. N., Shaked I., Hubbeling H. G., Punt J. A., Wu R., Herrley E., Zaugg C., Pei H., Geissmann F., Ley K., Hedrick C. C. (2012) NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ. Res. 110, 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boring L., Gosling J., Cleary M., Charo I. F. (1998) Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394, 894–897. [DOI] [PubMed] [Google Scholar]
- 49.Dawson T. C., Kuziel W. A., Osahar T. A., Maeda N. (1999) Absence of CC chemokine receptor-2 reduces atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 143, 205–211. [DOI] [PubMed] [Google Scholar]
- 50.Huo Y., Schober A., Forlow S. B., Smith D. F., Hyman M. C., Jung S., Littman D. R., Weber C., Ley K. (2003) Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat. Med. 9, 61–67. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi T., Tahara Y., Matsumoto M., Iguchi M., Sano H., Murayama T., Arai H., Oida H., Yurugi-Kobayashi T., Yamashita J. K., Katagiri H., Majima M., Yokode M., Kita T., Narumiya S. (2004) Roles of thromboxane A(2) and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J. Clin. Invest. 114, 784–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Von Brühl M. L., Stark K., Steinhart A., Chandraratne S., Konrad I., Lorenz M., Khandoga A., Tirniceriu A., Coletti R., Köllnberger M., Byrne R. A., Laitinen I., Walch A., Brill A., Pfeiler S., Manukyan D., Braun S., Lange P., Riegger J., Ware J., Eckart A., Haidari S., Rudelius M., Schulz C., Echtler K., Brinkmann V., Schwaiger M., Preissner K. T., Wagner D. D., Mackman N., Engelmann B., Massberg S. (2012) Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 209, 819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ittner L. M., Götz J. (2011) Amyloid-β and tau—a toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 12, 65–72. [DOI] [PubMed] [Google Scholar]
- 54.Michaud J. P., Bellavance M. A., Préfontaine P., Rivest S. (2013) Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Reports 5, 646–653. [DOI] [PubMed] [Google Scholar]
- 55.Naert G., Rivest S. (2013) A deficiency in CCR2+ monocytes: the hidden side of Alzheimer’s disease. J. Mol. Cell Biol. 5, 284–293. [DOI] [PubMed] [Google Scholar]
- 56.Yeaman M. R. (2014) Platelets: at the nexus of antimicrobial defence. Nat. Rev. Microbiol. 12, 426–437. [DOI] [PubMed] [Google Scholar]
- 57.Wong C. H., Jenne C. N., Petri B., Chrobok N. L., Kubes P. (2013) Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat. Immunol. 14, 785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jenne C. N., Kubes P. (2013) Immune surveillance by the liver. Nat. Immunol. 14, 996–1006. 24048121 [Google Scholar]
- 59.De Moraes L. V., Tadokoro C. E., Gómez-Conde I., Olivieri D. N., Penha-Gonçalves C. (2013) Intravital placenta imaging reveals microcirculatory dynamics impact on sequestration and phagocytosis of Plasmodium-infected erythrocytes. PLoS Pathog. 9, e1003154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murray P. J., Wynn T. A. (2011) Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maloney D. G. (2012) Anti-CD20 antibody therapy for B-cell lymphomas. N. Engl. J. Med. 366, 2008–2016. [DOI] [PubMed] [Google Scholar]
- 62.Montalvao F., Garcia Z., Celli S., Breart B., Deguine J., Van Rooijen N., Bousso P. (2013) The mechanism of anti-CD20-mediated B cell depletion revealed by intravital imaging. J. Clin. Invest. 123, 5098–5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gül N., Babes L., Siegmund K., Korthouwer R., Bögels M., Braster R., Vidarsson G., ten Hagen T. L., Kubes P., van Egmond M. (2014) Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. J. Clin. Invest. 124, 812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matzinger P. (1994) Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045. [DOI] [PubMed] [Google Scholar]
- 65.Bianchi M. E. (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5. [DOI] [PubMed] [Google Scholar]
- 66.Corps K. N., Roth T. L., McGavern D. B. (2015) Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 72, 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roth T. L., Nayak D., Atanasijevic T., Koretsky A. P., Latour L. L., McGavern D. B. (2014) Transcranial amelioration of inflammation and cell death after brain injury. Nature 505, 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nimmerjahn A., Kirchhoff F., Helmchen F. (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. [DOI] [PubMed] [Google Scholar]
