Abstract
Background
Because chronic lymphocytic leukemia (CLL) typically follows an indolent course, many patients do not need to initiate therapy until they reach a relatively advanced age, when frailty and reduced organ function can make some of the standard treatments difficult to tolerate and less effective. However, recent advances in the understanding of CLL biology and the approval of agents in novel treatment classes have offered significant advances in the management of the disease.
Methods
The author reviewed current treatment goals in CLL management, including issues surrounding complete remission (CR) and minimal residual disease (MRD); the findings of trials of treatments from novel drug classes, primarily kinase inhibitors and monoclonal antibodies; and current strategies for use of standard and novel therapies for treatment of individuals diagnosed with CLL, particularly elderly patients.
Results
Several agents and regimens featuring improved clinical outcomes and tolerability are now available or in advanced development for the management of CLL patients, including the elderly and those with high-risk disease. These include ibrutinib, idelalisib plus rituximab, and obinutuzumab plus chlorambucil.
Conclusion
The availability of Bruton’s tyrosine kinase inhibitors and phosphatidylinositol 3-kinase inhibitors and other novel therapies will allow elderly CLL patients to receive more efficacious treatment with greater tolerability than available with traditional approaches for management of the disease.
Introduction
Chronic lymphocytic leukemia (CLL) is the most prevalent adult leukemia in the Western world, accounting for approximately 30% of all leukemias diagnosed in the United States. Approximately 14,600 new cases of CLL are expected to be diagnosed in the United States in 2015.1 CLL primarily affects the elderly, with the majority of patients being > 65 years of age at diagnosis.2 Following diagnosis, most patients are monitored through a “watch and wait” approach, and therapy typically is not initiated until symptoms develop. Manifestations of CLL include fevers, night sweats, weight loss, symptomatic lymphadenopathy, or bone marrow failure (as evidenced by worsening anemia or thrombocytopenia).3 By the time most patients require therapy, the majority have multiple chronic comorbidities, including hypertension, arrhythmias, renal insufficiency, or other conditions that limit their quality of life and performance status.4,5 Therefore, patients typically receive their first therapy at an age when they may be too frail to tolerate a regimen that may be associated with severe toxicities.
Over the last decade, the understanding of CLL biology has advanced considerably with the discovery of chromosomal abnormalities and genetic mutations that contribute to the heterogeneity of the disorder and help predict its clinical course.6 Similarly, the discovery of the role of the microenvironment and of the signaling factors that play a key role in CLL pathogenesis has advanced clinicians’ understanding of the condition and has led to the development of agents that specifically target dysregulated pathways.7,8 With the approval of several new targeted agents having unprecedented clinical activity (particularly in patients with high-risk disease, poor prognostic markers, and inability to tolerate cytotoxic chemotherapy regimens), a transformation is occurring in the treatment of patients with a CLL diagnosis.
Because of the aging of the population and increased life expectancy of the elderly, CLL will likely become a progressively more common cause of morbidity and mortality in older individuals. The goal of this review is to describe novel treatment approaches by highlighting agents recently approved by the US Food and Drug Administration (FDA) that will impact the management of CLL, particularly in the frail and the elderly.
Principles of Chronic Lymphocytic Leukemia Treatment Prognostic Factors
The clinical course of CLL is heterogeneous, hence the need for staging and prognostic assessment to determine the anticipated disease course. The prognosis of CLL is affected by disease stage, the patient’s cytogenetic and molecular profile, and the patient’s functional ability to tolerate therapy.9 There is no evidence that initiation of therapy for asymptomatic early-stage disease (Rai 0–2 or Binet A) improves survival. Outside of clinical trials, treatment of early disease is recommended only if a patient develops B symptoms (fever, night sweats, unintentional weight loss) or disease progression (eg, worsening lymphadenopathy or bone marrow failure).