- 69.Davalos D., Grutzendler J., Yang G., Kim J. V., Zuo Y., Jung S., Littman D. R., Dustin M. L., Gan W. B. (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758. [DOI] [PubMed] [Google Scholar]
- 70.Haynes S. E., Hollopeter G., Yang G., Kurpius D., Dailey M. E., Gan W. B., Julius D. (2006) The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 9, 1512–1519. [DOI] [PubMed] [Google Scholar]
- 71.Koizumi S., Shigemoto-Mogami Y., Nasu-Tada K., Shinozaki Y., Ohsawa K., Tsuda M., Joshi B. V., Jacobson K. A., Kohsaka S., Inoue K. (2007) UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 446, 1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ren Y., Young W. (2013) Managing inflammation after spinal cord injury through manipulation of macrophage function. Neural Plast. 2013, 945034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwartz M., Yoles E. (2006) Immune-based therapy for spinal cord repair: autologous macrophages and beyond. J. Neurotrauma 23, 360–370. [DOI] [PubMed] [Google Scholar]
- 74.Shechter R., London A., Varol C., Raposo C., Cusimano M., Yovel G., Rolls A., Mack M., Pluchino S., Martino G., Jung S., Schwartz M. (2009) Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 6, e1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shechter R., Miller O., Yovel G., Rosenzweig N., London A., Ruckh J., Kim K. W., Klein E., Kalchenko V., Bendel P., Lira S. A., Jung S., Schwartz M. (2013) Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity 38, 555–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saederup N., Cardona A. E., Croft K., Mizutani M., Cotleur A. C., Tsou C. L., Ransohoff R. M., Charo I. F. (2010) Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One 5, e13693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Evans T. A., Barkauskas D. S., Myers J. T., Hare E. G., You J. Q., Ransohoff R. M., Huang A. Y., Silver J. (2014) High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Exp. Neurol. 254, 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mildner A., Jung S. (2014) Development and function of dendritic cell subsets. Immunity 40, 642–656. [DOI] [PubMed] [Google Scholar]
- 79.Kuka M., Iannacone M. (2014) The role of lymph node sinus macrophages in host defense. Ann. N. Y. Acad. Sci. 1319, 38–46. [DOI] [PubMed] [Google Scholar]
- 80.Junt T., Moseman E. A., Iannacone M., Massberg S., Lang P. A., Boes M., Fink K., Henrickson S. E., Shayakhmetov D. M., Di Paolo N. C., van Rooijen N., Mempel T. R., Whelan S. P., von Andrian U. H. (2007) Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450, 110–114. [DOI] [PubMed] [Google Scholar]
- 81.Phan T. G., Grigorova I., Okada T., Cyster J. G. (2007) Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 8, 992–1000. [DOI] [PubMed] [Google Scholar]
- 82.Phan T. G., Green J. A., Gray E. E., Xu Y., Cyster J. G. (2009) Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat. Immunol. 10, 786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bousso P., Robey E. (2003) Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat. Immunol. 4, 579–585. [DOI] [PubMed] [Google Scholar]
- 84.Miller M. J., Wei S. H., Cahalan M. D., Parker I. (2003) Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc. Natl. Acad. Sci. USA 100, 2604–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mempel T. R., Henrickson S. E., Von Andrian U. H. (2004) T-Cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159. [DOI] [PubMed] [Google Scholar]
- 86.Hickman H. D., Takeda K., Skon C. N., Murray F. R., Hensley S. E., Loomis J., Barber G. N., Bennink J. R., Yewdell J. W. (2008) Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat. Immunol. 9, 155–165. [DOI] [PubMed] [Google Scholar]
- 87.Hickman H. D., Li L., Reynoso G. V., Rubin E. J., Skon C. N., Mays J. W., Gibbs J., Schwartz O., Bennink J. R., Yewdell J. W. (2011) Chemokines control naive CD8+ T cell selection of optimal lymph node antigen presenting cells. J. Exp. Med. 208, 2511–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chtanova T., Han S. J., Schaeffer M., van Dooren G. G., Herzmark P., Striepen B., Robey E. A. (2009) Dynamics of T cell, antigen-presenting cell, and pathogen interactions during recall responses in the lymph node. Immunity 31, 342–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.John B., Harris T. H., Tait E. D., Wilson E. H., Gregg B., Ng L. G., Mrass P., Roos D. S., Dzierszinski F., Weninger W., Hunter C. A. (2009) Dynamic imaging of CD8(+) T cells and dendritic cells during infection with Toxoplasma gondii. PLoS Pathog. 5, e1000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crispe I. N. (2009) The liver as a lymphoid organ. Annu. Rev. Immunol. 27, 147–163. [DOI] [PubMed] [Google Scholar]