Unfavorable genomic and molecular features include the presence of unmutated immunoglobulin heavy chain variable (IGHV) gene, CD38 overexpression, zeta-chain-associated protein kinase (ZAP)-70, and specific chromosomal aberrations, including 11q deletion, 17p deletion, and the presence of a TP53 mutation. A patient’s molecular profile affects treatment decisions: for patients with evidence of a 17p deletion. and/or a TP53 mutation, the treatment options are limited. The only FDA-approved agent to treat a 17p deletion CLL patient, regardless of previous therapy, is the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib,10 although other agents have shown clinical activity in this patient population, including the monoclonal antibody alemtuzumab and the phosphatidylinositol 3-kinase delta (PI3Kδ) inhibitor idelalisib.11–13
Treatment Approaches
Although early intervention is considered crucial in most malignant diseases, this is not the case in CLL. The lack of evidence that CLL can be cured with currently available modalities has resulted in a “watch and wait” approach for most patients. Except for allogeneic bone marrow transplant,14 which is not an option in the majority of individuals aged 70 years and older, current treatment approaches are not curative. The treatment of asymptomatic early-stage disease is not indicated even in the presence of high-risk disease (such as a 17p deletion or TP53 mutation). The addition of immunotherapy to combination regimens of cytotoxic chemotherapy has demonstrated superior response and survival.15 Indeed, because of treatment advances over the past few decades, CLL patients’ median survival from the time of diagnosis has increased from 96 months in 1980 to > 120 months in 2002.16
Although several treatments are available for CLL, the duration of response and of progression-free survival (PFS) decreases and the risk of complications increases with every successive line of treatment (Fig). Therefore, practitioners should employ their clinical judgment in making treatment decisions, with the goal of achieving the most durable remission possible with the initial therapy, especially in older individuals, who are more susceptible to treatment complications and may not tolerate a second treatment regimen.
Fig.
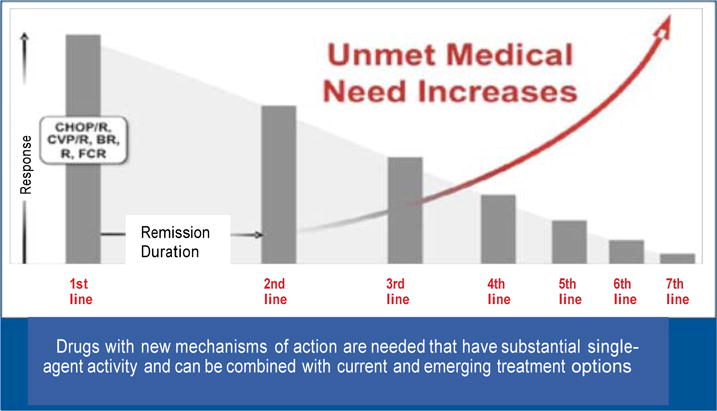
Regimens for chronic lymphocytic leukemia can have substantial toxicity and generally become less effective with recurrent treatment.
A remaining area of treatment controversy is whether minimal residual disease (MRD) should be pursued in addition to a clinical complete remission (CR). MRD is defined as < 0.1% of leukemic lymphocytes in the bone marrow detected by oligonucleotide polymerase chain reaction (PCR) and four-color flow cytometry. The absence of MRD after fludarabine- based chemoimmunotherapy is associated with a more prolonged PFS and overall survival (OS), although outcomes in the setting of novel targeted agents have not yet been established. The duration of response while using a novel targeted agent may not necessarily correlate with the depth of response, and a remission may not be necessary to obtain durable clinical benefit for as long as continuous therapy is offered.
Whereas in younger or fit patients the aim may be to achieve a CR and MRD negativity with the use of chemoimmunotherapy, this approach traditionally has been poorly tolerated in patients with multiple comorbidities. For elderly individuals, early detection of absence of MRD may prompt early chemoimmunotherapy cessation, thereby substantially reducing the risk of treatment-related toxicity.17 Alternatively, frail patients may benefit primarily from a low-intensity approach in which the therapeutic endpoint is not CR or MRD, but rather duration of remission, tolerability, and quality of life. There is a clear need for greater representation of elderly and frail patients in randomized CLL clinical trials to assess the therapeutic goals of CR and MRD negativity in this patient population, particularly in the era of kinase inhibitor therapy. MRD negativity should be regarded as a treatment goal only in the setting of a clinical trial.
Medications
Table 1 contains a list of the most common medications utilized in the treatment of CLL. Outlined here are targeted and immune-directed therapies that are impacting the way clinicians treat CLL.
Table 1.
Medications Approved in the United States for the Treatment of Chronic Lymphocytic Leukemia
| Drug Class | ||||
|---|---|---|---|---|
| Alkylating Agents | Purine Analogues | Monoclonal Antibodies | BTK Inhibitor | PI3Kδ Inhibitor |
| Chlorambucil | Fludarabine | Rituximab* (in combination with fludarabine and cyclophosphamide) | Ibrutinib | Idelalisib (in combination with rituximab) |
| Cyclophosphamide | Pentostatin | Ofatumumab (as monotherapy or in combination with chlorambucil) | ||
| Bendamustine | Obinutuzumab (in combination with chlorambucil) Alemtuzumab |
|||
BTK = Bruton’s tyrosine kinase, PI3Kδ = phosphatidylinositol-3-kinase delta.
Also used in combination with bendamustine (BR regimen), but not FDA-approved for this combination
The current CLL treatment paradigm is evolving based on the understanding of the disease’s pathophysiology. The B-cell receptor (BCR) regulates fundamental proliferation and survival mechanisms for malignant B-cells. These functions are mediated by signals that are transmitted intracellularly downstream through several kinases, including Lyn kinase, spleen tyrosine kinase (SYK), PI3K, BTK, and others. Targeting these kinases, in particular the BTK and the PI3K signaling pathways, has shown remarkable clinical activity in patients with CLL and with other B-cell malignancies.7,18
Ibrutinib
BTK is a cytoplasmic tyrosine kinase involved in signaling of the BCR and chemokine receptors. Ibrutinib is the first-in-class oral agent targeting the BTK pathway by forming a covalent bond with its active site, cysteine-481. It achieves target inhibition with once-daily oral dosing. In vitro and in vivo models demonstrate that ibrutinib inhibits survival, proliferation, and migration of CLL cells.18
Ibrutinib inhibits secretion of CCL3 and CCL4 by CLL cells, and at least part of this process occurs in a BCR-dependent manner.19 Use of ibrutinib (or any agent targeting the B-cell receptor pathway) results in rapid lymphocytosis accompanied by a marked reduction in lymphadenopathy, a “redistribution” phenomenon that is reversible upon temporary discontinuation of the targeted agent.18 In a phase 1b/2 clinical trial, treatment with ibrutinib monotherapy in patients with relapsed or refractory CLL resulted in an overall response rate (ORR) of 71% and durable remissions (estimated PFS at 26 months: 75%) for all patient groups, including elderly patients and those with high-risk disease.20 In the phase 3 RESONATE trial for patients with relapsed or refractory CLL, ibrutinib was evaluated against ofatumumab. Ibrutinib demonstrated improved PFS and OS vs ofatumumab, with outcomes independent of 17p deletion status.21
Minimal toxicities reported with ibrutinib included diarrhea, grades 1 and 2 pyrexia and infections, and grades 1 and 2 bleeding events.
Idelalisib
Idelalisib is a first-in-class inhibitor of PI3K, which plays a pivotal role in signal transduction involved in the growth, proliferation, differentiation, and survival of B-cells. A randomized, double-blind, placebo-controlled phase 3 trial of rituximab with or without idelalisib in CLL patients was discontinued early on the recommendation of an independent data and safety monitoring board after the combination regimen demonstrated clear superiority vs the monotherapy arm. The reported ORR was 81% in patients receiving idelalisib plus rituximab vs 13% in patients receiving rituximab plus placebo.22 The most common adverse events associated with idelalisib included pyrexia, fatigue, and nausea. It is important to note that, although the drug was initially well tolerated, a severe noninfectious secretory diarrhea (grade 3 or 4) and/or colitis was reported as a late toxicity in 14% of study participants.23
Both ibrutinib and idelalisib have shown clinical activity in deletion 17p and/or in TP53-mutated disease.10,13 Because of their excellent tolerability and effectiveness, both ibrutinib and idelalisib appear particularly suitable for the treatment of elderly individuals. Ongoing trials are evaluating these drugs’ usefulness both alone and in various combinations as frontline CLL treatment in frail and elderly patients.24,25
Obinutuzumab