- 91.Crispe I. N. (2011) Liver antigen-presenting cells. J. Hepatol. 54, 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Egen J. G., Rothfuchs A. G., Feng C. G., Winter N., Sher A., Germain R. N. (2008) Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity 28, 271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beattie L., Peltan A., Maroof A., Kirby A., Brown N., Coles M., Smith D. F., Kaye P. M. (2010) Dynamic imaging of experimental Leishmania donovani-induced hepatic granulomas detects Kupffer cell-restricted antigen presentation to antigen-specific CD8 T cells. PLoS Pathog. 6, e1000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee W. Y., Moriarty T. J., Wong C. H., Zhou H., Strieter R. M., van Rooijen N., Chaconas G., Kubes P. (2010) An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat. Immunol. 11, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frevert U., Engelmann S., Zougbédé S., Stange J., Ng B., Matuschewski K., Liebes L., Yee H. (2005) Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol. 3, e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Feuerer M., Beckhove P., Garbi N., Mahnke Y., Limmer A., Hommel M., Hämmerling G. J., Kyewski B., Hamann A., Umansky V., Schirrmacher V. (2003) Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat. Med. 9, 1151–1157. [DOI] [PubMed] [Google Scholar]
- 97.Milo I., Sapoznikov A., Kalchenko V., Tal O., Krauthgamer R., van Rooijen N., Dudziak D., Jung S., Shakhar G. (2013) Dynamic imaging reveals promiscuous crosspresentation of blood-borne antigens to naive CD8+ T cells in the bone marrow. Blood 122, 193–208. [DOI] [PubMed] [Google Scholar]
- 98.Mathis D. (2013) Immunological goings-on in visceral adipose tissue. Cell Metab. 17, 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deng T., Lyon C. J., Minze L. J., Lin J., Zou J., Liu J. Z., Ren Y., Yin Z., Hamilton D. J., Reardon P. R., Sherman V., Wang H. Y., Phillips K. J., Webb P., Wong S. T., Wang R. F., Hsueh W. A. (2013) Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 17, 411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cho K. W., Morris D. L., DelProposto J. L., Geletka L., Zamarron B., Martinez-Santibanez G., Meyer K. A., Singer K., O’Rourke R. W., Lumeng C. N. (2014) An MHC II-dependent activation loop between adipose tissue macrophages and CD4+ T cells controls obesity-induced inflammation. Cell Reports 9, 605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Looney M. R., Thornton E. E., Sen D., Lamm W. J., Glenny R. W., Krummel M. F. (2011) Stabilized imaging of immune surveillance in the mouse lung. Nat. Methods 8, 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thornton, E. E., Krummel, M. F., Looney, M. R. (2012) Live imaging of the lung. Curr, Protoc, Cytom, Chapter 12, Unit 12 28. [DOI] [PubMed] [Google Scholar]
- 103.Fiole D., Deman P., Trescos Y., Mayol J. F., Mathieu J., Vial J. C., Douady J., Tournier J. N. (2014) Two-photon intravital imaging of lungs during anthrax infection reveals long-lasting macrophage-dendritic cell contacts. Infect. Immun. 82, 864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Looney M. R., Bhattacharya J. (2014) Live imaging of the lung. Annu. Rev. Physiol. 76, 431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee S., Vinegoni C., Feruglio P. F., Fexon L., Gorbatov R., Pivoravov M., Sbarbati A., Nahrendorf M., Weissleder R. (2012) Real-time in vivo imaging of the beating mouse heart at microscopic resolution. Nat. Commun. 3, 1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Presson R. G. Jr., Brown M. B., Fisher A. J., Sandoval R. M., Dunn K. W., Lorenz K. S., Delp E. J., Salama P., Molitoris B. A., Petrache I. (2011) Two-photon imaging within the murine thorax without respiratory and cardiac motion artifact. Am. J. Pathol. 179, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nayak D., Roth T. L., McGavern D. B. (2014) Microglia development and function. Annu. Rev. Immunol. 32, 367–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M. F., Conway S. J., Ng L. G., Stanley E. R., Samokhvalov I. M., Merad M. (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Elmore M. R., Najafi A. R., Koike M. A., Dagher N. N., Spangenberg E. E., Rice R. A., Kitazawa M., Matusow B., Nguyen H., West B. L., Green K. N. (2014) Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Perdiguero E. G., Klapproth K., Schulz C., Busch K., Azzoni E., Crozet L., Garner H., Trouillet C., de Bruijn M. F., Geissmann F., Rodewald H. (2015) Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518, 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nayak D., Zinselmeyer B. H., Corps K. N., McGavern D. B. (2012) In vivo dynamics of innate immune sentinels in the CNS. Intravital 1, 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hickey W. F., Kimura H. (1988) Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science 239, 290–292. [DOI] [PubMed] [Google Scholar]