Rituximab, ofatumumab, and obinutuzumab are monoclonal antibodies directed at the CD20 antigen and are used mainly in combination with other chemotherapeutic agents. Except for the possibility of severe or life-threatening infusion reactions and viral hepatitis reactivation, these agents are generally well tolerated. The most promising of these monoclonal antibodies that target CD20 is obinutuzumab. Obinutuzumab differs from previous anti- CD20 monoclonal antibodies with respect to its glyco-engineered crystallizable fragment (Fc) region and its type 2 CD20-binding mode. Glyco-engineering increases the binding affinity of the Fc portion of obinutuzumab to the Fcγ receptor III on innate immune effector cells (such as neutrophils, natural killer cells, and macrophages); in turn, this results in improved antibody-dependent cell-mediated cytotoxicity and antibody-dependent cellular phagocytosis.26 Obinutuzumab is indicated in combination with chlorambucil for use in treatment-naive CLL patients, based on the findings of a large phase 3 trial in which treatment- naive patients were randomized to receive either chlorambucil or chlorambucil plus rituximab or chlorambucil plus obinutuzumab.27 In elderly (median age 73 years) patients with multiple debilitating comorbidities, the combination of obinutuzumab with chlorambucil was associated with a significant and clinically meaningful prolongation of PFS, increased CR, and an increased rate of MRD negativity, compared with the results obtained with the rituximab plus chlorambucil combination. Moreover, treatment with obinutuzumab plus chlorambucil resulted in a significant OS benefit compared with chlorambucil monotherapy, suggesting that the induction of deeper remissions (ie, MRD negativity) could translate into a survival advantage even in frail patients.26 Combination studies of obinutuzumab with the novel inhibitors of B-cell receptors are planned.
Alemtuzumab
Alemtuzumab is a monoclonal antibody directed against the CD52 antigen and is approved for use as monotherapy in CLL. Data from clinical trials suggest that the drug has good clinical activity in patients with minimal nodal disease and in those with 17p or 11q deletions. Until the introduction of ibrutinib, alemtuzumab was the most commonly used agent in patients with 17p deletion or TP53-mutated disease.11,12 Alemtuzumab may still represent an option for patients whose CLL has failed to respond to other treatments. Alemtuzumab can have substantial toxicity, including fevers, pancytopenia, and severe viral or fungal infections, particularly reactivation of cytomegalo-virus. Concerns about its infectious complications contributed to its limited use in CLL patients. The drug was withdrawn from the US and European markets, but it is still available through manufacturer-sponsored patient access programs.
Lenalidomide
Although not FDA-approved, lenalidomide is another targeted agent that has shown promising clinical activity in CLL patients.28,29 Unfortunately, the frontline study evaluating lenalidomide vs chlorambucil in CLL patients older than 65 years (ORIGIN trial, NCT00910910) had to be halted due to safety concerns. The patients randomized to lenalidomide had a 92% increased risk for death compared with the patients receiving chlorambucil. Adverse effects of lenalidomide use include neuropathy, thrombocytopenia, and thrombotic events. At this time, the role of lenalidomide in the management of CLL is not well defined because of the availability of multiple other treatment options.
Treatment Strategies
Current recommended initial treatment of CLL includes a combination of cytotoxic chemotherapy plus a CD20 monoclonal antibody in young patients or fit elderly patients. The most common regimens are (1) fludarabine, cyclophosphamide, and rituximab (FCR) and (2) bendamustine plus rituximab (BR). Recently, a large European phase 3 trial demonstrated the PFS superiority of FCR vs BR in patients with unmutated IGHV.30
In addition, FCR was associated with increased rates of complete remission and MRD negativity; in the setting of chemoimmunotherapy, this finding correlates with longer remission duration and possibly survival. The investigators recommended that, in fit patients without 17p deletion or TP53 mutation, FCR should be the preferred frontline treatment. It is important to note that the median age of the patients in both arms was 61 years, which is a decade younger than the median age at CLL diagnosis and the patients that participated in the trials were fit with few comorbidities. The difference in PFS was not statistically significant between the arms in patients ≥ 65 years old, suggesting that fit elderly patients may benefit from treatment with BR rather than FCR. FCR use was associated with increased risk of neutropenia, febrile neutropenia, and other complications that may be particularly severe and potentially lethal in older individuals (treatment- related mortality: 3.9% [FCR] vs 2.1% [BR]).
Despite the availability of combination regimens like FCR and BR, until very recently the frontline management of older and unfit CLL patients had been limited to the use of chlorambucil or rituximab monotherapy. The use of chemoimmunotherapy regimens was not an option until the recent report by Goede et al.27 The pivotal phase 3 study demonstrated the superiority of the combination of chlorambucil plus obinutuzumab against chlorambucil alone in terms of PFS, CR, and OS. This registrational trial did not include age as an eligibility criterion; rather, it used the presence of a high cumulative illness rating scale (CIRS) score, which describes functional comorbidity and/or the presence of impaired renal function (patients of any age with a glomerular filtration rate of 30 mL to 69 mL/min). This study represents a major advance in the treatment of elderly patients with CLL who lack a 17p deletion and establishes a standard of care for the elderly and for frail patients with multiple medical conditions.
In another important phase 3 study evaluating the use of chemoimmunotherapy in elderly patients (median age 69 years) with multiple comorbidities, Hillmen et al31 compared chlorambucil in combination with the anti-CD20 monoclonal antibody ofatumumab vs chlorambucil alone in patients for whom fludarabine therapy was inappropriate based on age or comorbidities. Patients receiving the combination treatment experienced a substantial improvement in PFS.
The only agent currently approved for use in patients with a 17p deletion is ibrutinib. Although idelalisib (in combination with rituximab) has not been approved for this particular indication, clinical trials have shown clinical activity in patients with a 17p deletion,13 and this would be a reasonable treatment approach in patients unable to tolerate ibrutinib therapy. For eligible patients, evaluation for allogeneic bone marrow transplantation is recommended in any patient with high-risk disease. Clinicians should make a decision only after carefully deliberating the advantages and disadvantages of continued therapy vs stem cell transplant. Conditions potentially favoring transplant include younger age and availability of a donor for a high-risk-disease patient carrying the 17p deletion or 11q deletion.32 The longest follow-up data of ibrutinib-treated patients having a 17p deletion (with a median of four prior therapies) reported a median PFS of 28 months. It is important to recognize that PFS with ibrutinib varies by interphase cytogenetic abnormality, with 17p deletion patients having a 30-month estimated PFS rate of 48% (less than the 74% rate observed for 11q deletion patients and the 87% rate observed when neither of these genomic aberrations is present).33 More recent data suggest that complex karyotype may also be a risk factor.34,35 In fact, most patients with relapsed or refractory CLL who discontinued ibrutinib early were difficult to treat and had poor outcomes with relatively short survival.33,34 Published data regarding the sequencing of these new agents are relatively limited and anecdotal. Until greater clarity develops regarding which newer agents can be recommended as salvage therapy in patients whose disease progressed following ibrutinib therapy, transplantation will remain an important consideration for fit, transplant-eligible patients with a deletion 17p (or other high-risk characteristic), as transplant offers a potentially curative therapy.
For previously treated patients, ibrutinib as a single agent proved significantly more effective than ofatumumab in the open-label phase 3 RESONATE study in CLL patients with measurable nodal disease who were not eligible for treatment with purine analog-based therapy and who had received ≥ 1 prior therapies.21 The outcomes of the 391 patients (median age 67 years) with relapsed CLL who participated in this trial were recently updated. The ORR with ibrutinib was 90% vs 25% with ofatumumab; PFS was not reached at 15 months with ibrutinib and was reached at 8.1 months with ofatumumab.36 With longer follow-up, ibrutinib-treated patients maintained the improved OS. No significant difference in 12-month PFS was observed in ibrutinib-treated patients with or without 17p deletion or for those who developed lymphocytosis compared with those without lymphocytosis. These dramatic results, which did not appear to be influenced by patient age and were achieved with minimal toxicity, have established ibrutinib as the preferred second-line agent in CLL.
In another pivotal phase 3 study in patients ineligible for cytotoxic therapy, the combination of idelalisib plus rituximab proved superior to rituximab alone in relapsed CLL. Of the 220 patients enrolled, 78% were 65 years and older.22 The median PFS duration was 5.5 months for rituximab and > 15 months for idelalisib plus rituximab; OS was also greater with combination therapy (92% in the idelalisib plus rituximab group vs 80% in the rituximab plus placebo group at 12 months). The results were independent of patient age or 17p deletion.13 The combination of idelalisib plus rituximab was well tolerated; adverse events reported included diarrhea, grades 1 and 2 pyrexia and infections, and grades 1 and 2 transaminitis.