- 113.Bartholomäus I., Kawakami N., Odoardi F., Schläger C., Miljkovic D., Ellwart J. W., Klinkert W. E., Flügel-Koch C., Issekutz T. B., Wekerle H., Flügel A. (2009) Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462, 94–98. [DOI] [PubMed] [Google Scholar]
- 114.Lodygin D., Odoardi F., Schläger C., Körner H., Kitz A., Nosov M., van den Brandt J., Reichardt H. M., Haberl M., Flügel A. (2013) A combination of fluorescent NFAT and H2B sensors uncovers dynamics of T cell activation in real time during CNS autoimmunity. Nat. Med. 19, 784–790. [DOI] [PubMed] [Google Scholar]
- 115.Yamasaki R., Lu H., Butovsky O., Ohno N., Rietsch A. M., Cialic R., Wu P. M., Doykan C. E., Lin J., Cotleur A. C., Kidd G., Zorlu M. M., Sun N., Hu W., Liu L., Lee J. C., Taylor S. E., Uehlein L., Dixon D., Gu J., Floruta C. M., Zhu M., Charo I. F., Weiner H. L., Ransohoff R. M. (2014) Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 211, 1533–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin E. Y., Nguyen A. V., Russell R. G., Pollard J. W. (2001) Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J. Exp. Med. 193, 727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wyckoff J., Wang W., Lin E. Y., Wang Y., Pixley F., Stanley E. R., Graf T., Pollard J. W., Segall J., Condeelis J. (2004) A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 64, 7022–7029. [DOI] [PubMed] [Google Scholar]
- 118.Wyckoff J. B., Wang Y., Lin E. Y., Li J. F., Goswami S., Stanley E. R., Segall J. E., Pollard J. W., Condeelis J. (2007) Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 67, 2649–2656. [DOI] [PubMed] [Google Scholar]
- 119.Qian B. Z., Pollard J. W. (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qian B. Z., Li J., Zhang H., Kitamura T., Zhang J., Campion L. R., Kaiser E. A., Snyder L. A., Pollard J. W. (2011) CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ruffell B., Affara N. I., Coussens L. M. (2012) Differential macrophage programming in the tumor microenvironment. Trends Immunol. 33, 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dovas A., Patsialou A., Harney A. S., Condeelis J., Cox D. (2013) Imaging interactions between macrophages and tumour cells that are involved in metastasis in vivo and in vitro. J. Microsc. 251, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Beerling E., Ritsma L., Vrisekoop N., Derksen P. W., van Rheenen J. (2011) Intravital microscopy: new insights into metastasis of tumors. J. Cell Sci. 124, 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roussos E. T., Balsamo M., Alford S. K., Wyckoff J. B., Gligorijevic B., Wang Y., Pozzuto M., Stobezki R., Goswami S., Segall J. E., Lauffenburger D. A., Bresnick A. R., Gertler F. B., Condeelis J. S. (2011) Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. J. Cell Sci. 124, 2120–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roh-Johnson M., Bravo-Cordero J. J., Patsialou A., Sharma V. P., Guo P., Liu H., Hodgson L., Condeelis J. (2014) Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene 33, 4203–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nayak D., Johnson K. R., Heydari S., Roth T. L., Zinselmeyer B. H., McGavern D. B. (2013) Type I interferon programs innate myeloid dynamics and gene expression in the virally infected nervous system. PLoS Pathog. 9, e1003395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Choi M., Kwok S. J., Yun S. H. (2015) In vivo fluorescence microscopy: lessons from observing cell behavior in their native environment. Physiology (Bethesda) 30, 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Megason S. G., Fraser S. E. (2007) Imaging in systems biology. Cell 130, 784–795. [DOI] [PubMed] [Google Scholar]
- 129.Hamel E. J., Grewe B. F., Parker J. G., Schnitzer M. J. (2015) Cellular level brain imaging in behaving mammals: an engineering approach. Neuron 86, 140–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.