As described, a number of new therapeutic options are available in the frontline and second-line (or beyond) settings for CLL patients (Table 2). These novel agents are associated with prolonged survival in CLL patients, including those with high-risk disease who historically have had poor survival rates. These targeted therapies are particularly promising for patients 70 years and older, whose numbers will progressively increase and who already represent the largest cohort of CLL patients.
Table 2.
Frontline Regimens for Chronic Lymphocytic Leukemia
| Patient Population | Treatment Options |
|---|---|
| Patients < 70 years of age, or older patients without significant comorbidities and without del(17p) | Fludarabine + cyclophosphamide + rituximab (FCR) Bendamustine ± rituximab (BR) Fludarabine + rituximab (FR) Pentostatin + cyclophosphamide + rituximab (PCR) Obinutuzumab + chlorambucil Ofatumumab + chlorambucil |
| Patients ≥ 70 years of age or younger patients with comorbidities without del(17p) | Obinutuzumab + chlorambucil Ofatumumab + chlorambucil Bendamustine ± rituximab (BR) Cyclophosphamide ± rituximab ± corticosteroids Fludarabine ± rituximab (FR) Dose-reduced fludarabine + cyclophosphamide + rituximab (FCR) Chlorambucil ± rituximab |
| Patients with significant comorbidities who are unable to tolerate purine analogues | Obinutuzumab + chlorambucil Ofatumumab + chlorambucil Chlorambucil ± rituximab |
| Patients with del(17p) | Ibrutinib Idelalisib + rituximab Clinical trial Alemtuzumab |
Management of Older Individuals with Chronic Lymphocytic Leukemia
Based on the findings reviewed in this article, this author proposes that older CLL patients or patients with comorbidities should be considered for participation in a clinical trial if available or treated according to the therapy recommendations outlined in Tables 2 and 3. All individuals 70 years of age and older should undergo a comprehensive geriatric assessment before initiation of therapy to estimate cancer-independent mortality risk and tolerance of chemotherapy and to identify conditions that may interfere with the treatment. These may include the potential for drug-drug interactions due to polypharmacy, poor access to nutrition, inadequate social support, depression, memory disorders, and other coexisting illnesses or conditions that may necessitate interventions, as these may affect the therapeutic effect of the proposed treatment plan. It is very important to individualize the patient’s therapy based on the specific clinical presentation.
Table 3.
Salvage Therapy for Relapsed Chronic Lymphocytic Leukemia
| Patient Population | Treatment Options |
|---|---|
| Patients with short response to initial therapy who are < 70 years of age | Clinical trial Ibrutinib Idelalisib + rituximab Fludarabine + rituximab ± cyclophosphamide (if no prior FCR) Pentostatin + rituximab ± cyclophosphamide (if no prior FCR) Bendamustine ± rituximab Ofatumumab High-dose methylprednisolone + rituximab Lenalidomide ± rituximab Alemtuzumab ± rituximab OFAR (oxaliplatin, fludarabine, cytarabine, rituximab) R-CHOP (rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine, prednisone) |
| Patients with short response to initial therapy who are aged ≥ 70 years without del(17p) | Clinical trial Ibrutinib Idelalisib + rituximab Bendamustine ± rituximab Ofatumumab High-dose methylprednisolone + rituximab Alemtuzumab ± rituximab |
| Patients with del(17p) | Clinical trial Ibrutinib Idelalisib + rituximab High-dose methylprednisolone + rituximab Alemtuzumab ± rituximab |
In the near future, novel agents such as those discussed in this article may be shown to have comparable or even improved outcomes with greater tolerability compared with FCR. Two large cooperative group trials in the United States are currently evaluating whether the rational use of targeted agents such as ibrutinib in the frontline setting is superior in terms of duration of remission, survival, quality of life, and tolerability in fit patients up to the age of 70 years (FCR vs ibrutinib plus rituximab [NCT02048813]) and in older patients with comorbidities (BR vs ibrutinib vs ibrutinib plus rituximab [NCT01886872]). Several other on- going clinical trials are specifically accruing patients older than 65 years with the goal of improving current outcomes (more information can be obtained at www.clinicaltrials.gov).
Most importantly, in addition to the recently ap proved agents, an array of promising new therapeutic interventions are undergoing evaluation in clinical trials including second-generation BTK or PI3Kδ inhibitors, anti-apoptotic inhibitors, targeted tumor-specific cellular therapies, and others. The rational design of targeted agents appears to address elderly patients’ unmet needs with respect to improved efficacy and tolerability. With the introduction of these agents into the CLL armamentarium, clinicians may be able to exploit combination strategies that enable patients to achieve longer remissions, potentially altering the natural course of the disease. In fact, it may be possible to envision a future in which patients can receive chemotherapy-free treatment that is potentially curative.
Acknowledgments
Advances in knowledge of pathogenesis and availability of novel therapies can improve the management of chronic lymphocytic leukemia, particularly in the elderly. Dr Barrientos’ work is supported in part by NIH/NCATS Grant #UL-1TR00457
Dr Barrientos is on the Medical Advisory Board of Gilead, Celgene, Pharmacyclics, Janssen, and Genentech. She also receives grants/research support from AbbVie, Gilead, and Pharmacyclics. Dr Barrientos’ work is supported in part by NIH/NCATS Grant #UL- 1TR00457, the 2015 American Society of Hematology Harold Amos Medical Faculty Development Program (ASH-AMFDP) Fellowship, and the philanthropic contributions from the Karches Foundation, Marks Foundation, Jerome Levy Foundation, Leon Levy Foundation, and the Frank and Mildred Feinberg Foundation.
Footnotes
The author has disclosed that this article discusses unlabeled/unapproved uses of the drug l
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Gribben JG. Chronic lymphocytic leukemia: planning for an aging population. Expert Rev Anticancer Ther. 2010;10:1389–1394. doi: 10.1586/era.10.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montserrat E, Moreno C. Chronic lymphocytic leukaemia: a short overview. Ann Oncol. 2008;19(suppl 7):vii, 320–325. doi: 10.1093/annonc/mdn460. [DOI] [PubMed] [Google Scholar]
- 5.Baumann T, Delgado J, Santacruz R, et al. Chronic lymphocytic leukemia in the elderly: clinico-biological features, outcomes, and proposal of a prognostic model. Haematologica. 2014;99:1599–1604. doi: 10.3324/haematol.2014.107326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiorazzi N, Rai KR, Ferrarini M. Mechanism of disease: chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 7.Wiestner A. Emerging role of kinase-targeted strategies in chronic lymphocytic leukemia. Blood. 2012;120:4684–4691. doi: 10.1182/blood-2012-05-423194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dal Bo M, Bomben R, Zucchetto A, et al. Microenvironmental interactions in chronic lymphocytic leukemia: hints for pathogenesis and identification of targets for rational therapy. Curr Pharm Des. 2012;18:3323–3334. doi: 10.2174/138161212801227078. [DOI] [PubMed] [Google Scholar]
- 9.Hallek M. Chronic lymphocytic leukemia: 2015 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2015;90:446–460. doi: 10.1002/ajh.23979. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien S, Jones JA, Coutre S, et al. Efficacy and safety of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic leukemia with 17p deletion: results from the phase II RESONATE™-17 Trial. Program and abstracts of the 56th ASH Annual Meeting and Exposition; December 6–9, 2014; San Francisco, California. Abstract 327. [Google Scholar]
- 11.Stilgenbauer S, Döhner H. Campath-1H-induced complete remission of chronic lymphocytic leukemia despite p53 gene mutation and resistance to chemotherapy. N Engl J Med. 2002;347:452–453. doi: 10.1056/NEJM200208083470619. [DOI] [PubMed] [Google Scholar]
- 12.Lozanski G, Heerema NA, Flinn IW, et al. Alemtuzumab is an effective therapy for chronic lymphocytic leukemia with p53 mutations and deletions. Blood. 2004;103:3278–3281. doi: 10.1182/blood-2003-10-3729. [DOI] [PubMed] [Google Scholar]
- 13.Sharman JP, Coutre SE, Furman RR, et al. Second interim analysis of a phase 3 study of idelalisib (ZYDELIG®) plus rituximab (R) for relapsed chronic lymphocytic leukemia (CLL): efficacy analysis in patient subpopulations with del(17p) and other adverse prognostic factors. Abstract; Program and abstracts of the 56th ASH Annual Meeting and Exposition; December 6–9, 2014; San Francisco, California. [Google Scholar]
- 14.Cassaday RD, Storer BE, Sorror ML, et al. Long-term outcome of patients with persistent indolent B cell malignancies undergoing non-myeloablative bone marrow transplantation. Biol Blood Marrow Transpl. 2015;21:281–287. doi: 10.1016/j.bbmt.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallek M, Fischer K, Fingerele-Rowson C, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukemia: a randomized, open-label phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 16.Thompson PA, Shpall EJ, Keating MJ. Shifting paradigms in the treatment of chronic lymphocytic leukemia. Future Oncol. 2015;11:641–657. doi: 10.2217/fon.14.288. [DOI] [PubMed] [Google Scholar]
- 17.Strati P, Keating MJ, O’Brien S, et al. Eradication of bone marrow minimal residual disease may prompt early treatment discontinuation in CLL. Blood. 2014;123:3727–3732. doi: 10.1182/blood-2013-11-538116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrientos J, Rai K. Ibrutinib: a novel Bruton’s tyrosine kinase inhibitor with outstanding responses in patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2013;54:1817–1820. doi: 10.3109/10428194.2013.796049. [DOI] [PubMed] [Google Scholar]
- 19.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coutré SE, Barrientos JC, Brown JR, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma. 2015 May;19:1–8. doi: 10.3109/10428194.2015.1022770. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Brien S, Lamanna N, Kipps TJ, et al. Update of a phase 2 study of idelalisib in combination with rituximab in treatment-naïve patients ≥65 years with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). Program and abstracts of the 56th ASH Annual Meeting and Exposition; December 6–9, 2014; San Francisco, California. Abstract 1994. [Google Scholar]
- 25.O’Brien S, Furman RR, Coutré SE, et al. Ibrutinib as initial therapy in elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open label, multicentre, phase 1b/2 study. Lancet Oncol. 2014;15:48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rai KR, Barrientos JC. Movement toward optimization of CLL therapy. N Engl J Med. 2014;370:1160–1162. doi: 10.1056/NEJMe1400599. [DOI] [PubMed] [Google Scholar]
- 27.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 28.Strati P, Keating MJ, Wierda WG, et al. Lenalidomide induces long- lasting responses in elderly patients with chronic lymphocytic leukemia. Blood. 2013;122:734–737. doi: 10.1182/blood-2013-04-495341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.James DF, Werner L, Brown JR, et al. Lenalidomide and rituximab for the initial treatment of patients with chronic lymphocytic leukemia: a multicenter clinical-translational study from the chronic lymphocytic leukemia research consortium. J Clin Oncol. 2014;32:2067–2073. doi: 10.1200/JCO.2013.51.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eichhorst B, Fink AM, Busch R, et al. Frontline chemo-immunotherapy with fludarabine (F), cyclophosphamide (C) and rituximab (R) (FCR) shows superior efficacy in comparison of bendamustine and rituximab (BR) in previously untreated and physically fit patients (pts) with advanced chronic lymphocytic leukemia (CLL): Final analysis of the German CLL study group (GCLLSG) (CLL10 study). Program and abstracts of the 56th ASH Annual Meeting and Exposition; December 6–9, 2014; San Francisco, California. Abstract 19. [Google Scholar]
- 31.Hillmen P, Robak T, Janssens A, et al. Chlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukaemia (CLL) (COMPLEMENT 1): a randomized multicentre open-label phase 3 study. Lancet. 2015;385:1873–1883. doi: 10.1016/S0140-6736(15)60027-7. [DOI] [PubMed] [Google Scholar]
- 32.Dreger P, Schetelig J, Andersen N, et al. Managing high-risk CLL during transition to a new treatment era: stem cell transplantation or novel agents? Blood. 2014;124:3841–3849. doi: 10.1182/blood-2014-07-586826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrd JC, Furman RR, Coutre SE. Three-year follow-up of treatment naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497–2506. doi: 10.1182/blood-2014-10-606038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125:2062–2067. doi: 10.1182/blood-2014-09-603670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1:80–87. doi: 10.1001/jamaoncol.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown JR, Hillmen P, O’Brien S, et al. Updated efficacy including genetic and clinical subgroup analysis and overall safety in the phase 3 RESONATE™ trial of ibrutinib versus ofatumumab in previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma. Program and abstracts of the 56th ASH Annual Meeting and Exposition; December 6–9, 2014; San Francisco, California. Abstract 3331. [Google Scholar]


